Abstract
Background
Positive sentinel lymph node (SLN) findings in DCIS range from 1–22% but have unknown biologic significance. We sought to identify predictors of positive SLNs and to assess their clinical significance in patients initially diagnosed with DCIS.
Methods
We identified 1234 patients with an initial diagnosis of DCIS who underwent SLN dissection (SLND) at our institution (1997–2011). Positive SLN findings were categorized as isolated tumor cells (ITCs) (≤0.2mm), micrometastases (>0.2–2mm), or macrometastases (>2mm). Predictors of positive SLNs were analyzed, and survival outcomes examined.
Results
Positive SLN findings were identified in 132 patients (10.7%): ITCs 66 (5.4%), micrometastases 36 (2.9%), and macrometastases 30 (2.4%). Upstaging to microinvasive (n=68 [5.5%]) or invasive (n=259 [21.0%]) cancer occurred in 327 patients (26.5%). Factors predicting positive SLNs included diagnosis by excisional biopsy (OR 1.90, P=.007), papillary histology (OR 1.77, P=.006), DCIS >2cm (OR 1.55, P=.030), >3 interventions before SLND (OR 2.04, P=.022 [4 interventions]; OR 3.87, P<.001 [≥5 interventions]), and occult invasion (OR 3.44, P=.001 [microinvasive]; OR 6.21, P<.001 [invasive]). Median follow-up was 61.7 months. Patients with pure DCIS with and without positive SLNs had equivalent survival (100.0% vs 99.7%, P=.679). Patients with occult invasion and positive SLNs had the worst survival (91.7%, P<.001).
Conclusions
Occult invasion and more than 3 total interventions were the strongest predictors of positive SLN findings in patients initially diagnosed with DCIS. This supports the theory of benign mechanical transport of breast epithelial cells. Other than patients at high risk for invasive disease, routine use of SLND in DCIS is not warranted.
Introduction
Due to widespread implementation of screening mammography, the incidence of ductal carcinoma in situ (DCIS) has increased. By definition, DCIS lacks the ability to metastasize and early series where the axilla was evaluated by axillary lymph node dissection (ALND) reported a metastases rate of <1%.1 However, in the sentinel lymph node (SLN) era, where fewer nodes are resected and subjected to serial sectioning and often to immunohistochemical evaluation, the reported incidence of positive SLNs in DCIS ranges from 1%–22%.1–14 The majority of these metastases are isolated tumor cells (ITCs) or small-volume metastases.2–4,7,14
Benign mechanical transport of cells through the lymphatics as a result of preoperative manipulation of the primary tumor has been cited as a potential reason for positive lymph node findings.15–29 It is also possible that a positive SLN reflects true metastatic disease in patients with a preoperative diagnosis of DCIS found to have occult invasion at the time of definitive surgery. This surgical upstaging has led some to advocate SLN dissection (SLND) in DCIS patients deemed to be at high risk for an invasive component or in patients for whom total mastectomy is planned.30–32
The biologic significance of positive SLN findings in patients with DCIS is largely unknown, and whether clinicians should change management in response to positive SLN findings is debated. In the current study, we sought to identify predictors of positive SLN findings and to assess their clinical significance in a cohort of patients with an initial diagnosis of DCIS who underwent SLND.
Methods
The Breast Surgical Oncology Database at The University of Texas MD Anderson Cancer Center was queried to identify 2918 patients with an initial diagnosis of DCIS treated from 1997 through 2011 including 1386 who underwent SLND. Patients with concurrent contralateral invasive breast cancer (n=96, 6.9%), history of prior ipsilateral breast cancer (n=40, 2.9%), or failed SLND (n=16, 1.2%) were excluded, resulting in a final study population of 1234 patients. The study was approved by the Institutional Review Board.
Sentinel Lymph Node Dissection
Patients underwent SLND at the discretion of the treating surgeon; reasons for performing SLND in patients with DCIS included planned mastectomy and the presence of features suggesting high risk for an invasive component (high grade, DCIS size >2 cm, palpable tumor, microinvasion suspected on biopsy). The reason for SLND was generally cited by the operating surgeon in the operative report; however, preoperatiave breast imaging results and pathology results were also used to determine all potential tumor characteristics that prompted the need for SLND. SLND was performed using filtered technetium Tc 99m-labeled sulfur colloid alone, 1% isosulfan blue dye alone (Lymphazurin, US Surgical Corporation, Norwalk, CT), or a combination of these agents.33 Dual tracers were utilized in 58.8% (725/1234) of patients. Mapping agents were injected subdermally, subareolar, or peritumorally. 99mTc-labeled sulfur colloid was injected on the day before (2.5 mCi) or on the day of surgery (0.5 mCi); blue dye was injected at the time of surgery at a volume of 3 to 5mL. SLNs were detected intraoperatively by visualization of blue dye, detection of radiolabelled colloid using a handheld gamma detection probe (Neoprobe, US Surgical Corporation), or both. A node was judged to be a SLN if it stained blue, had radioactivity, or both.
Pathologic Assessment of Sentinel Nodes
Harvested SLNs were sectioned at 2- to 3-mm intervals along the short axis of the lymph node as previously described.33 Nodal tissue was formalin fixed and paraffin embedded. Prior to 2000, a single section from each block was examined with hematoxylin and eosin (H&E). Beginning in 2000, paraffin blocks were sectioned at 5-μm intervals to yield 2 sections that were examined with H&E. If H&E-stained sections were negative for metastasis, a single section was examined using immunohistochemistry for cytokeratin. A positive SLN finding was defined as a SLN with tumor cells on histologic assessment of tissue sections with standard H&E staining or immunohistochemical analysis. Positive SLNs were categorized according to the American Joint Committee on Cancer (AJCC) staging system, seventh edition, as containing ITCs (pN0[i+]: ≤0.2 mm), micrometastases (pN1mi: >0.2 mm–2 mm), or macrometastases (pN1–3: >2 mm).34 The median number of SLNs removed during SLND was 2 (range 1–10).
