Abstract
Data on markers of hepatitis C virus (HCV) disease in HIV-HCV co-infected patients in resource-limited settings are scarce. We assessed HCV-RNA, HCV genotype (GT), IL28B GT, and liver fibrosis (FibroScan®) in 480 HIV-infected patients with positive HCV antibody in four HIV treatment centers in South East Asia. We enrolled 165 (34.4%) patients in Jakarta, 158 (32.9%) in Bangkok, 110 (22.9%) in Hanoi, and 47 (9.8%) in Kuala Lumpur. Overall, 426 (88.8%) were male, the median (IQR) age was 38.1 (34.7–42.5) years, 365 (76.0%) reported HCV exposure through injecting drug use, and 453 (94.4%) were on combination antiretroviral therapy. The median (IQR) CD4 count was 446 (325–614) cells/mm3 and 208 (94.1%) of 221 patients tested had HIV-1 RNA <400 copies/ml. A total of 412 (85.8%) had detectable HCV-RNA, at a median (IQR) of 6.2 (5.4-6.6) log10 IU/mL. Among 380 patients with HCV GT, 223 (58.7%) had GT1, 97 (25.5%) had GT3, 43 (11.3%) had GT6, 8 (2.1%) had GT4, 2 (0.5%) had GT2, and 7 (1.8%) had indeterminate GT. Of 222 patients with IL28B testing, 189 (85.1%) had rs12979860 CC genotype, and 199 (89.6%) had rs8099917 TT genotype. Of 380 patients with FibroScan®, 143 (37.6%) had no/mild liver fibrosis (F0-F1), 83 (21.8%) had moderate fibrosis (F2), 74 (19.5%) had severe fibrosis (F3), and 79 (20.8%) had cirrhosis (F4). One patient (0.3%) had FibroScan® failure. A high proportion of HIV-HCV co-infected patients had chronic HCV infection. HCV GT1 was predominant, and 62% of patients had liver disease warranting prompt treatment (>=F2).
Keywords: HCV, chronic infection, fibrosis, HIV, Asia
Introduction
It is estimated that approximately 80 million people may be chronically-infected with the hepatitis C virus (HCV) globally, and that 33 million of them live in Asia [1]. HCV and HIV share similar routes of transmission, albeit with different forces of infection [2]. Of the 37 million people living with HIV worldwide, around 2.3 million are thought to be co-infected with HCV [3]. In HIV-HCV co-infection, the harms caused by each virus are aggravated. The risk of developing HCV-related liver cirrhosis are substantially higher in case of HIV co-infection [4], and HIV patients have a 2-4 fold increased risk of mortality if co-infected with HCV [5,6].
While HIV antiretroviral treatment has been rolled out to reach 17 million people by the end of 2015 as a result of unprecedented global mobilization and initiatives [7], Treatment of hepatitis C is rarely provided in resource-limited settings (RLS) [8,9]. This applies to new direct antiviral agents (DAAs), but also treatment with pegylated-interferon (Peg-IFN) and ribavirin (RBV), which was previously the standard of care in hepatitis C treatment. Therapy is expensive, it has been deemed complex for implementation in RLS, and therefore only few national HCV treatment programs have been implemented in these settings, with little international support. As a consequence of lack of treatment, people with HCV, with or without HIV co-infection, rarely benefit from assessment of HCV disease [10]. In these instances, patients lack awareness on the status and effect of their infection, and the importance of reducing risks of further liver damage (e.g., through hepatotoxic medications and alcohol use). Moreover, knowledge is also lacking on the treatment needs at the population level.
In the present study, we aimed to study the prevalence of chronic infection, genotype distribution, and extent of liver disease in a cohort of HIV-infected patients from South East Asia with known positive HCV antibody (HCV Ab).
Patients with liver fibrosis of moderate or higher severity were provided HCV treatment in a separate study component that will be reported elsewhere (>= F2).
Methods
This study was conducted at four HIV treatment centers participating in the TREAT Asia HIV research network: HIV-NAT/the Thai Red Cross AIDS Research Center in Bangkok, Thailand, the National Hospital for Tropical Diseases in Hanoi, Vietnam, Cipto Mangukusumo General Hospital in Jakarta, Indonesia, and the University of Malaya Medical Center (UMMC) in Kuala Lumpur, Malaysia. The UMMC site collaborated with the Kerinchi Cure and Care Center, a community opioid substitution treatment (OST) center, serving as a sub-site in Kuala Lumpur. Referral to the study sites from other local centers was also permitted.
Study Population
Study candidates were HIV-infected patients under routine HIV care at the participating sites, and in whom positive HCV Ab tests were documented at least six months prior to study enrollment. Other eligibility criteria included age >18 years old and CD4 count >200 cells/μl. Patients were excluded if they had a history of a Child-Pugh score greater than A, ascites, encephalopathy, bleeding esophageal varices, liver cancer, were currently pregnant or breastfeeding, and if a female partner of a male subject was currently pregnant. As this study was linked to a treatment component using Peg-IFN plus RBV, the protocol discouraged inclusion into the disease assessment component of patients with known laboratory abnormalities and clinical conditions that would likely preclude use of this therapy.
Study Procedures
Study procedures were divided into three consecutive steps. First, quantitative HCV RNA testing was done for all patients. Those with negative HCV RNA were considered to have cleared HCV infection and ended participation. Those with positive HCV RNA tests then had HCV genotype (HCV GT) testing, IL28B testing, and liver fibrosis assessment with FibroScan® (Echosense, Paris, France). Patients with moderate or more advanced liver fibrosis (>F2, or 7.5 kPa) had further testing that included a full blood count, ALT, AST, total bilirubin, albumin, prothrombin time/INR, and hepatitis B surface antigen (HBs Ag) and antibody (HBs Ab).. Patients with liver cirrhosis had also abdominal ultrasound and alpha-fetoprotein testing.
HCV molecular tests were conducted locally at the study site laboratories (Bangkok, Hanoi) or in external private laboratories (Jakarta, Kuala Lumpur) on the Abbott Molecular m2000 platform (Abbott Laboratories, IL, USA). HCV RNA testing was done with the Abbott RealTime HCV® assay with a range of detection 12-100,000,000 IU/ml. HCV GT testing consisted in the Abbott RealTime HCV Genotype II®, targeting the HCV 5'UTR & NS5b regions. IL28B testing was done with the Abbott RealTime IL28B Investigational Use assay, for detection of the single nucleotide polymorphism (SNP) rs12979860 CC, CT, and TT genotypes, and rs8099917 TT, GT, GG genotypes. HCV GT that could not be identified with the Abbott genotype assay were further sequenced at each study site. FibroScan® was conducted locally at each of the study sites by trained personnel.
