Abstract
Ethylene (ET) signal transduction may regulate plant growth and defense, depending on which components are recruited into the pathway in response to different stimuli. We report here that the ET pathway controls both insect resistance (IR) and plant growth enhancement (PGE) in Arabidopsis (Arabidopsis thaliana) plants responding to harpin, a protein produced by a plant pathogenic bacterium. PGE may result from spraying plant tops with harpin or by soaking seeds in harpin solution; the latter especially enhances root growth. Plants treated similarly develop resistance to the green peach aphid (Myzus persicae). The salicylic acid pathway, although activated by harpin, does not lead to PGE and IR. By contrast, PGE and IR are induced in both wild-type plants and genotypes that have defects in salicylic acid signaling. In response to harpin, levels of jasmonic acid (JA) decrease, and the COI1 gene, which is indispensable for JA signal transduction, is not expressed in wild-type plants. However, PGE and IR are stimulated in the JA-resistant mutant jar1-1. In the wild type, PGE and IR develop coincidently with increases in ET levels and the expression of several genes essential for ET signaling. The ET receptor gene ETR1 is required because both phenotypes are arrested in the etr1-1 mutant. Consistently, inhibition of ET perception nullifies the induction of both PGE and IR. The signal transducer EIN2 is required for IR, and EIN5 is required for PGE because IR and PGE are impaired correspondingly in the ein2-1 and ein5-1 mutants. Therefore, harpin activates ET signaling while conscribing EIN2 and EIN5 to confer IR and PGE, respectively.
Ethylene (ET) plays important roles in plant defense (Dong, 1998, 2001; Wang et al., 2002) and in growth and development (Price et al., 2003; Guo and Ecker, 2004). Action by ET in these processes essentially depends on its perception by plants, which determines how the pathway is executed. In the absence of an ET signal, ET receptors, like ETR1 and ERS1 (Gamble et al., 2002), activate the Raf-like kinase CTR1, which in turn inhibits the downstream pathway (Clark et al., 1998). When ET is present, its binding inactivates the receptors, causing deactivation of CTR1, which allows the metal-transporter EIN2 protein (Alonso et al., 1999) or the uncharacterized component EIN5 (Roman et al., 1995) to positively regulate the pathway. Downstream, the EIN3 or ERF1 families of transcription factors may regulate the transcription of effector genes (Kieber, 1997; Chao et al., 1997). According to epistatic analysis, EIN3 and EIN5 act allelically, while EIN2 and EIN3 may act sequentially (Bleecker and Kende, 2000). EIN3 can bind to the promoter region of ERF1 (Chao et al., 1997; Solano et al., 1998). Consistently, expression of ERF genes confers constitutive ET response in ein3 backgrounds (Solano et al., 1998). Thus, EIN2 and EIN3 or EIN5 may act through ERF1 to regulate ET-dependent processes (Guzmán and Ecker, 1990; Wang et al., 2002; Li et al., 2003, Traw and Bergelson, 2003). However, EIN3 also interacts with specific F boxes, which function to suppress ET action and promote plant growth (Potuschak et al., 2003; Gagne et al., 2004). How the downstream divergences in the ET pathway occur and subsequently lead to the diverse plant phenotypes is unclear.
The type of stimuli or elicitors critically affects which particular pathway is activated and which pathways intersect. Activation of salicylic acid (SA)-mediated systemic acquired resistance (SAR) suppresses jasmonic acid (JA)-dependent induced systemic resistance and insect resistance (IR; Felton et al., 1999); nevertheless, both pathways cross-talk under modulation by the NPR1 protein (Spoel et al., 2003). Application of SA or its analogs to tomato (Lycopersicon esculentum) induces SAR but suppresses IR (Doares et al., 1995; Doherty et al., 1998). In Arabidopsis (Arabidopsis thaliana), blocking SA accumulation causes increase in JA levels and expression of JA-inducible genes (Spoel et al., 2003). Expression of a G protein in transgenic tobacco plants causes elevation in JA levels but abolishes SA production (Yoda and Sano, 2003). The JA/ET synergism also is affected by the type of stimuli. In Arabidopsis, attack by aphids and mechanical wounding activate both SA- and JA-inducible genes (Reymond et al., 2000; Moran and Thompson, 2001). In rice (Oryza sativa), treatment with the SA analog dichloro-isonicotinic acid (INA) and JA results in greater expression of defense genes than treatment with only INA (Schweizer et al., 1997). Interestingly, SA inhibits JA-induced generation of Arabidopsis trichomes (Traw and Bergelson, 2003), suggesting that JA and SA also antagonistically act on morphogenesis. Moreover, Glc antagonizes ET in modulating action by EIN3 and thereby affects the downstream portion of the ET pathway. Whereas ET appears to promote EIN3 stability, Glc promotes its breakdown (Yanagisawa et al., 2003). Quite intriguingly, several signals that are diverse in properties often are engaged in the same processes. For example, reactive oxygen species mediate cell expansion and plant growth (Neill et al., 2002; Laloi et al., 2004), which requires ET (Li et al., 2003). Clearly, characterization of how the sophisticated signaling networks function in response to different stimuli or elicitors is a prodigious challenge. Thus far, studies with specific elicitors have resulted in scant information concerning how plant defense pathways coordinate with growth regulation.
Harpins, proteins produced by several Gram-negative plant pathogenic bacteria, cause multiple effects in plants (Kim and Beer, 2000). Harpin or harpinEa from Erwinia amylovora independently triggers hypersensitive cell death and the SAR pathway (Dong et al., 1999; Peng et al., 2003). The protein also stimulates ion channels (El-Maarouf et al., 2001) and kinases (Desikan et al., 2001), which have not been related to any particular plant phenotypes. HarpinPsph from Pseudomonas syringae pv phaseolicola may require a kinase localized on cell membranes for pathogen defense in tobacco (Nicotiana tabacum). Binding of harpinPsph to membranes is correlated with defense gene expression, which, however, is abolished by a kinase inhibitor (Lee et al., 2001a). HarpinPsph makes pores in artificial membranes and activates Ca2+-channel responses (Lee et al., 2001b). If similar responses occur in planta during bacterial infection, they could facilitate production and transportation of some virulence effectors that function intracellularly. Therefore, the several different effects of harpins in plants may be attributed to activation of several distinct pathways.
We have studied effects of harpins on plants to understand some aspects of the signaling networks underlying plant defense and growth regulation (Dong et al., 1999; Kim et al., 1999; Kim and Beer, 2000; Peng et al., 2003, 2004a, 2004b). Here, we describe how ET responds to the treatment with harpin and how the hormone signaling simultaneously regulates IR and plant growth enhancement (PGE).
RESULTS
Characterization of Harpin-Induced PGE and IR
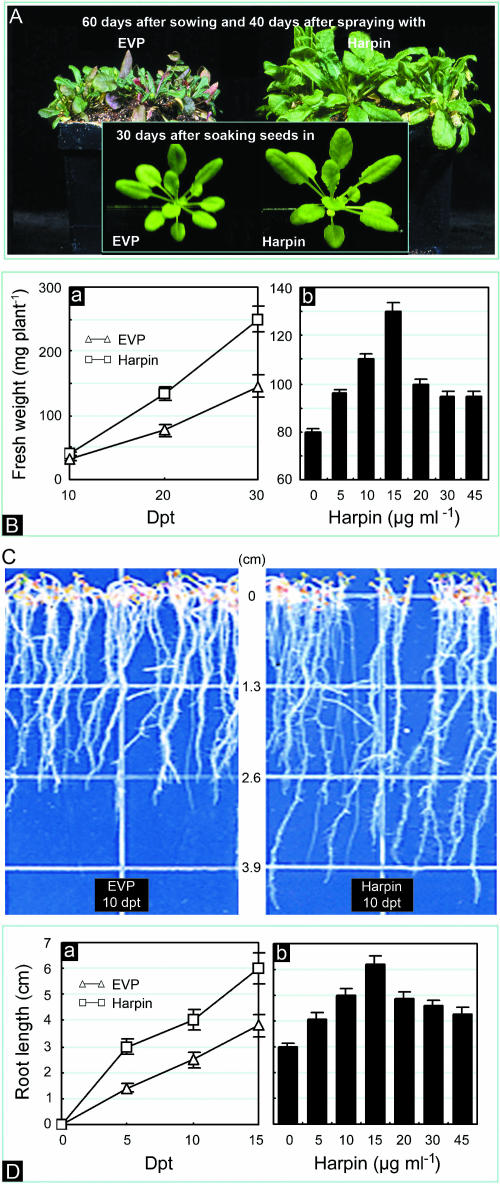
Figure 1A shows differences in morphology of plants incubated under vegetative growth conditions between treatments with 15 μg mL−1 harpin and empty vector preparation (EVP), which contained 15 μg mL−1 of inactive proteins. Seedlings sprayed with harpin were obviously larger at 40 d posttreatment (dpt) than similarly grown plants that were treated with EVP. Plants treated with EVP showed signs of senescence 60 d after sowing, while plants treated with harpin appeared vigorous at the same time (Fig. 1A, plants grown in pots). The fresh weight of seedlings sprayed with harpin was approximately 1.6-fold greater than seedlings sprayed with EVP by 20 dpt, and this trend continued for at least another 10 d (Fig. 1B, a). Concentrations of harpin greater than 15 μg mL−1 did not result in more growth than treatment with 15 μg mL−1 harpin (Fig. 1B, b).
