Abstract
The alleviating effect of silicon (Si) supply on aluminum (Al) toxicity was suggested to be based on ex or in planta mechanisms. In our experiments with the Al-sensitive maize (Zea mays) cultivar Lixis, Si treatment but not Si pretreatment ameliorated Al-induced root injury as revealed by less root-growth inhibition and callose formation. Si treatment did not affect monomeric Al concentrations in the nutrient solution, suggesting an in planta effect of Si on Al resistance. A fractionated analysis of Si and Al in the 1-cm root apices revealed that more than 85% of the root-tip Al was bound in the cell wall. Al contents in the apoplastic sap, the symplastic sap, and the cell wall did not differ between −Si and +Si plants. Si did not affect the Al-induced exudation of organic acid anions and phenols from the root apices. However, Al treatment greatly enhanced Si accumulation in the cell wall fraction, reducing the mobility of apoplastic Al. From our data we conclude that Si treatment leads to the formation of hydroxyaluminumsilicates in the apoplast of the root apex, thus detoxifying Al.
Aluminum (Al) toxicity is one of the main factors limiting plant growth and crop yields in acid soils. Although much progress has been made during recent years, the mechanisms of Al-induced inhibition of root elongation and Al resistance are still not well understood. There are a number of excellent reviews in recent years summarizing the state of knowledge and addressing knowledge gaps (Kochian, 1995; Taylor, 1995; Delhaize and Ryan, 1995; Matsumoto, 2000; Kochian et al., 2002). Particularly, the relative importance of symplastic versus apoplastic lesions of Al toxicity remains a matter of debate. Rengel (1996) and especially Horst (1995) focused the attention on the role of the apoplast in Al toxicity regarding short-term inhibition of root elongation by Al.
Silicon (Si) is a beneficial mineral element for plants and even a plant nutrient for some plant species (Epstein, 1999). The role of Si in plant resistance against biotic and abiotic stresses has been attributed particularly to modification of cell wall properties (Chérif et al., 1992; Horst et al., 1999a; Fawe et al., 2001; Lux et al., 2002). Iwasaki et al. (2002a, 2002b) and Rogalla and Römheld (2002) showed that Si-enhanced manganese-leaf tolerance is related to a reduction in the concentration of Mn2+ in the leaf apoplastic washing fluid in cowpea (Vigna unguiculata) and cucumber (Cucumis sativus), respectively. Si has been reported to alleviate Al toxicity in conifers (Ryder et al., 2003), barley (Hordeum vulgare; Hammond et al., 1995), soybean (Glycine max; Baylis et al., 1994), maize (Zea mays; Barceló et al., 1993), and sorghum (Sorghum bicolor; Galvez et al., 1987). Little or no effect of Si on Al resistance has been found in wheat (Triticum aestivum), pea (Pisum sativum; Hodson and Evans, 1995), and cotton (Gossypium hirsutum; Li et al., 1989), but this may have been due to methodological shortcomings (Ryder et al., 2003). The beneficial role of Si has been suggested to be based on two aspects: solution chemistry and in planta mechanisms (Cocker et al., 1998a). Ma et al. (1997) suggested that the ameliorative effect of Si on Al toxicity resulted from decreasing the toxic Al3+ concentration in solution by forming Al-Si complexes. On the other hand, some researchers observed in planta effects of Si on Al resistance (Hammond et al., 1995; Corrales et al., 1997; Kidd et al., 2001). Kidd et al. (2001) suggested that an enhanced exudation of phenolic compounds leading to complexation and thus detoxification of Al is responsible for the Si-mediated enhanced Al resistance in an Al-resistant maize cultivar. More recently, Ryder et al. (2003) concluded from their work with Norway spruce (Picea abies) seedlings that the amelioration of Al toxicity by Si could be best explained by a combination of both bulk solution and in planta effects.
The majority of the work on Si effects on plant Al resistance has been focused on the whole root and/or shoot system with relative long Al treatment periods, usually several days (Hodson and Evans, 1995). However, Al phytotoxicity expresses within minutes and hours in the root apices (Sivaguru and Horst, 1998). Therefore, the objective of this study was to better understand short-term effects of Al on root injury with special emphasis on Al/Si interaction in the root apoplast, which is the primary target of Al (Horst, 1995; Horst et al., 1999b). An Al-sensitive maize cultivar was chosen for the main study because we expected that an in planta amelioration of Al toxicity by Si would be more clearly expressed than in an Al-resistant cultivar, which requires a much higher Al concentration in the external medium for Al injury to occur.
RESULTS
Short-Term Experiments
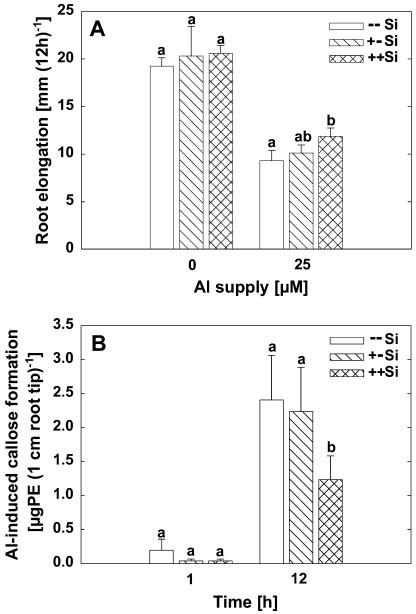
Al inhibited root elongation to about 50% within 12 h of Al treatment (Fig. 1A). Si supply during pretreatment and the Al treatment period significantly reduced the impact of Al on root elongation, whereas Si supply only during the pretreatment did not. Al greatly stimulated callose formation in the root apices (Fig. 1B). Al-induced callose formation reflected the ameliorative effect of Si supply during pretreatment and Al treatment on root injury even more clearly. Again, Si supply only during pretreatment did not enhance plant Al resistance as revealed by the nonaffected callose formation.
Figure 1.
Effect of Si on root elongation (A) and on Al-induced callose formation (B) of maize cv Lixis supplied without or with 25 μm Al in a solution containing 500 μm CaCl2 and 8 μm H3BO3, pH 4.3. Plants were precultured for 36 h without or with 1.4 mm Si and then treated without or with 25 μm Al for 12 h in the presence or absence of 1.4 mm Si. Si, Without Si during preculture and Al treatment; +Si, with Si during preculture, without Si during Al treatment; ++Si, with Si during preculture and Al treatment. Bars show sd. Significant differences between mean values are indicated by different letters at the P < 0.05 level (Tukey test). n = 3 for root elongation, n = 5 for callose formation.