Enrollment into the study and all study procedures were conducted between December 2013 and January 2015. The study was approved by an Ethics Committee (EC) registered with the US Office for Human Research Protections at each participating clinical site, and for the data management and analysis center (Kirby Institute, UNSW Australia) and the coordinating center (TREAT Asia, Bangkok, Thailand). All patients gave written informed consent to participate in the study.
Statistical analysis
Differences in baseline data between sites were evaluated using Kruskal-Wallis for continuous variables and chi-square for proportions. Logistic regression stratified by study site was used to evaluate factors associated with undetectable HCV RNA and advanced liver disease (i.e., severe fibrosis or cirrhosis). Study site odds ratios (OR) were calculated separately using ordinary logistic regression to illustrate site differences. In all regression analyses, variables that exhibited a univariate p-value <0.20 were considered for inclusion in the multivariate model, and multivariate p-values ≤0.05 were considered statistically significant. At the Bangkok site, 43 of the patients were found after inclusion to have had chronic HCV infection documented prior to study enrollment. Therefore, we performed a sensitivity analysis to evaluate how our results pertaining to HCV RNA detectability were altered when these patients were excluded.
Results
Patient characteristics
A total of 480 patients were enrolled: 165 (34.4%) in Jakarta, 158 (32.9%) in Bangkok, 110 (22.9%) in Hanoi, and 47 (9.8%) in Kuala Lumpur (Table 1). Overall, the majority of patients were males (88.8%), the median (IQR) age was 38.1 (34.7-42.5) years, and 365 (76.0%) reported injecting drug use (IDU) as the main route of HCV exposure. Fifty-one patients (10.6%) reported currently using OST (by methadone) and 11 (2.3%) still injecting heroin. Although 214 (44.6%) patients reported daily or regular alcohol drinking in the past, 15 (3.1%) reported doing so at the time of study testing. A total of 453 (94.4%) patients were on combination antiretroviral therapy (ART). The median (IQR) last CD4 count was 446 (325–614) cells/mm3 and 208 of 221 (94.1%) patients with testing available had HIV-1 RNA <400 copies/ml. Of those tested for HBs Ag, 10/229 (4.4%) had a positive result indicative of a triple HIV-HCV-HBV infection.
Table 1.
Patient characteristics
| Overall (n=480) | Jakarta (n=165) | Bangkok (n=158) | Hanoi (n=110) | Kuala Lumpur (n=47) | p-value* | |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Male | 426 (88.8%) | 147 (89.1%) | 133 (84.2%) | 99 (90.0%) | 47 (100.0%) | 0.02 |
| Age (years) | ||||||
| Median (IQR) | 38.1 (34.7-42.5) | 34.9 (32.6-38.0) | 42.1 (37.0-48.1) | 37.2 (34.9-39.5) | 42.3 (39.7-46.1) | <0.01 |
| HCV exposure | <0.01 | |||||
| IDU | 365 (76.0%) | 154 (93.3%) | 94 (59.5%) | 73 (66.4%) | 44 (93.6%) | |
| Heterosexual | 59 (12.3%) | 3 (1.8%) | 21 (13.3%) | 34 (30.9%) | 1 (2.1%) | |
| MSM | 28 (5.8%) | 1 (0.6%) | 25 (15.8%) | 1 (0.9%) | 1 (2.1%) | |
| Piercing and/or tattooing | 13 (2.7%) | 5 (3.0%) | 8 (5.1%) | 0 (0.0%) | 0 (0.0%) | |
| Blood products | 8 (1.7%) | 2 (1.2%) | 6 (3.8%) | 0 (0.0%) | 0 (0.0%) | |
| Other/unknown | 7 (1.5%) | 0 (0.0%) | 4 (2.5%) | 2 (1.8%) | 1 (2.1%) | |
| Body mass index (kg/m2) | ||||||
| Median (IQR) | 21.3 (19.5-23.7) | 22.2 (20.0-24.9) | 21.3 (18.7-23.6) | 20.5 (19.3-22.1) | 21.7 (19.7-23.8) | <0.01 |
| Current IDU or OST | ||||||
| IDU | 11 (2.3%) | 2 (1.2%) | 8 (5.1%) | 1 (0.9%) | 0 (0.0%) | 0.04 |
| OST (methadone) | 51 (10.6%) | 4 (2.4%) | 10 (6.3%) | 2 (1.8%) | 35 (74.5%) | <0.01 |
| Past alcohol use | ||||||
| Daily or regular | 214 (44.6%) | 95 (57.6%) | 85 (53.8%) | 20 (18.2%) | 14 (29.8%) | <0.01 |
| Current alcohol use | ||||||
| Daily or regular | 15 (3.1%) | 2 (1.2%) | 10 (6.3%) | 3 (2.7%) | 0 (0.0%) | 0.03 |
| Antiretroviral therapy | <0.01 | |||||
| None/monotherapy | 27 (5.6%) | 1 (0.6%) | 4 (2.5%) | 6 (5.5%) | 16 (34.0%) | |
| Combination | 453 (94.4%) | 164 (99.4%) | 154 (97.5%) | 104 (94.5%) | 31 (66.0%) | |
| CD4 cell count (cells/mm3) | ||||||
| Median (IQR) | 446 (325-614) | 461 (354-635) | 526 (363-679) | 359 (281-454) | 413 (341-529) | <0.01 |
| HIV viral load (copies/ml) | ||||||