Figure 1.
Effects of harpin on the growth of Arabidopsis. A, Appearance of plants grown in pots. B, Quantification of plant growth in pots. Subsection a, Increase in plant weight with time. Subsection b, Effects of harpin dose on growth. C, Appearance of roots grown on agar medium. D, Quantification of root growth on agar medium. Subsection a, Increase of root length with time. Subsection b, Effects of harpin dose on root growth. Plants in A and B were grown in pots in a controlled-environment chamber. Harpin at 15 μg mL−1, or the indicated dose, and 15 μg mL−1 EVP were applied 20 d after sowing by spaying plants to runoff. The plants shown in the insets in A are representative of those grown from seeds that were soaked in 15 μg mL−1 harpin or 15 μg mL−1 EVP for 6 h. Plants shown in the pots in the main photo of A were grown from untreated seeds. In B, plant weight was determined at the indicated times (a) or at 25 dpt (b). In C, plants shown are representative of those that grew from seeds soaked in 15 μg mL−1 harpin or 15 μg mL−1 EVP for 6 h prior to sowing on agar medium. In D, root length was measured at indicated times (a) or at 15 dpt (b). Quantitative assays were done 20 (A and B) or 10 (C and D) times; each assay was done with 3 replicates (B–D); each replicate involved 5 plants. In graphs and histograms (B and D), bars refer to statistical deviation.
Soaking seeds in solutions of harpin also resulted in enhanced growth of seedlings, although the effect was not evident until 20 to 25 dpt (Fig. 1A, insets). We determined whether harpin promotes seedling growth through affecting seed germination. Untreated seeds that were chilled for 4 to 5 d usually germinated within 24 h; those chilled for 2 d, or not chilled, generally required 6 to 7 d to germinate. Roots were evident 24 h after seed germination. Roots of plants grown from seeds soaked in harpin solution (15 μg mL−1) for 6 h prior to germination were longer than the controls (Fig. 1C). The effect was evident in 5 to 7 dpt and increased with time (Fig. 1D, a). Soaking seeds in concentrations of harpin greater than 15 μg mL−1 did not increase root length more than soaking in 15 μg mL−1 of harpin.
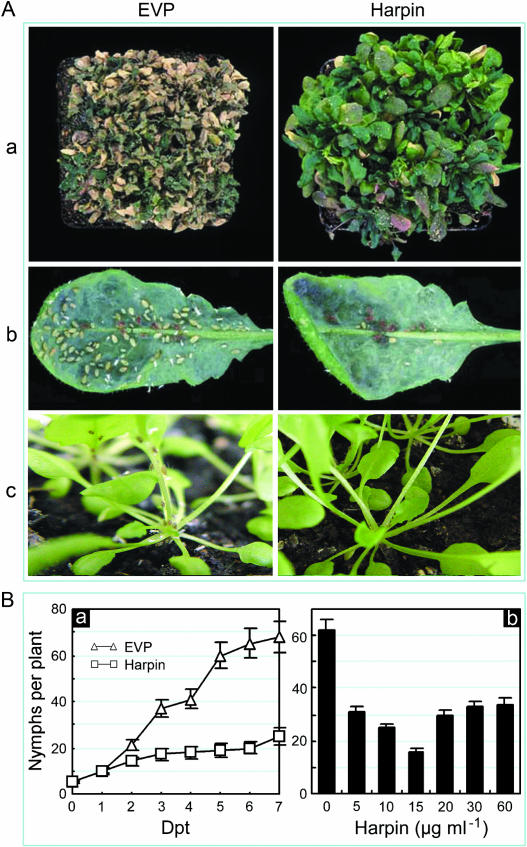
Harpin-induced resistance to insects first was suggested based on observations of field-grown peppers. Plants that had been treated with harpin incurred fewer injuries from the European corn borer than comparable untreated plants. Insect repellency was observed in harpin-treated cucumber; striped cucumber beetles preferred to colonize control plants rather than harpin-treated plants (Zitter and Beer, 1998). We studied effects of harpin treatment on the green peach aphid (Myzus persicae). The effect of colonization on plants by approximately 200 aphids per pot was evaluated based on plant vigor (Fig. 2A, a). After 30 d of insect colonization, plants treated with EVP were seriously damaged; most plants were dead. By contrast, damage to harpin-treated plants was much less severe and most plants still were growing (Fig. 2A, a). To quantify IR effects, 5 to 10 mature aphids were moved from nurse seedlings to the lower surfaces of leaves of treated plants; aphid reproductive rate was evaluated based on counting the numbers of nymphs reproduced on leaves. The insects seemed to prefer the undersides of leaves (Fig. 2A, b); sometimes, they moved to other parts of plants (Fig. 2A, c). Spraying plants with harpin markedly reduced subsequent aphid reproduction. The effect became apparent at 3 to 5 dpt (Fig. 2B, a). The greatest inhibition of aphid reproduction resulted from a moderate dose of harpin (Fig. 2B, b).
Figure 2.
Effects of harpin on colonization of Arabidopsis by green peach aphids. A, Appearance of plants following treatment and infestation with aphids. Subsection a, Overall damage by the insects to densely grown plants. Subsection b, Insect colonies on lower surfaces of single leaves from treated plants. Subsection c, Insects on single plants. B, Quantification of insect reproduction. Subsection a, Insect multiplication progressed with time following treatment. Subsection b, Effects of harpin concentration on insect reproduction. Twenty-day-old plants had been sprayed separately with solutions of EVP and harpin at 15 μg mL−1 or the indicated concentrations. At 5 dpt, aphids were moved from nurse plants to the treated plants. Plants in A, subsection a, were photographed 30 d after colonization by approximately 200 aphids per pot. In A, b and c, and B, mature aphids were placed on the lower sides of two leaves of a plant, 10 insects per leaf for A, b and c, and 5 insects per leaf for B. Leaves and plants were photographed 7 d later. The number of nymphs per plant was counted at 5 dpt or at the indicated times. Quantitative assays were done five times. For each assay, five plants were treated and evaluated. The data are presented as means ± sd.
T test (P = 0.05) indicated that amounts of plant growth and rates of insect multiplication were significantly different between treatments with EVP and harpin. Apparently, as the concentration of harpin applied increased above 15 μg mL−1, the effects on PGE and IR decreased proportionally (Figs. 1, B and D, and 2B). The Mann-Whitney U test (U test) at P = 0.05 suggested that levels of PGE and IR were significantly different for the treatment with harpin at 15 μg mL−1 versus treatment with all other concentrations, which, however, were not significantly different one another for both phenotypes.
Suppression of JA Signaling and Activation of the ET and SA Pathways
SA, ET, and JA mediate basal plant defenses (Ryals et al., 1996; Dong, 1998, 2001), affect plant development (Traw and Bergelson, 2003), and may play roles in the induction of PGE and IR. We reported previously that harpin activates the SA-mediated SAR pathway (Dong et al., 1999; Peng et al., 2003, 2004a). Here, we present evidence that harpin stimulates ET and SA while it suppresses JA signaling during the induction of PGE and IR.