Study Variables
Study variables included preoperative clinicopathologic factors (age at diagnosis, biopsy method, histologic subtype, and reason for SLND [higher histologic grade, DCIS >2 cm, palpable tumor, microinvasion suspected on biopsy, or planned total mastectomy]) and postoperative clinicopathologic factors (estrogen receptor status, progesterone receptor status, evidence of microinvasive or invasive cancer on final pathology, and larger pathologic size of DCIS). Occult invasion was classified as microinvasive carcinoma (≤1 mm in greatest dimension) or invasive carcinoma (>1 mm in greatest dimension) identified on final pathology.34 To evaluate the theory of benign mechanical transport of cells, variables that indicate the extent of preoperative tumor manipulation, including total number of biopsies, surgeries, and interventions (total biopsies and surgeries performed prior to SLND) and total needle localizations were interrogated. All procedures performed in the breast within 6 months prior to SLND were recorded. For example, a patient having undergone 4 biopsies or 3 biopsies plus partial mastectomy followed by total mastectomy and SLND would both be considered to have undergone 4 total interventions; the procedure performed in conjunction with the SLND was not counted towards the number of total interventions.
Statistical Analyses
The primary outcome was positive SLN findings. The secondary outcome was clinical significance of positive SLN findings as evidenced by patient survival and changes in management, such as completion ALND, nodal radiation therapy, or adjuvant chemotherapy. Univariate analysis was performed to examine the association between variables and positive SLN findings using the chi-squared test or Fisher’s exact test for categorical variables and Mann-Whitney U test for continuous variables. Variables with P<.25 were evaluated by multivariate analysis using the multiple logistic regression model after backward stepwise Wald elimination to identify independent predictors of positive SLN findings. Variables identified as independent predictors were subjected to within-response analysis to determine their individual contribution to specific subsets of positive SLN findings (ITCs and nodal metastases). Kaplan-Meier and actuarial methods were used to evaluate patient survival. P <.05 was considered significant. The statistical analyses were performed using SPSS software (version 15; SPSS, Inc., Chicago, IL).
Results
The median age was 54.0 years. Most patients were diagnosed by percutaneous biopsy (n=975, 79.0%) and treated with total mastectomy (n=948, 76.8%). The majority of tumors were composed of more than 1 histologic subtype, and comedonecrosis was common (n=896, 72.6%). Among patients tested, estrogen receptor was positive in 74.3% (n=776/1044), and progesterone receptor was positive in 56.0% (n=582/1040). Reasons for performing SLND included high-grade disease (n=622, 50.4%), DCIS size >2 cm (n=632, 51.2%), a clinically palpable tumor (n=160, 13.0%), and microinvasion suspected on biopsy (n=238, 19.3%). Some patients had more than 1 reason for SLND. Median follow-up time was 61.7 months.
A positive SLN was identified in 132 (10.7%) patients: 66 (5.4%) had ITCs, 36 (2.9%) had micrometastases, and 30 (2.4%) had macrometastases. Of these patients, the majority (n=114, 86.4%) had positive SLN findings in a single SLN. A total of 327 patients (26.5%) were upstaged to microinvasive (n=68, 5.5%) or invasive cancer (n=259, 21.0%). The rate of upstaging depended on the biopsy method used: 36.0% (173/480) for small-bore percutaneous biopsy (needle size >11 gauge), 24.0% (119/495) for large-bore percutaneous biopsy (needle size ≤11 gauge), and 13.5% (35/259) for excisional biopsy. The probability of a positive SLN finding was correlated with the extent of invasion evident on final pathology. Positive SLN findings occurred in 6.2% (56/907) of patients with pure DCIS, 16.2% (11/68) of patients with microinvasive cancer, and 25.1% (65/259) of patients with invasive cancer. Patients with pure DCIS had the lowest volume of SLN disease: 4.9% (44/907) had ITCs, 1.3% (12/907) had micrometastases and none had macrometastases. Among the patients with microinvasive cancer, 8.8% (6/68) had ITCs, 4.4% (3/68) had micrometastases and 2.9% (2/68) had macrometastses. In patients with invasive cancer, 6.2% (16/259) had ITCs, 8.1% (n=21/259) had micrometastases, and 10.8% (n=28/259) had macrometastases. Three patients with negative SLNs were found to have non-sentinel intramammary lymph node metastases and had a final pathologic nodal stage of pN1a.
Various clinical and pathologic factors were examined to determine predictors of positive SLN findings (Table 1). On univariate analysis, preoperative factors predictive of positive SLN findings included papillary histologic subtype, clinically palpable tumor, DCIS size >2 cm, and microinvasion suspected on biopsy. The total number of biopsies, total number of surgeries, and total number of interventions also correlated with positive SLN findings. Pathologic factors associated with positive SLN findings are shown in table 2. Within-response analysis of these factors demonstrated that excisional biopsy, DCIS size >2 cm, more than 3 total interventions, and occult invasion were predictive of ITCs, while only papillary histologic subtype and occult invasion remained predictive of SLN metastases.
Table 1.