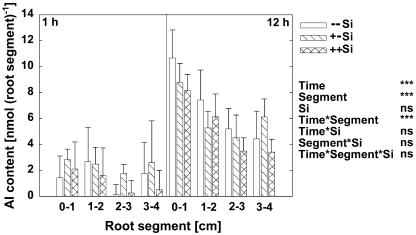
Al contents in the root segments of the primary root tip were measured after 1 and 12 h of Al treatment (Fig. 2). Overall, there was no significant difference between Si treatments. Al contents in root segments increased with prolonged Al treatment. There was a significant difference between root segments. Root segments closer to the root apex accumulated higher amounts of Al.
Figure 2.
Al contents of apical root segments of maize cv Lixis as affected by Si and Al supply grown in a solution containing 500 μm CaCl2 and 8 μm H3BO3, pH 4.3. Plants were precultured for 36 h without or with 1.4 mm Si and then treated without or with 25 μm Al for 1 h or 12 h in the presence or absence of 1.4 mm Si. Al contents at 0 μm Al supply were subtracted from the 25 μm Al treatment. Bars show sd, n = 5. ***, Significance at the P < 0.001 level according to the F test. ns, Not significant.
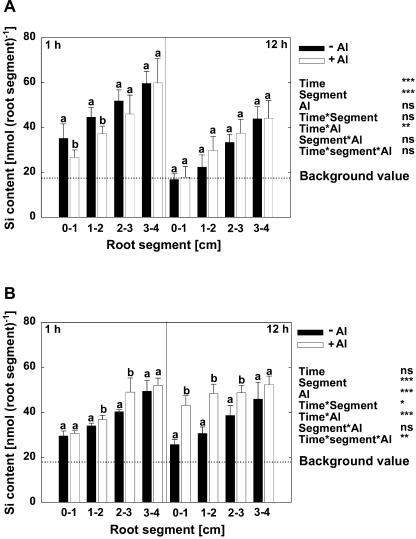
After 1 and 12 h of Al supply, Si contents in 1-cm root segments were measured (Fig. 3). The Si contents of the root tips of control plants (−Si treatment) were considered as background value. Si contents of the root segments of Si-treated plants gradually increased from the apical to the more basal root sections in all treatments (Fig. 3). After 1 h of growth in Si-free solution, the Si contents of all root sections were significantly above the background level (Fig. 3A). However, after 12 h of growth, the Si contents of all root sections decreased, in the apical 1 cm even to the background level. This shows that Si accumulated during the pretreatment period could not be transferred apically to the newly formed root tips. In the presence of Si also during the Al treatment period (Fig. 3B), the Si contents in all root segments were well above the background level. After 1 h of Al treatment, Si contents of root segments were slightly higher in presence of Al (significant only for the root zones 1–2 and 2–3 cm). But after 12 h, the Si contents of all Al-treated root zones, particularly the root apex, were clearly higher than in the root sections not treated with Al. Thus, it appears that the presence of elevated Si contents in the root apex is a prerequisite for the ameliorative effect of Si on Al toxicity.
Figure 3.
Si contents of root segments of maize cv Lixis as affected by Si and Al supply grown in a solution containing 500 μm CaCl2 and 8 μm H3BO3, pH 4.3. Plants were precultured for 36 h without or with 1.4 mm Si and then treated without or with 25 μm Al for 1 h or 12 h in the absence (A) or presence (B) of 1.4 mm Si. The background value (dashed line) presents the mean Si content of the root segments without Si treatment. Bars show sd. Significant differences between mean values are indicated by different letters at the P < 0.05 level (Tukey test), n = 5. *, **, and ***, Significance at the P < 0.05, 0.01, and 0.001 levels according to the F test. ns, Not significant.
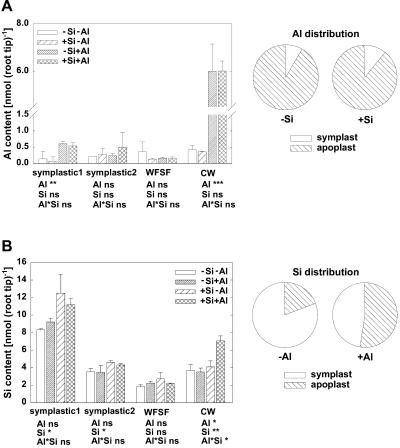
Since total root-tissue contents of Al and Si do not reveal their cellular distribution, their contents in different fractions of the apical 1-cm root tips were determined (Fig. 4). In Al-treated plants, only slightly higher Al contents could be found in the symplastic fraction. More than 85% of the root-tip Al was detected in the cell wall and, thus, the root apoplast (Fig. 4A). There was no significant difference between −Si and +Si plants in the Al content and its distribution. This indicates that the ameliorative effect of Si was not due to lower Al uptake into the root apex of maize.
Figure 4.
The contents (left) and relative distribution (right) of Al (A) and Si (B) in the symplast (symplastic1, 2), WFSF, and cell walls (CW) of 1-cm root tips of maize cv Lixis as affected by Si and Al supply in a solution containing 500 μm CaCl2 and 8 μm H3BO3, pH 4.3. Plants were precultured for 36 h without or with 1.4 mm Si and then treated without or with 25 μm Al for 12 h in the absence or presence of 1.4 mm Si. Relative distribution after subtracting the background Al or Si contents in −Al or −Si treatments, respectively. Bars show sd, n = 3. *, **, and ***, Significance at the P < 0.05, 0.01, and 0.001 levels according to the F test. ns, Not significant.
Si treatment significantly enhanced Si contents in the symplastic fractions but not in the water free space fluid (WFSF) (Fig. 4B). Whereas the Si content of the cell walls was only slightly affected by Si supply in absence of Al, it was greatly increased in Al-treated plants. This is particularly well illustrated by the change of the relative distribution of Si between symplast and apoplast. In −Al plants, 81% of the total Si was localized in the symplast and only 19% in the apoplast, while in +Al plants, 53% of the total Si was in the apoplast and 47% in the symplast. This indicates that Si modifies Al binding to the cell walls of root apices.