| <400/total tested | 208/221 (94.1%) | 20/22 (90.9%) | 89/95 (93.7%) | 87/87 (100.0%) | 12/17 (70.6%) | <0.01 |
| Hepatitis B co-infection | ||||||
| Positive HBsAg/total tested | 10/229 (4.4%) | 1/58 (1.7%) | 5/109 (4.6%) | 2/48 (4.2%) | 2/14 (14.3%) | 0.23 |
| Time since HCV Ab test (y) | ||||||
| Median (IQR) | 4.7 (2.3-7.5) | 6.9 (4.2-8.8) | 3.5 (1.6-6.5) | 3.3 (2.1-6.2) | 2.9 (1.6-5.9) | <0.01 |
p-values represent difference between study sites. IQR: interquartile range. IDU: injecting drug use. MSM: men who have sex with men. OST: opioid substitution therapy. HBsAg: hepatitis B surface antigen. Ab: antibody. y: years
HCV RNA detection
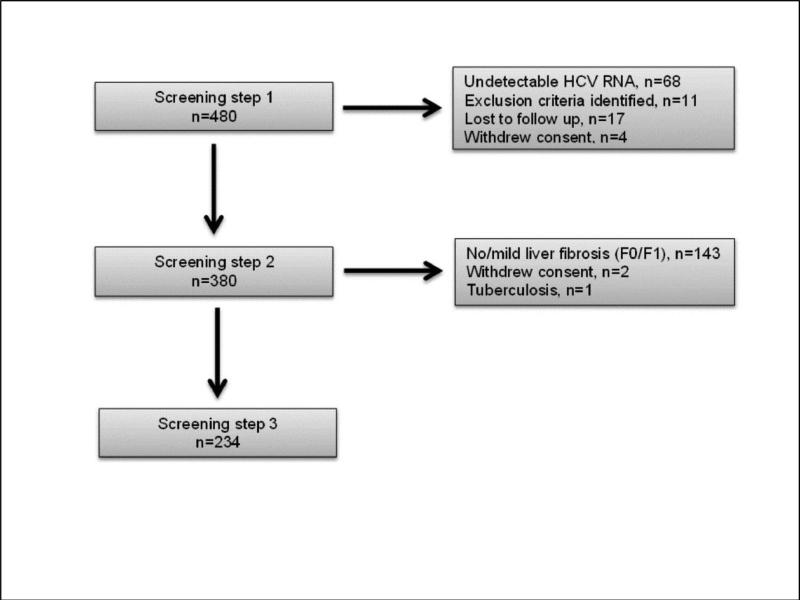
All 480 study patients and subsequently 380 and 234 patients undertook the step 1, 2, and 3 study procedures respectively (Figure 1). A total of 412 (85.8%) had a positive HCV RNA confirming chronic HCV infection. Among those, the median (IQR) viral load was 6.2 (5.4-6.6) log10 IU/ml (Table 2). A significant difference in the proportion of patients with chronic infection was noted between sites, ranging from 72.3% at the Kuala Lumpur site to 91.1% at the Bangkok site. However, upon sensitivity analysis excluding the 43 patients from Bangkok with a history of prior HCV RNA testing, the difference was no longer significant (Bangkok 101/115 (87.8%); across all sites 369/437 (84.4%); p=0.10).
Figure 1.
Patient Screening Steps Participation
Table 2.
HCV RNA, HCV genotypes, and IL28B
| Overall | Jakarta | Bangkok | Hanoi | Kuala Lumpur | p-value* | |
|---|---|---|---|---|---|---|
| HCV RNA | (n=480) | (n=165) | (n=158) | (n=110) | (n=47) | |
| N (%) with detectable HCV RNA | 412 (85.8%) | 140 (84.8%) | 144 (91.1%) | 94 (85.5%) | 34 (72.3%) | 0.01 |
| Median (IQR) HCV RNA (log10 IU/ml) | 6.2 (5.4-6.6) | 6.2 (5.6-6.6) | 6.1 (5.4-6.6) | 6.2 (5.4-6.6) | 6.0 (5.1-6.7) | 0.7 |
| N (%) with detectable HCV RNA (sensitivity analysis) | 369/437 (84.4%) | 140 (84.8%) | 101/115 (87.8%) | 94 (85.5%) | 34 (72.3%) | 0.10 |
| HCV GT | (n=380) | (n=137) | (n=126) | (n=93) | (n=24) | |
|---|---|---|---|---|---|---|
| GT1 | 223 (58.7%) | 94 (68.6%) | 57 (45.2%) | 62 (66.7%) | 10 (41.7%) | <0.01 |
| GT1a | 149/223 (66.8%) | 74/94 (78.7%) | 38/57 (66.7%) | 29/62 (46.8%) | 8/10 (80.0%) | <0.01 |
| GT1b | 33/223 (14.8%) | 5/94 (5.3%) | 12/57 (21.1%) | 15/62 (24.2%) | 1/10 (10.0%) | <0.01 |
| GT1 + other GT (mixed) | 17/223 (7.6%) | 3/94 (3.2%) | 7/57 (12.3%) | 6/62 (9.7%) | 1/10 (10.0%) | 0.41 |
| GT2 | 2 (0.5%) | 0 (0.0%) | 0 (0.0%) | 2 (2.2%) | 0 (0.0%) | 0.10 |
| GT3 | 97 (25.5%) | 30 (21.9%) | 52 (41.3%) | 2 (2.2%) | 13 (54.2%) | <0.01 |
| GT4 | 8 (2.1%) | 8 (5.8%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | <0.01 |
| GT6 | 43 (11.3%) | 1 (0.7%) | 16 (12.7%) | 25 (26.9%) | 1 (4.2%) | <0.01 |
| Indeterminate GT | 7 (1.8%) | 4 (2.9%) | 1 (0.8%) | 2 (2.2%) | 0 (0.0%) | 0.54 |
| IL28 B host GT | (n=222) | (n=66) | (n=67) | (n=70) | (n=19) | |
|---|---|---|---|---|---|---|
| rs12979860 CC | 189 (85.1%) | 58 (87.9%) | 56 (83.6%) | 61 (87.1%) | 14 (73.7%) | |
| rs12979860 CT | 33 (14.9%) | 8 (12.1%) | 11 (16.4%) | 9 (12.9%) | 5 (26.3%) | 0.44 |
| rs12979860 TT | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| rs8099917 TT | 199 (89.6%) | 61 (92.4%) | 60 (89.6%) | 62 (88.6%) | 16 (84.2%) | |
| rs8099917 TG | 23 (10.4%) | 5 (7.6%) | 7 (10.4%) | 8 (11.4%) | 3 (15.8%) | 0.74 |
| rs8099917 GG | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
p-values represent difference between study sites. IQR: interquartile range. GT: genotype
Factors associated with undetectable HCV RNA in multivariate analysis were HCV exposure category, BMI, and study site (Table 3). Men who have sex with men (MSM) had an odds ratio (OR) of undetectable HCV RNA of 3.4 (95% CI 1.1-10.3) as compared to IDUs. Patients with a BMI ≥25 kg/m2 had an OR of 3.1 (95% CI 1.1-9.2) compared to those with a BMI <18.5, and patients at the Bangkok and Kuala Lumpur sites had an OR of 0.4 (95% CI 0.2-0.9) and 2.2 (95% CI 1.0-4.8), respectively, as compared to patients from Jakarta. However, upon sensitivity analysis excluding the 43 patients from Bangkok with prior HCV RNA testing, only patients from Kuala Lumpur had a significantly different OR of undetectable HCV RNA.
