Abstract
Fronto-limbic brain networks involved in regulation of impulsivity and aggression are abnormal in Borderline Personality Disorder (BPD). However, it is unclear whether, or to what extent, these personality traits actually modulate brain responses during cognitive processing. Using fMRI, we examined the effects of trait impulsivity, aggression, and depressed mood on regional brain responses in 31 female BPD and 25 control subjects during a Go No-Go task using Ekman faces as targets. First-level contrasts modeled effects of negative emotional context. Second-level regression models used trait impulsivity, aggression and depressed mood as predictor variables of regional brain activations. In BPD, trait impulsivity was positively correlated with activation in the dorsal anterior cingulate cortex, orbital frontal cortex (OFC), basal ganglia (BG), and dorsolateral prefrontal cortex, with no areas of negative correlation. In contrast, aggression was negatively correlated with activation in OFC, hippocampus, and BG, with no areas of positive correlation. Depressed mood had a generally dampening effect on activations. Effects of trait impulsivity on healthy controls differed from effects in BPD, suggesting a disorder-specific response. Negative emotional context and trait impulsivity, but not aggression or depression, diminished task performance across both groups. Negative emotional context may interfere with cognitive functioning in BPD through interaction with the neurobiology of personality traits.
Keywords: neuroimaging, personality traits, affective interference, executive cognitive functioning, inhibition
1.0 Introduction
Impulsivity and aggressiveness are personality dimensions associated with suicidal and self-injurious behaviors independent of psychiatric diagnoses (Brezo et al., 2006; Perroud et al., 2013). They are also core diagnostic criteria for Borderline Personality Disorder (BPD), where they contribute a diathesis to impaired cognitive function at times of stress (Fertuck et al., 2006; Zanarini, 2005). With an estimated community prevalence of 1.6% (Lenzenweger et al., 2007; Paris, 2010), and a suicide rate of 3%-10% (Black et al., 2004), BPD is a clinically relevant model for studying the neural basis of impulsivity and aggressiveness and their effects on cognitive functioning. Impulsive and aggressive behaviors in BPD are often precipitated by negative affective stressors in a clinical context of affective instability (Brodsky et al., 2006). BPD subjects experience negative affects more strongly than healthy controls, and are slower to return to baseline once aroused (Jacob et al., 2008; Levine et al., 1997). Affective instability, negative affectivity, and impulsivity predict suicidal behavior in longitudinal studies of patients with personality disorders, including BPD (Yen et al., 2004, Soloff and Chiappetta, in press). Therefore, it is important to assess the effects of impulsivity and aggressiveness on cognitive function under conditions which model negative affective interference.
Impulsivity is not inherently pathologic, and is widely represented in the population. It is is a multifactorial construct of separable but related factors, expressed in cognitive and motor behavior. In the current study, we use a standard measure of trait impulsivity, the Barratt Impulsiveness Scale-version 11 (BIS-11), a self-reported measure which assesses three components of impulsiveness: attention/cognitive impulsiveness (i.e. inability to focus), non-planning impulsiveness (i.e. lack of regard for the future), and motor impulsiveness (i.e. action without reflection) (Barratt & Stanford, 1995). Laboratory measures of behavioral impulsivity do not correlate closely with self-rated measures of trait impulsivity (Stahl et al., 2014, Cyders & Coskunpinar, 2011, Reynolds et al. 2006, Reynolds et al. 2008, White et al. 1994). e.g. BIS-11 scores are correlated with, but not identical to the Go No-Go task, a behavioral measure of impulse control frequently used in fMRI studies, and modified for use in this study (Winstanley et al. 2010)
Within behavioral studies, impulse control is also a multifaceted executive function which includes motor response inhibition, cognitive decisional control (e.g. over premature decisions), and, motivational impulse control (e.g. discounting delayed rewards). Recent experimental studies have further extended the concept of impulse control to include: cognitive suppression of interfering stimuli, inhibition of irrelevant information, and suppression of irrelevant responses (Stahl et al 2014, Sebastian et al. 2013, 2014). In neuropsychological testing, BPD subjects appear more impaired in ability to suppress interfering stimuli (as in the Stroop test), and less impaired in emotionally neutral tasks that test response inhibition (as in the Stop Signal test). Deficits in controlling stimulus interference, but not response inhibition, predicted suicide risk in one study of female BPD subjects, (LeGris et al. 2012).
In fMRI studies, specific components of impulse control activate differing prefrontal neural networks. Under neutral, stress-free conditions, fMRI activation associated with response inhibition in BPD differs little from healthy controls; however, if modulated by negative emotions, fronto-limbic dysfunction is demonstrated (Jacob et al., 2013, Sebastian et al., 2014, van Eijk et al., 2015). Negative emotions also accentuate deficits in decisional and motivational components of impulse control (Sebastian et al. 2013 for review). In our study, affectively valenced Ekman faces, including negative stimuli, are used to motivate impulse control in a Go No-Go task.
Negative affect interferes with brain responses sub-serving attention and impulse control in participants with BPD compared to healthy controls (Soloff et al., 2015). In a recent study, attention-driven fMRI tasks (Go No-Go, X-CPT) were modified to evoke affective interference by replacing the standard cognitive tokens with negative, positive and neutral Ekman faces (Ekman and Friesen, 1976). Directing attention to negative affective stimuli resulted in increased modulation of fMRI responses in BPD subjects compared to controls in fronto-limbic pathways, including the orbital frontal cortex (OFC) and the anterior cingulate cortex (ACC), areas critically involved in control of emotion and impulsive behavior (Soloff et al., 2015). Negative affective interference during cognitive processing in these regulatory areas may contribute to emotion dysregulation, impulsive and aggressive behavior in patients with BPD (Siever, 2008). However, it is unclear whether, or to what extent, the borderline patient’s underlying personality traits (i.e. trait impulsivity and aggressiveness) actually modulate brain responses during cognitive processing under negative affective conditions.
1.2. fMRI and affective interference in BPD
Neuroimaging studies in subjects with BPD have described structural, metabolic, and functional abnormalities involving fronto-limbic networks, suggesting a neural basis for the borderline patient’s emotion dysregulation, impulsive and aggressive behavior (Krause-Utz et al., 2014; Schmahl and Bremner, 2006; Soloff et al., 2012). Affected regions include regulatory networks in the orbital frontal cortex (OFC), medial frontal cortex, dorsolateral prefrontal cortex (DLPFC), and anterior cingulate cortex (ACC), as well as limbic structures involved in emotional appraisal and arousal, including amygdala (AMY) and fusiform gyrus (Donegan et al., 2003; Herpertz et al., 2001; Minzenberg et al., 2007), hippocampus (HIP) and insula (Soloff et al., 2014). The failure of “top down” cortical regulation in the face of “bottom up” limbic hyper-arousal has been proposed as a model for affective interference with cognitive function in BPD, and for the borderline patient’s emotion dysregulation, impulsivity and aggression (Silbersweig et al., 2007, Siever, 2008). fMRI studies which pair emotion with cognitive tasks, demonstrate affective interference with neural processing in brain networks previously reported to have structural, metabolic or functional abnormalities (Soloff et al., 2015).
This study assessed the effects of personality traits, impulsivity and aggression, on neural processing and cognitive function during an fMRI task of response inhibition and motor impulsiveness (Go No-Go), modified using affectively valenced Ekman faces as targets. We hypothesized an interactive effect between the neurobiology of the personality traits and affective context that would interfere with neural processing and potentially exert effects on task performance. Specifically, in the negative context, the personality traits of impulsivity and aggression would each have effects on fMRI activation and task performance which differ significantly from healthy control subjects. As discrete neurobiologic dimensions, we hypothesize that effects of trait impulsivity on neural processing will differ from effects of aggression, though both dimensions may have adverse effects on task performance, compared to healthy control subjects.