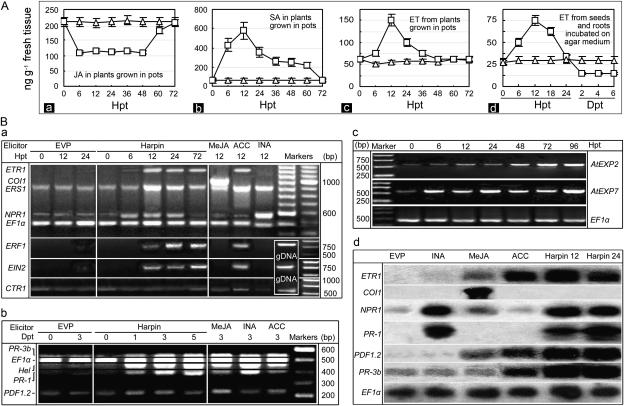
First, we studied the three signals. A basal level of free JA (approximately 200 ng g−1) was detected in control plants that had been treated with EVP (Fig. 3A, a). In harpin-treated plants, JA decreased to approximately one-half the basal level within 12 h posttreatment (hpt) and remained at that level until it again reached the basal level between 60 and 72 hpt. Thus, treatment with harpin resulted in reduced JA production. By contrast, as depicted in Figure 3A, subsection b, SA accumulated markedly in harpin-treated plants, peaking at 12 hpt, and declined to basal levels of approximately 55 ng g−1 fresh tissue by 72 hpt. Figure 3A, subsection c, shows that ET accumulation was transiently induced by harpin in tissues of potted plants, increasing at 12 hpt to 148 ng g−1 and falling to basal levels of 48 to 51 ng g−1 at 48 hpt. SA, ET, and JA from plants treated with EVP remained at basal levels. At each interval of assays, contents of three hormones in harpin-treated plants were all significantly higher than the basal levels observed in EVP-treated plants (T test, P = 0.05).
Figure 3.
Effects of harpin on the JA-, SA-, and ET-signaling pathways in Arabidopsis. A, Quantification of the hormones. Subsections a and b, Endogenous levels of free JA and SA. Subsections c and d, Amounts of ET released. B, The effects of harpin and known elicitors on expression of genes involved in the pathways. Subsections a to c, RT-PCR analysis of signaling regulatory genes, defense genes, and expansin genes. Subsection d, RNA gel-blot analysis to confirm RT-PCR results. In A, a solution containing 15 μg mL−1 harpin (rectangles) or 15 μg mL−1 EVP (triangles) was applied by spraying the seedlings (A, a–c) or by soaking seeds for 6 h before incubation on agar medium (A, d). Levels of JA, SA, and ET were quantified as described in the text based on fresh weight of tissues (A, a–c) or germinated seeds (A, d). The data points indicate means ± sd of results. In B, lower leaves of plants were sprayed separately with solutions containing the indicated compounds. Twelve hours later, or at the indicated times, RNA was extracted from untreated upper leaves. The constitutively expressed gene EF1α was used as a standard in the RT-PCR protocol. RT-PCR products of genes in B, a and b, were loaded to gel separately; products of genes in B, subsection c, were loaded in a mixture of equal amounts. In B, subsection d, harpin 12 and harpin 24 refer to leaf sampling at 12 and 24 hpt with harpin. Treatment with harpin resulted in increased expression of ETR1 and ERS1 encoding the ET receptors ERF1 and NPR1 that are required, respectively, for ET and SA signal transduction. Harpin did not induce expression of CTR1, which functions in the absence of an ET signal. Harpin also did not induce expression of COI1, which is required for JA signaling.
ET levels also were determined after soaking seeds and placing them on agar medium (Fig. 3A, d). Assays were done until differences in root length between plants treated with EVP and harpin were evident. ET fluctuated significantly (T test, P = 0.05) from a basal level of 23 to 26 ng g−1 in both germinating seeds and growing roots, similar to that in potted plants. ET from the harpin-soaked seeds increased to 48 ng g−1 by 6 hpt when germination started, peaked at 72 ng g−1 by 12 hpt, and then returned to the basal level at approximately 24 hpt. After that, ET levels declined to 13 to 14 ng g−1 in tissues of subsequently growing roots.
Next, we conducted reverse transcription (RT)-PCR analyses to determine if expression of the genes associated with the SA, JA, and ET pathways is coincident with levels of the signals in response to treatment with harpin. The effects of harpin were compared with those of the known elicitors, INA, methyl jasmonate (MeJA), and 1-aminocyclopropane-1-carboxylic acid (ACC), which activate the SA, JA, and ET pathways, respectively (Ryals et al., 1996; Dong, 1998). The constitutively expressed gene EF1α was used as a standard in the RT-PCR protocol. Products were dissolved in gels and stained with ethidium bromide; the staining intensity of the EF1α product was used to confirm uniform loading of RNA in different samples, which also helped us to evaluate relative levels of gene expression.
Figure 3B, subsection a, shows the behavior of the genes that regulate the SA, JA, and ET pathways in response to harpin and control solutions. Expression of the NPR1 gene was induced in harpin-treated plants within 6 hpt; in 12 to 24 hpt, the expression levels were comparable to that induced by INA. By contrast, few transcripts of NPR1 were detected in plants treated with EVP, MeJA, or ACC. In the same experiment, we studied the ETR1, ERS1, CTR1, EIN2, and ERF1 genes, which require induction for expression and participation in an ET-signaling circuit (Bleecker and Kende, 2000; Wang et al., 2002). The expression levels of ETR1 and ERS1 were greatly enhanced by treatment with harpin at 12 to 24 hpt; the levels were similar to those induced by ACC. EIN2 and ERF1 were expressed in harpin-treated plants markedly, just as in ACC-treated plants, but EIN2 and ERF1 were not expressed in plants treated with EVP, INA, or MeJA. CTR1, by contrast, was not induced by treatment with harpin, but it was slightly induced by ACC. Moreover, we assessed COI1, since COI1 positively regulates the JA pathway (Xie et al., 1998). The gene was expressed in plants treated with MeJA but not in harpin-treated plants. In addition, except for ERS1 and CTR1, which showed low levels of constitutive expression, other genes were not expressed in plants treated with EVP. These results suggest that only genes that positively regulate the SA and ET pathways are enhanced in expression by treatment with harpin.
Next, we investigated whether treatment with harpin affects the expression of relevant effector genes. Transcription of acidic PR genes, like PR-1 in Arabidopsis, is mediated by SA (Ryals et al., 1996); Hel is mediated by ET and weakly by SA, and PDF1.2 and basic PR genes, like PR-3b, are mediated by JA or ET, depending on exogenous stimulation (Pieterse et al., 1998; Clarke et al., 2000; Spoel et al., 2003). Figure 3B, subsection b, shows that PR-1, PR-3b, and Hel were all expressed in harpin-treated plants by 3 dpt, similarly as in plants treated with the known elicitors. Transcription of PDF1.2 was enhanced by treatment with harpin in 1 to 5 dpt. PR-3b and PDF1.2 were weakly expressed constitutively; their expression was enhanced by INA and MeJA or ACC, respectively. These results are consistent with previous results that ET and JA signaling affects activation of the two genes (Penninckx et al., 1998; Thomma et al., 1998; Andi et al., 2001). Thus, expression patterns of these genes are consistent with the behavior of the signals and regulatory genes. The expression of PDF1.2 and PR-3b seems mediated by ET rather than JA, inasmuch as JA production and COI1 expression were suppressed in harpin-treated plants.
The effector genes AtEXP2 and AtEXP7 encode the Arabidopsis expansins EXP2 and EXP7, which function to loosen cell walls and promote cell division and extension, mediated by ET, thereby promoting plant growth (Choi et al., 2003; Li et al., 2003). Figure 3B, subsection c, shows that expression of both genes was greatly enhanced by treatment with harpin. The accumulation of AtEXP2 transcripts increased by 12 hpt and reached higher levels in 2 to 3 d. Comparatively, AtEXP7 was activated earlier and more strongly; its expression started by 6 hpt and increased thereafter.
Results of RNA gel-blot analyses for AtEXP2 and AtEXP7 (data not shown) and other genes (Fig. 3B, d) confirmed the results of the RT-PCR analyses. For example, expression of NPR1 and defense genes was induced by harpin to higher levels, while only MeJA activated COI1. Responses of these genes to known elicitors also were consistent with the RT-PCR results and previous studies (Ryals et al., 1996; Clarke et al., 2000). ETR1 evidently was activated by MeJA or ACC but not by INA. NPR1 was activated slightly by ACC and strongly by INA. MeJA and ACC induced PDF1.2 expression, while INA did not. PR-1 and PR-3b were induced only by treatment with INA or ACC.