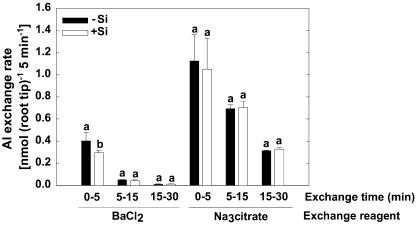
The binding stage of Al in the cell walls of the root apex was studied using a fractionated desorption procedure with BaCl2 followed by Na3citrate as extractants. With the exception of the first 5-min BaCl2-exchangable Al fraction, there were no differences between Si treatments (Fig. 5). The amount of readily BaCl2-exchangable cell wall Al in −Si plants was higher than in +Si plants.
Figure 5.
Al exchange rate of cell walls isolated from 1-cm root tips of maize cv Lixis. Cell walls were desorbed sequentially in 50 mm BaCl2, pH 4.3, for 5, 10, and 15 min, followed by desorption in 33 mm Na3citrate, pH 5.8, for 5, 10, and 15 min. Plants were precultured in a solution containing 500 μm CaCl2 and 8 μm H3BO3, pH 4.3, for 36 h without or with 1.4 mm Si and then treated without or with 25 μm Al for 12 h in the presence or absence of 1.4 mm Si. Bars show sd, n = 4. Significant differences between mean values are indicated by different letters at the P < 0.05 level (Tukey test).
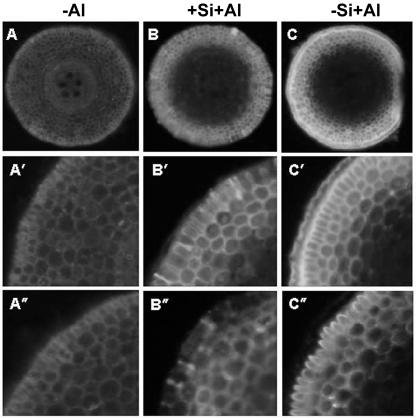
Distribution and biological activity of Al was also studied using morin as a stain for Al in root cross-sections (Fig. 6). After 1 h of Al treatment, Al entered up to 3 layers of cortical cells. Bright fluorescence in the apoplast showed that the cell walls were the main sites of Al localization. Clear differences in Al distribution were visible between the Si treatments. Without Si supply, Al treatment resulted in a bright morin-Al fluorescence of the outer tangential wall of all epidermal cells. In the +Si treatments, many epidermal cells were not fluorescent, with the exception of some radial cell walls of epidermal cells.
Figure 6.
Fluorescence of the morin-Al complex in root cross-sections of maize cv Lixis 1 to 3 mm behind the root tip. Plants were precultured in a solution containing 500 μm CaCl2 and 8 μm H3BO3, pH 4.3, for 36 h without or with 1.4 mm Si and then treated without or with 25 μm Al for 1 h in the presence or absence of 1.4 mm Si. Excitation filter 395 to 440 nm, barrier filter 470 nm. A to C, Cross-sections from the root zone 1 to 2 mm behind the root tip. A′ to C′, Close-up of Al staining of epidermal and cortical cells in A to C, respectively. A″ to C″, Close-up of Al staining of epidermal and cortical cells in cross-sections from the root zone 2 to 3 mm behind the root tip.
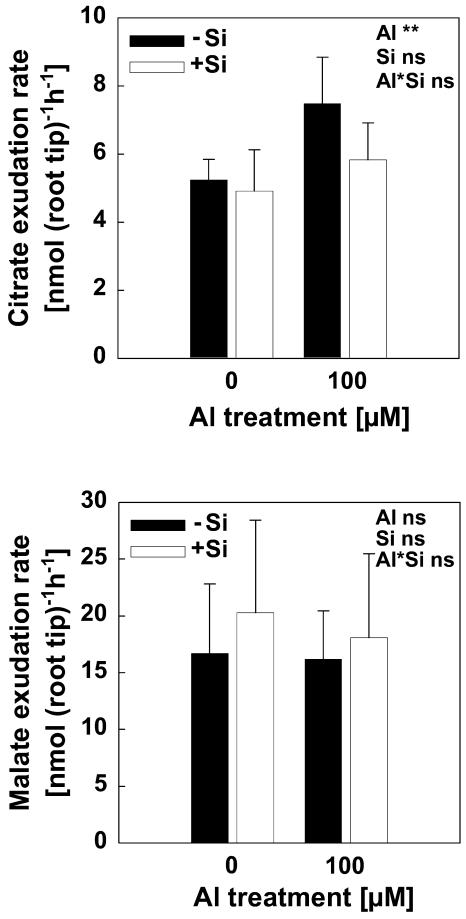
Organic acid anion exudation is a well-documented Al resistance mechanism in maize. In order to determine whether either Si or Al and Si together interfere with this resistance mechanism, we determined the release of organic acid anions from root apices during short-term (2-h) Al treatment (Fig. 7). Al induced citrate exudation, but Si did not show a significant effect on citrate excretion of the root tips. There was even a trend of lower citrate release in Al- and Si-treated plants that may reflect less Al stress in the presence of Si (see above). Malate exudation was not affected either by Al or Si. No oxalate exudation was detected in this experiment.
Figure 7.
Citrate and malate exudation of 1-cm root tips of intact plants of maize cv Lixis. Plants were precultured in 500 μm CaCl2 and 8 μm H3BO3 solution at pH 4.3 for 24 h without or with 1.4 mm Si. Then roots of 10 plants were bundled and the tips (1 cm) incubated for 2 h in 5 mL of the same solution with different Al and Si levels according to the treatments. Bars show sd. Results of two experiments were combined, and data are means of six replicates.
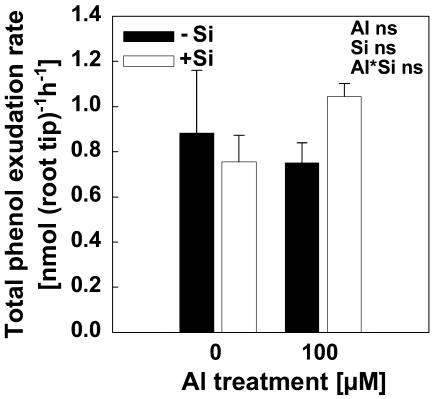
Because phenol exudation was reported to confer Si-induced Al resistance in maize (Kidd et al., 2001), we also investigated the effect of Si and Al on phenol exudation from root apices in our short-term experiments. The result showed that neither Al nor Si induced phenol exudation (Fig. 8).
Figure 8.