2. Methods
2.1 Subjects
The study was approved by the University of Pittsburgh Institutional Review Board. Fifty-six female subjects, 31 cases and 25 controls, 18 – 45 years of age, were recruited from the PI’s ongoing longitudinal studies of BPD, from psychiatric outpatient clinics, and by advertisement from the surrounding community. The study was restricted to females as they comprise 75% of BPD patients in clinical settings, avoiding any confounds due to gender (American Psychiatric Association, 2013). All subjects gave written informed consent. To be included in the BPD sample, subjects were required to meet criteria for a probable or definite lifetime diagnosis of BPD on the International Personality Disorders Examination (IPDE) (Loranger, 1999), and a definite current diagnosis of BPD on the Diagnostic Interview for Borderline Patients-Revised (DIB-R), using a two-year timeframe (Zanarini et al., 1989). Co-morbidity on Axis I was determined by the Structured Clinical Interview for DSM-IV (SCID), for current and lifetime diagnoses (First et al., 2005). Impulsivity was assessed on the Barratt Impulsiveness Scale (BIS-11) (Barratt and Stanford, 1995), aggressiveness on the Brown Goodwin Lifetime History of Aggression (LHA) (Brown and Goodwin, 1986). As depressed mood is highly prevalent in BPD, we also assessed its potential effects on activation, using the Hamilton Rating Scale for Depression-24 item format (HAM-D) (Guy, 1976). There was no statistically significant correlation between any paired combination of HAM-D, BIS-11, and LHA. Therefore, each variable may have effects on fMRI activation independent of the others. Childhood abuse was ascertained by interview on a structured Abuse History (Soloff et al. 2002); suicide attempts on the Columbia Suicide History (Oquendo et al. 2003). Control subjects did not meet criteria for any current or lifetime Axis I or II disorders and were free of psychoactive medication. BPD subjects on psychoactive medication were permitted to remain on their medication. Immediately preceding the scan, all subjects had negative urine toxicology for drugs of abuse (MedTox) and negative pregnancy tests. This sample includes some subjects who participated in a previous analysis, unrelated to impulsivity and aggression (Soloff et al., 2015).
Exclusion criteria included: 1.) a current or lifetime diagnosis of schizophrenia, delusional (paranoid) disorder, schizoaffective disorder, bipolar disorder, or psychotic depression; 2). a current diagnosis of Substance Dependence or any current drug and/or alcohol related CNS deficits (A diagnosis of Substance Abuse was permitted so long as the subject had been abstinent for one week, showed no signs of withdrawal, and had a clean urine toxicology drug screen at the time of the scan.); 3.) CNS pathology of any etiology, including acquired or developmental deficits or seizure disorder; 4.) Physical disorders or treatments with known psychiatric consequence (e.g. hypothyroidism, steroid medications); 5.) Mental Retardation (IQ <70 by WAIS); 6.) standard exclusion criteria for MRI scans (i.e. ferromagnetic artifacts, inability to fit in the scanner, claustrophobia, inability to co-operate with instructions.)
2.2 Procedures
2.2.1. Imaging specifications
Anatomical images were acquired on the 3.0T Siemens Trio system in the axial plane parallel to the AC-PC line using a 3D MPRAGE sequence (TE/TI/TR=3.29ms/900ms/2200ms, flip angle=9, isotropic 1mm3 voxel, 192 axial slices, matrix size=256×192). fMRI data were acquired in the axial plane using gradient echo EPI (TR=2000 ms, TE=30 ms, flip angle=70 deg, 30 slices, slice thickness=3.1 mm, 3 mm × 3 mm in-plane, matrix size=64×64).
2.2.2. fMRI paradigm
The Go No-Go test is a neuropsychological measure of response inhibition and motor impulsiveness which requires subjects to inhibit a prepotent response and respond based on target class. The traditional version of the Go No-Go test was modified by incorporating negative (angry, sad, fearful), positive (happy), and neutral Ekman faces as stimuli (Ekman and Friesen, 1976; Soloff et al., 2015). Before a block of trials, subjects were instructed on the affective context of the upcoming block of rapidly presented faces. Subjects were instructed to make a response only if a presented face was consistent with the instructed affective context. Thus, by gating responses to the affective valence of the Ekman faces, the task induced a psychological interaction between affective context, personality traits, and neural processing in the specific cognitive domain (i.e., impulsivity). During a block of trials, Ekman faces were presented briefly (500 ms) in a mixed jittered event-related design (Inter-Stimulus interval range: 500–1500 ms in 250 ms increments) (Amaro and Barker, 2006; Donaldson et al., 2001). The affective context for target stimuli was signaled at block onset and subjects responded if the affect in the face was consistent with the affective context (67% targets). Four block types were employed (three repetitions, 30 s block length): negative, neutral and positive valence and distorted blocks (in which target images were scrambled faces), with three fixation blocks interspersed. A schematic depiction is provided in Supplementary Figure 2.
2.2.3. Image and fMRI data analyses
fMRI data were processed with Statistical Parametric Mapping (SPM8) using standardized methods, with block-design analyses employed to model conditions of interest. Serial correlations were corrected using an auto-regression (AR(1)) filter, with an expanded high-pass filter (256 s) applied to remove low frequency fluctuations. Realignment was performed to correct for head motion artifact. Normalization parameters, achieved after normalizing each subjects’ high-resolution anatomical image to the template image, were applied to each acquired EPI image. The resultant normalized images were resliced (8 mm3 voxels) and smoothed (8 mm FWHM). Across all analyses, the six motion parameters were modeled as regressors of no interest to model statistical artifacts associated with motion. In first-level analyses, epochs were modeled as separate regressors by convolving with the canonical hemodynamic response function.
Given the preferential role of negative (over positive) valence, the first level contrasts of interest focused on responses during the negative (relative to positive) context (Soloff et al. 2015). For the BPD participants, these were then submitted to second level regression models where the effects of each of the BIS-11, LHA and HAM-D scores were modeled as predictor variables on fMRI responses. This statistical approach was motivated by the statistical independence of each of the 3 scales within the BPD sample (see Methods). To account for potential age-related effects on brain responses associated with impulsivity and aggression, age was employed as an additional covariate in the regression models. From these regression models contrasts were employed to uncover both positive and negative relationships between clinical variables and activation profiles.
Because trait impulsivity (as defined by the BIS-11) is a temperamental trait widely distributed in the population, and not defined as pathological, the relationships between impulsivity and activation metrics were separately explored in the healthy control sample. This analysis was not extended to aggression and depression, because each is defined as an inherently pathological trait, and, therefore, the LHA and HAM-D scores in controls are negligible.
To identify significant fMRI results, Monte Carlo simulations based on the observed smoothness of the data were conducted to derive the minimum cluster extent to be deemed significant for a contiguous set of supra-threshold voxels (p<0.01 cluster forming threshold) in the a priori anatomically defined regions of interest (Maldjian et al., 2003; Tzourio-Mazoyer et al., 2002). The regions of interests were selected for their associations with executive functions of emotion regulation, attention and memory, as described in previous anatomical studies of BPD. They included the orbital frontal cortex, dorsal prefrontal cortex, cingulate gyrus, parietal lobe, basal ganglia, the amygdala, hippocampus and parahippocampus (Soloff et al., 2012; Soloff et al., 2014). This chosen approach performs a Monte Carlo alpha probability simulation, thus computing the probability of a random field of noise (after taking into account the spatial correlations of voxels based on the image smoothness within each region of interest estimated directly from the data set) to produce a cluster of a given size (p<0.01, cluster level), after the noise is thresholded at a given level. Thus, instead of using the individual voxel probability threshold alone in achieving the desired overall significance level, the method uses a combination of both probability thresholding and minimum cluster size thresholding. The underlying principle is that true regions of activation will tend to occur over contiguous voxels within a region of relative functional homogeneity, whereas noise has much less of a tendency to form clusters of activated voxels. We report the results of cluster level corrections in Table 1 while also reporting peak locations within each significant cluster. Because inference is cluster based we provide peak voxel coordinates and p values under the peak within the significant cluster.
Table 1a.