Clinical and pathologic characteristics of patients with DCIS who underwent SLNDa
| Negative SLN (n=1102) |
Positive SLN findings (n=132) |
P valuec (Neg vs pos SLN) |
P valued (ITCs vs mets) |
||
|---|---|---|---|---|---|
| ITCs (n=66) |
Metastases b (n=66) |
||||
| Age, years | .001 | .168 | |||
| Mean | 54.9 | 52.8 | 50.4 | ||
| Median (range) | 54.0 (21–90) | 51.5 (32–76) | 49.0 (29–80) | ||
| Biopsy method e | .099 | .010 | |||
| Percutaneous only | 878 (90.1) | 42 (4.3) | 55 (5.6) | ||
| Excisional | 224 (86.5) | 24 (9.3) | 11 (4.2) | ||
| Biopsy histologic subtype | |||||
| Solid | 683 (90.1) | 35 (4.6) | 40 (5.3) | .250 | .380 |
| Cribriform | 563 (88.1) | 43 (6.7) | 33 (5.2) | .159 | .078 |
| Papillary | 276 (83.9) | 27 (8.2) | 26 (7.9) | <.001 | .859 |
| Comedonecrosis | 798 (89.1) | 52 (5.8) | 46 (5.1) | .656 | .232 |
| Reason for SLND | |||||
| High grade | 559 (89.9) | 30 (4.8) | 33 (5.3) | .515 | .601 |
| DCIS size >2 cm f | 548 (86.7) | 47 (7.4) | 37 (5.9) | .003 | .070 |
| Palpable tumor | 131 (81.9) | 13 (8.1) | 16 (10.0) | .001 | .528 |
| Microinvasion suspicion | 196 (82.4) | 19 (8.0) | 23 (9.7) | <.001 | .455 |
| Total mastectomy | 841 (88.7) | 52 (5.5) | 55 (5.8) | .222 | .505 |
| Total no. of biopsies g | .030 | .878 | |||
| Mean | 1.29 | 1.52 | 1.52 | ||
| Median (range) | 1 (0–5) | 1 (0–4) | 1 (0–4) | ||
| Total no. of surgeries h | .007 | <.001 | |||
| Mean | 1.31 | 1.65 | 1.23 | ||
| Median (range) | 1 (1–4) | 2 (1–3) | 1 (1–3) | ||
| Total no. of interventions i | <.001 | .011 | |||
| Mean | 2.60 | 3.17 | 2.74 | ||
| Median (range) | 2 (1–8) | 3 (2–6) | 2 (1–7) | ||
| Total needle localizations | .519 | .114 | |||
| Mean | 0.5 | 0.6 | 0.4 | ||
| Median (range) | 0 (0–4) | 0 (0–3) | 0 (0–3) | ||
| Final pathologic diagnosis | <.001 | <.001 | |||
| Pure DCIS | 851 (93.8) | 44 (4.9) | 12 (1.3) | ||
| Microinvasive cancer | 57 (83.8) | 6 (8.8) | 5 (7.4) | ||
| Invasive cancer | 194 (74.9) | 16 (6.2) | 49 (18.9) | ||
| Pathologic size of DCIS * | <.001 | .258 | |||
| Mean | 3.8 | 5.6 | 6.6 | ||
| Median (range) | 3.0 (0–19.0) | 4.3 (0.3–18.0) | 6.5 (0.1–20.0) | ||
| ER status * | .033 | .405 | |||
| Positive | 695 (89.6) | 37 (4.8) | 44 (5.7) | ||
| Negative | 227 (84.7) | 22 (8.2) | 19 (7.1) | ||
| Unknown | 180 (94.7) | 7 (3.7) | 3 (1.6) | ||
| PR status * | .029 | .837 | |||
| Positive | 525 (90.2) | 27 (4.6) | 30 (5.2) | ||
| Negative | 393 (85.8) | 32 (7.0) | 33 (7.2) | ||
| Unknown | 184 (94.8) | 7 (3.6) | 3 (1.5) | ||
| Modified BNG j* | .808 | .862 | |||
| I | 61 (91.0) | 3 (4.5) | 3 (4.5) | ||
| II | 349 (88.8) | 24 (6.1) | 20 (5.1) | ||
| III | 580 (88.4) | 36 (5.5) | 40 (6.1) | ||
| Unknown | 112 (94.9) | 3 (2.5) | 3 (2.5) | ||
| Follow-up time, months | .799 | .061 | |||
| Mean | 68.0 | 62.1 | 75.7 | ||
| Median (range) | 61.8 (0.7–171) | 55.7 (8.7–149) | 71.9 (8.6–165) | ||
BNG, Black’s Nuclear Grade; DCIS, ductal carcinoma in situ; ER, estrogen receptor; ITCs, isolated tumor cells; mets, metastases; no., number; PR, progesterone receptor; SLN, sentinel lymph node; SLND, SLN dissection.
Values in table are number of patients (percentage) unless otherwise indicated.
Metastases includes micrometastases and macrometastases.
Comparison between negative SLN group versus positive SLN group.
Comparison within group with positive SLN findings (isolated tumor cells versus SLN metastases).
Method was classified as excisional if the patient underwent 1 or more excisional or incisional biopsies.
Size is greatest measurable diameter of lesion or calcifications on mammography or ultrasonography.
All percutaneous biopsies.
All excisional biopsies and surgeries.
Sum of total biopsies and total surgeries.
Grade I, well differentiated; grade II, moderately differentiated; grade III, poorly differentiated.
Excluded unknown values in statistics calculations.
Table 2.