Table 3.
Factors associated with undetectable HCV RNA
| Total patients | N with HCV RNA <LOD | Multivariate OR (95% CI) | Specific p-value | Indicator p-value | |
|---|---|---|---|---|---|
| HCV exposure | 0.13 | ||||
| IDU | 365 | 49 (13.4%) | 1.0 | ||
| Heterosexual | 59 | 8 (13.6%) | 1.2 (0.5-3.0) | 0.63 | |
| MSM | 28 | 6 (21.4%) | 3.4 (1.1-10.3) | 0.03 | |
| Others* | 28 | 5 (17.9%) | 2.1 (0.7-6.2) | 0.17 | |
| Body mass index (kg/m2) | 0.03 | ||||
| <18.5 | 73 | 5 (6.8%) | 1.0 | ||
| 18.5-24.9 | 332 | 47 (14.2%) | 1.9 (0.7-5.0) | 0.19 | |
| ≥25 | 75 | 16 (21.3%) | 3.1 (1.1-9.2) | 0.04 | |
| Study site | 0.01 | ||||
| Jakarta | 165 | 25 (15.2%) | 1.0 | ||
| Hanoi | 110 | 16 (14.5%) | 1.0 (0.5-2.2) | 0.95 | |
| Bangkok | 158 | 14 (8.9%) | 0.4 (0.2-0.9) | 0.03 | |
| Kuala Lumpur | 47 | 13 (27.7%) | 2.2 (1.0-4.8) | 0.05 | |
| Study site (upon sensitivity analysis) | 0.06 | ||||
| Jakarta | 165 | 25 (15.2%) | 1.0 | ||
| Hanoi | 110 | 16 (14.5%) | 1.0 (0.5-2.2) | 0.91 | |
| Bangkok | 115 | 14 (12.2%) | 0.6 (0.3-1.4) | 0.25 | |
| Kuala Lumpur | 47 | 13 (27.7%) | 2.2 (1.0-4.9) | 0.04 |
Sex, age, ART, WHO stage, CD4 count, HIV viral load, current IDU or OST, past alcohol use, and current alcohol use were not associated with having undetectable HCV RNA. HBs Ag was not included in the model as too few patients (N=10) had a positive test result.
Others= piercing or tattooing, blood products.
LOD = limit of detection. OR: odds ratio. IDU: injecting drug use. MSM: men who have sex with men. OST: opioid substitution therapy.
HCV genotypes
Of 380 patients tested for HCV GT, a majority (N=223, 58.7%) had a GT1 infection (Table 2). GT3 was second most common (97; 25.5%), followed by GT6 (43; 11.3%). Very few cases of GT2 and GT4, and no GT5 were identified. Another 38 patients were found to have an indeterminate GT, before sequencing analysis revealed GT6 and GT3 infection in 22 and 16 patients, respectively. In seven patients the GT remained unidentified at the end of the study. Significant differences in the proportion of different GTs were also noted between sites. While GT1 was most common in Jakarta (68.6%) and Hanoi (66.7%), GT1 and GT3 were found in comparable proportions in Bangkok (45.2% and 41.3%), and Kuala Lumpur (41.7% and 54.2%). GT6 was mainly found in Hanoi (26.9%) and Bangkok (12.7%). Among all GT1 cases, the GT1a subtype was most common (66.8% across all sites), and 17 (7.6%) of the GT1 infections were found to be mixed infections with at least one other GT.
IL28B host genotype
IL28B testing was done for 222 of the 380 participants undertaking the second screening step, due to limited availability of testing reagents (Table 2). For the SNP rs12979860, the CC and CT alleles were identified in 85.1% and 14.9% of the patients, whereas 89.6% and 10.4% had SNP rs8099917 TT and TG genotypes. No differences were noted between study sites.
Liver fibrosis
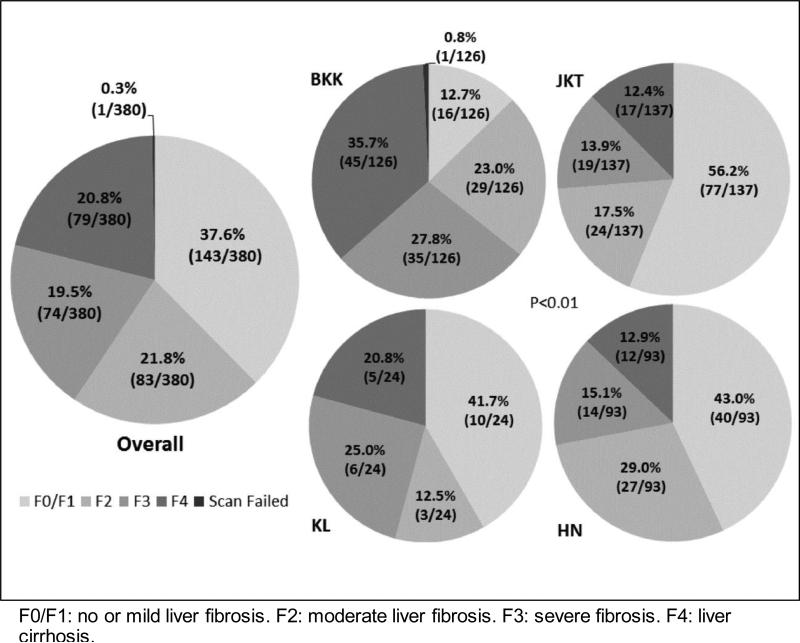
FibroScan® assessment was done in 380 patients, of whom 143 (37.6%) had no or mild liver fibrosis (F0/F1, <7.5 kPa), 83 (21.8%) had moderate fibrosis (F2, 7.5-9.4 kPa), 74 (19.5%) had severe fibrosis (F3, 9.5-12.9 kPa), and 79 (20.8%) had liver cirrhosis (F4, ≥13.0 kPa) (Figure 2). One (0.3%) patient had a FibroScan® test failure. No liver biopsy was done in any patient. The proportions of patients in different stages of liver fibrosis were significantly different across all study sites, with notably a smaller proportion of patients with no/mild liver fibrosis (12.7%) and a higher proportion of patients with liver cirrhosis (35.7%) observed at the Bangkok site.
Figure 2.