Effects of trait impulsivity, aggression and depression on activation in BPD subjects during the Go No-Go task (Neg.>Pos.)
| Clinical Factor | Anat ROI | Cluster Ext | Actual Ext | P Uncorr. | Voxel Peak (MNI) | Region |
|---|---|---|---|---|---|---|
| Go No-Go Positive Correlations
| ||||||
| AGG | - | - | - | - | - | - |
| BIS | dACC | 201 | 619 | 0001 | 3 38 15 | R. ant. cingulate BA 32 |
| OFC | 140 | 542 | 0.001 | −34 14 −20 | L. inf. frontal BA 13 | |
| BG | 172 | 458 | 0.001 | 14 4 21 | R. caudate body | |
| dPFC | 26 | 75 | 0.001 | −42 9 31 | L. dlPFC, BA 9 | |
| HAM-D | OFC | 66 | 87 | 0.011 | −14 54 -0 | L. sup_med. frontal BA10 |
| Go No-Go Negative Correlations | ||||||
| AGG | HIP-Parahip | 218 | 1041 | <0.001 | −32 −21 −14 | L-hippocampus |
| OFC | 95 | 404 | 0.003 | 8 58 1 | R. sup. frontal, BA 10 | |
| BG | 344 | 379 | 0.004 | −22 9 6 | L-putamen | |
| BIS | - | - | - | - | - | - |
| HAM-D | Parietal | 600 | 1962 | 0.001 | 57 −42 30 | R. inf. parietal, BA40 |
| OFC | 104 | 1514 | 0.001 | 32 60 13 | R. mid. frontal, BA10 | |
| dACC | 97 | 119 | 0.007 | −9 8 27 | L. ant. cingulate BA33 | |
| dPFC | 20 | 97 | 0.001 | 39 17 36 | R. dlPFC, BA8 | |
2.2.4. Behavioral data analyses
The effects of Condition (Negative, Neutral, or Positive valenced faces) on behavioral performance were modeled within subjects in repeated measures analyses of variance, with Condition as the single factor. Behavioral performance is reported as Hit Rates (correct responses during Go conditions) and False Alarm Rates (errors of commission during No-Go conditions). Effects of impulsivity, aggression and depression on Hit Rates and False Alarm Rates were examined individually (as BIS-11, LHA and HAM-D scores).
2.2.5. Effects of medication
Subjects were told to remain on current medication regimens throughout the assessment period, including the fMRI scan. The time from intake to scan availability averaged 4 or more weeks. Effects of medication status (yes/no) on clinical variables, BIS-11, LHA, and HAM-D, were assessed using logistic regression, comparing medicated (n = 15) and non-medicated BPD subjects (n = 16). Effects of psychoactive medication on fMRI signal values were assessed for the voxel peaks depicted in Figures 1 and 2, using independent t-tests.
Figure 1.
(a) The sagittal view depicts significant clusters in the dorsal anterior cingulate (dACC) showing a positive relationship between fMRI activation in BPD, and the degree of impulsivity measured by the Barratt Impulsiveness Scale-version 11 (BIS-11). The fMRI response under the significant peak (cross-hairs) is depicted in the adjoining graph (95% confidence intervals are depicted around the regression function) as a function of the BIS-11. (b) The axial slice depicts significant clusters in the orbitofrontal cortex (OFC) showing a positive relationship between fMRI activation in BPD, and the degree of impulsivity measured by the (BIS-11). The fMRI response under the significant peak (cross-hairs) is depicted in the adjoining graph (95% confidence intervals are depicted around the regression function), as a function of the BIS-11.
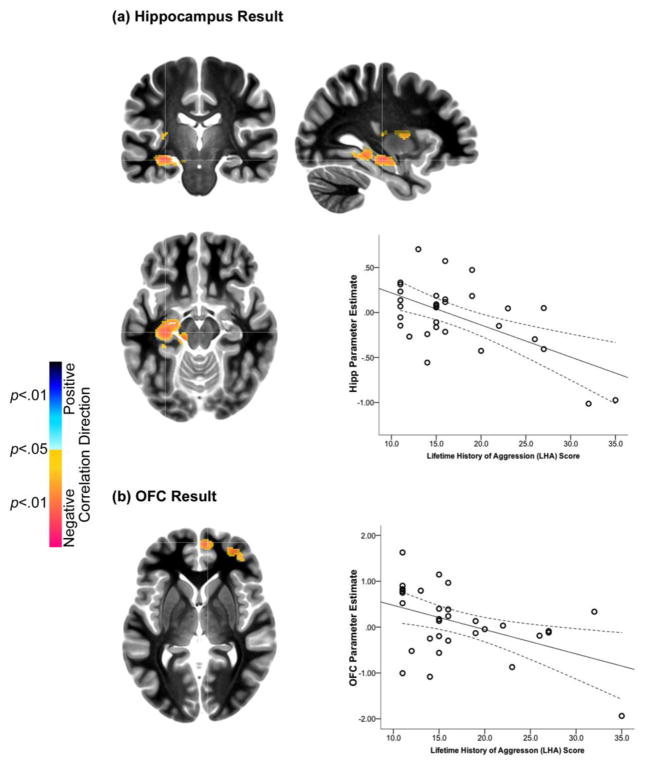
Figure 2.
(a). The orthoview depicts significant clusters in the hippocampus (Hipp) showing a negative relationship between fMRI activation in BPD, and the degree of aggression measured by the Lifetime History of Aggression (LHA) scale. The fMRI response under the significant peak (cross-hairs) is depicted in the adjoining graph (95% confidence intervals are depicted around the regression function), as a function of the LHA. (b). The axial view depicts significant clusters in orbitofrontal cortex (OFC) showing a negative relationship between fMRI activation in BPD, and the degree of aggression measured by the Lifetime History of Aggression (LHA) scale. The fMRI response under the significant peak (cross-hairs) was extracted and is depicted in the adjoining graph (95% confidence intervals are depicted around the regression function), as a function of the LHA.
3. Results
3.1. Subject Characteristics
The sample included 31 female BPD and 25 female control subjects. The mean (s.d.) age of the BPD sample was 30 (8.2) years, compared to 24.5 (5.5) years for healthy controls, (t = 3.00, df = 52.4, p = 0.004). At the time of the scan, current co-morbid Axis I diagnoses were noted in 27 subjects (87.1%), the most frequent being Major Depressive Disorder (MDD) (in 19 subjects (61.3%)) and Generalized Anxiety Disorder (in 11 (35.5%)), with some overlap. A current Substance Use Disorder was noted in only 2 subjects (6.5%). A past history of Attention-Deficit/Hyperactivity Disorder (ADHD) was found in only 2 (6.5%) subjects. Additional Axis II co-morbidity was diagnosed in 18 BPD subjects (58.1%), the most frequent being Paranoid PD (in 5 subjects (16.1%)). Antisocial Personality Disorder (ASPD) was present in 2 (6.5%) subjects. Nineteen BPD subjects (61.3%) had histories of childhood abuse (14 sexually abused). Twenty-two (71%) BPD subjects had past histories of suicide attempts; 9 were non-attempters. Fifteen BPD subjects (48.8%) were taking one or more psychotropic medications (antidepressants (11), anxiolytics (6), neuroleptics (2), mood stabilizers (3), stimulants (1)).
Among BPD subjects, there were no significant correlations between BIS-11, LHA and HAM-D scores. All were normally distributed (by one-sample Chi Square Test (SPSS 22) with mean (s.d.) values as follows: BIS-11: 75.5 (5.1), LHA: 17.3 (6.1), HAM-D: 14.1 (10.6). Among healthy controls, scores for the BIS were normally distributed, with a mean (s.d.) of 72.0 (3.2), significantly less than the BPD sample (t = 3.1, 51.4 df, p = 0.003). HAM-D and LHA assess clinically pathological symptoms and behaviors infrequent among healthy subjects. Among control females, these scores were negligible and not normally distributed: HAM-D = 0.28 (0.54), LHA = 12.5 (3.1) (where 11 is the minimum score). Correlating these scores with fMRI metrics would not be meaningful.
Depressed mood was highly prevalent in subjects with BPD. There was no significant difference in HAM-D scores between subjects with BPD+MDD: HAM-D = 15.5 (11.1) vs. BPD no MDD: HAM-D = 12.0 (9.7), t = 0.890, df = 29, p = 0.38.
3.2. Impulsivity and fMRI activations in BPD subjects (Table 1a, Figure 1)
Impulsivity was positively correlated with activation in four brain regions during the Affective Go No-Go task. In order of observed extent, these clusters included: 1) the dorsal ACC, 2) OFC, 3) basal ganglia (BG), and 4) a small area in dPFC. (Voxel peaks are noted in Table 1a.) There were no brain regions where impulsivity was negatively related to activation.
3.3. Aggression and fMRI activations in BPD subjects (Table 1a, Figure 2)
The effects of aggression were largely opposite of trait impulsivity. Aggression was negatively correlated with activation in three ROIs: 1) HIP/ParaHip, 2) OFC, and, 3) BG. Aggression had no significant positive associations with activation in any brain region.
3.4. Depression and fMRI activations in BPD subjects (Table 1a)
Depression (HAM-D) had a general dampening effect on activations, and was negatively associated with activations in four ROIs: 1) Parietal lobe, 2) OFC, and, 3) dACC, 4.) and dPFC. There was one small area of positive correlation in OFC, anatomically separate from the larger negatively associated area in OFC.