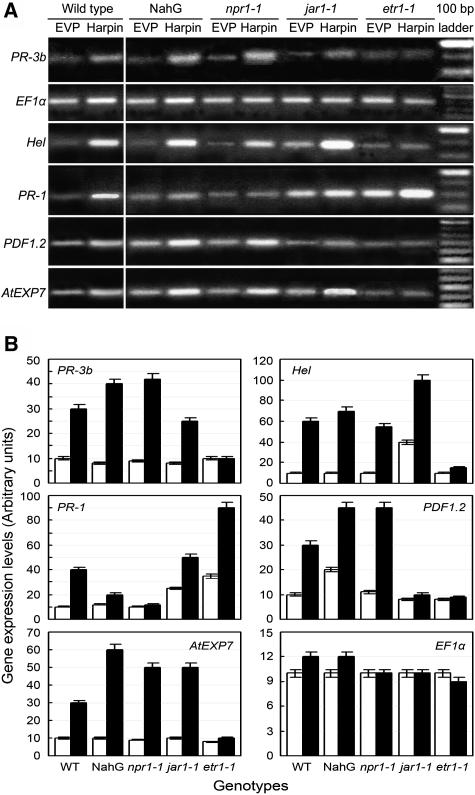
Representative genotypes with defects in the SA, ET, or JA pathways (Ryals et al., 1996; Dong, 1998) were compared with wild-type plants for expression of defense and EXP7 genes, in response to harpin. Gene expression patterns varied with genotypes and treatments (Fig. 4A). Transcript levels of a gene in other genotypes treated differently were relative to the level in wild-type plants treated with EVP (Fig. 4B). Expression levels were similar for most of the genes evaluated in EVP-treated plants of different genotypes, while differences primarily were provided by the treatment with harpin. We first assayed NahG plants that fail to accumulate SA (Delaney et al., 1994) and the npr1-1 mutant that contains a nonfunctional NPR1 gene (Cao et al., 1994, 1997). In both phenotypes, patterns of PR-3b, Hel, PDF1.2, and EXP7 expression were similar as in the wild type. Expression levels of these genes were greatly increased to different extents by treatment with harpin, as compared to EVP. However, PR-1 behaved differently. In the wild type, PR-1 transcript evidently accumulated, but its expression did not increase substantially in NahG and npr1-1 plants, following the application of harpin. Then, we tested the MeJA-insensitive mutant jar1-1. Levels of PDF1.2 expression were lower in jar1-1 compared to wild type, irrespective of treatments, and little increased in the mutant plants treated with harpin in contrast to EVP. Other genes were all expressed at higher levels in jar1-1 plants following the application of harpin, relative to EVP. Noticeably, harpin-induced expression of Hel, PR-1, and AtExp7 was greater in jar1-1 than wild type. Finally, we investigated the ET-insensitive mutant etr1-1, which possesses an ETR1 locus with a dominant mutation that prevents ET binding to the receptor ETR1 (Schaller and Bleecker, 1995; Gamble et al., 2002). In ert1-1 plants, expression of all the genes studied was suppressed, except PR-1, which was strongly expressed. These results suggest that harpin-induced expression of PR-1a is suppressed only by defects in the SA pathway, while that of others is impaired by insensitivity to ET instead of JA. Thus, with the exception of PR-1, ET signaling is critical for harpin to stimulate expression of the expansin and defense genes.
Figure 4.
Effects of Arabidopsis genotypes on expression of several effector genes in SA, ET, and JA signaling in response to harpin. A, Gel patterns of gene expression. B, Relative levels of gene expression. RT-PCR was conducted with RNA isolated at 3 dpt from untreated upper leaves of plants that had been sprayed on lower leaves with EVP or harpin. In gel photos, the brightest band of the 100-bp ladder is 600 bp. Gene expression levels are presented as white and black bars, respectively, for treatments with EVP (control) and harpin. For each gene, the arbitrary units were determined by verification based on defining the expression level in controls as 10. Data are given as means ± sd from three replicates.
Effects of Genetic Blocking of ET Signaling on PGE and IR
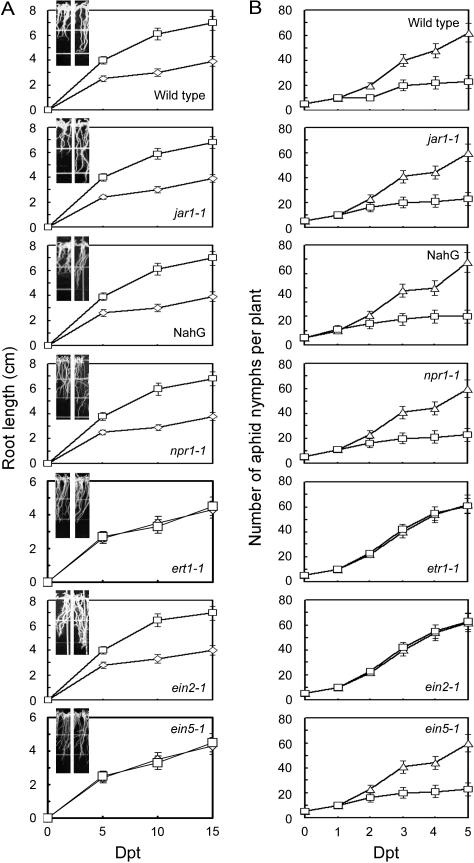
To attribute PGE and IR to particular pathways, we determined if harpin could induce the phenotypes in the same mutants as tested above. Based on the lengths of roots grown on agar medium (Fig. 5A) and the number of aphid nymphs reproduced on potted plants (Fig. 5B), PGE and IR were differently affected by some genotypes of Arabidopsis. After treatment with harpin, roots of the wild-type, jar1-1, and npr1-1 mutants and NahG transgenics were significantly longer than the controls treated with EVP (T test, P = 0.05). Similarly when these four genotypes were treated with harpin, their ability to support multiplication of aphids was greatly reduced relative to plants treated with EVP. Thus, the JA and SA pathways are not likely to result in PGE and IR in response to harpin. By contrast, harpin did not affect plant growth and IR relative to the effect of EVP in the etr1-1 mutant. The roots that grew from harpin-soaked seeds of the mutant were not longer than those from EVP-soaked seeds. The number of reproduced nymphs was similar in harpin-treated and control plants. These results confirm that ET signaling is essential to harpin-induced PGE and IR.
Figure 5.
Effects of Arabidopsis genotypes on the development of PGE and IR in response to harpin. A, Root length of seedlings grown on agar medium. B, The number of aphids on leaves of potted plants. Plants were observed at the indicated times after soaking seeds (A) or spraying seedlings (B) with EVP (triangles and left insets) or harpin (rectangles and right insets). Seedlings in insets were photographed when the effects were evident by 5 dpt for the wild type, jar1-1, and NahG and 8 dpt for others. The data points indicate means ± sd of results from three replicates each containing approximately 120 seedlings in A and 15 plants in B.
The ET signal transducers EIN2 and EIN5 act downstream of ET receptors and lead to stress resistance and root elongation in Arabidopsis (Bleecker and Kende, 2000). The ein2-1 and ein5-1 mutants were assayed to determine if they differ from the wild type in the induction of IR and PGE. In ein2-1 plants, IR was impaired while PGE was not impaired in response to harpin, based on aphid multiplication on the plants grown in pots and growth of seedlings from the seeds incubated on agar medium. Soaking ein2-1 seeds in a harpin solution resulted in significantly more seedling and root growth (T test, P = 0.05) than similar plants treated with EVP (Fig. 5A). However, aphids multiplied equally well in potted plants treated with harpin and EVP (Fig. 5B). In contrast with ein2-1 plants, ein5-1 plants displayed IR (Fig. 5B) but not PGE (Fig. 5A) after treatment with harpin. Clearly, harpin recruits EIN2 and EIN5 to confer IR and PGE, respectively.
Pharmacological Inhibition of PGE and IR
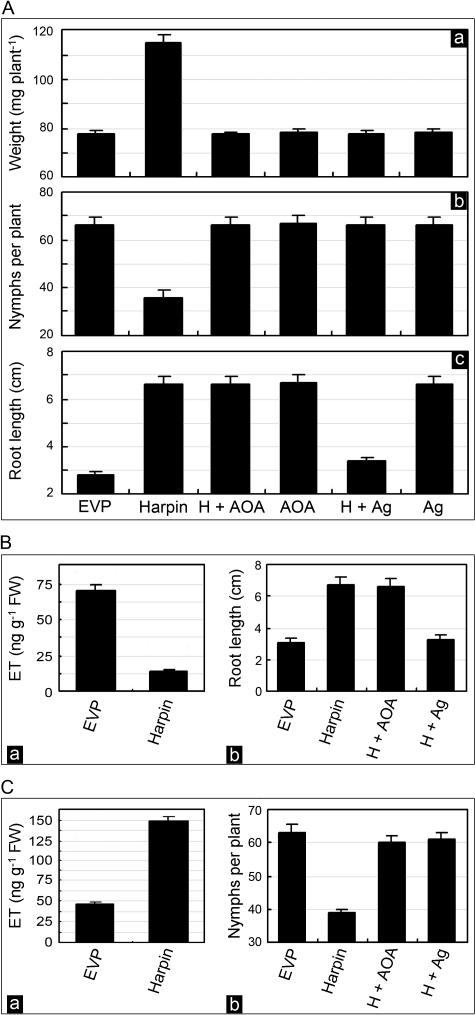
Amonooxyacetic acid (AOA) and AgNO3 inhibit synthesis and perception of ET, respectively (Ghassemian et al., 2000). We determined whether the inhibitors are active in eliminating PGE and IR in Arabidopsis plants treated with harpin. When both inhibitors were applied alone or with EVP to wild-type plants, there was no effect on PGE or IR (Fig. 6A, a–c). However, when either of the two inhibitors was applied together with harpin to plants growing in pots, PGE and IR, which were observed in the absence of the inhibitors, did not occur (Figs. 6A, a and b). In potted plants treated with harpin, an approximately 1.5-fold increase in growth (Fig. 6A, a) and an approximately 2.4-fold reduction in the number of nymphs occurred (Fig. 6A, b). These effects were eliminated by including AOA or AgNO3 in the treatment. However, only AgNO3 abolished the enhancement of root growth on agar medium (Fig. 6A, c). Thus, root growth promotion requires ET perception, while the induction of PGE and IR in potted plants requires ET production as well.