Multivariate logistic regression analysis of predictors of positive SLN findings
| POSITIVE SLN FINDINGS | ||||
|---|---|---|---|---|
|
| ||||
| Factor | N | OR | (95% CI) | P value |
| Biopsy method | 0.007 | |||
| Percutaneous (Ref) | 975 | 1.00 | ||
| Excisional | 259 | 1.90 | (1.20–3.01) | 0.007 |
|
| ||||
| Papillary histologic subtype | 0.006 | |||
| No (Ref) | 905 | 1.00 | ||
| Yes | 329 | 1.77 | (1.18–2.67) | 0.006 |
|
| ||||
| DCIS size >2 cm a | 0.030 | |||
| No (Ref) | 602 | 1.00 | ||
| Yes | 632 | 1.55 | (1.04–2.31) | 0.030 |
|
| ||||
| Total no. of interventions b | 0.001 | |||
| 1–2 (Ref) | 681 | 1.00 | ||
| 3 | 384 | 1.43 | (0.93–2.22) | 0.107 |
| 4 | 112 | 2.04 | (1.11–3.75) | 0.022 |
| ≥5 | 57 | 3.87 | (1.88–7.95) | <0.001 |
|
| ||||
| Final pathologic diagnosis | <0.001 | |||
| Pure DCIS (Ref) | 907 | 1.00 | ||
| Microinvasive cancer | 68 | 3.44 | (1.66–7.12) | 0.001 |
| Invasive cancer | 259 | 6.21 | (4.08–9.45) | <0.001 |
|
| ||||
| ISOLATED TUMOR CELLS c | ||||
|
| ||||
| Biopsy method | 0.001 | |||
| Percutaneous (Ref) | 920 | 1.00 | ||
| Excisional | 248 | 2.55 | (1.46–4.44) | 0.004 |
|
| ||||
| DCIS size >2 cm | 0.002 | |||
| No (Ref) | 573 | 1.00 | ||
| Yes | 595 | 2.38 | (1.37–4.16) | 0.001 |
|
| ||||
| Total no. of interventions | <0.001 | |||
| 1–2 (Ref) | 646 | 1.00 | ||
| 3 | 367 | 2.82 | (1.54–5.14) | 0.001 |
| 4 | 101 | 2.65 | (1.12–6.32) | 0.027 |
| ≥ 5 | 54 | 7.62 | (3.27–17.74) | <0.001 |
|
| ||||
| Final pathologic diagnosis | 0.047 | |||
| Pure DCIS (Ref) | 895 | 1.00 | ||
| Microinvasive cancer | 63 | 2.28 | (0.90–5.79) | 0.084 |
| Invasive cancer | 210 | 1.95 | (1.04–3.66) | 0.037 |
|
| ||||
| METASTASES c | ||||
|
| ||||
| Papillary histologic subtype | 0.008 | |||
| No (Ref) | 866 | 1.00 | ||
| Yes | 302 | 2.11 | (1.21–3.66) | 0.008 |
|
| ||||
| Final pathologic diagnosis | <0.001 | |||
| Pure DCIS (Ref) | 863 | 1.00 | ||
| Microinvasive cancer | 62 | 6.37 | (2.16–18.78) | 0.001 |
| Invasive cancer | 243 | 18.30 | (9.52–35.16) | <0.001 |
DCIS, ductal carcinoma in situ; OR, odds ratio; no., number; SLN, sentinel lymph node; SLND, sentinel lymph node dissection.
We chose to use the preoperative categorical variable rather than the postoperative continuous variable to go into the final regression model.
We chose to use total number of interventions rather than total number of biopsies and total number of surgeries in the final model as the first variable is a composite of the other two.
Only variables found to be predictive of positive SLN findings were chosen for entry into the models for isolated tumor cells and metastases to determine their individual contributions.
We further examined the relationship between total number of interventions and positive SLN findings by subdividing the cohort into patients with pure DCIS and patients with microinvasive or invasive cancer on final pathology. The probability of positive SLN findings increased with total number of interventions independent of whether the patient had pure DCIS or evidence of occult invasion (Figure 1). This phenomenon is likely a result of the association between increasing number of interventions and finding ITCs (P<.001) (Table 2).
Figure 1.
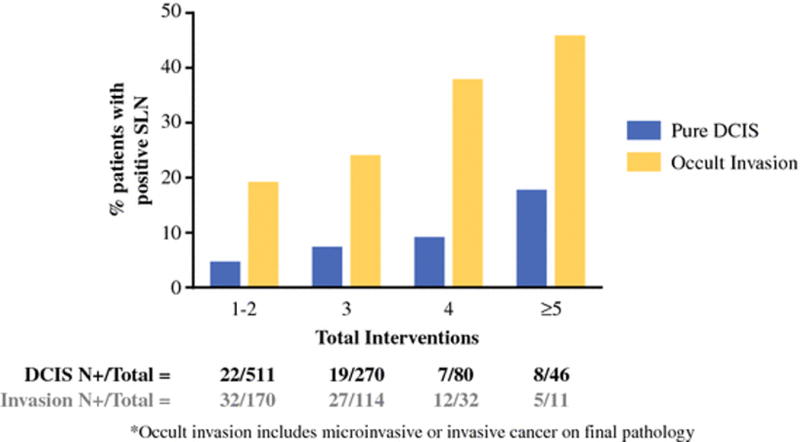
Correlation between number of pre-SLND interventions and positive SLN findings, comparing patients with pure DCIS to patients with occult invasion on final pathology.
The impact of positive SLN findings on clinical management and patient survival is summarized in Table 3. Of the 132 patients with positive SLN findings, 51 (38.6%) had completion ALND (6 for ITCs and 45 for metastases). ALND identified additional positive nodes in 8 (15.7%) patients; all 8 had occult invasion and SLN metastases (1 micrometastasis, 7 macrometastases). The majority of patients with SLN macrometastases (N=27/30, 90.0%) had a final pathologic nodal stage of pN1a. Among patients with positive SLN findings, adjuvant chemotherapy was administered more frequently to patients with occult invasion than to patients with pure DCIS (47/76, 61.8% vs 7/56, 12.5%, P<.001). Patients with ITCs (n=66) experienced few recurrence events: in this group, there were 2 local recurrences (3%), 1 regional recurrence (1.5%), and no distant recurrences.
Table 3.
