The WHO and UNAIDS have recommended using “treatment as prevention” (TasP) for the global elimination of HIV.1,2 Treatment suppresses an individuals’ viral load, making them less infectious.3 The higher the coverage of treatment, the lower the viral load in the population, and the lower the incidence of new infections. Incidence is very difficult to measure directly as it requires frequent testing of the entire population. Therefore population viral load, which can be used as an indirect measure of the effectiveness of treatment as prevention, has been proposed as a proxy for incidence.4,5 PVL is the average (mean5 or median6) viral load, calculated from the distribution of viral loads in diagnosed and undiagnosed individuals. To date, only one HIV epidemic has been shown to be close to elimination: the Danish HIV epidemic in men who have sex with men (MSM).7 HIV incidence in Danish MSM began to decrease soon after the introduction of effective therapies in 1996; by 2013, incidence was close to the WHO elimination threshold of one new HIV infection per 1,000 individuals per year. Here we show how the PVL changed, from 1996 onwards, as coverage increased and incidence decreased.
We calculated the PVL, each year, between 1996 and 2013. To make these calculations we determined (each year) the number of diagnosed and undiagnosed MSM who were living with HIV, and their viral loads. We used data from the Danish HIV Cohort Study (DHCS),8,9 an ongoing population-based study, to make these determinations for diagnosed individuals. Diagnosed individuals were either: 1) not yet on treatment, 2) on treatment but not virally suppressed, or 3) on treatment and virally suppressed (defined as <200 copies per mL). For the PVL calculation we used the exact viral load measurement for individuals who were untreated, or who were on treatment but not virally suppressed. In the first two years of the study (1996 and 1997), we used a value of 199 copies per mL for virally suppressed individuals. For virally suppressed individuals from 1998 onwards (as the test sensitivity had increased), we used the exact viral load measurement for individuals with a viral load between 200 and 20 copies per mL; we used a value of 19 copies per mL for individuals with a viral load below 20 copies per mL.
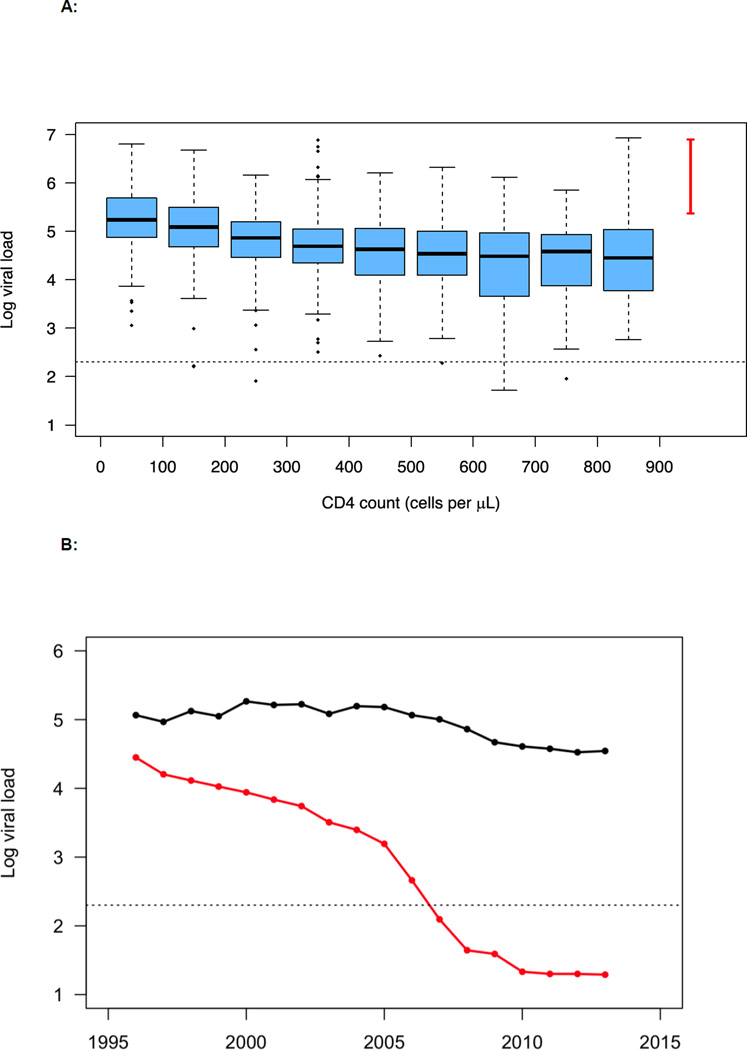
To specify the number of undiagnosed individuals (each year), we used CD4-cell stratified estimates from our previous analysis of the DHCS data.7 We assigned viral loads to these undiagnosed individuals by using a bootstrap sample of CD4-cell stratified viral load data from 1,059 treatment-naïve diagnosed individuals who participated in the DHCS (figure 1A). Since very few individuals in the DHCS had recently been infected (and were therefore in the acute stage of infection), we sampled from a distribution that was based on other studies to assign viral loads to undiagnosed individuals who were in the acute stage.10–12 The range for this distribution is shown in figure 1A. The viral load in the acute stage is a characteristic of the natural history of HIV infection; consequently, it is appropriate to use data from other studies to specify a range of values for the viral load in the acute stage. We note that the small sample of patients in the DHCS that are characterized as being in the acute stage of infection have viral loads that fall within the range of values from the studies that we used.
