Abstract
The metastasis of cancer to the central nervous system (CNS) remains a devastating clinical reality, carrying an estimated survival time of less than one year in spite of recent therapeutic breakthroughs for other disease contexts. Advances in brain metastasis research are hindered by a number of reasons, including its complicated nature and the difficulty of modeling metastatic cancer growth in the unique brain microenvironment. In this review, we will discuss the clinical challenge, and compare the values and limitations of the available models for brain metastasis research. Additionally, we will specifically address current knowledge on how brain metastases take advantage of the unique brain environment to benefit their own growth. Finally, we will explore the distinctive metabolic and nutrient characteristics of the brain; how these paradoxically represent barriers to establishment of brain metastasis, but also provide ample supplies for metastatic cells’ growth in the brain. We envision that multi-disciplinary innovative approaches will open opportunities for the field to make breakthroughs in tackling unique challenges of brain metastasis.
Keywords: Brain metastasis, Central nervous system, Cancer models, Metabolism, Tumor-microenvironment interaction
1. Overview of brain metastasis
1.1 Imposing clinical challenges
In the United States, it is estimated that between 6% [1] and 14% [2] of all newly diagnosed cancers will ultimately metastasize to the brain; based on the 1.7 million new diagnoses of cancer expected in 2016 [3], between 100,000 and 240,000 cases are expected to eventually lead to brain metastasis. These numbers represent a conservative projection, for 3 principal reasons: 1) autopsies of patients dying due to metastatic cancer are rare, making comprehensive studies difficult; therefore, the apparent numbers seen thus far may be a result of under sampling [4], 2) as improved systemic therapies have lengthened the survival of patients with metastases to other organs, they may develop brain metastasis later, surpassing projections based on prior incidence [4], and 3) increased detection capability of clinical imaging modalities may allow for the identification of brain metastases that would not have been found previously [5]. The primary cancers that most frequently metastasize to the brain are lung, breast, melanoma, and colorectal, accounting for ~45%, ~15%, ~10%, and ~5% of brain metastases, respectively [4, 6].
The natural history of primary cancers’ progression to brain metastasis varies according to primary tumor site. Lung cancer brain metastasis diagnosis occurs at an average of 4.5 months following the diagnosis of primary cancer, and frequently occurs synchronously with primary tumor growth [7], while melanoma and breast cancer brain metastases are diagnosed at an average of 24–30.5 months [8, 9] and 41 months [10] later than their corresponding primary tumors, respectively. Once brain metastasis has occurred, the outcomes are dismal. If left untreated, the average survival is less than 2 months [11]; palliative therapies including corticosteroids, chemotherapy, and radiotherapy can extend survival, in the best case, to an average of less than one year [12]. The lethality of brain metastasis follows severe deterioration of patients’ quality of life, and symptoms can include headaches, seizures, cognitive or motor dysfunction, and coma [13, 14]. When only a single brain metastatic tumor is detected, surgery or stereotactic radiosurgery can be performed; however, these therapies only have modest effects on survival with life extended merely a few months as other metastases spring forth from untreated brain regions [6]. Moreover, at the time of detection, more than 80% of patients already have multiple brain metastases, disallowing surgery as a therapeutic option [15, 16]. As brain metastasis is almost universally chemo-resistant, chemotherapy is usually only used for treating systemic (non-brain) metastasis growing synchronously with the brain metastasis or as salvage therapy [17, 18]. Some primary tumors or tumor subtypes are more responsive to therapies targeting specific molecular or functional pathways; non-small cell lung cancer (NSCLC) frequently expresses epidermal growth factor receptor (EGFR) mutations [19] and anaplastic lymphoma kinase (ALK) rearrangements [20], melanoma frequently presents with BRAF V600 mutations [21], and breast cancer can be driven by the human epidermal growth factor receptor 2 (HER2) [22]; the presence of these mutations or hyperactivity of the associated pathways in the associated