Treatment characteristics and survival outcomes in patients with DCIS who underwent SLNDa
| ENTIRE COHORT | Negative SLN (n=1102) |
Positive SLN findings (n=432) |
P valuec (Neg vs pos SLN) |
P valued (ITCs vs mets) |
|
|---|---|---|---|---|---|
| ITCs (n=66) |
Metastasesb (n=66) |
||||
| Adjuvant chemotherapy e | 60 (5.4) | 7 (11) | 47 (71) | <.001 | <.001 |
| Adjuvant hormonal therapy | 357 (32.4) | 23 (35) | 41 (62) | <.001 | .002 |
| Adjuvant radiation therapy | 228 (20.7) | 11 (17) | 12 (18) | .363 | .819 |
| Boost to nodal basins | 0 (0.0) | 2 (3) | 4 (6) | <.001 | .440 |
| Completion ALND | ~ | 6 (9) | 45 (68) | ~ | <.001 |
| Positive node on ALND | ~ | 0 (0) | 8 (18) | ~ | .572 |
| 5-year disease-free survival | 99.3% | 100.0% | 90.9% | <.001 | .014 |
| 5-year overall survival | 96.7% | 93.9% | 89.2% | .014 | .383 |
|
| |||||
| PURE DCIS | Negative SLN (n=851) |
Positive SLN findings (n=56) |
P valuec (Neg vs pos SLN) |
P valued (ITCs vs mets) |
|
| ITCs (n=44) |
Metastasesb (n=12) |
||||
|
| |||||
| Adjuvant chemotherapy e | 3 (0.4) | 1 (2) | 6 (50) | <.001 | <.001 |
| Adjuvant hormonal therapy | 217 (25.5) | 11 (25) | 8 (67) | .172 | .015 |
| Adjuvant radiation therapy | 158 (18.6) | 5 (11) | 0 (0) | .067 | .574 |
| Boost to nodal basins | 0 (0.0) | 0 (0) | 0 (0) | * | * |
| Completion ALND | ~ | 1 (2) | 3 (25) | ~ | .028 |
| Positive node on ALND | ~ | 0 (0) | 0 (0) | ~ | * |
| 5-year disease-free survival | 99.7% | 100.0% | 100.0% | .679 | * |
| 5-year overall survival | 96.8% | 96.9% | 90.9% | .637 | .342 |
|
| |||||
| OCCULT INVASION | Negative SLN (n=251) |
Positive SLN findings (n=76) |
P valuec (Neg vs pos SLN) |
P valued (ITCs vs mets) |
|
| ITCs (n=22) |
Metastasesb (n=54) |
||||
|
| |||||
| Adjuvant chemotherapy e | 57 (22.7) | 6 (27) | 41 (76) | <.001 | <.001 |
| Adjuvant hormonal therapy | 140 (55.8) | 12 (55) | 33 (61) | .540 | .534 |
| Adjuvant radiation therapy | 70 (27.9) | 6 (27) | 12 (22) | .447 | .639 |
| Boost to nodal basins | 0 (0.0) | 2 (9) | 4 (7) | <.001 | 1.000 |
| Completion ALND | ~ | 5 (23) | 42 (78) | ~ | <.001 |
| Positive node on ALND | ~ | 0 (0) | 8 (19) | ~ | .571 |
| 5-year disease-free survival | 97.9% | 100.0% | 88.8% | .008 | .129 |
| 5-year overall survival | 96.4% | 87.9% | 88.8% | .026 | .876 |
ALND, axillary lymph node dissection; DCIS, ductal carcinoma in situ; ITCs, isolated tumor cells; mets, metastases; SLN, sentinel lymph node.
Values in table are number of patients (percentage) unless otherwise indicated.
Metastases includes micrometastases and macrometastases.
Comparison between negative SLN group versus positive SLN group.
Comparison within positive SLN group (isolated tumor cells versus SLN metastases).
Patients who received adjuvant chemotherapy for a concurrent other cancer were included in the analysis (n=3 in the pure DCIS, negative SLN group; n=1 in the occult invasion, negative SLN group).
Unable to compute statistics because groups are exactly equivalent.
Kaplan-Meier analysis showed that patients with pure DCIS with and without positive SLN findings had equivalent 5-year disease-free survival (DFS) (100.0% and 99.7%, respectively, P=.679) (Figure 2). The 5-year DFS was 99.7% in patients with pure DCIS compared to 96.5% in patients with occult invasion (P<.001). Patients with occult invasion with positive SLN findings had the lowest 5-year actuarial DFS (91.7%, P<.001).
Figure 2.
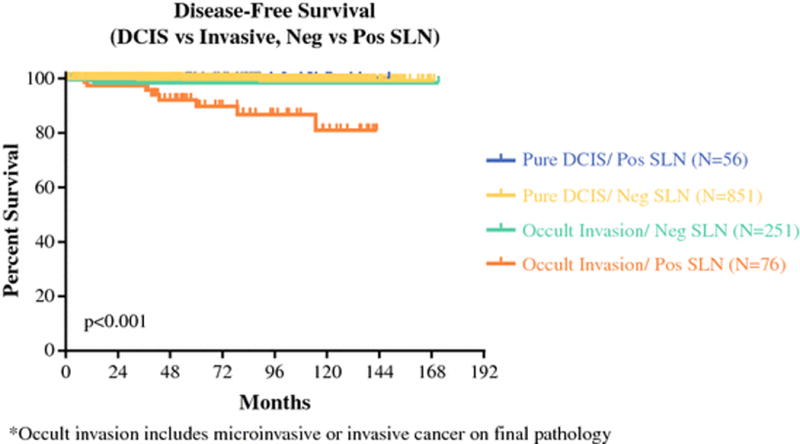
Disease-free survival as a function of invasive status on final pathology and SLN status.