Patient Liver Fibrosis Stages
Factors associated with advanced liver disease (F3, F4) in multivariate analysis were age, current IDU or OST, BMI, HIV-1 RNA, IL28B rs12979860 and study site (Table 4). Patients above 50 years of age had a 9.4 OR (95% CI 2.7-32.8) of advanced disease compared to patients aged 31-40 years. Those reporting IDU or OST had an OR of 0.1 (95% CI 0.0-0.3) compared to those not reporting these use, and those with a BMI ≥25 kg/m2 had an OR of 3.6 (95% CI 1.4-9.2) relative to those with a BMI <18.5. Patients with a HIV-1 RNA >400 copies/ml had a 6.6 OR (95% CI 1.0-44.2) of the outcome compared to those with undetectable HIV-1 RNA, whereas the rs12979860 CT host genotype showed a 0.3 OR (95% CI 0.1-0.6) as opposed to those with the CC genotype. Finally, patients at the Bangkok and Kuala Lumpur sites had respectively a 7.2 (95% CI 3.2-15.8) and 5.2 (95% CI 1.1-24.1) OR of advanced liver disease as compared to participants in Jakarta. Each log10 increase in HCV RNA level tended to be associated with a decreased odds of advanced disease, but this did not reach statistical significance. All other factors analyzed, including past or current alcohol use, AST elevation above the UNL, HCV GT and IL28B rs8099917 were not associated with the risk of advanced liver disease.
Table 4.
Factors associated with severe liver disease
| Total patients | N with fibrosis >F2 | Multivariate OR (95% CI) | Specific p-value | Indicator p-value | |
|---|---|---|---|---|---|
| Age (years) | <0.01 | ||||
| ≤30 | 14 | 2 (14.3%) | 0.5 (0.1-2.7) | 0.45 | |
| 31-40 | 230 | 69 (30.0%) | 1.0 | ||
| 41-50 | 107 | 59 (55.1%) | 2.3 (1.2-4.2) | 0.01 | |
| >50 | 28 | 23 (82.1%) | 9.4 (2.7-32.8) | <0.01 | |
| Current IDU or OST | |||||
| No | 344 | 143 (41.6%) | 1.0 | ||
| Yes | 35 | 10 (28.6%) | 0.1 (0.0-0.3) | <0.01 | |
| Body mass index (kg/m2) | 0.01 | ||||
| <18.5 | 64 | 25 (39.1%) | 1.0 | ||
| 18.5-24.9 | 262 | 98 (37.4%) | 1.4 (0.7-2.9) | 0.32 | |
| ≥25 | 53 | 30 (56.6%) | 3.6 (1.4-9.2) | 0.01 | |
| HIV-1 RNA (copies/ml) | |||||
| <400 | 172 | 73 (42.4%) | 1.0 | ||
| ≥400 | 10 | 7 (70.0%) | 6.6 (1.0-44.2) | 0.05 | |
| HCV RNA - every log10 IU/ml increase | 379 | 153 (40.4%) | 0.8 (0.6-1.0) | 0.08 | |
| HCV GT | 0.68 | ||||
| GT1 | 222 | 82 (36.9%) | 1.0 | ||
| GT3 | 97 | 49 (50.5%) | 0.7 (0.3-1.5) | 0.40 | |
| Other GT | 60 | 22 (36.7%) | 0.9 (0.5-1.7) | 0.72 | |
| IL28B – rs12979860 | |||||
| CC | 189 | 103 (54.5%) | 1.0 | ||
| CT | 33 | 12 (36.4%) | 0.3 (0.1-0.6) | <0.01 | |
| IL28B – rs8099917 | |||||
| TT | 199 | 105 (52.8%) | 1.0 | ||
| TG | 23 | 10 (43.5%) | 2.7 (0.6-12.4) | 0.21 | |
| Study site | <0.01 | ||||
| Jakarta | 137 | 36 (26.3%) | 1.0 | ||
| Hanoi | 93 | 26 (28.0%) | 1.4 (0.6-3.1) | 0.40 | |
| Bangkok | 125 | 80 (64.0%) | 7.2 (3.2-15.8) | <0.01 | |
| Kuala Lumpur | 24 | 11 (45.8%) | 5.2 (1.1-24.1) | 0.04 |
Sex, HCV exposure, past alcohol use, current alcohol use, WHO stage, ART, CD4 count and liver transaminase (AST) above upper normal limit were not associated with having severe liver disease. HBs Ag was not included in the model as too few patients (N=10) had a positive test result.
Severe liver disease = severe fibrosis or cirrhosis.
OR: odds ratio. IDU: injecting drug use. OST: opioid substitution therapy. GT: genotype
Other clinical conditions
During the study step 3 assessments, a total of 33 of 234 (14.1%) patients assessed were found to have contra-indication to treatment with Peg-IFN and RBV. Specifically, 13 (5.6%) had anemia (hemoglobin <12g/dl), 6 (2.6%) had thrombocytopenia (platelets <90,000/mm3), 3 (1.3%) had neutropenia (neutrophils <1500/mm3), 3 (1.3%) had functionally compromised or decompensated liver cirrhosis (Child-Pugh score greater than grade A), 1 (0.4%) had a hepatocellular carcinoma, and 7 (3.0%) had other clinical contraindications.
Discussion
To our knowledge, this is the first regional study of comprehensive assessment of HCV infection and HCV-related liver disease in HIV-HCV co-infected patients in Asia. Among the 480 patients with known positive HCV Ab enrolled, 85.8% had chronic HCV infection. GT1 infection was overall most common (58.7%), followed by GT3 (25.5%) and GT6 (11.3%). A total of 85.1% of patients had an IL28B rs12979860 CC genotype, and 89.6% an rs8099917 TT genotype. Importantly, 62.1% had liver disease in the form of moderate liver fibrosis (F2, 21.8%), severe fibrosis (F3, 19.5%), and liver cirrhosis (F4, 20.8%). Among those, 14% had a contraindication to Peg-IFN and RBV therapy.
The proportion of patients in this cohort having liver disease is concerning, as HCV treatment is usually not available or accessible for patients under routine HIV care in South East Asia. This applies to both Peg-IFN plus RBV, and DAA-based therapy. The median time interval that we observed between the time of HCV Ab testing and entry into this study was 4.7 years, which illustrates gaps that exist in assessment of HCV infection in routine HIV care services, due notably to lack of treatment and high cost of diagnostic assessments.