Figure 6.
Effects of inhibitors and inducers of ET synthesis and perception on the induction of PGE and IR by harpin in wild-type Arabidopsis and ein mutants. A, Assays of wild-type plants in response to different treatments. Subsection a, Fresh weight of plants grown in pots. Subsection b, Numbers of aphids on leaves of plants grown in pots. Subsection c, Length of roots grown on the agar medium. B, Assays of ein2-1 plants grown on the medium. Subsection a, ET levels from roots. Subsection b, Root length. C, Assays of ein5-1 plants grown in pots. Subsection a, ET levels from leaves. Subsection b, Numbers of aphids on leaves. Solutions that contained the indicated compounds were applied separately by spraying 20-d-old potted plants or soaking seeds for 6 h before germination. Soaked seeds were placed on the agar medium for root growth. AOA and AgNO3 (Ag) were applied together with harpin and labeled as H + AOA and H + Ag, respectively. Fresh weight of potted plants was determined at 20 dpt and is shown as weight per plant. Nymphs present on each plant were counted at 7 dpt and are shown as the number of nymphs per plant. Lengths of roots grown on medium were measured at 10 dpt. At 12 hpt, ET was quantified with fresh weight (FW) of leaves. Bars in the histograms represent sd.
We similarly assayed ein2-1 and ein5-1 mutants. Although ET declined 5-fold in ein2-1 roots grown on agar medium from seeds soaked in harpin solution (Fig. 6B, a), these roots still were 56% longer than control roots (Fig. 6B, b). AgNO3 applied together with harpin eliminated the effect, but AOA applied together with harpin did not (Fig. 6B, b). In potted ein5-1 plants, the ET level increased 3.3-fold at 12 hpt with harpin (Fig. 6C, a), while insect multiplication was only 58% on these plants relative to control plants at 7 dpt (Fig. 6C, b). AOA or AgNO3 applied with harpin abolished the induction of IR (Fig. 6C, b). Clearly, both synthesis and perception of ET are required for the induction of IR in potted ein5-1 plants. Nevertheless, enhanced root growth in ein2-1 plants grown on agar medium requires only perception of ET.
DISCUSSION
Previous studies have shown that harpins activate several plant defense pathways (Strobel et al., 1996; Dong et al., 1999; Lee et al., 2001a; Peng et al., 2003). However, how multifunctional elicitors affect the pathways that regulate plant growth is unclear. In Arabidopsis plants treated with harpin, both PGE and IR (Figs. 1 and 2) result from the activation of ET signaling. The increase in ET levels (Fig. 3A) and the absence of CTR1 expression (Fig. 3B) in harpin-treated plants suggest that the elicitor activates an ET-signaling circuit that does not involve CTR1, which is active when ET is absent (Wang et al., 2002). Expression of ETR1 and ERS1 (Fig. 3B) predicts that the stimulation by harpin is transduced to ETR1 and ERS1, two of the five functionally redundant ET receptors (Hua and Meyerowitz, 1998) identified based on loss of ET responses in any single-receptor mutant (Bleecker and Kende, 2000). The dominant etr1-1 mutation prevents ET binding to ETR1 at the receiver domain (Schaller and Bleecker, 1995), which resides at the C terminus of ETR1 and is absent in ERS1 (Gamble et al., 2002). The failure of etr1-1 mutant plants to express critical defense and expansin genes (Fig. 4), in response to harpin, indicates that perception of ET is required for the induced expression of the genes. Relationally, the expression of ERF1, concomitant with that of defense genes (Fig. 3B), is reminiscent of the role that ERF1 plays in transcriptional regulation of defense genes when under attack by pathogens or insects (Berrocal-Lobo et al., 2002). The impairment of AtEXP7 expression and the absence of PGE in harpin-treated etr1-1 plants (Fig. 5) suggest the recruitment of expansins into the process. This possibility is supported by recent findings that expansins promote growth of plant cells and plants under mediation by ET (Cho and Cosgrove, 2002; Choi et al., 2003; Li et al., 2003). The failure of etr1-1 plants to develop both PGE and IR following treatment with harpin (Fig. 5) suggest that ET sensing is required for both processes. Pharmacological studies with wild-type plants growing in pots bear out that ET synthesis is required as well (Fig. 6A). However, similar studies with roots grown on agar medium indicate that promotion of root growth requires ET sensing but is not affected by inhibition of ET synthesis (Figs. 3A and 6A). Therefore, sensing ET is critical to the coordinate induction of PGE and IR.
We have demonstrated how the same pathway regulates the two distinct processes, by assessing the ein2-1 and ein5-1 mutants (Guzmán and Ecker, 1990; Roman et al., 1995). The ein2-1 mutant has a defect in EIN2, which positively regulates the ET pathway; thus, the mutant lacks ET responsiveness throughout plant development (Alonso et al., 1999). The ein2-1 mutation also impairs stress response, alters sensitivity to pathogens (Roman et al., 1995; Wang et al., 2002), and, presumably, affects IR similarly. In ein2-1 plants, harpin induced PGE but not IR (Fig. 5), which suggests that EIN2 is required for IR but not for PGE. The EIN5 locus is epistatic to CTR1; the ein5-1 mutant is insensitive to ET (Roman et al., 1995). As such, EIN5 is believed to positively regulate an ET-signaling circuit following the deactivation of CTR1 (Wang et al., 2002). In ein5-1 plants, harpin induced IR but did not induce PGE (Fig. 5). Therefore, EPG rather than IR requires EIN5. Pharmacological studies with both ein2-1 and ein5-1 confirmed the participation of ET signaling in both PGE and IR. Growth promotion of ein2-1 roots requires ET perception but does not require induced synthesis of ET (Fig. 6B), although IR development in the aerial parts of ein5-1 plants requires ET synthesis in addition (Fig. 6C). This is coincident with changes in ET levels in ein2-1 roots grown on medium (Fig. 6B) and in leaves of ein5-1 plants grown in pots (Fig. 6C). These results constitute convincing evidence that EIN2 and EIN5 are recruited to confer IR and PGE, respectively, in response to harpin.
The signal transduction that leads to PGE may be different in the aerial parts of plants and in roots. Regulation of root growth by the ET receptors depends on ET levels and putatively the proportion of the receptors that are bound (Ghassemian et al., 2000; Finkelstein et al., 2002). Application of 0.3 μg mL−1 or less of ET to seeds and roots stimulates germination and growth, but higher doses inhibit both processes (Ghassemian et al., 2000). ET still is required to maintain root growth, but the concentration is lower than that required to break dormancy and stimulate germination (Leo-Kloosterziel et al., 1996; Kepczynski and Kepczyska, 1997). In our experiments with harpin, ET was modulated to a level that favors root growth (Figs. 1C, 3A, and 6B). In aerial parts of the plants, however, the increase in ET levels is coincident with the induction of PGE and IR (Fig. 6, A and C). Actually, ET levels can be elevated by tens to 1,000-fold in leaves while responding to environmental stress and during phase transition like flower initiation (Wilkins, 1984). More studies are needed to better define how stimulation of plants by harpin is transduced to the ET pathway.