Regression analyses for each of the BIS-11, the LHA and the HAM-D performed for the Negative > Neutral contrast in BPD subjects were not sensitive (Supplementary Figure 1). These results are also consistent with activation profiles in each of the groups (Supplementary Figure 3).
3.5. Trait impulsivity and fMRI activations in healthy controls (Table 1b)
Table 1b.
Effects of trait impulsivity on activation in healthy control subjects during the Go No-Go task (Neg.> Pos.)
| Clinical Factor | Anat ROI | Cluster Ext | Actual Ext | P Uncorr. | Voxel Peak (MNI) | Region |
|---|---|---|---|---|---|---|
| Go No-Go Positive Correlations | ||||||
| BIS | Parietal | 381 | 4655 | 0.001 | −24 −40 49 | L_inf. parietal, BA3 |
| HIP-Parahip | 159 | 508 | 0.002 | −36 −21 −12 | L_hippocampus | |
| OFC | 66 | 98 | 0.007 | 24 57 −12 | R. sup. frontal, BA11 | |
| Go No-Go Negative Correlations | ||||||
| BIS | Parietal | 381 | 1381 | 0.001 | 39 −72 30 | R. inf. parietal, BA39 |
| BG | 195 | 310 | 0.001 | −9 −6 15 | L. caudate body | |
| OFC | 106 | 306 | 0.001 | −38 21 −18 | L. inf. frontal BA47 | |
| AMY | 66 | 282 | 0.001 | −21 −1 −15 | L amygdala | |
| dPFC | 19 | 95 | 0.001 | 45 33 13 | R. dlPFC, BA 46 | |
Trait impulsivity exerted markedly different effects on activation in healthy controls compared to BPD, with no overlap in affected regions. Trait impulsivity scores were positively correlated with activation in three ROIs: 1) the Parietal lobe, 2) HIP, and 3) a small area of OFC. Negative correlations were found in 5 ROIs: 1.) Parietal lobe, 2) BG, 3) OFC, 4) amygdala (AMY), and, 5.) a small area of dPFC.
3.6. Behavioral results (Figure 3)
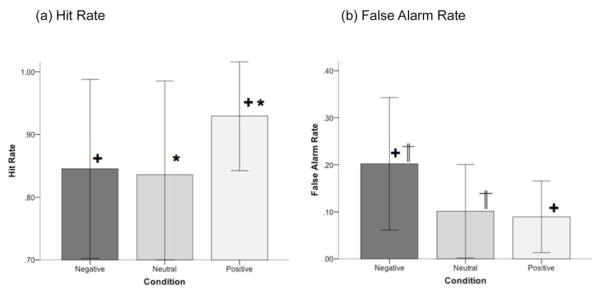
Figure 3.
Mean hit rates (a) and false alarm rates (b) are depicted for BPD (Error bars are ± sd.). Pairwise differences (Bonferroni adjustment for multiple comparisons, p<.05) for each are denoted. As seen, condition exerted significant effects on behavioral sensitivity (see text for statistical information) implying a pattern of selective interference of non-positive stimuli on hit rates and false alarm rates. The impulsivity score (based on the Barratt Impulsiveness Scale-version 11, BIS-11) exerted marginal effects on behavioral performance. Regardless of condition, hit rates decreased with increased impulsivity (negative relationship) and false alarm rates increased with increased impulsivity (positive relationship).
The effects of Condition on each of the Hit Rates and False Alarm rates were analyzed in repeated measures analyses of variance with Condition (Negative, Neutral or Positive) as the single factor. Notable effects of Condition were observed for both behavioral measures: Hit Rates, F2,56 = 15.43, p < 0.001, MSe = 0.016; False Alarm Rates, F2,56 = 8.97, p < 0.001, MSe = 0.008. Post-hoc analyses (with Bonferroni adjustment for multiple comparisons, p< 0.05) were conducted to assess inter-condition differences on Hit and False Alarm rates respectively. Hit Rates for both Negative and Neutral conditions were significantly lower than for the Positive Condition. False Alarm rates for the Negative Condition were significantly higher than the Neutral and Positive Conditions. These effects are visually depicted in Figure 3.
In addition, we investigated the effects of trait impulsivity (BIS-11), aggression (LHA), and depression (HAM-D) on behavioral performance (Hit Rate and False Alarm Rate), modeling each dimension as covariates interacting with Condition. Only trait impulsivity (BIS-11), exerted significant effects on the behavioral measures, with Hit Rates decreasing with an increase in impulsivity, F1,99 = 7.78, p < 0.01, MSe = 0.04, and False Alarm rates increasing with an increase in impulsivity F1,99 = 5.58, p < 0.05, MSe = 0.012. No other effects reached significance.
3.7. Effects of psychoactive medication
Using logistic regression analyses, we found that medication status (yes/no) was not predicted by any of the three clinical variables (BIS-11, LHA, HAM-D): [BIS: Exp(B) = 1.02, Wald = 0.73, 1 df, p = 0.79; AGG: Exp(B) = 0.94, Wald = 0.74, 1 df, p = 0.39; HAM-D: Exp(B) = 1.00, Wald = 0.00, 1 df, p.= 1.00].
Effects of medication status (yes/no) on fMRI signal values were assessed for the voxel peaks depicted in Figures 1 and 2 using independent samples t tests. Psychoactive medications had no effects on fMRI metrics (p > 0.2 on all tests).
4. Discussion
Among BPD subjects, trait impulsivity and aggression had significant but demonstrably different effects on neural processing under negative affective conditions. Each personality trait exerted significant effects on fMRI activation metrics but in opposite directions and in differing anatomical regions. Additionally, the effects of trait impulsivity on neural processing in BPD subjects involved different brain regions and opposite direction of correlations than effects in healthy controls, suggesting a disorder-specific interaction of this trait on brain responses. Healthy controls do not have the structural, metabolic or functional abnormalities associated with emotion dysregulation and impulsive-aggression in BPD. Notably, the performance effects depending on emotional context did not differ between groups (explored further below.) These results are notable for being the first to correlate personality traits of impulsivity and aggression with BOLD responses in BPD during a test of impulse control.
The “top down/bottom up” model of emotion dysregulation in BPD describes the neural basis of affective interference with cognitive functioning, but does not address effects of specific personality dimensions on this process. As a modification of the “top-down, bottom-up” model, we propose that negative emotion, arising from situational stressors, interacts with the pre-existing neurobiology of personality traits, resulting in affective interference with neural processing of cognitive functions. The neurobiology of personality traits refers to the structural, metabolic and functional abnormalities associated with impulsivity and aggression in subjects with BPD (and other impulsive personality disorders). Hyper-activation or hypo-activation of specific brain networks is the end result of this interaction (Soloff et al., 2015). In our Affective Go No-Go task, higher trait impulsivity is associated with hyper-activation in BPD subjects, while higher aggression is associated with hypo-activation of specific brain networks. We propose that behavioral outcomes of negative affective stress, including emotion dysregulation, impulsive-aggression and suicidal behavior, are directly related to the specific brain networks and cognitive functions involved in this modulation.
4.1. Impulsivity and response inhibition
Go No-Go is a test of response inhibition and motor impulsiveness. In fMRI studies, standard Go No-Go paradigms have been shown to activate bilateral OFC, dPFC, ACC, and right inferior frontal gyrus in normal subjects (Asahi et al., 2004; Casey et al., 1997; Fineberg et al., 2014; Horn et al., 2003; Rubia et al., 2003). Recent meta-analyses also describe activation in right inferior frontal gyrus, anterior insula, pre-supplemental motor area (SMA), and basal ganglia in response inhibition studies (Aron 2011, Cai et al 2014, Swick et al 2011.) Some studies, though not all, report correlations between self-report measures of impulsiveness and neural activation in specific ROIs during response inhibition. For example, a positive correlation was reported between high scores on the impulsivity subscale of Eysenck’s Impulsivity, Venturesomeness, and Empathy Inventory (Eysenck and Eysenck, 1991), and activation in right posterior OFC, right inferior frontal gyrus, and right insula during a Go No-Go study in healthy subjects (Horn et al., 2003). Activation of OFC and ACC correlate with behavioral performance on the Go No-Go test. The greater the activation of the OFC, the greater the inhibition (Casey et al., 1997). This is consistent with the regulatory functions ascribed to the OFC and ACC, which include response inhibition and impulse control. Our study supports this view. Trait impulsivity in BPD subjects was positively associated with activation in both dACC and OFC while performing the Affective Go No-Go task under negative affective conditions, suggesting increased cortical regulation in impulsive subjects during task performance. Hyper-activation of OFC and dACC among BPD subjects in response to the negative emotional context during the Affective Go No-Go task, suggests enhanced engagement of “top down” inhibitory controls to compensate for the subject’s own trait impulsivity. (The opposite effect is seen in healthy controls).