The proportion of patients with chronic HCV infection that we found was higher than that commonly observed in HIV-uninfected patients [11], and on the higher end of what has been reported in HIV co-infected patients [12]. These data support that spontaneous HCV clearance in HIV-infected persons in very uncommon, and that in settings where HCV RNA testing is challenging, HIV-infected patients with positive HCV Ab should be considered chronically infected until proven otherwise, and given care and counseling accordingly (including liver-friendly lifestyle recommendations and medications). Our observation that MSM were more likely than IDU to have undetectable HCV RNA is consistent with studies that have reported more common spontaneous HCV clearance in MSM than IDU [13] and MSM with no history of IDU [14]. It could also relate to the possibility of more exposures in IDU leading to higher likelihood of eventual chronicity. The association that we found between higher BMI and increased odds of undetectable HCV RNA may be surprising, given that HCV overweight patients tend to have higher progression of liver fibrosis, as previous reports have shown [15,16] and as we ourselves identified in this study. Yet, others have also reported lower HCV chronicity in people with positive HCV Ab and higher body weight [17], and it is possible that increased inflammatory markers in obese patients with HCV infection [18,19] could play a role in both of these observations. Sex was not associated with having undetectable HCV RNA in our cohort, and while this is not common [11,20], similar findings have been reported in several population-based surveys [21].
HCV GT distributions in Asian settings have mainly been reported from HCV mono-infected patients [22]. Our data from HIV co-infected patients provide new information, which shows some consistency with epidemiological knowledge in mono-infected populations. We found substantial diversity in HCV GT infections between the different cities involved. Such diversity in GT distribution has been documented between and within countries in the region in HCV mono-infected patients. In our study, HCV GT1 was largely predominant in Indonesia, whereas GT3 was most or more common in Malaysia and Thailand. This is consistent with other findings [22-28]. In contrast with several studies, GT6 was only the second most common GT at our study site in Vietnam, after GT1. Several reports from HCV mono-infected patients indicate that GT6 is most common in Vietnam [29,30], but different findings exist, and GT1 may perhaps be predominant in particular groups, including IDUs [31,32] who represented most patients in our study. Within GT1 infections, the GT1a subtype was consistently more common in all our sites, and similar findings have been reported in mono-infected populations in Thailand, Malaysia and Vietnam [12,23,27,31-33]. The proportion of mixed GT infections that we identified was within the range of mixed infections reported elsewhere [34,35].
Regarding IL28 B, both the rs12979860 CC and the rs8099917 TT genotypes were largely predominant, as expected in Asia [36]. As we only performed IL28B testing in patients with confirmed chronic infection, we could not analyze the association between IL28B and spontaneous HCV clearance. Yet, we found that rs12979860 CC was associated with higher odds of advanced liver disease, and although differences exist [37], others have also observed this association [38,39]. As expected, increasing age showed a strong association with advanced liver disease [40,41]. The association that we found between detectable HIV RNA and increased risk of liver disease also concurred with previous findings in co-infected patients [42,43], and provides additional evidence supporting recommendations of prompt initiation of ART in HIV patients with HCV co-infection [44]. The fact that patients reporting ongoing heroin use or OST had a 90% lower odds of advanced liver disease could possibly result from a shorter history of drug use and HCV infection in this group. We could not explain why patients in Bangkok and Kuala Lumpur had a higher risk of advanced liver disease after adjusting for other risk factors. In another cohort of HIV-HCV co-infected patients in Thailand, Avihingsanon, et al, found that extent of exposure to stavudine and hypovitaminosis D were strongly associated with advanced liver disease, but we could not collect such information in our study [45]. These site differences should also be interpreted with caution, as some differences or bias in entry into care or selection into the study could also exist between sites.
Among study limitations, participants were indeed not randomly selected from the pool of patients with documented HCV Ab at the sites. Specifically though, as we excluded patients with known decompensated cirrhosis and discouraged inclusion of patients with conditions not compatible with Peg-IFN and RBV therapy, our description of stages of liver disease could still include an underestimation of the frequency of most advanced liver disease in the population of interest. Instead, inclusion was based on additional clinical criteria that may have limited the inclusion of patients with either more or less severe liver disease. At the Bangkok site, some patients had already been found to have positive HCV RNA prior to this study, which slightly increased the proportion of patients with chronic infection in our cohort, but was addressed in a sensitivity analysis. As the study candidates were patients already known to have HCV Ab and this test is administered at the study sites to a majority but not all patients with HIV, they did not represent the total population of patients infected with HCV at the study sites. In addition, there were a limited number of study sites, and therefore our findings may not be generalizable to the broader HIV-HCV co-infected population in these countries. Finally, we only tested for IL28B in 58% of patients with chronic HCV, but despite this limitation, the proportions of IL28B genotypes that we found were very similar to these that were reported in Asian populations with chronic HCV in a meta-analysis [36].
In conclusion, our study provides novel information at a regional level in Asia on HCV infection and HCV-related disease in HIV-infected patients, which is of timely importance for the advocacy and planning of HCV treatment rollout in this population. In our cohort, most patients with HCV Ab had chronic infection, and 62% of those chronically infected had previously undiagnosed liver disease (including 20.8% with liver cirrhosis). This finding and the HCV GT distribution diversity observed emphasize that creating access to DAA-based HCV therapy is urgent, and needs to incorporate pan-genotypic regimens with good efficacy for GT3, including in patients with liver cirrhosis.
Acknowledgement and Disclosures
Abbott RealTime HCV assay and RealTime HCV GT II assay were procured from Abbott Molecular at a discounted rate. IL28B Investigational Use Only assay was donated by Abbott Molecular.
The study group has received drug donations (Peg-IFN and RBV) from Merck Sharp and Dohme for a related study of treatment of HCV infection.
DB is supported by a research grant from Gilead Sciences.
GM has received grants support from Abbvie, Gilead Sciences, Janssen-Cilag, Merck Sharp & Dohme.
ML has received grants support from Boehringer Ingelhiem, Bristol-Myers Squibb, Gilead Sciences, Janssen-Cilag, Merck Sharp & Dohme, ViiV HealthCare. He has received DSMB sitting fees from Sirtex pty Ltd.
Funding
This study is an initiative of TREAT Asia, a program of amfAR, The Foundation for AIDS Research, with support from the U.S. National Institutes of Health's National Institute of Allergy and Infectious Diseases, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and National Cancer Institute, as part of the International Epidemiologic Databases to Evaluate AIDS (IeDEA; U01AI069907), and amfAR. The Kirby Institute is funded by the Australian Government Department of Health and Ageing, and is affiliated with the Faculty of Medicine, UNSW Australia (The University of New South Wales). The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of any of the governments or institutions mentioned above.
