Abstract
Increasing antimicrobial resistance has continued to plague successful anti-Helicobacter pylori eradication therapy. With rare exception, clarithromycin-containing triple therapy now provides unacceptably low treatment success. Here we discuss the factors that influence treatment outcome, how to predict outcome with new regimens, the 4-drug regimens that are currently effective in the West and their limitations, considerations about the approach to treatment failures and finally, based on the experience in the West, provide recommendations for choosing an empiric regimen in Japan. The dictum “use what works best locally” is probably the best advice for clinicians with a corollary that this dictum overrides results published by consensus conferences and advice from experts from elsewhere.
Introduction
H. pylori is a treatable infectious disease and, as with other common bacterial diseases, the outcome of antimicrobial therapy should be predictable provided one has reliable data regarding the effectiveness with the chosen antimicrobial regimen in both susceptible and resistant infections as well as the proportion of the subject population that has resistant infections. With antimicrobial therapies there are a limited number of factors that must be taken into consideration when choosing a regimen and predicting outcome of the therapy (Table 1). As a general rule, one should only prescribe regimens where the expected outcome is success in at least 90% for patients who complete the regimen (i.e., per protocol results). One should strive to use regimes that reliable provide 95 % or greater success (i.e., grade A results)1–3).
Table 1.
Factors that may influence the outcome of antimicrobial therapy
| Regimen-specific factor | Drugs used and their manufacturer’s |
| Drug dose, frequency of administration, formulation, duration of therapy | |
| Relation of drug administration in relation to meals | |
| Type and dose of adjuvant therapies (e.g., PPI, mucolytics, etc) | |
| Study-specific factors | Method to detect infection and confirm eradication |
| Prevalence of antimicrobial resistance | |
| Odds of reinfection | |
| Patient specific factors | Compliance with instructions |
| Side effects of medications | |
| Genetic polymorphisms that affect drug metabolism |
Adapted from17
Predicting treatment outcome
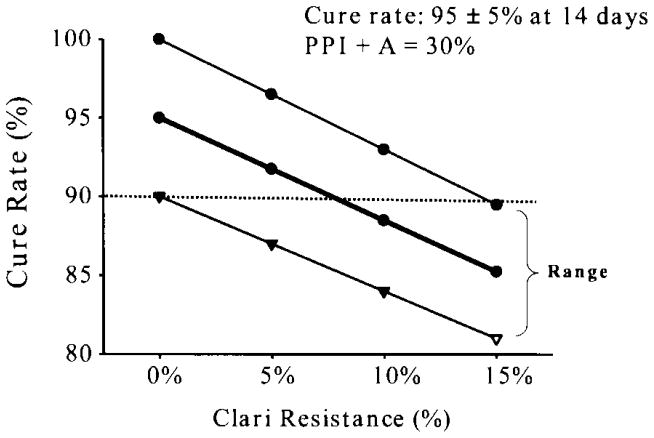
The outcome of a treatment will be the sum of the result in the subgroup with susceptible infections plus the outcome in those with resistant infection 2). For example, if a 14 day three drug (triple) therapy consisting of twice daily PPI, amoxicillin, and clarithromycin provided 95 ± 5% success when given for 14 days to individuals with susceptible infections and 30 ± 5% success for those with clarithromycin resistant infections, the outcome of therapy would be predicted using the formula (the proportion with susceptible infections × 0.95) plus (the proportion with resistant infections × 0.3). At a clarithromycin resistance prevalence of between 5 and 10% the result in the best scenario (per protocol) would fall below 90% and should be avoided if better options are available (Figure 1)4).
Figure 1.
The range of outcomes in relation to the proportion of the population with clarithromycin resistant infections. The regimen depicted is a cure rate of 95% ± 5% for the three drugs, and 30% for the PPI plus amoxicillin dual therapy given for 14 days. From reference 4) with permission.