To address the viability of this explanation, we analyzed hit rates and false alarm rates for each of the affective contexts, negative, positive and neutral. These were compared between groups (BPD and HC) to assess whether groups differed on either behavioral measure of interest. For hit rates, no significant effects were observed for Negative (F1,57= 0.59, p> 0.10), Positive (F1,57=1.88, p> 0.10) or Neutral (F1,57=2.57, p> 0.10) contexts. Effect sizes for these analyses were small (Partial η2= 0.01 to 0.04). For false alarm rates, no significant effects were observed for Negative (F1,57= 0.01, p>0.10), Positive (F1,57=2.0, p>0.10) or Neutral (F1,57=1.4, p>0.10) contexts. Effect sizes for these analyses were also small (Partial η2= 0.00 to 0.035). These results provide evidence for comparable performance between groups suggesting that increased responses in frontal striatal regions with impulsivity may reflect a compensatory neurobiological process to maintain performance in the range comparable to healthy control participants.
Enhanced activation of prefrontal regions during Go No-Go was reported by Vollm et al. (2004) in a small sample of male in-patients with BPD and/or ASPD compared to healthy controls. During a standard Go No-Go task, healthy control subjects activated left OFC and right dPFC, while BPD/ASPD subjects activated a much wider prefrontal area, including bilateral medial, superior, and inferior frontal gyri and ACC. Behaviorally, there were no significant group differences in reaction times or errors of omission. The authors suggested that the enhanced activations in the BPD/ASPD patients during response inhibition reflected network recruitment.
The ACC has many executive functions, including selective attention and effortful control, error detection, conflict resolution, decision-making, and learning (Botvinick et al., 2001; Carter et al., 2000; Tana et al., 2010). In concert with the amygdala, ACC is involved in the regulation of negative emotion, which is directly relevant to our affective task (Ruocco et al., 2013). It has been shown that BPD subjects have diminished metabolic connectivity between right OFC, right sub-genual ACC (BA 25) and ventral AMY compared to healthy controls (New et al., 2007), possibly contributing to dysregulation of affect and impulsive behavior.
ACC may also be involved in the mechanism of emotional interference with executive cognitive functioning. The ACC is a key component of the attention network (which also includes middle frontal gyrus (MFG), inferior frontal gyrus (IFG), and anterior insula), and is engaged by emotional stimuli (such as threat) and motivational states (like reward). A “ dual competition model” has been proposed which describes competition for limited information processing resources posed by strong emotion during executive task performance, thereby impairing performance (Pessoa (2009).
Activation in basal ganglia (BG) was positively correlated with impulsivity in BPD subjects and negatively in healthy controls. The BG are involved in inhibitory control (as reactive stopping or motor response inhibition in the Go No-Go or Stop Signal Tests) through a fronto-striatal network. Reviews and meta-analyses of inhibitory control studies using these paradigms demonstrate activation of the right inferior frontal cortex, right anterior insula, dorsomedial frontal cortex (pre-supplemental motor area), subthalamic nucleus and globus pallidus pars interna in response inhibition. Activation of this network in healthy control subjects results in thalamo-cortical output to the primary motor area which inhibits motor action (Aron et al. 2011, Cai et al. 2014, Swick et al. 2011). Abnormal functioning of this network in BPD subjects would impair inhibitory control.
The BG also have extensive connectivity with the prefrontal cortex (PFC) and participate in executive cognitive functions such as procedural learning, delay discounting, and other reward-based decisions (Dombrovski et al., 2012). The globus pallidus, dorsolateral caudate, and thalamus are involved in learning new sequences (Middleton and Strick, 2000). In highly impulsive BPD subjects, activation of these task-related regions may also serve a compensatory function to assist cognitive performance during a response inhibition task.
In healthy subjects, the effects of trait impulsivity on neural responses to the Go No-Go task were markedly different from those of BPD subjects in both anatomical localization and in the direction of correlations. It is unlikely that these extensive differences were simply due to increased severity of the impulsive trait in BPD, as the absolute difference in mean BIS-11 scores between groups, though statistically significant, was actually quite small (HC:72.0 (3.2) vs. BPD: 75.5 (5.1)). Instead, these differences suggest a disorder-specific response in BPD. Among healthy subjects, higher levels of trait impulsivity resulted in diminished activation in both cortical control areas (OFC, dlPFC), and limbic regions (AMY). This effect on the fronto-limbic network is absent in BPD subjects. Higher degrees of trait impulsivity in healthy subjects modulate both increases and decreases in activation of task processing areas in parietal cortex as the most robust effects; however, the affected regions differ markedly in laterality and anatomical specificity. Activation of parietal regions in healthy control subjects may serve to facilitate task performance, a response not seen in BPD subjects in relation to trait impulsivity or aggression. Activation of the BG is also noted in both BPD and control subjects in relation to trait impulsivity, but with opposite direction of correlation and laterality. Taken together, these results suggest a disorder-specific response in BPD; i.e., trait impulsivity in BPD may be mediated by an entirely different neural pathway compared to healthy subjects, a pathway affected by the neurobiologic abnormalities that characterize BPD.
4.2. Aggression and response inhibition
Aggression in BPD subjects had a negative effect on brain activations during response inhibition in a negative affective context, markedly different from the effects of trait impulsivity. Higher degrees of aggression were associated with diminished activation in OFC, BG, and HIP, a response with potentially important clinical implications. BPD subjects are more likely to respond aggressively to a negative emotional stimulus if the inhibitory function of the OFC is diminished (Berlin et al., 2005; Siever LJ, 2008). Similarly, behavioral aggression is more likely if the social decision making functions of the BG are diminished, as well as the ability to recall episodic memories of past experience, a function of the HIP. (Delayed recall of episodic autobiographic memory has been demonstrated among suicide attempters independent of diagnosis (Williams and Broadbent, 1986)). The net effect of high degrees of aggression on neural processing during response inhibition would be to impair adaptive responding.
We found no limbic arousal associated with aggression. The negative affective stimuli used in our study, angry, sad and fearful Ekman faces, do activate the limbic system in BPD during passive viewing (Donegan et al., 2003; Herpertz et al., 2001); however, limbic activation may be diminished when the faces are presented in the context of a competing cognitive task. Jacob et al. (2013) paired a more aversive negative stimulus, an anger induction paradigm, with a standard Go No-Go test, and reported differences in activation metrics between healthy control and BPD subjects. Among healthy controls, increased activity was noted in left inferior frontal cortex during response inhibition, while BPD subjects increased activation in right sub-thalamic nucleus, which was interpreted as a compensatory response. Even with limbic arousal, behavioral performance on the standard Go No-Go test did not differ between groups.
Studies of emotional interference with cognitive function in subjects with BPD have incorporated emotional words into Stroop and Go No-Go designs (Wingenfeld et al 2009, Silberzweig et al. 2007), used aversive pictures and faces as task-irrelevant disractors in working memory tasks (Krause-Utz et al. 2012), flanker tasks (Holtmann et al. 2013), in n-back tasks (Prehn et al 2013), and reward processing tasks (Enzi et al. 2013). Soloff et al (2015) demonstrated emotional interference with executive functions by incorporating affectively valenced Ekman faces in Go Go-Go, and X-CPT paradigms as task relevant targets, and emotional IAPS pictures into a Memory encoding and recall task. These studies document disruptions in neural processing by emotional stressors in BPD compared to control subjects. However, emotional interference with behavioral task performance depends, in part, on the intensity and relevance of the negative stimulus.
4.3 Depressed mood and response inhibition
Depressed mood had a general dampening effect on neural responsiveness in OFC, dACC, dPFC, and large areas of the parietal cortex, suggesting diminished cortical regulation and efficiency of task-related functions. Depressed mood (HAM-D) is a frequent symptom in patients with BPD, related in many, though not all, to co-morbid MDD. We found no significant difference between HAM-D scores in BPD subjects with and without co-morbid MDD. Depressed mood is a component of the borderline patient’s “negative affectivity”, a personality dimension independent of formal Axis I affective diagnoses. Negative affectivity includes chronic attitudes of low self-esteem, pessimism and hopelessness, and contributes a personality vulnerability to suicidal behavior in patients with mood disorders (Oquendo et al., 2004). Negative affectivity predicts suicide attempts in prospective studies of patients with personality disorders, including BPD (Yen et al., 2009, Soloff and Chiappetta, in press).