Discussion
In this cohort of 1234 patients with an initial diagnosis of DCIS who underwent SLND, we found a 10.7% (n=132) incidence of positive SLN findings. Occult invasion in the primary tumor was identified in 327 patients (26.5%). Independent predictors of positive SLN findings included excisional biopsy, papillary histologic subtype, DCIS size >2 cm, more than 3 interventions, and evidence of occult invasion. Of these, more than 3 interventions and occult invasion were the strongest risk factors for positive SLN findings in patients with an initial diagnosis of DCIS. The association of positive SLN findings with increased number of interventions in both patients with pure DCIS and patients with DCIS with occult invasion supports the theory of benign mechanical transport of breast epithelial cells due to manipulation of the primary tumor. The patients with ITCs in SLNs had a 100% DFS at 5 years with few changes in management (median follow-up 55.7 months). We also found that positive SLN findings were associated with reduced 5-year DFS among patients with occult invasion but not among those with pure DCIS, suggesting that positive SLN findings impact prognosis only if occult invasion is identified.
Despite the inherently low metastatic potential of DCIS, previous studies have reported positive SLNs in 1% to 22% of patients with pure DCIS; our rate of 6.2% (ITCs in 4.9% and metastases in 1.3%) is consistent with rates in previous reports.1–14 The 2 largest series to date investigating SLND in pure DCIS, the series of Veronesi et al.8 and Intra et al. from the European Institute of Oncology,35 reported SLN metastasis rates of 1.8% (9/508) and 1.4% (12/854), respectively. These rates are concordant with our findings. Intra et al., however, reported a significantly lower ITC rate (0.8%, 4/508) than the rate reported in our study, likely as a result of the less routine use of immunohistochemical analysis at their institution.
Our study identified more than 3 interventions and occult invasion as the strongest predictors of positive SLN findings in patients with DCIS. The relationship between occult invasion and increased risk of positive SLNs has previously been demonstrated.36,37 The theory of benign mechanical transport of cells secondary to preoperative biopsy, surgical excision, or tumor massage has been proposed as a potential source for positive nodal findings.15–29 To our knowledge, however, the present study is the first to investigate the total number of interventions (total number of biopsies and surgeries prior to SLND) as a predictor of positive SLN findings. Our data suggest that increasing number of interventions is associated with higher incidence of positive SLN findings in DCIS; this finding may support the theory of benign mechanical transport of breast epithelial cells.
In our study, we specifically sought to examine a cohort of patients with an initial diagnosis of DCIS undergoing SLND. Few studies have taken this approach. Most previous studies have chosen to investigate a more heterogeneous group of patients with an initial diagnosis of either DCIS or DCIS with microinvasion or have chosen to exclude patients with evidence of occult invasion on final pathology. This latter patient group is not insubstantial and traditionally accounts for 10% to 25% of patients with an initial diagnosis of DCIS depending on the biopsy method used; in fact, they accounted for 26.5% of patients in our study (36.0% for small-bore percutaneous biopsy, 24.0% for large-bore percutaneous biopsy and 13.5% for excisional biopsy). We designed this study to closely mimic the decision-making process that clinicians are faced with when treating patients with a presumptive diagnosis of DCIS because we wished to determine whether all, none, or a select group of patients with DCIS should undergo SLND. As demonstrated in our study, it is the select group of DCIS patients found to have evidence of occult invasion on final pathology for whom positive SLN findings impact prognosis and who thus may benefit from SLND and adjunct therapies. While larger-bore percutaneous biopsy has decreased the rate of upstaging, upstaging does still occur and the question remains how to best identify patients at high risk for invasive disease to determine who will benefit from SLND. In patients undergoing breast-preserving surgery, one reasonable approach may be to avoid SLND unless invasive disease is discovered on final pathology. This would save 66% of patients undergoing breast-preserving surgery an unnecessary procedure and may reduce overall costs of treatment. Another approach is to avoid the use of immunohistochemical analysis of SLNs. This would likely reduce the finding of ITCs which can introduce difficulty in determining the need for ALND and systemic therapy in patients with pure DCIS.
Our study has certain limitations including the retrospective study design and its inherent biases. Because this was a single-institution study, external validation is needed. Most of the patients in our series were treated with mastectomy due to large tumor size or extensive microcalcifications. We included only DCIS patients undergoing SLND; we did not examine DCIS patients selected not to undergo SLND, and thus, we were unable to examine the possibility that the SLND procedure itself had an impact on survival in patients with positive SLN findings. Using a propensity-score-based analysis to compare survival between patients who did and did not undergo SLND could provide additional evidence regarding the utility of SLND in DCIS.
Overall, our study demonstrates that occult invasion and more than 3 interventions are the strongest risk factors for positive SLN findings in patients with an initial diagnosis of DCIS. This may support the theory of benign mechanical transport of breast epithelial cells. Positive SLN findings appear to impact prognosis only when occult invasion is present in the final resected specimen, suggesting that routine use of SLND in patients with DCIS is not warranted other than in patients at high risk for invasive disease.
Synopsis.
Occult invasion and more than 3 total interventions were the strongest risk factors for positive SLN findings in DCIS. Positive SLN findings impact prognosis only when occult invasion is present. Other than in patients at high risk for invasive disease, routine use of SLND in patients with DCIS is not warranted.
Acknowledgments
This work was supported by grants from the National Institutes of Health, the Cancer Center Support Grant CA016672 (Ronald DePinho) and the T32 grant CA009599 (Funda Meric-Bernstam). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. We thank Stephanie Deming for editorial assistance.