Total phenol exudation of 2-cm root tips of intact plants of maize cv Lixis. Plants were precultured in 500 μm CaCl2 and 8 μm H3BO3 solution at pH 4.3 for 24 h without or with 1.4 mm Si. Then the roots of 10 plants were bundled and the tips (2 cm) were incubated for 2 h in 5 mL of the same solution with different Al and Si levels according to the treatments. Bars show sd, n = 4.
Long-Term Experiments
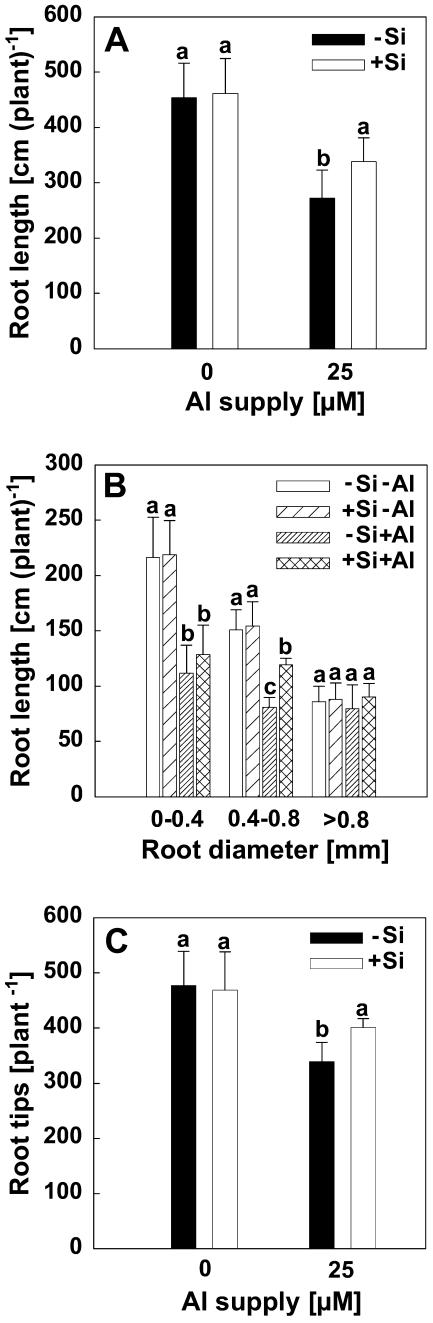
The ameliorative effect of Si on Al toxicity was even clearer in long-term experiments (44 h of Al treatment). Al supply significantly decreased the root growth either in the presence or absence of Si as shown in Figure 9A. Without Al supply, there was no difference between Si treatments. For the 25 μm Al treatment, total root length of +Si plants was higher than that of −Si plants, which was mainly due to higher lateral root length of +Si plants (Fig. 9B). The number of root tips was decreased by Al supply. Si alone had no effect on the number of root tips, but Si enhanced the number of root tips under conditions of Al toxicity (Fig. 9C).
Figure 9.
Effect of Si on total root length (A), root length of different root classes (B), and the number of root tips (C) of maize cv Lixis exposed to 0 and 25 μm Al in a basic solution containing 500 μm CaCl2 and 8 μm H3BO3 at pH 4.3. Plants were precultured for 36 h without or with 1.4 mm Si and then treated without or with 25 μm Al for 44 h in the presence or absence of 1.4 mm Si. Bars show sd, n = 6. Significant differences between mean values are indicated by different letters at the P < 0.05 level (Tukey test).
DISCUSSION
The ameliorative effect of Si on Al toxicity in plants was attributed to a decreased availability of phytotoxic Al in the culture media by some authors (Baylis et al., 1994; Ma et al., 1997). This decrease in Al concentration is supposed to be due to the formation of biologically inactive complexes of hydroxyaluminumsilicates (HAS). Besides the chemical reaction in solution, in planta detoxification was suggested based on experiments where amelioration was observed but HAS formation was minimal (Corrales et al., 1997; Kidd et al., 2001).
A major problem in investigating Al and Si interactions in hydroponic culture over the last 15 years has been uncertainties concerning the chemistry of Al and Si in the solution in which the plants were grown (Ryder et al., 2003). It is well known that at neutral and moderately acid solution pH, Al and Si will form HAS, but Al toxicity mainly occurs at pH values below 5. Doucet et al. (2001) found that HAS were not identified in any solution in which the precipitation of Al hydroxide was not predicted. A review by Exley et al. (2002) demonstrated that the formation of an Al hydroxide template was a prerequisite for HAS formation. These findings suggest that in acid solutions (pH 4.3), HAS formation is not a major factor because only low concentrations of Al hydroxide exist in low-pH solutions. Under our experimental conditions, it can be assumed that the HAS formation in the Al treatment solution was low. The assumption is supported by the fact that we could not detect any changes in the concentration of inorganic monomeric Al, which has been shown to be the most physiologically active phytotoxic form of Al (Kerven et al., 1989). Therefore, we consider an in planta effect as the main contributing factor to the amelioration of Al toxicity by Si, under our experimental conditions.
Horst (1995) proposed that the apoplast of the root tip is the primary site of Al phytotoxicity. Al binds rapidly to the negatively charged binding sites in the cell wall, altering cell wall properties and thus affecting root growth. Different mechanisms are discussed on how Si could exert its positive effect on Al resistance. Corrales et al. (1997) suggested that esterification of cell wall components by Si reduces the binding of Al to the cell wall. Kidd et al. (2001) suggested that an enhanced exudation of phenolic compounds is responsible for the Si-induced Al resistance in maize. Both mechanisms would lead to reduce Al concentrations in the apoplast. Cocker et al. (1998b) proposed the formation of HAS in the apoplast, so that Al would be transferred into a nonphytotoxic form, without reducing the Al content. This conclusion is supported by the results of Hodson and Sangster (1993). Using x-ray microanalysis, they showed the copresence of Si and Al in epidermal cells of sorghum roots treated with both Al and Si. Later, in roots of Al- and Si-treated wheat, Cocker et al. (1997) found Al and Si colocalized in epidermal and hypodermal cells.