4.4. Effects of negative emotional contexts
Negative faces, compared to positive, adversely affected behavioral performance in this test of response inhibition. In prior studies using the Affective Go No-Go paradigm, the magnitude of neural effects in BPD subjects compared to controls was also greatest for negative stimuli, followed by neutral, then positive stimuli (Soloff et al., 2015). (BPD subjects tend to project negative attributes onto neutral faces in fMRI studies, and demonstrate hyper-arousal of amygdala to neutral Ekman faces (Donegan et al., 2003)) In the clinical setting, negative affective stress and impaired impulse control in BPD subjects would pre-dispose to impulsive, aggressive and self-destructive behaviors. We have proposed that negative emotional contexts interfere with neural processing in BPD, and that this interference results from an interaction of affective stimuli with the underlying neurobiology of temperamental traits. Furthermore, impulsivity and aggression modulate activation in specific, but differing, anatomical regions and directions, suggesting separate neural pathways for these two important personality dimensions.
4.5. Limitations
Significant differences in the effects of impulsivity on activation in BPD and healthy control subjects suggest “disorder-specific” findings in BPD. This interpretation is limited by the potential effects of co-morbidities or adverse life events (e.g. childhood abuse) which may contribute to fMRI activation. Clinical control groups may be helpful in this regard, though unlikely to address every possibility.
This study was restricted to female subjects with BPD. Female BPD subjects tend to internalize emotional behavior, and present with more identity disturbance, eating disorders and post-traumatic stress disorders compared to their male counterparts. Male BPD subjects are more prone to externalizing behaviors, and more comorbidity with substance use disorders and antisocial personality disorder (Johnson et al. 2003). Gender differences in impulsivity and aggression would likely result in different findings in a male BPD sample.
In our analysis of medication effects, it is important to note that small sample sizes may lead to false negative results and limit interpretation.
Supplementary Material
Supplementary Figure 1. Regression analyses for each of the BIS-11, the LHA and the HAM-D performed for the Negative > Neutral contrast in BPD subjects. As seen, activations under contrast are not well predicted by any of the clinical scales (contrasted against those under the Negative > Positive contrast). Data are shown at p<0.01, cluster level.
Supplementary Figure 2. A schematic depiction of the modified Go-No-Go task is provided. An overall mixed-block design was employed. Within a block of trials, subjects were asked to respond if the presented face depicted an affect consistent with the framing instruction (example of a negative block is shown).
Supplementary Figure 3. Significant activations for each group (BPD and HC) and contrast (Negative > Positive and Negative > Neutral) are rendered on a mosaic of axial slices (p<0.05, cluster level). Activations are observed in the OFC, the dPFC, the dACC, the basal ganglia, the medial temporal lobe and the parietal lobe. Labels for the clusters are left out of the figure for ease of readability.
Highlights.
We assessed effects of impulsivity and aggression on neural processing in subjects with BPD using an Affective Go No-Go task.
With negative affect, impulsivity was positively correlated with activation in dACC, OFC, and BG, with no areas of negative correlation.
Aggression was negatively correlated with activation in OFC, HIP, and BG with no areas of positive correlation.
Negative affect and impulsivity, but not aggression, diminished task performance.
Interference with cognitive function results from an interaction between personality traits and affective context.
Acknowledgments
Funding: This work was supported by the National Institute of Mental Health (grant number MH 048063). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health, which had no role in the conduct of the research.
Footnotes
Paper presented at the International Society for the Study of Personality Disorders (ISSPD XIV), Montreal, Canada, Oct.16, 2015
Contributors:
Paul Soloff was involved in the study design, analysis, interpretation and manuscript writing. Kristy Abraham, Ashley Burgess, Karthik Ramaseshan, and Asadur Chowdury were involved in data analysis. Vaibhav A. Diwadkar was involved in the study design, analysis, interpretation, and manuscript writing.
Conflict of interest:
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amaro E, Barker GJ. Study design in fMRI: Basic principles. Brain Cogn. 2006;60:220–232. doi: 10.1016/j.bandc.2005.11.009. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Borderline personality disorder, Diagnostic and Statistical Manual of Mental Disorders, DSM-5. 5. American Psychiatric Publishing; Washington, DC: 2013. pp. 663–666. [Google Scholar]
- Aron AR. From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biol Psychiatry. 2011;69(12):55–68. doi: 10.1016/j.biopsych.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahi S, Okamoto Y, Okada G, Yamawaki S, Yokota N. Negative correlation between right prefrontal activity during response inhibition and impulsiveness: a fMRI study. Eur Arch Psychiatry Clin Neurosci. 2004;254:245–251. doi: 10.1007/s00406-004-0488-z. [DOI] [PubMed] [Google Scholar]
- Barratt ES, Stanford MS. Impulsiveness. In: Costello CG, editor. Personality Characteristics of the Personality Disordered. Wiley; New York: 1995. pp. 91–118. [Google Scholar]
- Berlin HA, Rolls ET, Iverson SD. Borderline personality disorder, impulsivity and the orbitofrontal cortex. Am J Psychiatry. 2005;162:2360–2373. doi: 10.1176/appi.ajp.162.12.2360. [DOI] [PubMed] [Google Scholar]
- Black DW, Blum N, Pfohl B, Hale N. Suicidal behavior in borderline personality disorder: prevalence, risk factors, prediction, and prevention. J Pers Disord. 2004;18:226–239. doi: 10.1521/pedi.18.3.226.35445. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Brezo J, Paris J, Turecki G. Personality traits as correlates of suicidal ideation, suicide attempts, and suicide completions: a systematic review. Acta Psychiatr Scand. 2006;113:180–206. doi: 10.1111/j.1600-0447.2005.00702.x. [DOI] [PubMed] [Google Scholar]
- Brodsky BS, Groves SA, Oquendo MA, Mann JJ, Stanley B. Interpersonal precipitants and suicide attempts in borderline personality disorder. Suicide Life Threat Behav. 2006;36:313–322. doi: 10.1521/suli.2006.36.3.313. [DOI] [PubMed] [Google Scholar]
- Brown GL, Goodwin FK. Cerebrospinal fluid correlates of suicide attempts and aggression. Ann NY Acad Sci. 1986;487:175–188. doi: 10.1111/j.1749-6632.1986.tb27897.x. [DOI] [PubMed] [Google Scholar]
- Cai W, Ryali S, Chen T, Li CS, Menon V. Dissociable roles of right inferior frontal cortex and anterior insula in inhibitory control: evidence from intrinsic and task-related functional parcellation, connectrivity, and response profile analyses across multiple datasets. J Neurosci. 2014;34(44):14652–14667. doi: 10.1523/JNEUROSCI.3048-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Macdonald AM, Botvinick M, Ross LL, Stenger VA, Noll D, Cohen JD. Parsing executive processes: strategic vs. evaluative functions of the anterior cingulate cortex. Proc Natl Acad Sci U S A. 2000;97:1944–1948. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Trainor RJ, Orendi JL, Schubert AB, Nystrom LE, Giedd JN, Castellanos FX, Haxby JV, Noll DC, Cohen JD, Forman SD, Dahl RE, Rapoport JL. A developmental functional MRI study of prefrontal activation during performance of a Go-No-Go task. J Cogn Neurosci. 1997;9:835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- Cyders MA, Coskunpinar A. Measurement of constructs using self-report and behavioral lab tasks: is there overlap in nomothetic span and construct representation for impulsivity? Clin Psychol Rev. 2011;31(6):965–82. doi: 10.1016/j.cpr.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Dombrovski AY, Siegle GJ, Szanto K, Clark L, Reynolds CF, Aizenstein H. The temptation of suicide: striatal gray matter, discounting of delayed rewards, and suicide attempts in late-life depression. Psychological Med. 2012;42:1203–1215. doi: 10.1017/S0033291711002133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson DI, Petersen SE, Ollinger JM, Buckner RL. Dissociating state and item components of recognition memory using fMRI. NeuroImage. 2001;13:129–142. doi: 10.1006/nimg.2000.0664. [DOI] [PubMed] [Google Scholar]