References
- 1.Gower E, Estes C, Blach S, et al. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014 Nov;61(1 Suppl):S45–57. doi: 10.1016/j.jhep.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 2.Sutton AJ, Hope VD, Mathei C, et al. A comparison between the force of infection estimates for blood-borne viruses in injecting drug user populations across the European Union: a modelling study. J Viral Hepat. 2008 Nov;15(11):809–16. doi: 10.1111/j.1365-2893.2008.01041.x. [DOI] [PubMed] [Google Scholar]
- 3.Platt L, Easterbrook P, Gower E, et al. Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect Dis. 2016 Jul;16(7):797–808. doi: 10.1016/S1473-3099(15)00485-5. [DOI] [PubMed] [Google Scholar]
- 4.Soto B, Sánchez-Quijano A, Rodrigo L, et al. Human immunodeficiency virus infection modifies the natural history of chronic parenterally-acquired hepatitis C with an unusually rapid progression to cirrhosis. J Hepatol. 1997 Jan;26(1):1–5. doi: 10.1016/s0168-8278(97)80001-3. [DOI] [PubMed] [Google Scholar]
- 5.Branch AD, Van Natta ML, Vachon ML, et al. Mortality in HCV-infected Patients with a Diagnosis of AIDS in the Era of Combination Anti-retroviral Therapy. Clin Infect Dis. 2012 Jul;55(1):137–44. doi: 10.1093/cid/cis404. Epub 2012 Apr 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hernando V, Perez-Cachafeiro S, Lewden C, et al. All-cause and liver-related mortality in HIV positive subjects compared to the general population: differences by HCV co-infection. J Hepatol. 2012 Oct;57(4):743–51. doi: 10.1016/j.jhep.2012.06.010. Epub 2012 Jun 16. [DOI] [PubMed] [Google Scholar]
- 7.UNAIDS Global AIDS. update 2016. Available at: http://www.unaids.org/sites/default/files/media_asset/global-AIDS-update-2016_en.pdf.
- 8.Lemoine M, Thursz M. Hepatitis C, a global issue: access to care and new therapeutic and preventive approaches in resource-constrained areas. Semin Liver Dis. 2014 Feb;34(1):89–97. doi: 10.1055/s-0034-1371082. [DOI] [PubMed] [Google Scholar]
- 9.Ford N, Swan T, Beyer P, Hirnschall G, Easterbrook P, Wiktor S. Simplification of antiviral hepatitis C virus therapy to support expanded access in resource-limited settings. J Hepatol. 2014 Nov;61(1 Suppl):S132–8. doi: 10.1016/j.jhep.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 10.Solomon SS, Mehta SH, Srikrishnan AK, et al. Burden of hepatitis C virus disease and access to hepatitis C virus services in people who inject drugs in India: a cross-sectional study. Lancet Infect Dis. 2015 Jan;15(1):36–45. doi: 10.1016/S1473-3099(14)71045-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Micallef JM, Kaldor JM, Dore GJ. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J Viral Hepat. 2006 Jan;13(1):34–41. doi: 10.1111/j.1365-2893.2005.00651.x. [DOI] [PubMed] [Google Scholar]
- 12.Thomson EC, Fleming VM, Main J, et al. Predicting spontaneous clearance of acute hepatitis C virus in a large cohort of HIV-1-infected men. Gut. 2011 Jun;60(6):837–45. doi: 10.1136/gut.2010.217166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soriano V, Mocroft A, Rockstroh J, et al. Spontaneous viral clearance, viral load, and genotype distribution of hepatitis C virus (HCV) in HIV-infected patients with anti-HCV antibodies in Europe. J Infect Dis. 2008 Nov 1;198(9):1337–44. doi: 10.1086/592171. [DOI] [PubMed] [Google Scholar]
- 14.Seaberg EC, Witt MD, Jacobson LP, et al. Spontaneous Clearance of the Hepatitis C Virus Among Men Who Have Sex With Men. Clin Infect Dis. 2015 Nov 1;61(9):1381–8. doi: 10.1093/cid/civ562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dyal HK, Aguilar M, Bhuket T, et al. Concurrent Obesity, Diabetes, and Steatosis Increase Risk of Advanced Fibrosis Among HCV Patients: A Systematic Review. Dig Dis Sci. 2015 Sep;60(9):2813–24. doi: 10.1007/s10620-015-3760-3. [DOI] [PubMed] [Google Scholar]
- 16.Konerman MA, Mehta SH, Sutcliffe CG, et al. Fibrosis progression in human immunodeficiency virus/hepatitis C virus coinfected adults: prospective analysis of 435 liver biopsy pairs. Hepatology. 2014 Mar;59(3):767–75. doi: 10.1002/hep.26741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy EL, Fang J, Tu Y, et al. Hepatitis C virus prevalence and clearance among US blood donors, 2006-2007: associations with birth cohort, multiple pregnancies, and body mass index. J Infect Dis. 2010 Aug 15;202(4):576–84. doi: 10.1086/654882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palmer C, Corpuz T, Guirguis M, et al. The effect of obesity on intrahepatic cytokine and chemokine expression in chronic hepatitis C infection. Gut. 2010 Mar;59(3):397–404. doi: 10.1136/gut.2008.165316. [DOI] [PubMed] [Google Scholar]
- 19.Jonsson JR, Barrie HD, O'Rourke P, Clouston AD, Powell EE. Obesity and steatosis influence serum and hepatic inflammatory markers in chronic hepatitis C. Hepatology. 2008 Jul;48(1):80–7. doi: 10.1002/hep.22311. [DOI] [PubMed] [Google Scholar]
- 20.Bakr I, Rekacewicz C, El Hosseiny M, et al. Higher clearance of hepatitis C virus infection in females compared with males. Gut. 2006 Aug;55(8):1183–7. doi: 10.1136/gut.2005.078147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stroffolini T, Rapicetta M, Di Stefano R. Hepatitis C virus clearance and gender. Gut. 2007 Jun;56(6):884. [PMC free article] [PubMed] [Google Scholar]
- 22.Wasitthankasem R, Vongpunsawad S, Siripon N, et al. Genotypic distribution of hepatitis C virus in Thailand and Southeast Asia. PLoS One. 2015 May 11;10(5):e0126764. doi: 10.1371/journal.pone.0126764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akkarathamrongsin S, Hacharoen P, Tangkijvanich P, et al. Molecular epidemiology and genetic history of hepatitis C virus subtype 3a infection in Thailand. Intervirology. 2013;56(5):284–94. doi: 10.1159/000351621. [DOI] [PubMed] [Google Scholar]