In the experiments presented here, the total amount of Al in the cell wall, as well as in any other cell fraction, was not changed by Si treatment (Fig. 4A). But the exchangeability of the cell wall-bound Al changed. The easily exchangeable Al fraction was reduced by Si (Fig. 5). Concomitant with this modification of Al binding, we found a change in the cellular distribution of Si (Fig. 4B). Al treatment shifted the cellular Si distribution from the cytoplasmic to the cell wall fraction. These findings support the hypothesis that the formation of Al-Si complexes is responsible for the ameliorative effect of Si and are in agreement with the observation of Cocker (1997). In her study with wheat, the fluorescent dye morin was employed to localize Al. She showed that roots treated with both Al and Si were less fluorescent than roots treated with Al alone. Morin is believed to bind only to biologically active Al (Browne et al., 1990). Therefore, her results suggested that although Si did not reduce the concentration of total Al, it might have reduced the concentration of biologically active Al within the cell wall. The morin staining method was also applied in this study (Fig. 6). Instead of staining whole root tips after long-time Al treatment (Cocker, 1997), cross-sections from the root apical 1 to 3 mm behind the root tip were examined after 1 h of Al treatment. The results were generally in accordance with the results of Cocker (1997). After 1 h of Al treatment, Al entered up to 3 layers of cortical cells, and the most fluorescent compartment was the cell wall. In the presence of Si, the general staining of Al with morin was less intense, with the exception of a few radial walls of the epidermis. A possible explanation for this phenomenon could be that silicic acid on the root surface retarded Al accumulating on the root surface. The other possible explanation is the formation of HAS. High Al concentrations in the epidermis (Marienfeld et al., 2000) and the relative high pH (compared to the bulk solution) on the root surface of the distal transition zone (Kollmeier et al., 2000) will favor HAS formation, thus reducing the biologically active Al concentration. These results are consistent with the results of Cocker et al. (1997), who detected Al and Si codeposits in the outer tangential walls of the root epidermis of wheat.
The morin technique cannot provide quantitative information on Al localization and binding stage in planta. Therefore, we applied a fractionation technique. In Si-treated roots, the most mobile cell wall Al fraction that could be desorbed with BaCl2 within 5 min was significantly reduced by Si treatment. However, this fraction represented only a 2% difference between the Si treatments when related to the total Al content of the root tip. So the question arises whether these 2% less loosely bound Al in the cell wall of Si-treated plants can account for the Si-amelioration effect observed. Obviously, this fraction and the WFSF fraction are characterized by a particularly high mobility in the apoplast. Therefore, it can be expected that part of these fractions were recovered in the symplastic1 fraction during the extraction/centrifugation steps, which then is overestimated at the expense of the apoplastic fractions. The mobile apoplastic fractions are expected to determine the Al activity at the plasma membrane and thus Al toxicity (Kinraide, 1994) as revealed by enhanced callose formation in the presence of Al (Fig. 1B).
Hodson and Wilkins (1991) localized Al in the roots of Norway spruce using x-ray microanalysis. They found that Si concentrations in the cortical cell walls of the Al-resistant plants increased in response to Al treatment. Using an Al-sensitive maize cultivar, we found a similar response: The Si content of the apical 1-cm primary root increased with Al treatment. The results of this study support the proposition that Si exerts its beneficial effect on the expression of Al toxicity through the formation of nonphytotoxic HAS in the apoplast. This assumption is based on the fact that Si treatment leads to similar Al contents but less loosely bond Al in the cell walls of Al-treated root tips. Additionally, the ameliorative effect of Si on Al toxicity occurred only when sufficient Si was present in the root tips. When Si was applied to the plants only during pretreatment and the Si content of the 1-cm root apex was diluted by growth to the background Si level (Fig. 3A), no ameliorative effect of Si was found (Fig. 1). This finding conflicts with the results of Corrales et al. (1997). They showed that an Al-sensitive maize variety pretreated with Si and then exposed to Al for 24 h in the absence of Si showed higher root growth rates than plants not pretreated with Si, and the ameliorative effect of Si was due to lower Al uptake of the whole root system or mature root zones (approximately 5 cm from the root tip). The difference between these results may come from the different root zones that have been investigated in the two studies. We believe using root tips as the target for Al and Si interaction in plants is preferable because the primary site of Al toxicity in maize is the root apex. Ryan et al. (1993) have shown that in maize, root elongation is inhibited only when apices are exposed to Al, whereas exposing the remainder of the root does not inhibit elongation. In this study, we used the same maize cv Lixis, which has been intensively investigated by Sivaguru and Horst (1998), who showed that the distal transition zone (1–2 mm from the root apex) is the primary target of Al.
Kidd et al. (2001), too, observed the effect of Si pretreatment on Al resistance in maize. Under their experimental conditions, phenol exudation was a major factor contributing to Si-enhanced Al resistance. Al and Si triggered the release of catechol and of the flavonoid-type phenols catechin and quercetin. In an Al-resistant variety, Si-pretreated plants exuded more phenols than plants not pretreated with Si. In our short-term experiments, neither Al nor Si induced phenol exudation. It cannot be ruled out that in our experiments Al treatment time (2 h) was too short for the effects to occur. But considering that in Si-pretreated root tips, elevated Si contents could not be measured after the roots had been growing in a Si-free solution for only 12 h, the enhanced phenol exudation reported by Kidd et al. (2001) after 24 h of growth in Si-free solution might be due to the release of phenols from more mature root zones. Even though a genotype-specific response can still not be excluded, a comparative study of the Al-sensitive cv Lixis with the Al-resistant cvs ATP-Y (Kollmeier et al., 2000) and Sikuani (Kidd et al., 2001) did not reveal a different response pattern regarding the effect of Si on Al-induced inhibition of root elongation and callose formation (data not shown). Therefore, an interaction between Si nutrition and the Al-induced Al resistance mechanism, such as the release of organic acid anions, appears less probable.
With regards to organic acid anions, Al stimulated root exudation of citrate within 2 h (Fig. 7), but the exudation was not affected by Si. This result is consistent with Cocker et al. (1998b). In their experiments to assess exudation of malate by roots of the wheat cv Atlas 66 treated with 100 μm Al, the presence of Si was found to have a negligible effect on exudation after 24 h of Al treatment. In the study of Kidd et al. (2001), Al stimulated root exudation of oxalic acid greatly in three maize varieties after 24 h of Al treatment; this exudation was also not affected by Si pretreatment. It appears that organic acid anions do not play a significant role in Si-mediated amelioration of Al toxicity.