Although SA and JA mediate plant defense and growth under many circumstances (Ryals et al., 1996; Dong, 1998; Wang et al., 2002; Traw and Bergelson, 2003), the two hormones do not mediate PGE and IR. Activation of the SA pathway (Figs. 3 and 4), which regulates harpin-induced resistance to pathogens (Strobel et al., 1996; Dong et al., 1999; Peng et al., 2003, 2004a), did not lead to IR and PGE. Conversely, IR and PGE were induced in NahG and npr1-1 plants (Fig. 5) that have defects in SA signaling (Cao et al., 1994; Delaney et al., 1994). JA, often synergizing ET, is essential for insect resistance in plants that respond to insect feeding and some elicitors (Penninckx et al., 1998; Moran and Thompson, 2001). Rather surprisingly, we found that harpin deactivates JA signaling while inducing IR (Figs. 1–5). The decrease in JA levels and nonexpression of the COI1 gene, which is indispensable for JA signal transduction (Xie et al., 1998; Xu et al., 2002), did not affect activation of relevant defense genes in wild-type plants (Figs. 3, A and B, and 4). By contrast, PGE and IR were induced in the jar1-1 mutant similarly as in wild type (Fig. 5). In consistence, MeJA inhibits harpin-induced defense response in tobacco (Andi et al., 2001). Apparently, stimulation of plants by harpin separates roles of ET and JA in insect defense. In harpin-treated plants, ET no longer synergizes JA as does it in plants under attack by insects (Moran and Thompson, 2001), for example, but instead acts itself to mediate IR development. Thus, ET, JA, and SA may be synergistic (McConn et al., 1997; Reymond et al., 2000), antagonistic (Niki et al., 1998; Spoel et al., 2003), or independent (Thomma et al., 1998) in controlling different processes. The basis for such different interactions for the hormones in plant defense and growth regulation remains to be studied.
MATERIALS AND METHODS
Plant Growth and Insect Maintenance
Arabidopsis (Arabidopsis thaliana) plants used included the ecotype Columbia (Col-O) and several mutants, npr1-1, jar1-1, etr1-1, ein2-1, and ein5-1 (CS1092, CS3726, CS8072, CS237, CS3071, CS8054), the ecotype Landsberg erecta (Ler-O), and its mutants abi1-1 and abi2-1 (CS20, CS22, CS23), obtained from the Arabidopsis Biological Research Center, Columbus, Ohio (http://Arabidopsis.org). Transgenic NahG plants in the Col-O background were also included in this study. Seeds were disinfested in a 1.5% (w/v) solution of sodium hypochlorite for 10 min and chilled at 4°C for 5 d. Plants for assays of PGE and IR induced in aerial potions of the plants were grown in 60-mL pots containing a mixture of sand and potting soil for 20 d before use, except as specified otherwise. Seedlings for growth of roots were incubated in 10-cm square plates containing an agar medium, composed of 0.8% (w/v) Phytagar (Invitrogen Life Technologies, Carlsbad, CA) and 0.44% (w/v) Arabidopsis germination medium (Beta Technologies, Ghent, Belgium). Plants were incubated in chambers with a 14-h-day (200 μE m−2 s−1 at 24°C) and 10-h-night (20°C) cycle. The green peach aphids (Myzus persicae) were collected near Ithaca, New York, and near Nanjing in China for the experiments done earlier (Figs. 1 and 2) and later (Figs. 5 and 6), respectively. Aphids were cultured in nursery Arabidopsis seedlings and were transferred to fresh plants every 2 weeks.
Plant Treatment, PGE and IR Scoring, Hormone Determination, and Data Analysis
Preparation and quantification of harpin and the EVP that contains inactive proteins followed methods described previously (Bauer et al., 1995; Dong et al., 1999). Both EVP and harpin were used at 15 μg mL−1, except as otherwise specified, based on different responses of genotypes of Arabidopsis to various doses of harpin (Peng et al., 2003). MeJA, ACC, and INA (Sigma, St. Louis) of 10 μm, 1 mm, and 1 mm were used as positive controls for assays of IR and PGE. Pharmacological studies were done with two compounds. An aqueous solution of AgNO3 was used at 20 μm. AOA was maintained in a 100 mm aqueous stock at 4°C and used at the final concentrations of 0.5 mm. AOA or AgNO3 was mixed with a solution of harpin immediately prior to application to plants by methods appropriate to purposes of the individual assays.
Disinfested and chilled seeds were soaked in filter-sterilized solution of each compound for 6 h before placing the seeds on the agar medium. Subsequent to growth on the medium, root length was determined. Plants growing in pots were sprayed with each compound to be evaluated for IR and PGE. PGE in potted plants was judged based on weight per plant. To study IR, mature nymphs of aphids were moved from nurse Arabidopsis seedlings to young leaves of the plants 5 dpt, except as otherwise specified. The extent of IR was expressed as the percentage decrease in number of aphid nymphs reproduced over time on plants treated with harpin, relative to controls.
ET concentrations were determined by gas chromatography (Guzmán and Ecker, 1990). Gas was collected from the environment of seedlings growing in pots, in sealed glass boxes or from imbibed seeds and growing roots on the agar medium contained in sealed 5-mL glass bottles. Free forms of SA or JA were extracted, as described (Penninckx et al., 1998; Clarke et al., 2000) and quantified by HPLC (Meuwly and Mètraux, 1993) and gas-liquid chromatography-mass spectroscopy (Rao et al., 2000), respectively.
Each experiment was carried out three times, and each treatment was applied to 15 plants, except as specified in figure legends. For quantitative determination, data were treated statistically using the statistical analysis tools in Microsoft Excel version 2003 (Microsoft, Beijing). The T test at P = 0.05 was applied for significance in the difference between each induction treatment and the treatment with EVP. Multiple comparisons were done by U test at P = 0.05 for significance in differences among different induction treatments.
Gene Expression Analysis
RNA was isolated from leaves as described (Clark, 1997; Dong and Beer, 2000). RT-PCR was performed using RT-PCR Beads (Amersham Pharmacia Biotech, Piscataway, NJ), as per the manufacturer's protocol. The EF1α gene, which is highly conserved and constitutively expressed in eukaryotes (Gallie et al., 1998), was used as a standard. The RT-PCR protocol has proven valid for estimating levels of gene expression (Peng et al., 2003, 2004a, 2004b). Genes tested were amplified for 25 to 30 cycles using specific primers. RT-PCR products were cloned into the pGEM-T Easy Vector (Promega, Madison, WI), sequenced (Takara Biotech, Dalian, China), confirmed by BLAST searches, and visualized by staining with ethidium bromide in agarose gels following electrophoresis. Levels of gene expression were evaluated by quantifying RT-PCR products based on ethidium bromide-staining densities in bands 5 × 2.5 mm2 in gels, determined by a scanner attached to the Image System SX-100 (Shanghai Sixing, Shanghai, China). Northern-blot hybridization was done as described previously (Dong et al., 1999; Peng et al., 2003). Replicates of RNA blotted to nylon membranes were hybridized with [32P]dCTP-labeled cDNA produced by PCR, after confirmation of sequences similarly for RT-PCR products. Uniform loading of RNA in gels was checked by probing blots with a probe specific for EF1α.
Primers specific for genes studied and sizes (bp) of the gene products are as follows: NPR1, 5′-TACTCTCTATCAGAGGCACTTATTGGACGT-3′/5′-CCATAGTGGCTTGTTTTGCGATCATGA-3′, 506; COI1, 5′-ATGCCTGAGAAGTACATGAATCTGGTTT-3′/5′-AGTAAACAGACCCCTGAGGAAAAATAAAGA-3′, 1,001; ERS1, 5′-ATGGAGTCATGCGATTGTTTTGAGAC-3′/5′-CTGATCCGCCACGTTTTCTACAA-3′, 906; CTR1, 5′-ATGGAAATGCCCGGTA-3′/5′-CCAGAAACGATGTGAAAC-3′, 648; EIN2, 5′-GATTCACTGAAGCAGCAGAGGAC-3′/5′-CTGTGGCAAACTGTAGGCATCTC-3′, 766; ERF1, 5′-CAATCCACTAACGATCCCTAA-3′/5′-CGCCAAGTATCACAAAAGTAC-3′, 850; PR-1, 5′-CAAGATAGCCCACAAGATTATCTAAGGGTT-3′/5′-GGCTTCTCGTTCACATAATTCCCACG-3′, 408; PR-3b 5′-CTACAGCACCAGACGGACCATA-3′, 5′-CTAAATAGCAGCTTCGAGGAGGCC-3′, 539; Hel, 5′-AGACTTAGCATAACCATCATACTTTT-3′/5′-CATTGGTCCACTATTCTCACAG-3′, 455; PDF1.2, 5′-AGAAATATGCATGTCATAAAGTTACTCAT-3′/5′-CAATGGTGGAAGCACAGAAG-3′, 244; AtEXP2, 5′-ACGGTAACTTACACAGCCAAGGC-3′/5′-GCACAACATCGTAGCTCACAACAG-3′, 557; AtEXP7, 5′-CATGGAGATATGCTCACGCCAC-3′/5′-GCTTATCCAATTCGTCCGGCTA-3′, 512; ABH1, 5′-AGAGCATTGAGAATGCGACT-3′/5′-CAAGTATCTCCCATGGCTGA-3′, 500; and EF1α, 5′-AGACCACCAAGTACTACTGCAC-3′/5′-CCACCAATCTTGTACACATCC-3′, 495.