primary tumor frequently makes these pathways targets of choice in brain metastasis [23]. While several targeted therapies do indeed show activity in the brain and are delivered into the brain metastatic tumors, their accumulation in the brain generally occurs at dramatically lower concentrations than outside the brain [24], raising the question of whether efficacious concentrations of the drugs are ever reached or maintained. One notable and recent exception is the antibody-drug conjugate ado-trastuzumab emtansine (T-DM1), which targets HER2 in HER2-overexpressing breast cancer; T-DM1 has shown clinical efficacy in HER2+ brain metastasis both as a single agent and in combination, positively impacting both progression-free and overall survival [25, 26]. Yet even in patients showing responses to T-DM1, the metastases ultimately relapse. Currently, there are over 100 clinical trials ongoing or in active recruitment to evaluate the efficacy of targeted therapies in brain metastasis (compiled in clinicaltrials.gov). Most of these trials involve the use of targeted therapies in combination with radiotherapy or immune checkpoint blockade (ICB). Notably, the investigational targeted therapies are also based upon mutations and driver alterations found in the primary tumors from which brain metastases were derived, with ALK and EGFR inhibitors being tested in lung cancer brain metastasis, BRAF inhibitors in BRAF-mutant melanoma, and HER2 inhibitors in HER2-high breast cancer. It is unclear whether these drivers are also key modulators of brain metastasis. In an examination of brain metastases from breast, lung, melanoma, and esophageal cancers, it was noted that in addition to mutations frequently seen in TP53, NRAS, and KRAS in primary cancers, DSC2, ST7, PIK3R1 and SMC5 mutations were also observed in brain metastases [27]. Enrichment of activation of the DNA repair, ERBB-HER signaling, axon guidance, and protein kinase-A signaling pathways were also seen in brain metastases, showing that molecular enrichment for certain functional phenotypes are seen in the brain [27]. Based on the enriched molecular alterations seen in brain metastasis compared to primary tumors, it is possible that targeting brain metastasis-specific alterations could yield improved therapeutic efficacy.
1.2 Obstacles to advancing research
To make breakthroughs in tackling the clinical challenge of brain metastasis, we need a clear understanding of its biological mechanisms. Unfortunately, the vast majority of cancer funding and public attention are directed towards the treatment and prevention of primary cancer [28, 29], even though the reality is that 90% of cancer mortality is due to metastasis [30]. This disparity in funding represents a pragmatic yet frustrating trend. Although primary cancer survival rates have continuously improved for the past 30 years, such progress has largely evaded metastatic cancer patients, whose outcomes remain dismal [31]. This ever-widening gap demands attention. Funding for research is the first step to making progress in the understanding of cancer metastasis, especially, brain metastasis.
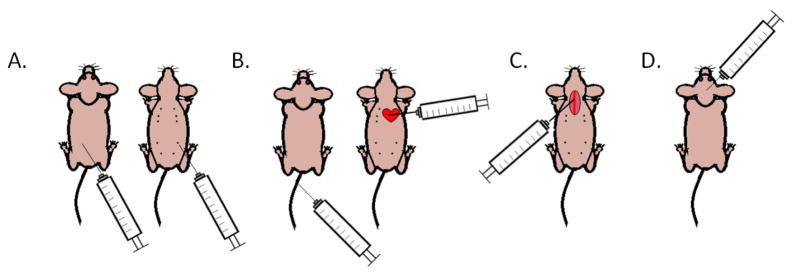
Brain metastasis research is surrounded by a number of practical scientific barriers. First, the unique nature of the brain environment (see Section 2) and how it interacts with metastatic cancer cells makes the study of brain metastasis using simple in vitro models minimally relevant, and unlikely to reveal impactful therapeutic vulnerabilities. Second, the in vivo models currently used to study brain metastasis are subject to their own limitations. Depending on the focus of the study, a spontaneous or/and an experimental brain metastasis model in mice can be used. Cancer cells need to perform a litany of complex biological functions to succeed in the multi-step metastatic cascade, from growing at the primary tumor site, to obtaining their own blood supply, entering and surviving within the circulation, arresting within secondary organs, and extravasating and colonizing the secondary organs [32]. Spontaneous metastasis models are the best at reflecting the entire process (Fig. 1A, Table 1). However, due to the timing of metastatic latency, the limited lifespan of mice, and the potential of mice to succumb to metastasis to other organ sites prior to the overt manifestation of brain metastasis, studies involving spontaneous modeling of brain metastasis are rarely reported. One such study investigating the spontaneous metastasis of mammary tumors generated from patient-derived xenografts (PDXs) of breast cancer specifically