Abstract
We report a procedure to detect electrical activity in cultured neurons by changes in their intrinsic optical properties. Using dark-field microscopy to detect scattered light, we observe an optical signal that is linearly proportional to the change in the membrane potential. Action potentials can be recorded without signal averaging. We use the dark-field method to show that there are substantial time delays between activity in the soma and in fine distal processes of identified Aplysia neurons. The biophysical basis for the change in optical properties of the neuron was deduced from measurements of the angular distribution of scattered laser light. An analysis of the data indicates that the radial component of the index of refraction of the membrane increases and the tangential components decrease concomitant with an increase in membrane potential. This is suggestive of a rapid reorientation of dipoles in the membrane during an action potential.
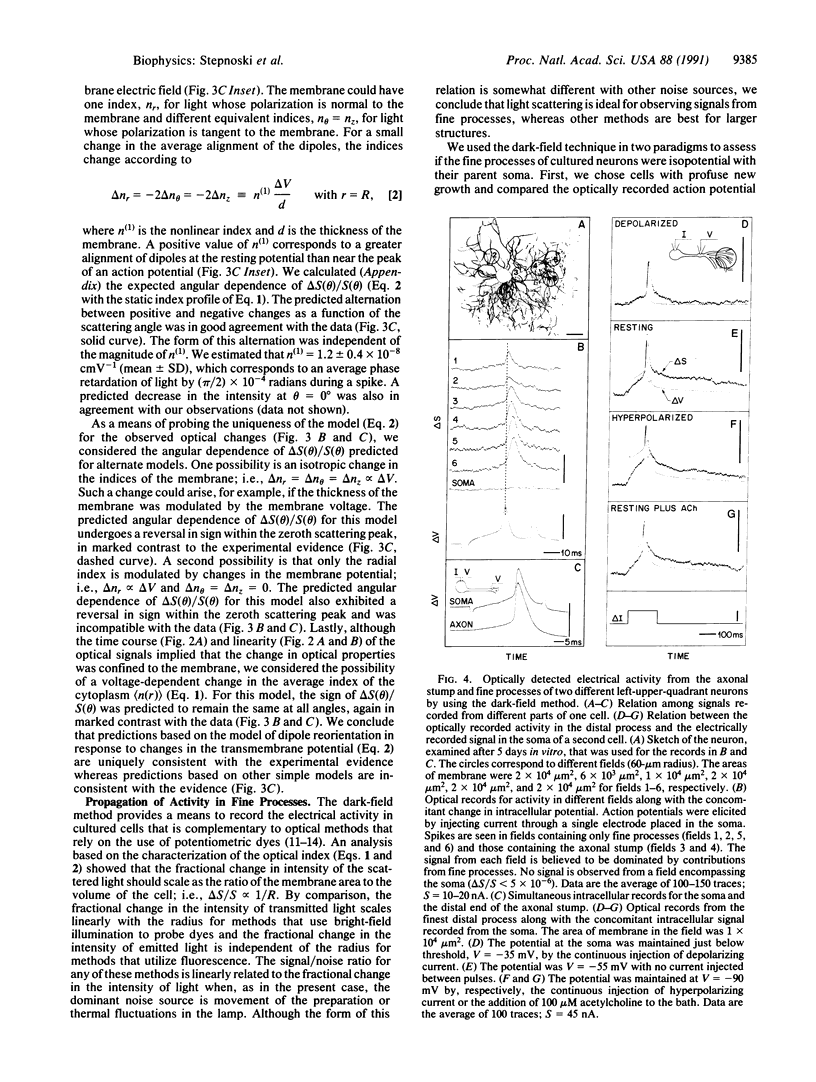
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Camardo J., Proshansky E., Schacher S. Identified Aplysia neurons form specific chemical synapses in culture. J Neurosci. 1983 Dec;3(12):2614–2620. doi: 10.1523/JNEUROSCI.03-12-02614.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien C. B., Pine J. Voltage-sensitive dye recording of action potentials and synaptic potentials from sympathetic microcultures. Biophys J. 1991 Sep;60(3):697–711. doi: 10.1016/S0006-3495(91)82099-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. B., Keynes R. D., Hille B. Light scattering and birefringence changes during nerve activity. Nature. 1968 May 4;218(5140):438–441. doi: 10.1038/218438a0. [DOI] [PubMed] [Google Scholar]
- Cohen L. B., Keynes R. D., Landowne D. Changes in axon light scattering that accompany the action potential: current-dependent components. J Physiol. 1972 Aug;224(3):727–752. doi: 10.1113/jphysiol.1972.sp009920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. B., Keynes R. D., Landowne D. Changes in light scattering that accompany the action potential in squid giant axons: potential-dependent components. J Physiol. 1972 Aug;224(3):701–725. doi: 10.1113/jphysiol.1972.sp009919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinvald A., Ross W. N., Farber I. Simultaneous optical measurements of electrical activity from multiple sites on processes of cultured neurons. Proc Natl Acad Sci U S A. 1981 May;78(5):3245–3249. doi: 10.1073/pnas.78.5.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL D. K. The effect of stimulation on the opacity of a crustacean nerve trunk and its relation to fibre diameter. J Physiol. 1950 Oct 16;111(3-4):283–303. doi: 10.1113/jphysiol.1950.sp004480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill D. K., Keynes R. D. Opacity changes in stimulated nerve. J Physiol. 1949 May 15;108(3):278–281. [PMC free article] [PubMed] [Google Scholar]
- Huang C., Thompson T. E. Properties of lipid bilayer membranes separating two aqueous phases: determination of membrane thickness. J Mol Biol. 1965 Aug;13(1):183–193. doi: 10.1016/s0022-2836(65)80088-2. [DOI] [PubMed] [Google Scholar]
- Kleinfeld D., Raccuia-Behling F., Chiel H. J. Circuits constructed from identified Aplysia neurons exhibit multiple patterns of persistent activity. Biophys J. 1990 Apr;57(4):697–715. doi: 10.1016/S0006-3495(90)82591-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinfeld D, Raccuia-Behling F, Blonder GE. Comment on "Physical mechanisms underlying neurite outgrowth: A quantitative analysis of neuronal shape". Phys Rev Lett. 1990 Dec 10;65(24):3064–3064. doi: 10.1103/PhysRevLett.65.3064. [DOI] [PubMed] [Google Scholar]
- Kriegstein A. R., Castellucci V., Kandel E. R. Metamorphosis of Aplysia californica in laboratory culture. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3654–3658. doi: 10.1073/pnas.71.9.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons T. D., Salzberg B. M., Obaid A. L., Raccuia-Behling F., Kleinfeld D. Long-term optical recording of patterns of electrical activity in ensembles of cultured Aplysia neurons. J Neurophysiol. 1991 Jul;66(1):316–333. doi: 10.1152/jn.1991.66.1.316. [DOI] [PubMed] [Google Scholar]
- Ross W. N., Arechiga H., Nicholls J. G. Optical recording of calcium and voltage transients following impulses in cell bodies and processes of identified leech neurons in culture. J Neurosci. 1987 Dec;7(12):3877–3887. doi: 10.1523/JNEUROSCI.07-12-03877.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacher S., Proshansky E. Neurite regeneration by Aplysia neurons in dissociated cell culture: modulation by Aplysia hemolymph and the presence of the initial axonal segment. J Neurosci. 1983 Dec;3(12):2403–2413. doi: 10.1523/JNEUROSCI.03-12-02403.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]