Significant advances have been made in understanding the complex chemistry of Al and Si interaction in solution. However, little is known of Al reactions in the root apoplast, and Al and Si interactions in this compartment are likely to be even more complex (Cocker et al., 1998a). The formation of an Al-Si complex is dependent on pH, and Al and Si concentration. In the nutrient solution we used, low pH and low Al concentration were not favorable for Al-Si complex formation (see above). But within the apoplast of the root apex, higher pH combined with high Al and Si concentrations (considering the small volume of the apoplast in the root tip) could promote HAS formation. Kollmeier et al. (2000) showed that the root surface pH of the root apex of maize cv Lixis grown in bulk solution with pH 4.5 was as high as 5.3 without Al supply. With Al supply, the surface pH of the same root zone was decreased to 4.9 and 4.7 after 15 and 60 min, respectively. Peters and Felle (1999) also observed that the complete profile of root surface pH of the maize root apex was more alkaline than the bulk at medium pH 4.2. It may be assumed that under our experimental conditions, the apoplastic pH in the root tip was also higher than that of the root surface because we observed an increase in the bulk solution pH, if the pH was not kept constant using a pH-stat during the experiment (data not shown). Cocker et al. (1998a) suggested that the concentrations of Al and Si and pH within the apoplast are likely to decide on HAS formation. The fact that no ameliorative effect of Si on Al toxicity was observed after 1 h of Al treatment, according to Al-induced callose formation, but a clear effect could be detected after 12 h Al treatment may reflect such dose requirement in cortical cell walls. In addition, the beneficial effect of Si on Al resistance was more pronounced in the long-term experiment (Fig. 9).
In conclusion, the ameliorative effect of Si on Al toxicity described here can be attributed to an in planta effect. This effect is most likely due to the formation of HAS in the apoplast, which transforms Al into a nonphytotoxic form in the apoplast of the root apex.
MATERIALS AND METHODS
Seeds of an Al-sensitive maize (Zea mays) cv Lixis were soaked in tap water overnight, then placed between filter paper moistened with basic solution containing 500 μm CaCl2 and 8 μm H3BO3 and kept in a vertical position for 3 d. Uniform seedlings were transferred to plastic pots containing the above-mentioned solution. Half the number of plants was supplemented with 1.4 mm H4SiO4. Silicic acid was prepared by passing potassium silicate through a column filled with a cation exchange resin (AG 50W-X8, 100–200 mesh; Bio-Rad, Hercules, CA).
One day after transplanting, the pH of the nutrient solution was stepwise adjusted (using a pH-stat system) to pH 4.3 within 12 h. Then plants from both Si treatments were exposed to 0 or 25 μm AlCl3 for 1 h or 12 h without Si [Si, +Si] or with Si [++Si], and solution pH was maintained at 4.3 ± 0.1, thus avoiding Al precipitation.
All experiments were conducted in a growth chamber under controlled environmental conditions of a 16/8 h day/night cycle, 30/27°C day/night temperature, 75% relative air humidity, and a photon flux density of 230 μmol m−2 s−1 photosynthetic active radiation at plant height.
At harvest, the culture solutions were filtered immediately through 0.025-μm nitrocellulose membranes. Monomeric Al (Almono) concentrations were measured colorimetrically using the aluminon method according to Kerven et al. (1989). The Almono concentration of the nominal 25 μm Al treatment solution was 20 μm after the 12-h Al treatment. There was no difference between the Si treatments (data not shown), suggesting that Si application did not lead to precipitation of Al in the treatment solution.
Root tips of 1 cm were excised and stored at 4°C for Al or Si analysis or frozen immediately in liquid nitrogen for callose determination. The apoplastic and symplastic saps of the root tips were collected by centrifugation, according to the method described by Yu et al. (1999) with some modifications (Iwasaki et al., 2002c). Briefly, freshly excised 1-cm root tips from 20 seedlings were arranged in a filter unit (Ultrafree-MC, 0.45 μm; Millipore, Bedford, MA) with the cut ends facing down, and the WFSF was collected by centrifugation at 3,000g at 4°C for 15 min. After collecting the WFSF, the root tips were frozen at −20°C. The symplastic1 fraction was recovered from the frozen-thawed samples by centrifugation at 3,000g at 4°C for 15 min. The residue was transferred to Eppendorf vials, and then the samples were homogenized in 1 mL of ethanol with a mixer mill (MM200; Retsch, Haan, Germany) at a speed of 30/s for 3 min. After centrifugation, the supernatant and pellet were separated, and the pellet was washed again with ethanol. The combined two supernatants represented the symplastic2 fraction. The pellet consisted of the cell wall material (CW). Al was extracted from the CW on a Millipore filtration unit by a sequential procedure using solutions of 50 mm BaCl2, pH 4.3, for 5, 10, and 15 min, followed by 33 mm Na3citrate, pH 5.8, for 5, 10, and 15 min. The Al contents in the BaCl2 and Na3citrate solution were determined by GFAAS (Unicam 939 QZ; Analytical Technology, Cambridge, UK).
For short-term root-elongation measurement, plant roots were stained in 0.5% neutral red, pH 5.6, for 10 min before Al treatment. At harvest, the length of the unstained part of the root tip was measured as root elongation during the treatment. For long-term root-length measurement, all culture procedures were the same as described above except extending the Al treatment to 44 h, and the solution was renewed once during the Al treatment period. At harvest, the whole root system was scanned. The root length and the number of root tips were measured using the software WinRHIZO image analysis (WIN MAC; Regent Instruments, Quebec City, Canada).
For callose determination, three 1-cm root tips were homogenized in 500 μL of 1 m NaOH at a speed of 20/s for 2 min with a mixer mill. After homogenization, another 500 μL of 1 m NaOH was added, and callose was extracted for 30 min at 80°C in a water bath. Callose was quantified fluorometrically according to Köhle et al. (1985), using aniline blue as color reagent (Hitachi f2000; Hitachi, Tokyo; excitation 393 nm and emission 484 nm).
For Al analysis, the root segments were wet digested with ultrapure concentrated HNO3 at 135°C for 35 min in a microwave oven (MLS-ETHOS plus; Mikrowellen-Laborsystem, Leutkirch, Germany). Al concentrations in the solutions were quantified by ICP-OES (Spektro Analytical Instruments, Kleve, Germany) or GFAAS (Unicam 939 QZ; Analytical Technology).
Si in the root segments or in different fractions of root tips was extracted by a mixture of 1 m HCl and 2.3 m HF (1:2, v/v). Si concentrations in the extract were determined colorimetrically (Van der Vorm, 1987).