investigated the incidence and gene profiles of metastases to different organs [33]. At the experimental endpoint, the mice were euthanized due to high lung metastatic burden while brain metastasis was rare and brain micro-metastatic cancer cells expressed a dormancy-associated gene signature [33]. Other spontaneous metastasis models have shown a similarly lower metastatic incidence and metastatic burden in the brain relative to other organs, again reducing the opportunity to study brain metastasis [34]. In order to increase the brain metastasis-forming efficiency, experimental metastasis assays to introduce cancer cells directly into the circulation can be utilized. These include injections to either the tail vein (intravenous) [35, 36] or left ventricle of the heart (intracardiac) [37]. However, while these models do allow for rare brain metastasis generation, they still support the dissemination of cancer cells throughout the organism. This frequently leads to animals succumbing to pulmonary or bone metastasis prior to macro-metastasis formation in the brain (Fig. 1B, Table 1).
Figure 1.
Methods of studying brain metastasis in vivo. A. Spontaneous brain metastasis assays involve injection of cancer cells into their associated site of primary tumor origin. Left: intradermal injection for study of spontaneous melanoma metastasis. Right: mammary fat pad injection for study of spontaneous breast cancer metastasis. B. Experimental brain metastasis assays by direct inoculation of cancer cells into the circulation. Left: tail vein injection of cancer cells introduces them into the general circulation, but results in inefficient brain metastasis formation as most cells are trapped in the lungs. Right: intracardiac injection of cancer cells distributes them throughout the mouse body, but dispersal can be unpredictable. Mice frequently die of metastasis to other organ sites prior to overt brain metastasis outgrowth. Experimental brain metastasis can be achieved more efficiently by serial in vivo selection of tumors formed in this way, so that cells will home more specifically to the brain in later experiments. C. Experimental brain metastasis by intracarotid arterial injection of cancer cells. This procedure limits the dissemination of cancer cells to the brain vascular bed, resulting in high experimental consistency but is technically challenging. D. Direct, stereotactic intracerebral injection. This method is the most well-controlled and reproducible way to assay cancer cell growth in the brain, as cancer cells are confined to one region of the brain. However, this assay limits the study to tumor cell outgrowth at the site of implantation, not spread and dissemination to secondary locations.
Table 1.
Advantages and disadvantages of in vivo brain metastasis assays
| In vivo brain metastasis method | Advantages | Disadvantages |
|---|---|---|
| Spontaneous metastasis assays |
|
|
| Experimental brain metastasis by intracardiac or tail vein injection of cancer cells into general circulation |
|
|
| Experimental brain metastasis by intracarotid arterial injection of cancer cells |
|
|
| Cancer cell brain outgrowth assay by stereotactic intracerebral injection of cancer cells |
|
|
In order to avoid some of the complications of the aforementioned metastasis assays, such as timing and consistency, an experimental brain metastasis assay injecting cancer cells into the carotid artery of immunocompromised mice has been developed and extensively used for studying brain metastasis [38]. This assay allows the interrogation of the brain-specific requirements for cancer cell metastasis, from adhesion to the brain vasculature, extravasation, obtaining oxygen and nutrients, and outgrowth in the brain parenchyma, all major barriers to establishment of brain metastasis (Fig. 1C, Table 1). However, the intracarotid injection skips the steps of cancer cells leaving the primary tumor and entering the circulation in the metastatic cascade. Additionally, intracarotid injection of cancer cells is a technically challenging procedure, involving a microsurgery to expose the carotid artery prior to injection; mastering the technique is labor intensive and time consuming, representing another hurdle to brain metastasis research. As an alternative, many investigators are turning to intracerebral injection of cancer cells to study their outgrowth in the brain (Fig. 1D, Table 1) [39]. This method, originally developed to orthotopically model primary brain tumor growth [40], allows researchers to study tumor cell outgrowth in the brain, the last step of the metastatic cascade [32].