Sequence data from this article have been deposited with the GenBank data libraries under accession numbers U76707 and AF036340 (NPR1), AF002109 (COI1), U21952 (ERS1), L08790 (CTR1), AF141203 (EIN2), AF076277 (ERF1), M90508 (PR-1), AB023463 (PR-3b), U01880 (Hel), T04323 (PDF1.2), NM120611 (AtEXP2), NM101127 (AtEXP7), AF27289 (ABH1), AJ223969, AF120093, AF181492, and X97131 (EF1α).
Acknowledgments
We thank K. Lawton (Syngenta, Research Triangle, NC) for the gift of INA and NahG plant seeds, and the Arabidopsis Biological Resource Center (Ohio State University) for supplying other seeds used in this study. We thank X. Dong (Duke University, Durham, NC), A. Collmer (Cornell University, Ithaca, NY), and T.P. Delaney (University of Vermont, Burlington, VT) for critical comments on an earlier version of this article, and two anonymous reviewers for important suggestions on the present version.
This work was supported by the U.S. Department of Agriculture (grants to S.V.B.) and a royalty income fund (to S.V.B.), the China National Natural Science Foundation (grant no. 30370969), the Ministry of Education of China (Century-Across Talent award no. 2002–48), and the China National 863 Plan (award no. 2002AA245011 to H.D.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.048900.
References
- Alonso JM, Hirayama T, Roman G, Nourizadehm S, Ecker JR (1999) EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 284: 2148–2152 [DOI] [PubMed] [Google Scholar]
- Andi S, Taguchi F, Toyoda K, Shiraishi T, Ichinose Y (2001) Effect of methyl jasmonate on harpin-induced hypersensitive cell death, generation of hydrogen peroxide and expression of PAL mRNA in tobacco suspension cultured BY-2 cells Plant Cell Physiol 42: 446–449 [DOI] [PubMed] [Google Scholar]
- Bauer DW, Wei ZM, Beer SV, Collmer A (1995) Erwinia chrysanthemi harpinEch: an elicitor of the hypersensitive response that contributes to soft-rot pathogenesis. Mol Plant Microbe Interact 8: 484–491 [DOI] [PubMed] [Google Scholar]
- Berrocal-Lobo M, Molina A, Solano R (2002) Constitutive expression of ETHYLENE-RESPONSE-FACTOR1 in Arabidopsis confers resistance to several necrotrophic fungi. Plant J 29: 23–32 [DOI] [PubMed] [Google Scholar]
- Bleecker AB, Kende H (2000) Ethylene: a gaseous signal molecule in plants. Annu Rev Cell Dev Biol 16: 1–18 [DOI] [PubMed] [Google Scholar]
- Cao H, Bowling SA, Dong X (1994) Characterization of an Arabidopsis mutant that is nonresponsive to inducers of systemic acquired resistance. Plant Cell 6: 1583–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Glazebrook J, Clarke JD, Volko S, Dong X (1997) The Arabidopsis NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell 88: 57–63 [DOI] [PubMed] [Google Scholar]
- Chao Q, Rothenberg M, Solano R, Roman G, Terzaghi W, Ecker JR (1997) Activation of the ethylene gas response pathway in Arabidopsis by the nuclear protein ETHYLENE-INSENSITIVE3 and related proteins. Cell 89: 1133–1144 [DOI] [PubMed] [Google Scholar]
- Cho HT, Cosgrove DJ (2002) Regulation of root hair initiation and expansin gene expression in Arabidopsis. Plant Cell 14: 3237–3253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D, Lee Y, Cho HT, Kende H (2003) Regulation of expansin gene expression affects growth and development in transgenic rice plants. Plant Cell 15: 1386–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark KL, Larsen PB, Wang X, Chang C (1998) Association of the Arabidopsis CTR1 Raf-like kinase with the ETR1 and ERS ethylene receptors. Proc Natl Acad Sci USA 95: 5401–5406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MS (1997) Plant Molecular Biology, A Laboratory Manual. Springer, Berlin
- Clarke JD, Aarts N, Feys BJ, Dong X, Parker JE (2000) Roles of salicylic acid, jasmonic acid, and ethylene in cpr-induced resistance in Arabidopsis. Plant Cell 12: 2175–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney TP, Uknes S, Vernooij B, Friedrich L, Weymann K, Negrotto D, Gaffney T, Gut-Rella M, Kessmann H, Ward E, et al (1994) A central role of salicylic acid in plant disease resistance. Science 266: 1247–1250 [DOI] [PubMed] [Google Scholar]
- Desikan R, Hancock JT, Ichimura K, Shinozaki K, Neill SJ (2001) Harpin induces activation of the Arabidopsis mitogen-activated protein kinases ATMPK4 and ATMPK6. Plant Physiol 126: 1579–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doares SH, Narvaez-Vasquez J, Conconi A, Ryan CA (1995) Salicylic acid inhibits synthesis of proteinase inhibitors in tomato leaves induced by systemin and jasmonic acid. Plant Physiol 108: 1741–1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty HM, Selvendran RR, Bowles DJ (1998) The wound response of tomato plants can be inhibited by aspirin and related hydroxy-benzoic acids. Physiol Mol Plant Pathol 33: 377–384 [Google Scholar]
- Dong H, Beer SV (2000) Riboflavin induces disease resistance in plants by activating a novel signal transduction pathway. Phytopathology 90: 801–811 [DOI] [PubMed] [Google Scholar]
- Dong H, Delaney TP, Bauer DW, Beer SV (1999) Harpin induces disease resistance in Arabidopsis through the systemic acquired resistance pathway mediated by salicylic acid and the NIM1 gene. Plant J 20: 207–215 [DOI] [PubMed] [Google Scholar]
- Dong X (1998) SA, JA, ethylene, and disease resistance in plants. Curr Opin Plant Biol 1: 316–323 [DOI] [PubMed] [Google Scholar]
- Dong X (2001) Genetic dissection of systemic acquired resistance. Curr Opin Plant Biol 4: 309–314 [DOI] [PubMed] [Google Scholar]
- El-Maarouf H, Barny MA, Rona JP, Bouteau F (2001) Harpin, a hypersensitive response elicitor from Erwinia amylovora, regulates ion channel activities in Arabidopsis thaliana suspension cells. FEBS Lett 25: 82–84 [DOI] [PubMed] [Google Scholar]
- Felton GW, Korth KL, Bi JL, Wesley SV, Huhman DV, Mathews MC, Murphy JB, Lamb C, Dixon RA (1999) Inverse relationship between systemic resistance of plants to microorganisms and to insect herbivory. Curr Biol 9: 317–320 [DOI] [PubMed] [Google Scholar]
- Finkelstein RR, Gampala SSL, Rock CD (2002) Abscisic acid signaling in seeds and seedlings. Plant Cell (Suppl) 14: S15–S45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagne JM, Smalle J, Gingerich DJ, Walker JM, Yoo SD, Yanagisawa S, Vierstra RD (2004) Arabidopsis EIN3-binding F-box 1 and 2 form ubiquitin-protein ligases that repress ethylene action and promote growth by directing EIN3 degradation. Proc Natl Acad Sci USA 101: 6803–6808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallie DR, Le H, Caldwell C, Browning KS (1998) Analysis of translation elongation factors from wheat during development and flowering heat shock. Biochem Biophys Res Commun 245: 295–300 [DOI] [PubMed] [Google Scholar]
- Gamble RL, Qu X, Schaller GE (2002) Mutational analysis of the ethylene receptor ETR1. Role of the histidine kinase domain in dominant ethylene insensitivity. Plant Physiol 128: 1428–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghassemian M, Nambara E, Cutler S, Kawaide H, Kamiya Y, McCourt P (2000) Regulation of abscisic acid signaling by the ethylene response pathway in Arabidopsis. Plant Cell 12: 1117–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Ecker JR (2004) The ethylene signaling pathway: new insights. Curr Opin Plant Biol 7: 40–49 [DOI] [PubMed] [Google Scholar]
- Guzmán P, Ecker JR (1990) Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell 2: 513–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua J, Meyerowitz EM (1998) Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell 94: 261–271 [DOI] [PubMed] [Google Scholar]
- Kepczynski J, Kepczyska E (1997) Ethylene in seed dormancy and germination. Physiol Plant 101: 720–726 [Google Scholar]
- Kieber JJ (1997) The ethylene response pathway in Arabidopsis. Annu Rev Plant Physiol Plant Mol Biol 48: 277–296 [DOI] [PubMed] [Google Scholar]