Grading the outcome of anti-H. pylori infections
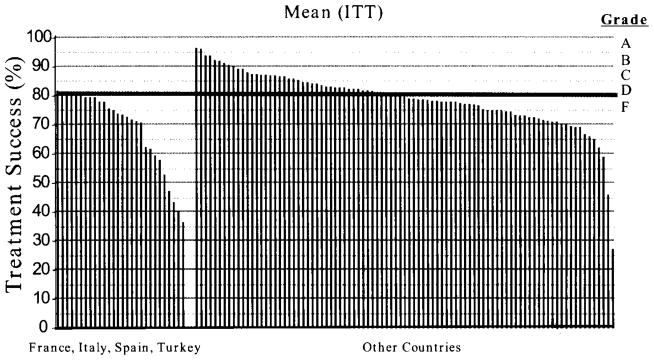
The outcome of a treatment regimen can also be scored as either acceptable or unacceptable (i.e., good or bad result). Here, we define good as 90% or greater per protocol success and bad as 85% or less. Values between 85% and 90% are considered acceptable but undesirable. In the West, with very few exceptions, clarithromycin resistance has increased to the point that traditional clarithromycin containing triple therapy is no longer an acceptable alternative (Figure 2)5) and 4 drug regimen are now preferred (see below)1,5). It is important to note that as a common treatable infectious disease, not only are the results of therapy predictable but that predictability also extends to other areas or regions that have similar patterns of resistance where the regimen will achieve identical or near identical results2).
Figure 2.
Percentage of intention-to-treat treatment success intervals for “Legacy Triple Therapy” in populations in southern Europe and other geographic areas.*
*Legacy triple therapy” contains a proton pump inhibitor (PPI), clarithromycin, and amoxicillin
†Southern European countries studied include France, Italy, Spain, and Turkey. From reference5), with permission.
Dealing with treatment failures
As a general rule, treatment failure should be followed by retreatment with antimicrobials not used in the first trial. While it is ideal to choose a second choice regimen based on susceptibility testing, that is often impractical and most must select a regimen based on using antimicrobials for which resistance is rare and has not been used before in that patient to replace the ones for which resistance was likely the cause of treatment failure (e.g., in Japan metronidazole for clarithromycin). However, the first choice regimen should always be the one expected to produce the highest expect yield locally. That choice should consider prior use of antimicrobial agents and this information should always be sought from the clinical history and patient record. Resistance is expected if the patient has previously used macrolides (e.g., clarithromycin, erythromycin, azithromycin), fluoroquinolones (ciprofloxacin, levofloxacin, etc) or metronidazole. Resistance is rare with tetracycline and amoxicillin and they can typically be reused. Metronidazole resistance is unusual in that it can be partially overcome especially in bismuth-containing 4 drug regimens by the use of higher doses (e.g., at least 1500 mg) and longer durations (at least 10 preferably 14 days).
Four drug combination therapies
Generally speaking there are 3 clarithromycin-containing regimens and one bismuth-containing regimen that are available (Table 2). All use the same doses and combinations of drugs (e.g., twice a day PPI, amoxicillin, clarithromycin and metronidazole or tinidazole). Sequential administers the PPI and amoxicillin as a dual therapy for 5 days followed by the triple combination of the PPI, clarithromycin and metronidazole for 5 additional days. Studies have not shown an improvement in treatment success when the duration was increased from 5 days plus 5 days to 7 days plus 7 days 6). In contrast, hybrid therapy consisting of dual PPI plus amoxicillin for 7 days followed by all four drugs (PPI, amoxicillin, clarithromycin, metronidazole) for 7 days gave a 99 % treatment success7). It is not yet known whether shortening the duration of hybrid therapy to 5 days plus 5 days would be equivalent and thus 14 days is recommended. The final clarithromycin-containing 4 drug combination is concomitant therapy in which all 4 drugs are given for 10 days instead of the more complicated sequential approach 8, 9). Concomitant therapy has been shown to be equivalent to sequential therapy in one head-to-head study and is less complex10). We prefer it over sequential unless the sequential approach is used as part of hybrid therapy. Success with sequential and concomitant therapies fall below 90 % when the prevalence of clarithromycin resistance increases above 20 % and/or metronidazole resistance above 40%2). It is not yet know how hybrid therapy would fare but theoretically is should do better.
Table 2.