For the collection of organic acid anions and total phenols exuded from root apices, we employed the method described by Kollmeier et al. (2001). Briefly, roots of 10 intact 5-d-old seedlings were bundled. The tips (10 mm or 20 mm) were incubated for 2 h in 5 mL of a solution containing 500 μm CaCl2 and 8 μm H3BO3 with different Al and Si levels, according to the treatments. The rest of the roots was kept moist by wrapping them in filter paper soaked with basic solution. For the quantification of the exudation of organic acid anions, the incubation was performed in filtration columns (Bakerbond SPE; J.T. Baber, Phillipsburg, PA) loaded with 1 g of an anion-exchange resin (AG 1-X8,100–200 mesh; Bio-Rad). After removing the roots, the incubation medium was passed through the exchange resin at a rate of 1 mL min−1. The organic acid anions adsorbed on the resin were eluted with 10 mL of 8 m formic acid. The formic acid was evaporated in a centrifugal evaporator (RCT 10-22T; Jouan, Saint-Herblain, France). The residue was dissolved in 1 mL of perchloric acid (10 mm) and was then filtered through 0.45-μm filtration units (Ultrafree-MC; Millipore, Eching, Germany). Samples were analyzed by isocratic HPLC (Kroma System 3000; Kontron Instruments, Munich) separated on an Aminex HPX-87H column (Bio-Rad), supplemented with a cation microguard cartridge, using 10 mm perchloric acid as eluent at a flow rate of 0.5 mL min−1 at 35°C. Total phenols in the root exudates were determined after concentration in a centrifugal evaporator using Folin-ciocalteu reagent (Swain and Hillis, 1959).
Al in the root tissue was localized by staining with morin. After 1 h of Al treatment, root tips from both Si treatments were excised and washed in a solution containing 500 μm CaCl2 and 8 μm H3BO3, pH 4.3. Free-hand sections from the 1- to 3-mm zone behind the root apex were stained with 25 μm morin, pH 5.6, for 30 min at room temperature. After washing in distilled water, the sections were observed under a fluorescence microscope (excitation filter 395–440 nm, barrier filter 470 nm). Images were taken by a digital camera (DSC-S85; Sony, Tokyo) and then exported to Adobe Photoshop 5.0 (Adobe Systems, Mountain View, CA).
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third-party owners of all or parts of the material. Obtaining any permission will be the responsibility of the requestor.
This work was supported by the European Union (within the INCO project ICA4 CT–2000 30017).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.045005.
References
- Barceló J, Guevara P, Poschenrieder C (1993) Silicon amelioration of aluminium toxicity in teosinte (Zea mays L. ssp. Mexicana). Plant Soil 154: 249–255 [Google Scholar]
- Baylis AD, Gragopoulou C, Davidson KJ, Birchall JD (1994) Effect of silicon on the toxicity of aluminium to soybean. Commun Soil Sci Plant Anal 25: 537–546 [Google Scholar]
- Browne BA, Driscoll CT, McColl JG (1990) Aluminum speciation using morin. II. Principles and procedures. J Environ Qual 19: 73–82 [Google Scholar]
- Chérif M, Benhamou N, Menzies JG, Bélanger RR (1992) Silicon induced resistance in cucumber plants against Pythium ultimum. Physiol Mol Plant Pathol 41: 411–425 [Google Scholar]
- Cocker KM (1997) Silicon amelioration of aluminium toxicity in wheat. PhD thesis. Oxford Brookes University, Oxford
- Cocker KM, Evans DE, Hodson MJ (1998. a) The amelioration of aluminium toxicity by silicon in higher plants: solution chemistry or an in planta mechanism? Physiol Plant 104: 608–614 [Google Scholar]
- Cocker KM, Evans DE, Hodson MJ (1998. b) The amelioration of aluminium toxicity by silicon in wheat (Triticum aestivum L.): malate exudation as evidence for an in planta mechanism? Planta 204: 318–323 [Google Scholar]
- Cocker KM, Hodson MJ, Evans DE, Sangster AG (1997) The interaction between silicon and aluminum in Triticum aestivum L. (cv. Celtic). Isr J Plant Sci 45: 289–292 [Google Scholar]
- Corrales I, Poschenrieder C, Barceló J (1997) Influence of silicon pretreatment on aluminium toxicity in maize roots. Plant Soil 190: 203–209 [Google Scholar]
- Delhaize E, Ryan PR (1995) Aluminum toxicity and tolerance in plants. Plant Physiol 107: 315–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet FJ, Rotov ME, Exley C (2001) Direct and indirect identification of the formation of hydroxyaluminosilicates in acid solutions. J Inorg Biochem 87: 71–79 [DOI] [PubMed] [Google Scholar]
- Epstein E (1999) Silicon. Annu Rev Plant Physiol Plant Mol Biol 50: 641–664 [DOI] [PubMed] [Google Scholar]
- Exley C, Schneider C, Doucet FJ (2002) The reaction of aluminium with silicic acid in acidic solution: an important mechanism in controlling the biological availability of aluminium? Coordin Chem Rev 228: 127–135 [Google Scholar]
- Fawe A, Menzies JG, Chérif M, Bélanger RR (2001) Silicon and disease resistance in dicotyledons. In LE Datnoff, GH Snyder, GH Korndörfer, eds, Silicon in Agriculture. Elsevier Science, Amsterdam, pp 159–170
- Galvez L, Clark RB, Gourley LM, Maranville JW (1987) Silicon interactions with manganese and aluminum toxicity in sorghum. J Plant Nutr 10: 1139–1147 [Google Scholar]
- Hammond KE, Evans DE, Hodson MJ (1995) Aluminium/silicon interactions in barley (Hordeum vulgare L.) seedlings. Plant Soil 173: 89–95 [Google Scholar]
- Hodson MJ, Evans DE (1995) Aluminium/silicon interactions in higher plants. J Exp Bot 46: 161–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodson MJ, Sangster AG (1993) The interaction between silicon and aluminium in Sorghum bicolor (L.) Moench: growth analysis and X-ray microanalysis. Ann Bot (Lond) 72: 389–400 [Google Scholar]
- Hodson MJ, Wilkins DA (1991) Localization of aluminium in the roots of Norway spruce (Picea abies L. Karst.) inoculated with Paxillus involutus Fr. New Phytol 118: 273–278 [DOI] [PubMed] [Google Scholar]