- Kim JF, Bauer DW, Bogdanove AJ, Dong H, Beer SV, Wei Z (1999) Secreted enigmatic proteins of Erwinia amylovora—for good and evil. Acta Hortic 489: 38–42 [Google Scholar]
- Kim JF, Beer SV (2000) hrp genes and harpins of Erwinia amylovora: a decade of discovery. In JL Vanneste, ed, Fire Blight and Its Causative Agent, Erwinia amylovora. CAB International, Wallingford, pp 141–162
- Laloi C, Apel K, Danon A (2004) Reactive oxygen signalling: the latest news. Curr Opin Plant Biol 7: 323–328 [DOI] [PubMed] [Google Scholar]
- Lee J, Klessig DF, Nurnberger T (2001. a) A harpin binding site in tobacco plasma membranes mediates activation of the pathogenesis-related gene HIN1 independent of extracellular calcium but dependent on mitogen-activated protein kinase activity. Plant Cell 13: 1079–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Klusener B, Tsiamis G, Stevens C, Neyt C, Tampakaki AP, Panopoulos NJ, Noller J, Weiler EW, Cornelis GR, et al (2001. b) HrpZPsph from the plant pathogen Pseudomonas syringae pv. phaseolicola binds to lipid bilayers and forms an ion-conducting pore in vitro. Proc Natl Acad Sci USA 98: 289–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo-Kloosterziel KM, van der Bunt GA, Zeevaart JAD, Koornneef M (1996) Arabidopsis mutants with reduced dormancy. Plant Physiol 110: 233–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Jones L, McQueen-Mason S (2003) Expansins and cell growth. Curr Opin Plant Biol 6: 603–610 [DOI] [PubMed] [Google Scholar]
- McConn M, Greelmann RA, Bell E, Mullet JE, Browse J (1997) Jasmonate is essential for insect defense in Arabidopsis. Proc Natl Acad Sci USA 94: 5473–5477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuwly P, Mètraux JP (1993) Ortho-anisic acid as internal standard for the simultaneous quantitation of salicylic acid and its putative biosynthetic precursors in cucumber leaves. Anal Biochem 214: 500–505 [DOI] [PubMed] [Google Scholar]
- Moran PJ, Thompson GA (2001) Molecular responses to aphid feeding in Arabidopsis in relation to plant defense pathways. Plant Physiol 125: 1074–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill S, Desikan R, Hancook J (2002) Hydrogen peroxide signaling. Curr Opin Plant Biol 5: 388–395 [DOI] [PubMed] [Google Scholar]
- Niki T, Mitsuhara I, Seo S, Ohtsubo N, Ohashi Y (1998) Antagonistic effect of salicylic acid and jasmonic acid on the expression of pathogenesis-related (PR) protein genes in wounded mature tomato leaves. Plant Cell Physiol 39: 500–507 [Google Scholar]
- Peng J-L, Bao Z-L, Ren H-Y, Wang J-S, Dong H-S (2004. a) Expression of harpinXoo in transgenic tobacco induces pathogen defense in the absence of hypersensitive cell death. Phytopathology 94: 1048–1055 [DOI] [PubMed] [Google Scholar]
- Peng J-L, Bao Z-L, Li P, Chen G-Y, Wang J-S, Dong H (2004. b) Harpinxoo and its functional domains activate pathogen-inducible plant promoters in Arabidopsis. Acta Bot Sin 21: 1083–1090 [Google Scholar]
- Peng J-L, Dong H-S, Dong H-P, Delaney TP, Bonasera BM, Beer SV (2003) Harpin-elicited hypersensitive cell death and pathogen resistance requires the NDR1 and EDS1 genes. Physiol Mol Plant Pathol 62: 317–326 [Google Scholar]
- Penninckx IA, Thomma BP, Buchala A, Metraux JP, Broekaert WF (1998) Concomitant activation of jasmonate and ethylene response pathways is required for induction of a plant defensin gene in Arabidopsis. Plant Cell 10: 2103–2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieterse CM, van Wees SC, van Pelt JA, Knoester M, Laan R, Gerrits H, Weisbeek PJ, van Loon LC (1998) A novel signaling pathway controlling induced systemic resistance in Arabidopsis. Plant Cell 10: 1571–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potuschak T, Lechner E, Parmentier Y, Yanagisawa S, Grava S, Koncz C, Genschik P (2003) EIN3-dependent regulation of plant ethylene hormone signaling by two Arabidopsis F box proteins: EBF1 and EBF2. Cell 115: 679–689 [DOI] [PubMed] [Google Scholar]
- Price J, Li TC, Kang SG, Na JK, Jang JC (2003) Mechanisms of glucose signaling during germination of Arabidopsis. Plant Physiol 132: 1424–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MV, Lee H-i, Greelman RA, Mullet JE, Davis KR (2000) Jasmonic acid signaling modulates ozone-induced hypersensitive cell death. Plant Cell 12: 1633–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymond P, Weber H, Damond M, Farmer EE (2000) Differential gene expression in response to mechanical wounding and insect feeding in Arabidopsis. Plant Cell 12: 707–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman G, Lubarsky B, Kieber JJ, Rothenberg M, Ecker JR (1995) Genetic analysis of ethylene signal transduction in Arabidopsis thaliana: five novel mutant loci integrated into a stress response pathway. Genetics 139: 1393–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryals JA, Neuenschwander UH, Willits MG, Molina A, Steiner H-Y, Hunt MD (1996) Systemic acquired resistance. Plant Cell 8: 1809–1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller GE, Bleecker AB (1995) Ethylene-binding sites generated in yeast expressing the Arabidopsis ETR1 gene. Science 270: 1809–1811 [DOI] [PubMed] [Google Scholar]
- Schweizer P, Buchala A, Silverman P, Seskar M, Raskin I, Metraux JP (1997) Jasmonate-inducible genes are activated in rice by pathogen attack without a concomitant increase in endogenous jasmonic acid levels. Plant Physiol 114: 79–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano R, Stepanova A, Chao QM, Ecker JR (1998) Nuclear events in ethylene signaling: a transduction cascade mediated by ETHYLENE INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes Dev 12: 3703–3714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoel SH, Koornneef A, Claessens SMC, Korzelius JP, Van Pelt JA, Mueller MJ, Buchala AJ, Metraux J-P, Brown R, Kazan K, et al (2003) NPR1 modulates cross-talking between salicylate- and jasmonate-dependent defense pathways through a novel function in the cytosol. Plant Cell 15: 760–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel RN, Gopalan JS, Kuc JA, He SY (1996) Induction of systemic acquired resistance in cucumber by Pseudomonas syringae pv. syringae 61 HrpZPss protein. Plant J 9: 431–439 [Google Scholar]
- Thomma BPHJ, Eggermont K, Penninckx IAMA, Mauch-Mani B, Vogelsang R, Cammue BPA, Broekaert WF (1998) Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc Natl Acad Sci USA 95: 15107–15111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traw MB, Bergelson J (2003) Interactive effects of jasmonic acid, salicylic acid, and gibberellin on induction of trichomes in Arabidopsis. Plant Physiol 133: 1367–1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KL-C, Li H, Ecker JR (2002) Ethylene biosynthesis and signaling networks. Plant Cell (Suppl) 14: S131–S151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins MB (1984) Advanced Plant Physiology. Pitman, London
- Xie DX, Feys BF, James S, Nieto-Rostro M, Turner JG (1998) COI1: an Arabidopsis gene required for jasmonate-regulated defense and fertility. Science 280: 1091–1094 [DOI] [PubMed] [Google Scholar]
- Xu L, Liu F, Lechner E, Genschik P, Crosby WL, Ma H, Peng W, Huang D, Xie D (2002) The SCFCOI1 ubiquitin-ligase complexes are required for jasmonate response in Arabidopsis. Plant Cell 14: 1919–1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa S, Yoo SD, Sheen J (2003) Differential regulation of EIN3 stability by glucose and ethylene signalling in plants. Nature 425: 521–525 [DOI] [PubMed] [Google Scholar]
- Yoda H, Sano H (2003) Activation of hypersensitive response genes in the absence of pathogens in transgenic tobacco plants expressing a rice small GTPase. Planta 217: 993–997 [DOI] [PubMed] [Google Scholar]
- Zitter TA, Beer SV (1998) Harpin for insect control. Phytopathology 88: S104–S105 [Google Scholar]