Recommended regimens for anti-H. pylori therapy
| 1. Recommended regimes for empiric therapy in the West |
| Concomitant therapy: 4 drugs: Amoxicillin 1 g, clarithromycin 500 mg, tinidazole or metronidazole 500 mg, a PPI all given b.i.d. for 14 days (eg, PrevPac® or generics with an additional metronidazole or tinidazole 500 mg b.i.d. for 14 days). (generics are much cheaper) |
| Sequential therapy: Amoxicillin 1 g plus a PPI b.i.d. for 5 days, then add clarithromycin 500 mg and tinidazole or metronidazole 500 mg b.i.d, to complete 10 to 14days. |
| Sequential-concomitant hybrid therapy: Amoxicillin 1 g plus a PPI b.i.d. for 7 days, then amoxicillin 1 gm bid, plus clarithromycin 500 mg and tinidazole or metronidazole 500 mg b.i.d for 7 days to complete 14days. |
| Bismuth quadruple therapy: Bismuth subsalicylate 2 tabs q.i.d,, tetracycline HCl 500 mg q.i.d. (with meals and bedtime), metronidazole or tinidazole 500 mg, t.i.d. (with meals) and a PPI b.i.d. |
| 2. Recommended regime for empiric therapy in Japan |
| Metronidazole-triple therapy: A PPI, 750 mg amoxicillin, and 250 mg metronidazole all b.i.d. for 14 days 16) |
Bismuth quadruple therapy
The first truly successful therapy was a combination of bismuth, tetracycline and metronidazole. It was subsequently found that the addition of a PPI, increasing the duration to at least 10 days (14 days is preferred), and the metronidazole dose to at least 1,500 mg would partially overcome the decrease in effectiveness associated with metronidazole resistance 1, 11) (Table 2). It is unknown whether there is any clinical advantage of one bismuth over another (e.g., subcitrate vs. subsalicylate) in terms of treatment success. Twice a day (noon and evening meal) administration has proven effective in three trials when given for 10 or 14 days 12–14). Bismuth-containing regimens are underutilized and when tested against other therapies are often given at less than optimum doses and durations. As with any regimen one should pay attention to what are parameters for optimum success and ensure that they are utilized unless there is proof that less is equally effective in terms of Grade of result (i.e., an A therapy does not become a B therapy).
Treatment as a package
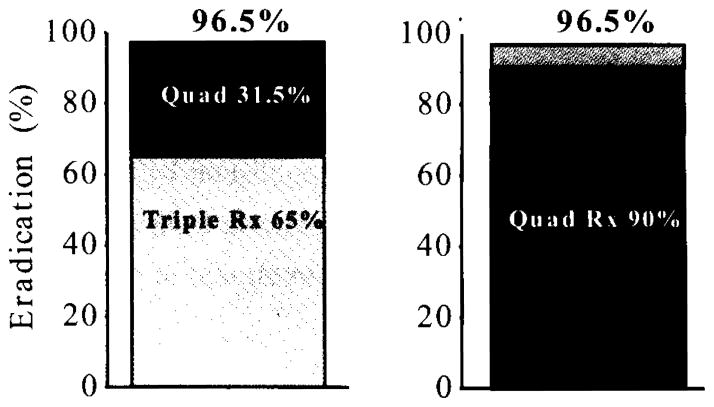
The Maastricht conferences have suggested that treatment be considered as a package of two different regimens such at if the first choice failed, one would then give the second choice. However, it is important to always start with the better of the two as this will reduce the proportion of patients who must be treated twice and thus reduces the risks associated with therapy including loss to follow-up. While it is possibly not intuitive, the final proportion cured is the same whether the first regimen has unacceptable or acceptable treatment success (Figure 3). However, the treatment success with use of the inferior regimen first is only 65% compared to 90% with the superior regimen such that the inferior then superior sequence results in 35 % of the patients being treated twice compared to only 10% in the superior then inferior sequence. It should be clear that it is both illogical and likely unethical the initiate therapy with the inferior regimen.
Figure 3.
Model comparison of outcome with a “package” of therapies such that treatment failure is followed by a different regimen in the West. We compare the sequence of therapy A (e.g., clarithromycin-containing triple therapy with 65% success) followed by treatment B (e.g., bismuth quadruple therapy with 90% success) and the opposite sequence (i.e., A+B, or B+A). While both sequences yield identical overall results (e.g., 96.5%), 35% of those who received triple therapy first required retreatment and its attendant risks compared to only 10% in the B+A sequence.