References
- 1.Silverstein MJ, Rosser RJ, Gierson ED, et al. Axillary lymph node dissection for intraductal breast carcinoma–is it indicated? Cancer. 1987 May 15;59(10):1819–1824. doi: 10.1002/1097-0142(19870515)59:10<1819::aid-cncr2820591023>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 2.Intra M, Veronesi P, Mazzarol G, et al. Axillary sentinel lymph node biopsy in patients with pure ductal carcinoma in situ of the breast. Archives of surgery. 2003 Mar;138(3):309–313. doi: 10.1001/archsurg.138.3.309. [DOI] [PubMed] [Google Scholar]
- 3.Cox CE, Nguyen K, Gray RJ, et al. Importance of lymphatic mapping in ductal carcinoma in situ (DCIS): why map DCIS? The American surgeon. 2001 Jun;67(6):513–519. discussion 519–521. [PubMed] [Google Scholar]
- 4.Klauber-DeMore N, Tan LK, Liberman L, et al. Sentinel lymph node biopsy: is it indicated in patients with high-risk ductal carcinoma-in-situ and ductal carcinoma-in-situ with microinvasion? Annals of surgical oncology. 2000 Oct;7(9):636–642. doi: 10.1007/s10434-000-0636-2. [DOI] [PubMed] [Google Scholar]
- 5.Kelly TA, Kim JA, Patrick R, Grundfest S, Crowe JP. Axillary lymph node metastases in patients with a final diagnosis of ductal carcinoma in situ. American journal of surgery. 2003 Oct;186(4):368–370. doi: 10.1016/s0002-9610(03)00276-9. [DOI] [PubMed] [Google Scholar]
- 6.Lara JF, Young SM, Velilla RE, Santoro EJ, Templeton SF. The relevance of occult axillary micrometastasis in ductal carcinoma in situ: a clinicopathologic study with long-term follow-up. Cancer. 2003 Nov 15;98(10):2105–2113. doi: 10.1002/cncr.11761. [DOI] [PubMed] [Google Scholar]
- 7.Pendas S, Dauway E, Giuliano R, Ku N, Cox CE, Reintgen DS. Sentinel node biopsy in ductal carcinoma in situ patients. Annals of surgical oncology. 2000 Jan-Feb;7(1):15–20. doi: 10.1007/s10434-000-0015-z. [DOI] [PubMed] [Google Scholar]
- 8.Veronesi P, Intra M, Vento AR, et al. Sentinel lymph node biopsy for localised ductal carcinoma in situ? Breast. 2005 Dec;14(6):520–522. doi: 10.1016/j.breast.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Zavotsky J, Hansen N, Brennan MB, Turner RR, Giuliano AE. Lymph node metastasis from ductal carcinoma in situ with microinvasion. Cancer. 1999 Jun 1;85(11):2439–2443. doi: 10.1002/(sici)1097-0142(19990601)85:11<2439::aid-cncr19>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 10.Camp R, Feezor R, Kasraeian A, et al. Sentinel lymph node biopsy for ductal carcinoma in situ: an evolving approach at the University of Florida. The breast journal. 2005 Nov-Dec;11(6):394–397. doi: 10.1111/j.1075-122X.2005.00128.x. [DOI] [PubMed] [Google Scholar]
- 11.Cserni G. Sentinel lymph node biopsy as a tool for the staging of ductal carcinoma in situ in patients with breast carcinoma. Surgery today. 2002;32(2):99–103. doi: 10.1007/s005950200000. [DOI] [PubMed] [Google Scholar]
- 12.Farkas EA, Stolier AJ, Teng SC, Bolton JS, Fuhrman GM. An argument against routine sentinel node mapping for DCIS. The American surgeon. 2004 Jan;70(1):13–17. discussion 17–18. [PubMed] [Google Scholar]
- 13.Rahusen FD, Meijer S, Taets van Amerongen AH, Pijpers R, van Diest PJ. Sentinel node biopsy for nonpalpable breast tumors requires a preoperative diagnosis of invasive breast cancer. The breast journal. 2003 Sep-Oct;9(5):380–384. doi: 10.1046/j.1524-4741.2003.09503.x. [DOI] [PubMed] [Google Scholar]
- 14.Mittendorf EA, Arciero CA, Gutchell V, Hooke J, Shriver CD. Core biopsy diagnosis of ductal carcinoma in situ: an indication for sentinel lymph node biopsy. Current surgery. 2005 Mar-Apr;62(2):253–257. doi: 10.1016/j.cursur.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 15.Carter BA, Jensen RA, Simpson JF, Page DL. Benign transport of breast epithelium into axillary lymph nodes after biopsy. American journal of clinical pathology. 2000 Feb;113(2):259–265. doi: 10.1309/7EF8-F1W7-YVNT-H8H5. [DOI] [PubMed] [Google Scholar]
- 16.Diaz LK, Wiley EL, Venta LA. Are malignant cells displaced by large-gauge needle core biopsy of the breast? AJR. American journal of roentgenology. 1999 Nov;173(5):1303–1313. doi: 10.2214/ajr.173.5.10541110. [DOI] [PubMed] [Google Scholar]
- 17.Diaz NM, Cox CE, Ebert M, et al. Benign mechanical transport of breast epithelial cells to sentinel lymph nodes. The American journal of surgical pathology. 2004 Dec;28(12):1641–1645. doi: 10.1097/00000478-200412000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Diaz NM, Mayes JR, Vrcel V. Breast epithelial cells in dermal angiolymphatic spaces: a manifestation of benign mechanical transport. Human pathology. 2005 Mar;36(3):310–313. doi: 10.1016/j.humpath.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Diaz NM, Vrcel V, Centeno BA, Muro-Cacho C. Modes of benign mechanical transport of breast epithelial cells to axillary lymph nodes. Advances in anatomic pathology. 2005 Jan;12(1):7–9. doi: 10.1097/01.pap.0000151267.34438.a1. [DOI] [PubMed] [Google Scholar]