- Donegan NH, Sanislow CA, Blumberg HP, Fulbright RK, Lacadie C, Skudlarski P, Gore JC, Olson IR, McGlashen TH, Wexler BE. Amygdala hyperreactivity in borderline personality disorder: implications for emotional dysregulation. Biol Psychiatry. 2003;54:1284–1293. doi: 10.1016/s0006-3223(03)00636-x. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Consult. Psychologists Press; Palo Alto, CA: 1976. Pictures of facial affect. [Google Scholar]
- Enzi B, Doering S, Faber C, Hinrichs J, Bahmer J, Northoff G. Reduced deactivation in reward circuitry and midline struictures during emotion processing in borderline personality disorder. World J Biol Psychiatry. 2013;14:45–56. doi: 10.3109/15622975.2011.579162. [DOI] [PubMed] [Google Scholar]
- Eysenck HJ, Eysenck SBG. Manual of the Eysenck personality scales (EPS adult) : comprising the EPQ-revised (EPQ-R) (including addiction and criminality scales), EPQ-R short scale, impulsiveness (IVE) questionnaire (impulsiveness/venturesomeness/empathy) Hodder & Stoughton; London: 1991. [Google Scholar]
- Fertuck EA, Lenzenweger MF, Clarkin JF, Hoermann S, Stanley B. Executive neurocognition, memory systems, and borderline personality disorder. Clin Psychol Rev. 2006;26:346–375. doi: 10.1016/j.cpr.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Fineberg NA, Chamberlain SR, Goudriaan AE, Stein DJ, Vanderschuren LJ, Gillan CM, Shekar S, Gorwood PA, Voon V, Morein-Zamir S, Denys D, Sahakian BJ, Moeller FG, Robbins TW, Potenza MN. New developments in human neurocognition: clinical, genetic, and brain imaging correlates of impulsivity and compulsivity. CNS spectrums. 2014;19:69–89. doi: 10.1017/S1092852913000801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders-Patient Edition (SCID-I/P, 4/2005 revision) Biometrics Research Department, New York State Psychiatric Institute; New York: 2005. [Google Scholar]
- Guy W. ECDEU Assessment Manual of Psychopharmacology-Revised. National Institute of Mental Health (U.S.). Psychopharmacology Research Branch; Rockville, Md: 1976. [Google Scholar]
- Herpertz SC, Dietrich TM, Wenning B, Krings T, Erberich SC, Willmes K, Thron A, Sass H. Evidence of abnormal amygdala functioning in borderline personality disorder: A functional MRI study. Biol Psychiatry. 2001;50:292–298. doi: 10.1016/s0006-3223(01)01075-7. [DOI] [PubMed] [Google Scholar]
- Holtmann J, Herbort MC, Wustenberg T, Soch J, Richter S, Walter H, Roepke S, Schott BH. Trait anxiety modulates fronto-limbic processing of emotional interference in borderline personality disorder. Front Hum Neurosci. 2013;7:1–21. doi: 10.3389/fnhum.2013.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn NR, Dolan M, Elliott R, Deakin JF, Woodruff PW. Response inhibition and impulsivity: an fMRI study. Neuropsychol. 2003;41:1959–1966. doi: 10.1016/s0028-3932(03)00077-0. [DOI] [PubMed] [Google Scholar]
- Jacob GA, Guenzler C, Zimmermann S, Scheel CN, Rusch N, Leonhart R, Nerb J, Lieb K. Time course of anger and other emotions in women with borderline personality disorder: A preliminary study. J Behav Ther Exp Psy. 2008;39:391–402. doi: 10.1016/j.jbtep.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Jacob GA, Zvonik K, Kamphausen S, Sebastian A, Maier S, Philipsen A, Tebartz van Elst L, Lieb K, Tuscher O. Emotional modulation of motor response inhibition in women with borderline personality disorder: an fMRI study. J Psychiatry Neurosci. 2013;38:164–172. doi: 10.1503/jpn.120029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DM, Shea MT, Yen S, Battle CL, Zlotnick C, Sanislow CA, Grilo CM, Skodol AE, Bender DS, McGlashan TH, Gunderson JG, Zanarini MC. Gender differences in borderline personality disorder: Findings from the Collaborative Longitudinal Personality Disoeders Study. Compr Psychiatry. 2003;44(4):284–292. doi: 10.1016/S0010-440X(03)00090-7. [DOI] [PubMed] [Google Scholar]
- Krause-Utz A, Oei NY, Niedtfeld I, Bohus M, Spinhoven P, Schmahl C, Elzinga BM. Influence of emotional distraction on working memory performance in borderline personality disorder. Psychol Med. 2012;42(10):2181–2192. doi: 10.1017/S0033291712000153. [DOI] [PubMed] [Google Scholar]
- Krause-Utz A, Winter D, Niedtfeld I, Schmahl C. The latest neuroimaging findings in borderline personality disorder. Curr Psychiatry Rep. 2014;16:438. doi: 10.1007/s11920-014-0438-z. [DOI] [PubMed] [Google Scholar]
- LeGris J, Links PS, van Reeekum R, Tannock R, Toplak M. Executive function and suicidal risk in women with biorderline personality disorder. Psychiatry Res. 2012;196:101–108. doi: 10.1016/j.psychres.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Lenzenweger MF, Lane MC, Loranger AW, Kessler RC. DSM-IV personality disorders in the National Comorbidity Survey Replication. Biol Psychiat. 2007;62:553–564. doi: 10.1016/j.biopsych.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine D, Marziali E, Hood J. Emotion processing in borderline personality disorders. J Nerv Ment Dis. 1997;185:240–246. doi: 10.1097/00005053-199704000-00004. [DOI] [PubMed] [Google Scholar]
- Loranger AW. DSM-IV and ICD-10 Interviews. Psychological Assessment Resources, Inc; Lutz, FL: 1999. International Personality Disorder Examination. [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia output and cognition: evidence from anatomical, behavioral, and clinical studies. Brain Cogn. 2000;42:183–200. doi: 10.1006/brcg.1999.1099. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Fan J, New AS, Tang CY, Siever LJ. Fronto-limbic dysfunction in response to facial emotion in borderline personality disorder: An event-related fMRI study. Psychiatry Res. 2007;155:231–243. doi: 10.1016/j.pscychresns.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New AS, Hazlett EA, Buchsbaum MS, Goodman M, Mitelman SA, Newmark R, Trisdorfer R, Haznedar MM, Koenigsberg HW, Flory J, Siever LJ. Amygdala-prefrontal disconnection in borderline personality disorder. Neuropsychopharmacology. 2007;32:1629–1640. doi: 10.1038/sj.npp.1301283. [DOI] [PubMed] [Google Scholar]
- Oquendo MA, Galfalvy H, Russo S, Ellis SP, Grunebaum MF, Burke A, Mann JJ. Prospective study of clinical predictors of suicidal acts after a major depressive episode in patients with major depressive disorder or bipolar disorder. Am J Psychiatry. 2004;161:1433–1441. doi: 10.1176/appi.ajp.161.8.1433. [DOI] [PubMed] [Google Scholar]
- Oquendo MA, Halberstam B, Mann JJ. Risk factors for suicidal behavior. The utility and limitations of research instruments. In: First MB, editor. Standardized Evaluation in Clinical Practice Review of Psychiatry. Vol. 8. American Psychiatric Publishing; Washington, D.C: 2003. pp. 103–130. [Google Scholar]
- Paris J. Estimating the prevalence of personality disorders in the community. J Pers Disord. 2010;24:405–411. doi: 10.1521/pedi.2010.24.4.405. [DOI] [PubMed] [Google Scholar]
- Perroud N, Baud P, Ardu S, Krejci I, Mouthon D, Vessaz M, Guillaume S, Jaussent I, Olie E, Malafosse A, Courtet P. Temperament personality profiles in suicidal behaviour: An investigation of associated demographic, clinical and genetic factors. J Affect Disord. 2013;146:246–253. doi: 10.1016/j.jad.2012.09.012. [DOI] [PubMed] [Google Scholar]