- 24.Utama A, Tania NP, Dhenni R, et al. Genotype diversity of hepatitis C virus (HCV) in HCV-associated liver disease patients in Indonesia. Liver Int. 2010 Sep;30(8):1152–60. doi: 10.1111/j.1478-3231.2010.02280.x. [DOI] [PubMed] [Google Scholar]
- 25.Inoue Y, Sulaiman HA, Matsubayashi K, et al. Genotypic analysis of hepatitis C virus in blood donors in Indonesia. Am J Trop Med Hyg. 2000 Jan;62(1):92–8. doi: 10.4269/ajtmh.2000.62.92. [DOI] [PubMed] [Google Scholar]
- 26.Ho SH, Ng KP, Kaur H, Goh KL. Genotype 3 is the predominant hepatitis C genotype in a multi-ethnic Asian population in Malaysia. Hepatobiliary Pancreat Dis Int. 2015 Jun;14(3):281–6. doi: 10.1016/s1499-3872(15)60363-0. [DOI] [PubMed] [Google Scholar]
- 27.Mohamed NA, Zainol Rashid Z, Wong KK, S A A, Rahman MM. Hepatitis C genotype and associated risks factors of patients at University Kebangsaan Malaysia Medical Centre. Pak J Med Sci. 2013 Sep;29(5):1142–6. doi: 10.12669/pjms.295.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sunanchaikarn S, Theamboonlers A, Chongsrisawat V, et al. Seroepidemiology and genotypes of hepatitis C virus in Thailand. Asian Pac J Allergy Immunol. 2007 Jun-Sep;25(2-3):175–82. [PubMed] [Google Scholar]
- 29.Pham VH, Nguyen HD, Ho PT, et al. Very high prevalence of hepatitis C virus genotype 6 variants in southern Vietnam: large-scale survey based on sequence determination. Jpn J Infect Dis. 2011;64(6):537–9. [PMC free article] [PubMed] [Google Scholar]
- 30.Li C, Yuan M, Lu L, et al. The genetic diversity and evolutionary history of hepatitis C virus in Vietnam. Virology. 2014 Nov;468-470:197–206. doi: 10.1016/j.virol.2014.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanimoto T, Nguyen HC, Ishizaki A, et al. Multiple routes of hepatitis C virus transmission among injection drug users in Hai Phong, Northern Vietnam. J Med Virol. 2010;82:1355–1363. doi: 10.1002/jmv.21787. [DOI] [PubMed] [Google Scholar]
- 32.Dunford L, Carr MJ, Dean J, et al. Hepatitis C virus in Vietnam: high prevalence of infection in dialysis and multi-transfused patients involving diverse and novel virus variants. PLoS One. 2012;7:e41266. doi: 10.1371/journal.pone.0041266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pham DA, Leuangwutiwong P, Jittmittraphap A, et al. High prevalence of Hepatitis C virus genotype 6 in Vietnam. Asian Pac J Allergy Immunol. 2009 Jun-Sep;27(2-3):153–60. [PubMed] [Google Scholar]
- 34.Yun H, Kim D, Kim S, et al. High prevalence of HBV and HCV infection among intravenous drug users in Korea. J Med Virol. 2008 Sep;80(9):1570–5. doi: 10.1002/jmv.21255. [DOI] [PubMed] [Google Scholar]
- 35.Pham ST, Bull RA, Bennett JM, et al. Frequent multiple hepatitis C virus infections among injection drug users in a prison setting. Hepatology. 2010 Nov;52(5):1564–72. doi: 10.1002/hep.23885. [DOI] [PubMed] [Google Scholar]
- 36.Jiménez-Sousa MA, Fernández-Rodríguez A, Guzmán-Fulgencio M, García-Álvarez M, Resino S. Meta-analysis: implications of interleukin-28B polymorphisms in spontaneous and treatment-related clearance for patients with hepatitis C. BMC Med. 2013 Jan 8;11:6. doi: 10.1186/1741-7015-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kitson MT, George J, Dore GJ, et al. Interleukin-28B rs12979860 C allele: Protective against advanced fibrosis in chronic hepatitis C genotype 1 infection. J Gastroenterol Hepatol. 2014;29(7):1458–62. doi: 10.1111/jgh.12544. [DOI] [PubMed] [Google Scholar]
- 38.Ydreborg M, Westin J, Rembeck K, et al. Impact of Il28b-related single nucleotide polymorphisms on liver transient elastography in chronic hepatitis C infection. PLoS One. 2013 Nov 14;8(11):e80172. doi: 10.1371/journal.pone.0080172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rembeck K, Alsiö A, Christensen PB, et al. Impact of IL28B-related single nucleotide polymorphisms on liver histopathology in chronic hepatitis C genotype 2 and 3. PLoS One. 2012;7(1):e29370. doi: 10.1371/journal.pone.0029370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thein HH, Yi Q, Dore GJ, Krahn MD. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology. 2008 Aug;48(2):418–31. doi: 10.1002/hep.22375. [DOI] [PubMed] [Google Scholar]
- 41.Smith DJ, Combellick J, Jordan AE, Hagan H. Hepatitis C virus (HCV) disease progression in people who inject drugs (PWID): A systematic review and meta-analysis. Int J Drug Policy. 2015 Oct;26(10):911–21. doi: 10.1016/j.drugpo.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bräu N, Salvatore M, Ríos-Bedoya CF, et al. Slower fibrosis progression in HIV/HCV-coinfected patients with successful HIV suppression using antiretroviral therapy. J Hepatol. 2006 Jan;44(1):47–55. doi: 10.1016/j.jhep.2005.07.006. Epub 2005 Jul 27. [DOI] [PubMed] [Google Scholar]
- 43.Cooper C, Rollet-Kurhajec KC, Young J, et al. HIV virological rebounds but not blips predict liver fibrosis progression in antiretroviral-treated HIV/hepatitis C virus-coinfected patients. HIV Med. 2015 Jan;16(1):24–31. doi: 10.1111/hiv.12168. Epub 2014 May 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. Available at: http://aidsinfo.nih.gov/guidelines. Accessed on 1/29/2016.
- 45.Avihingsanon A, Jitmitraparp S, Tangkijvanich P, et al. Advanced liver fibrosis by transient elastography, fibrosis 4, and alanine aminotransferase/platelet ratio index among Asian hepatitis C with and without human immunodeficiency virus infection: role of vitamin D levels. J Gastroenterol Hepatol. 2014 Sep;29(9):1706–14. doi: 10.1111/jgh.12613. [DOI] [PubMed] [Google Scholar]