H. pylori infections in Japan
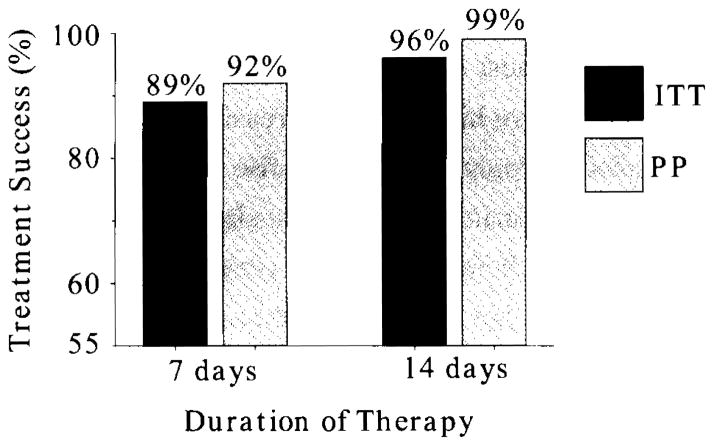
Japan is almost unique in that metronidazole resistance is still rare but is likely increasing as metronidazole is increasingly used 15). The rise in clarithromycin resistance in Japan has resulted in clarithromycin-containing triple therapy no longer providing acceptable results and in our opinion clarithromycin-containing triple therapy should be abandoned as an empiric choice for H. pylori therapy. However, pretreatment susceptibility testing allowing therapy targeted to only patients with susceptible infections remains an option1). A recent meta-analysis of metronidazole-containing triple therapy in Japan (Figure 4) showed unequivocally that 2 week therapy with a PPI, amoxicillin and metronidazole reliably provided excellent results even when given for treatment failures of clarithromycin-containing triple therapy 16). We propose that this 2 week triple therapy regimen should now be the treatment of choice as an empirically chosen anti-H. pylori eradication therapy (Table 2). However, because increasing use of metronidazole (e.g., for Clostridium difficile infections) will result in an increase in the prevalence of metronidazole resistance such that post treatment confirmatory testing is recommended as it provides an early warning system of the problem of increasing resistance.
Figure 4.
Meta-analysis of metronidazole-containing triple therapy in Japan. Adapted from reference16).
Conclusions
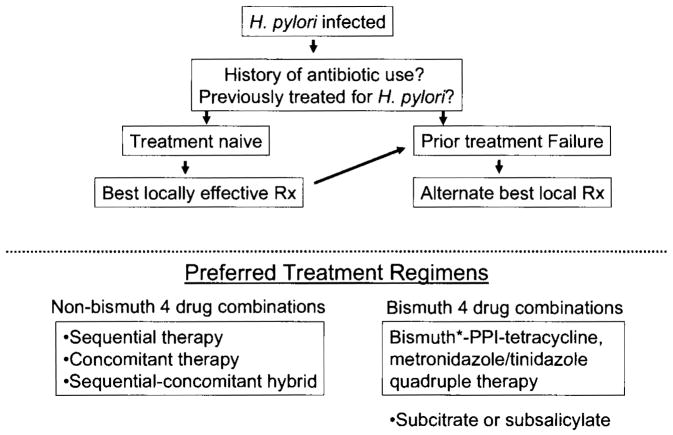
The dictum “use what works best locally” is probably the best advice for clinicians with a corollary that this dictum overrides results published by consensus conferences and advice from experts from elsewhere. A schema for the approach to treatment in the West is shown in Figure 5.
Figure 5.
Proposed schema for H. pylori therapy in the West.
Acknowledgments
Acknowledgments and potential conflicts
Dr. Graham is supported in part by the Office of Research and Development Medical Research Service Department of Veterans Affairs, Public Health Service grant DK56338. which funds the Texas Medical Center Digestive Diseases Center, DK067366 and CA116845. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the VA or NIH. Dr. Graham is a unpaid consultant for Novartis in relation to vaccine development for treatment or prevention of H. pylori infection. Dr. Graham is a also a paid consultant for Otsuka Pharmaceuticals regarding diagnostic testing until has received royalties on the Baylor College of Medicine patent covering materials related to 13C-urea breath test. Dr. Rimbara has nothing to declare.