- Horst WJ (1995) The role of the apoplast in aluminium toxicity and resistance of higher plants: a review. Z Pflanzenernahr Bodenkd 158: 419–428 [Google Scholar]
- Horst WJ, Fecht M, Naumann A, Wissemeier AH, Maier P (1999. a) Physiology of manganese toxicity and tolerance in Vigna unguiculata (L) Walp. Z Pflanzenernahr Bodenkd 162: 263–274 [Google Scholar]
- Horst WJ, Schmohl N, Kollmeier M, Baluška F, Sivaguru M (1999. b) Does aluminium affect root growth of maize through interaction with the cell wall-plasma membrane-cytoskeleton continuum? Plant Soil 215: 163–174 [Google Scholar]
- Iwasaki K, Maier P, Fecht M, Horst WJ (2002. a) Effects of silicon supply on apoplastic manganese concentrations in leaves and their relation to manganese tolerance in cowpea (Vigna unguiculata (L.) Walp.). Plant Soil 238: 281–288 [Google Scholar]
- Iwasaki K, Maier P, Fecht M, Horst WJ (2002. b) Influence of the apoplastic silicon concentration on the manganese tolerance of cowpea (Vigna unguiculata (L.) Walp.). J Plant Physiol 159: 167–173 [Google Scholar]
- Iwasaki K, Matsumura A, Sakai N, Takemoto K, Tanaka S (2002. c) Comparison of silicon uptake characteristics between two cultivars of pumpkin (Curcubita moschata Duch) [abstract]. In Second Silicon in Agriculture Conference, August 22–26, 2002. Tsuruoka, Yamagata, Japan, pp 114–117
- Kerven GL, Edwards DG, Asher CJ, Hallman PS, Kokot S (1989) Aluminium determination in soil solution. II. Short term colorimetric procedures for the measurement of inorganic monomeric aluminium in the presence of organic ligands. Aust J Soil Res 27: 91–102 [Google Scholar]
- Kidd PS, Llugany M, Poschenrieder C, Gunsé B, Barceló J (2001) The role of root exudates in aluminum resistance and silicon-induced amelioration of aluminum toxicity in three varieties of maize (Zea mays L.). J Exp Bot 52: 1339–1352 [PubMed] [Google Scholar]
- Kinraide TB (1994) Use of a Gouy-Chapman-Stern model for membrane-surface electrical potential to interpret some features of mineral rhizotoxicity. Plant Physiol 106: 1583–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochian LV (1995) Cellular mechanisms of aluminum toxicity and resistance in plants. Annu Rev Plant Physiol Plant Mol Biol 46: 237–260 [Google Scholar]
- Kochian LV, Pence NS, Letham DLD, Pineros MA, Magalhaes JV, Hoekenga OA, Garvin DF (2002) Mechanisms of metal resistance in plants: aluminum and heavy metals. Plant Soil 247: 109–119 [Google Scholar]
- Köhle H, Jeblick W, Poten F, Blaschek W, Kauss H (1985) Chitosan-elicited callose synthesis in soybean cells as a Ca2+-dependent process. Plant Physiol 77: 544–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollmeier M, Dietrich P, Bauer CS, Horst WJ, Hedrich R (2001) Aluminum activates a citrate-permeable anion channel in the aluminum-sensitive zone of the maize root apex. A comparison between an aluminum-sensitive and an aluminum-resistant cultivar. Plant Physiol 126: 397–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollmeier M, Felle H, Horst WJ (2000) Genotypical differences in aluminum resistance of maize are expressed in the distal part of the transition zone. Is reduced basipetal auxin flow involved in inhibition of root elongation by aluminum? Plant Physiol 122: 945–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YC, Alva AK, Sumner ME (1989) Response of cotton cultivars to aluminum in solutions with varying silicon concentrations. J Plant Nutr 12: 881–892 [Google Scholar]
- Lux A, Luxová M, Hattori T, Inanaga S, Sugimoto Y (2002) Silicification in sorghum (Sorghum bicolor) cultivars with different drought tolerance. Physiol Plant 115: 87–92 [DOI] [PubMed] [Google Scholar]
- Ma JF, Sasaki M, Matsumoto H (1997) Al-induced inhibition of root elongation in corn, Zea mays L. is overcome by Si addition. Plant Soil 188: 171–176 [Google Scholar]
- Marienfeld S, Schmohl N, Klein M, Schröder WH, Kuhn AJ, Horst WJ (2000) Localization of aluminium in root tips of Zea mays and Vicia faba. J Plant Physiol 156: 666–671 [Google Scholar]
- Matsumoto H (2000) Cell biology of aluminum toxicity and tolerance in higher plants. Int Rev Cytol 200: 1–45 [DOI] [PubMed] [Google Scholar]
- Peters WS, Felle HH (1999) The correlation of profiles of surface pH and elongation growth in maize roots. Plant Physiol 121: 905–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengel Z (1996) Uptake of aluminium by plant cells. New Phytol 134: 389–406 [Google Scholar]
- Rogalla H, Römheld V (2002) Role of leaf apoplast in silicon-mediated manganese tolerance of Cucumis sativus L. Plant Cell Environ 25: 549–555 [Google Scholar]
- Ryan PR, Ditomaso JM, Kochian LV (1993) Aluminum toxicity in roots: an investigation of special sensitivity and the role of the root cap. J Exp Bot 44: 437–446 [Google Scholar]
- Ryder M, Gérard F, Evans DE, Hodson MJ (2003) The use of root growth and modeling data to investigate amelioration of aluminium toxicity by silicon in Picea abies seedlings. J Inorg Biochem 97: 52–58 [DOI] [PubMed] [Google Scholar]
- Sivaguru M, Horst WJ (1998) The distal part of the transition zone is the most aluminum-sensitive apical root zone of Zea mays L. Plant Physiol 116: 155–163 [PMC free article] [Google Scholar]
- Swain T, Hillis WE (1959) The phenolic constituents of Prunes domestica I. The quantitative analysis of phenolic constituents. J Sci Food Agric 10: 63–68 [Google Scholar]
- Taylor GJ (1995) Overcoming barriers to understanding the cellular basis of aluminium resistance. Plant Soil 171: 89–103 [Google Scholar]
- Van der Vorm PDJ (1987) Dry ashing of plant material and dissolution of the ash in HF for the colorimetric determination of silicon. Commun Soil Sci Plant Anal 18: 1181–1189 [Google Scholar]
- Yu Q, Tang C, Chen Z, Kuo J (1999) Extraction of apoplastic sap from plant roots by centrifugation. New Phytol 143: 299–304 [Google Scholar]