Figure 1.
A: CD4-stratified viral load distributions are shown plotted on a logarithmic scale, log10 copies per mL. Distributions were constructed using data collected from 1,059 treatment-naïve HIV-infected MSM who participated in the Danish HIV Cohort Study. The horizontal line in each box plot represents the median, black dots show outliers, the dotted line denotes viral suppression (<200 copies per mL), and the red line shows the range of viral load estimates for acute infection.
B: Temporal trends in the mean (black) and median (red) values of the population viral load (PVL) as the Danish HIV epidemic in MSM was driven to the brink of elimination; PVL is shown on a logarithmic scale, log10 copies per mL. Data are from the DHCS; the dotted line denotes viral suppression (<200 copies per mL).
Figure 1B shows the temporal trends in the mean, and the median, PVL for the Danish HIV epidemic in MSM. In 1996, when effective therapies were introduced, the mean PVL was 116,100 (and the median was 28,171) copies per mL. These data are right-skewed, i.e., the mean PVL is always substantially higher than the median PVL. This implies that, every year, there were a few individuals with very high viral loads.
The mean, and the median, PVL provide different insights into the changes that occurred in the internal dynamics of the epidemic as it was driven to the brink of elimination (figure 1B). The median PVL decreased as the proportion of infected individuals who were on treatment, and virally suppressed, increased. The median fell below 200 copies per mL in 2007, reflecting the fact that just over 50% were virally suppressed. By 2013, the median had fallen below 20 copies per mL – the limit of detectable virus. The mean PVL also declined; this reflects a decreasing number of undiagnosed individuals, as well as the fact that the distribution of their viral loads became more concentrated at lower values. In 2007 the mean PVL was ~100,000 copies per mL; by 2013 it had fallen to ~35,000 copies per mL. This indicates (based on a function that transforms viral load into transmission risk)3 that the average per act probability of transmitting HIV decreased from 0·0041 (in 2007) to 0·0027 (in 2013). Notably, neither measure of PVL completely captures the risk of transmission, as they do not reflect potential changes in risk behavior. There are also other important issues that need to be considered when using PVL as a measure of ongoing transmission.4
Our results show, that as treatment coverage expands and the incidence of new infections decreases, the mean PVL is a more useful measure of the effectiveness of TasP than the median PVL. The median has little utility once more than 50% of HIV-infected individuals are virally suppressed. However the mean will always be useful, even when incidence is close to the WHO elimination threshold, as it provides a measure of the risk of ongoing transmission.