References
- 1.Rimbara E, Fischbach LA, Graham DY. Optimal therapy for Helicobacter pylori infections. Nat Rev Gastroenterol Hepatol. 2011;8:79–88. doi: 10.1038/nrgastro.2010.210. [DOI] [PubMed] [Google Scholar]
- 2.Graham DY, Rimbara E. Understanding and appreciating sequential therapy for Helicobacter pylori eradication. J Clin Gastroenterol. 2011;45:309–313. doi: 10.1097/MCG.0b013e31820ac05e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graham DY, Lu H, Yamaoka Y. A report card to grade Helicobacter pylori therapy. Helicobacter. 2007;12:275–278. doi: 10.1111/j.1523-5378.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- 4.Graham DY, Dore MP. Helicobacter pylori therapy demystified. Helicobacter. 2011;16:343–345. doi: 10.1111/j.1523-5378.2011.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59:1143–1153. doi: 10.1136/gut.2009.192757. [DOI] [PubMed] [Google Scholar]
- 6.Hsu PI, Wu DC, Wu JY, Graham DY. Is there a benefit to extending the duration of Helicobacter pylori sequential therapy to 14 days? Helicobacter. 2011;16:146–152. doi: 10.1111/j.1523-5378.2011.00829.x. [DOI] [PubMed] [Google Scholar]
- 7.Hsu PI, Wu DC, Wu JY, Graham DY. Modified sequential Helicobacter pylori therapy: proton pump inhibitor and amoxicillin for 14 days with clarithromycin and metronidazole added as a quadruple (Hybrid) therapy for the final 7 days. Helicobacter. 2011;16:139–145. doi: 10.1111/j.1523-5378.2011.00828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Essa AS, Kramer JR, Graham DY, Treiber G. Metaanalysis: four-drug, three-antibiotic, non-bismuth-containing “concomitant therapy” versus triple therapy for Helicobacter pylori eradication. Helicobacter. 2009;14:109–118. doi: 10.1111/j.1523-5378.2009.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gisbert JP, Calvet X. Review article: non-bismuth quadruple (concomitant) therapy for eradication of Helicobacter pylori. Aliment Pharmacol Ther. 2011;34:604–617. doi: 10.1111/j.1365-2036.2011.04770.x. [DOI] [PubMed] [Google Scholar]
- 10.Wu DC, Hsu PI, Wu JY, Opekun AR, Kuo CH, Wu IC, Wang SS, Chen A, Hung WC, Graham DY. Sequential and concomitant therapy with 4 drugs are equally effective for eradication of H. pylori infection. Clin Gastroenterol Hepatol. 2010;8:36–41. doi: 10.1016/j.cgh.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fischbach L, Evans EL. Meta-analysis: the effect of antibiotic resistance status on the efficacy of triple and quadruple first-line therapies for Helicobacter pylori. Aliment Pharmacol Ther. 2007;26:343–357. doi: 10.1111/j.1365-2036.2007.03386.x. [DOI] [PubMed] [Google Scholar]
- 12.Dore MP, Farina V, Cuccu M, Mameli L, Massarelli G, Graham DY. Twice-a-day bismuth-containing quadruple therapy for Helicobacter pylori eradication: A randomized trial of 10 and 14 days. Helicobacter. 2011;16:295–300. doi: 10.1111/j.1523-5378.2011.00857.x. [DOI] [PubMed] [Google Scholar]
- 13.Dore MP, Maragkoudakis E, Pironti A, Tadeu V, Tedde R, Realdi G, Delitala G. Twice-a-day quadruple therapy for eradication of Helicobacter pylori in the elderly. Helicobacter. 2006;11:52–55. doi: 10.1111/j.0083-8703.2006.00370.x. [DOI] [PubMed] [Google Scholar]
- 14.Graham DY, Belson G, Abudayyeh S, Osato MS, Dore MP, El-Zimaity HM. Twice daily (mid-day and evening) quadruple therapy for H. pylori infection in the United States. Dig Liver Dis. 2004;36:384–387. doi: 10.1016/j.dld.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi I, Murakami K, Kato M, Kato S, Azuma T, Takahashi S, Uemura N, Katsuyama T, Fukuda Y, Haruma K, Nasu M, Fujioka T. Changing antimicrobial susceptibility epidemiology of Helicobacter pylori strains in Japan between 2002 and 2005. J Clin Microbiol. 2007;45:4006–4010. doi: 10.1128/JCM.00740-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hori K, Miwa H, Matsumoto T. Efficacy of 2-week, second-line Helicobacter pylori eradication therapy using rabeprazole, amoxicillin, and metronidazole for the Japanese population. Helicobacter. 2011;16:234–240. doi: 10.1111/j.1523-5378.2011.00842.x. [DOI] [PubMed] [Google Scholar]
- 17.Graham DY. Helicobacter pylori eradication therapy research: Ethical issues and description of results. Clin Gastroenterol Hepatol. 2010;8:1032–1036. doi: 10.1016/j.cgh.2010.07.002. [DOI] [PubMed] [Google Scholar]