- 20.Hansen NM, Ye X, Grube BJ, Giuliano AE. Manipulation of the primary breast tumor and the incidence of sentinel node metastases from invasive breast cancer. Archives of surgery. 2004 Jun;139(6):634–639. doi: 10.1001/archsurg.139.6.634. discussion 639–640. [DOI] [PubMed] [Google Scholar]
- 21.Hoorntje LE, Schipper ME, Kaya A, Verkooijen HM, Klinkenbijl JG, Borel Rinkes IH. Tumour cell displacement after 14G breast biopsy. European journal of surgical oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2004 Jun;30(5):520–525. doi: 10.1016/j.ejso.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 22.Liberman L, Vuolo M, Dershaw DD, et al. Epithelial displacement after stereotactic 11-gauge directional vacuum-assisted breast biopsy. AJR. American journal of roentgenology. 1999 Mar;172(3):677–681. doi: 10.2214/ajr.172.3.10063859. [DOI] [PubMed] [Google Scholar]
- 23.Moore KH, Thaler HT, Tan LK, Borgen PI, Cody HS., 3rd Immunohistochemically detected tumor cells in the sentinel lymph nodes of patients with breast carcinoma: biologic metastasis or procedural artifact? Cancer. 2004 Mar 1;100(5):929–934. doi: 10.1002/cncr.20035. [DOI] [PubMed] [Google Scholar]
- 24.Nagi C, Bleiweiss I, Jaffer S. Epithelial displacement in breast lesions: a papillary phenomenon. Archives of pathology & laboratory medicine. 2005 Nov;129(11):1465–1469. doi: 10.5858/2005-129-1465-EDIBLA. [DOI] [PubMed] [Google Scholar]
- 25.Rosser RJ. A Point of View: Trauma is the Cause of Occult Micrometastatic Breast Cancer in Sentinel Axillary Lymph Nodes. The breast journal. 2000 May;6(3):209–212. doi: 10.1046/j.1524-4741.2000.20002.x. [DOI] [PubMed] [Google Scholar]
- 26.Tvedskov TF, Jensen MB, Kroman N, Balslev E. Iatrogenic displacement of tumor cells to the sentinel node after surgical excision in primary breast cancer. Breast cancer research and treatment. 2012 Jan;131(1):223–229. doi: 10.1007/s10549-011-1720-y. [DOI] [PubMed] [Google Scholar]
- 27.van Deurzen CH, Bult P, de Boer M, et al. Morphometry of isolated tumor cells in breast cancer sentinel lymph nodes: metastases or displacement? The American journal of surgical pathology. 2009 Jan;33(1):106–110. doi: 10.1097/PAS.0b013e31817eec40. [DOI] [PubMed] [Google Scholar]
- 28.Youngson BJ, Cranor M, Rosen PP. Epithelial displacement in surgical breast specimens following needling procedures. The American journal of surgical pathology. 1994 Sep;18(9):896–903. doi: 10.1097/00000478-199409000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Youngson BJ, Liberman L, Rosen PP. Displacement of carcinomatous epithelium in surgical breast specimens following stereotaxic core biopsy. American journal of clinical pathology. 1995 May;103(5):598–602. doi: 10.1093/ajcp/103.5.598. [DOI] [PubMed] [Google Scholar]
- 30.Tan JC, McCready DR, Easson AM, Leong WL. Role of sentinel lymph node biopsy in ductal carcinoma-in-situ treated by mastectomy. Annals of surgical oncology. 2007 Feb;14(2):638–645. doi: 10.1245/s10434-006-9211-9. [DOI] [PubMed] [Google Scholar]
- 31.Yen TW, Hunt KK, Ross MI, et al. Predictors of invasive breast cancer in patients with an initial diagnosis of ductal carcinoma in situ: a guide to selective use of sentinel lymph node biopsy in management of ductal carcinoma in situ. Journal of the American College of Surgeons. 2005 Apr;200(4):516–526. doi: 10.1016/j.jamcollsurg.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 32.Lyman GH, Temin S, Edge SB, et al. Sentinel lymph node biopsy for patients with early-stage breast cancer: american society of clinical oncology clinical practice guideline update. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2014 May 1;32(13):1365–1383. doi: 10.1200/JCO.2013.54.1177. [DOI] [PubMed] [Google Scholar]
- 33.Hunt KK, Yi M, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy is accurate and reduces the need for axillary dissection in breast cancer patients. Annals of surgery. 2009 Oct;250(4):558–566. doi: 10.1097/SLA.0b013e3181b8fd5e. [DOI] [PubMed] [Google Scholar]
- 34.Edge SB, American Joint Committee on Cancer . AJCC cancer staging manual. 7th. New York: Springer; 2010. [DOI] [PubMed] [Google Scholar]
- 35.Intra M, Rotmensz N, Veronesi P, et al. Sentinel node biopsy is not a standard procedure in ductal carcinoma in situ of the breast: the experience of the European institute of oncology on 854 patients in 10 years. Annals of surgery. 2008 Feb;247(2):315–319. doi: 10.1097/SLA.0b013e31815b446b. [DOI] [PubMed] [Google Scholar]
- 36.Cserni G, Boross G, Maraz R, et al. Multicentre validation of different predictive tools of non-sentinel lymph node involvement in breast cancer. Surgical oncology. 2012 Jun;21(2):59–65. doi: 10.1016/j.suronc.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 37.Yi M, Krishnamurthy S, Kuerer HM, et al. Role of primary tumor characteristics in predicting positive sentinel lymph nodes in patients with ductal carcinoma in situ or microinvasive breast cancer. American journal of surgery. 2008 Jul;196(1):81–87. doi: 10.1016/j.amjsurg.2007.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]