Acknowledgments
JG has received research funding from Gilead, ViiV, Abbott, Bristol-Myers Squibb, Merck Sharp & Dohme, Janssen, and Boehringer Ingelheim. NO has received research funding from Gilead, GlaxoSmithKline, Janssen, Bristol-Myers Squibb and Boehringer Ingelheim.
JTO and SB acknowledge the financial support of the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grants R01 AI116493, R56 AI041935 and R21 AI114478). JG and NO acknowledge support from Preben og Anna Simonsens Fond, NOVO Nordisk Foundation and the Danish AIDS Foundation. The Danish HIV Cohort Study includes patients from the Departments of Infectious Diseases at Copenhagen University Hospitals, Rigshospitalet (J. Gerstoft, N. Obel) and Hvidovre (G. Kronborg), Odense University Hospital (C. Pedersen), Aarhus University Hospitals, Skejby (C.S. Larsen) and Aalborg (G. Pedersen), Herning Hospital (R. Mohey), Hillerød Hospital (L. Nielsen), Roskilde Hospital (L. Weise), Kolding Hospital (J. Jensen) and Herlev Hospital (B. Kvinesdal).
Footnotes
Contributors
JTO, JG, NO and SB developed the concept. JG and NO contributed the data, and JTO made the figures. All authors drafted the manuscript together.
Declaration of interests
JTO and SB report no conflicts of interest.
References
- 1.UNAIDS. Strategy for 2016—2021: fast-tracking to zero. Geneva: UNAIDS; 2015. [Google Scholar]
- 2.World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. Geneva: World Health Organization; 2015. [PubMed] [Google Scholar]
- 3.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342(13):921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 4.Miller WC, Powers KA, Smith MK, Cohen MS. Community viral load as a measure for assessment of HIV treatment as prevention. Lancet Infect Dis. 2013;13(5):459–464. doi: 10.1016/S1473-3099(12)70314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Guidance on community viral load: a family of measures, definitions, and method for calculation. [accessed July 4, 2016];2011 http://www.ct.gov/dph/lib/dph/aids_and_chronic/surveillance/statewide/community_viralload_guidance.pdf.
- 6.Montaner JS, Lima VD, Barrios R, et al. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet. 2010;376(9740):532–539. doi: 10.1016/S0140-6736(10)60936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okano JT, Robbins D, Palk L, Gerstoft J, Obel N, Blower S. Testing the hypothesis that treatment can eliminate HIV: a nationwide, population-based study of the Danish HIV epidemic in men who have sex with men. Lancet Infect Dis. 2016;16(7):789–796. doi: 10.1016/S1473-3099(16)30022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Obel N, Engsig FN, Rasmussen LD, Larsen MV, Omland LH, Sorensen HT. Cohort profile: the Danish HIV cohort study. Int J Epidemiol. 2009;38(5):1202–1206. doi: 10.1093/ije/dyn192. [DOI] [PubMed] [Google Scholar]
- 9.Omland LH, Ahlstrom MG, Obel N. Cohort profile update: the Danish HIV cohort study (DHCS) Int J Epidemiol. 2014;43(6):1769—9e. doi: 10.1093/ije/dyu153. [DOI] [PubMed] [Google Scholar]
- 10.de Souza MS, Pinyakorn S, Akapirat S, et al. Initiation of Antiretroviral Therapy During Acute HIV-1 Infection Leads to a High Rate of Nonreactive HIV Serology. Clin Infect Dis. 2016;63(4):555–561. doi: 10.1093/cid/ciw365. [DOI] [PubMed] [Google Scholar]
- 11.Pilcher CD, Joaki G, Hoffman IF, et al. Amplified transmission of HIV-1: comparison of HIV-1 concentrations in semen and blood during acute and chronic infection. AIDS. 2007;21(13):1723–1730. doi: 10.1097/QAD.0b013e3281532c82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schacker TW, Hughes JP, Shea T, Coombs RW, Corey L. Biological and virologic characteristics of primary HIV infection. Ann Intern Med. 1998;128(8):613–620. doi: 10.7326/0003-4819-128-8-199804150-00001. [DOI] [PubMed] [Google Scholar]