- Pessoa L. How do emotion and motivation direct executive control? Trends Cogn Sci. 2009;13(4):160–166. doi: 10.1016/j.tics.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prehn K, Schulze L, Rossmann S, Berger C, Vohs K, Fleischer M, Hauenstein K, Keiper P, Domes G, Herpertz S. Effects of emotional stimuli on working memory processes in male criminal offenders with borderline and antisocial personality disorder. World J Biol Psychiatry. 2013;14:71–78. doi: 10.3109/15622975.2011.584906. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Ortengren A, Richards JB, de Wit H. Dimensions of impulsive behavior: Personality and behavioral measures. Personal Individ Differ. 2006;40:305–315. [Google Scholar]
- Rubia K, Smith AB, Brammer MJ, Taylor E. Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. NeuroImage. 2003;20:351–358. doi: 10.1016/s1053-8119(03)00275-1. [DOI] [PubMed] [Google Scholar]
- Ruocco AC, Amirthavasagam S, Choi-Kain LW, McMain SF. Neural correlates of negative emotionality in borderline personality disorder: An activation-likelihood-estimation meta-analysis. Biol Psychiatry. 2013;73:153–160. doi: 10.1016/j.biopsych.2012.07.014. [DOI] [PubMed] [Google Scholar]
- Schmahl CG, Bremner JD. Neuroimaging in borderline personality disorder. J Psychiatr Res. 2006;40:419–427. doi: 10.1016/j.jpsychires.2005.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian A, Jacob G, Lieb K. Impulsivity in borderline personality disorder: A matter of disturbed impulse control or a facet of emotional dysregulation? Curr Psychiatry Rep. 2013;15:339. doi: 10.1007/s11920-012-0339-y. [DOI] [PubMed] [Google Scholar]
- Sebastian A, Jung P, Krause-Utz A, Lieb K, Schmahl C, Tuscher O. Frontal dysfunctions of impulse control - a systematic review in borderline personality disorder and attention-deficit/hyperactivity disorder. Front Hum Neurosci. 2014;8:698. doi: 10.3389/fnhum.2014.00698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siever LJ. Neurobiology of aggression and violence. Am J Psychiatry. 2008;165:429–442. doi: 10.1176/appi.ajp.2008.07111774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbersweig D, Clarkin JF, Goldstein M, Kernberg OF, Tuescher O, Levy KN, Brendel G, Pan H, Beutel M, Pavony MT, Epstein J, Lenzenweger MF, Thomas KM, Posner MI, Stern E. Failure of frontolimbic inhibitory function in the context of negative emotion in borderline personality disorder. Am J Psychiatry. 2007;164:1832–1841. doi: 10.1176/appi.ajp.2007.06010126. [DOI] [PubMed] [Google Scholar]
- Soloff PH, Chiappetta L. Suicidal behavior and psychosocial outcome in borderline personality disorder at 8 year follow-up. J Pers Disod. doi: 10.1521/pedi_2017_31_280. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloff PH, Lynch KG, Kelly TM. Childhood abuse as a risk factor for suicidal behavior in borderline personality disorder. J Pers Disord. 2002;16(3):201–214. doi: 10.1521/pedi.16.3.201.22542. [DOI] [PubMed] [Google Scholar]
- Soloff PH, Pruitt P, Sharma M, Radwan J, White R, Diwadkar VA. Structural brain abnormalities and suicidal behavior in borderline personality disorder. J Psychiatr Res. 2012;46:516–525. doi: 10.1016/j.jpsychires.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloff PH, White R, Diwadkar VA. Impulsivity, aggression and brain structure in high and low lethality suicide attempters with borderline personality disorder. Psychiatry Research: Neuroimaging. 2014;222:131–139. doi: 10.1016/j.pscychresns.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloff PH, White R, Omari A, Ramaseshan K, Diwadkar VA. Affective context interferes with brain responses during cognitive processing in borderline personality disorder: fMRI evidence. Psychiatry Res. 2015;233:23–35. doi: 10.1016/j.pscychresns.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl C, Voss A, Schmitz F, Nuszbaum M, Tuscher O, Lieb K, Klauer KC. Behavioral components of impulsivity. J Exp Psychol Gen. 2014;143(2):850–886. doi: 10.1037/a0033981. [DOI] [PubMed] [Google Scholar]
- Swick D, Ashley V, Turken U. Are the neural correlates of stopping and not going identical? Quantitative meta-analyses of two response inhibition tasks. Neuroimage. 2011;56(3):1655–1665. doi: 10.1016/j.neuroimage.2011.02.070. [DOI] [PubMed] [Google Scholar]
- Tana MG, Montin E, Cerutti S, Bianchi AM. Exploring cortical attention system by using fMRI during a Continuous Perfomance Test. Comput Intell Neurosci. 2010:329213–329213. doi: 10.1155/2010/329213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- van Eijk J, Sebastian A, Krause-Utz A, Cackowski S, Demirakca T, Biedermann SV, Lieb K, Bohus M, Schmahl C, Ende G, Tuscher O. Women with borderline personality disorder do not show altered BOLD responses during response inhibition. Psychiatry Research: Neuroimaging. 2015;234:378–389. doi: 10.1016/j.pscychresns.2015.09.017. [DOI] [PubMed] [Google Scholar]
- Vollm B, Richardson P, Stirling J, Elliott R, Dolan M, Chaudhry I, Del Ben C, McKie S, Anderson I, Deakin B. Neurobiological substrates of antisocial and borderline personality disorder: preliminary results of a functional fMRI study. Criminal behaviour and mental health : CBMH. 2004;14:39–54. doi: 10.1002/cbm.559. [DOI] [PubMed] [Google Scholar]
- White JL, Moffitt TE, Caspi A, Bartusch DJ, Needles DJ, Stouthamer-Loeber M. Measuring impulsivity and examining its relationship to delinquency. J Abn Psychol. 1994;103(2):192–205. doi: 10.1037//0021-843x.103.2.192. [DOI] [PubMed] [Google Scholar]
- Williams JM, Broadbent K. Autobiographical memory in suicide attempters. J Abnorm Psychol. 1986;95:144–149. doi: 10.1037//0021-843x.95.2.144. [DOI] [PubMed] [Google Scholar]
- Wingenfeld K, Mensebach C, Rullkoetter N, Schlosser N, Schaffrath C, Woermann FG, Driessen M, Beblo T. Attention bias to personally relevant words in borderline personality disorder is strongly related to comorbid posttraumatic stress disorder. J Pers Disord. 2009;23:141–155. doi: 10.1521/pedi.2009.23.2.141. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Olausson P, Taylor JR, Jentsch JD. Insight into the relationship between impulsivity and substance abuse from studies using animal models. Alcohol Clin Exp Res. 2010;34(8):1306–1318. doi: 10.1111/j.1530-0277.2010.01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen S, Shea MT, Sanislow CA, Grilo CM, Skodol AE, Gunderson JG, McGlashan TH, Zanarini MC, Morey LC. Borderline personality disorder criteria associated with prospectively observed suicidal behavior. Am J Psychiatry. 2004;161:1296–1298. doi: 10.1176/appi.ajp.161.7.1296. [DOI] [PubMed] [Google Scholar]
- Yen S, Shea MT, Sanislow CA, Skodol AE, Grilo CM, Edelen MO, Stout RL, Morey LC, Zanarini MC, Markowitz JC, McGlashan TH, Daversa MT, Gunderson JG. Personality traits as prospective predictors of suicide attempts. Acta Psychiatr Scand. 2009;120:222–229. doi: 10.1111/j.1600-0447.2009.01366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanarini MC. The subsyndromal phenomenology of borderline personality disorder. In: Zanarini MC, editor. Borderline Personality Disorder. Taylor and Francis Group; New York: 2005. pp. 19–40. [Google Scholar]
- Zanarini MC, Gunderson JG, Frankenburg FR, Chauncey DL. The Revised Diagnostic Interview for Borderlines: Discriminating BPD from other Axis II disorders. J Pers Disord. 1989;3:10–18. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Regression analyses for each of the BIS-11, the LHA and the HAM-D performed for the Negative > Neutral contrast in BPD subjects. As seen, activations under contrast are not well predicted by any of the clinical scales (contrasted against those under the Negative > Positive contrast). Data are shown at p<0.01, cluster level.
Supplementary Figure 2. A schematic depiction of the modified Go-No-Go task is provided. An overall mixed-block design was employed. Within a block of trials, subjects were asked to respond if the presented face depicted an affect consistent with the framing instruction (example of a negative block is shown).
Supplementary Figure 3. Significant activations for each group (BPD and HC) and contrast (Negative > Positive and Negative > Neutral) are rendered on a mosaic of axial slices (p<0.05, cluster level). Activations are observed in the OFC, the dPFC, the dACC, the basal ganglia, the medial temporal lobe and the parietal lobe. Labels for the clusters are left out of the figure for ease of readability.