A common concern with the use of both spontaneous and experimental xenograft models of human cancer cell lines or PDXs grown in mice is that both systems require the use of immunocompromised animals, which ignore the impact of the adaptive immune system on preventing or promoting brain metastasis. To address this issue, it is possible to use either mouse tumor-derived cell lines or allografts in syngeneic hosts to perform either spontaneous or experimental brain metastasis assays. While mouse tumor models may not perfectly recapitulate the genomics or transcriptomics of human disease, complementary use of both murine tumor-derived allograft and human PDXs would allow more complete studies of brain metastasis. A recent study using a brain orthotopic model of HER2-positive breast cancer brain metastasis PDXs enabled the determination of remarkably effective targeted therapeutic combinations for brain metastasis, with three out of five brain metastasis models studied showing long-lasting complete remissions [41]. The brain microenvironment, specialized to support integrity of the CNS, is meant to maintain homeostasis and limit the action of pharmaceuticals in the brain. Therefore research efforts that take into consideration the role of the host brain in supporting metastatic growth in vivo are more likely to represent fruitful avenues in developing effective treatment strategies for brain metastasis than those that simply look for in vitro cytotoxicity.
2. The brain as a unique microenvironment for metastasis
The specialization of the brain to suit its functions as the processing and control center for the body has led to the evolution of a number of unique features and safeguards. As the primary role of the brain is to house and support the neurons that allow for signals to be transmitted and received throughout the whole body, many of its features are specifically involved in supporting and maintaining neuronal functions. The distinctive nature of the brain microenvironment extends to its resident cell populations [42], structural composition [43], blood flow [44], immune responses [45, 46], and nutrient and oxygen availability [47]. Each of these special characteristics represents a potential challenge to would-be metastatic tumor cells; at the same time, cancer cells with the right features to adapt and survive in such conditions can take advantage of the neuroprotective environment to gain a foothold that has proven difficult to broach by current therapies.
2.1 Brain structural and cellular features involved in metastasis
One of the most recognized brain-specific features is the presence of a blood-brain barrier (BBB) that separates the brain from the general circulation [48]. This ‘barrier’ is in reference to both form and function, as cells and molecules are excluded from entering the brain parenchyma by physical obstructions. Specifically, the brain endothelial cells are joined by tight junctions, and possess active efflux transporters to expel potentially harmful molecules that do penetrate the physical barrier. The main function of this barrier is the maintenance of homeostatic environment for neuronal function [49]. The BBB’s tight junctions, which are composed of proteins including claudins, occludins, and zona occludens proteins [50], possess high electrical resistance [51]. The abluminal (brain-facing) side of the BBB is surrounded by a thick basement membrane, which is in turn supported by pericytes. The tight junctions’ electrical resistance prevents free diffusion of charged molecules through the BBB, while pericytes physically support endothelial cells and promote their survival and maturation [52]. Finally, astrocytic end-feet form the outer layer of the BBB. Astrocytes express glucose transporters for allowing glucose into the brain and P-glycoprotein for pumping drugs/neuroactive compounds out of the brain, and possess the ability to alter the tightness of the BBB in response to stresses such as hypoxia [53]. Oxygen and other lipophilic molecules can directly diffuse into the brain through the endothelial cell membrane. Due to the brain’s high demand for oxygen, the partial pressure of oxygen (pO2) in the brain, at steady-state levels, is only 35 mmHg (equates to 4.6% oxygen) [54]; this generates a continuous gradient of oxygen into the brain [54]. Other molecules required for brain function, such as amino acids, are selectively admitted to the brain by specialized transporters [55].
As cancer cells arrive in the vasculature of the brain, they first have to arrest within the blood vessels prior to extravasation. The process of cell arrest in the brain is non-trivial, indicated by the finding that the top gene differentially enriched in cancer cells metastatic to the brain versus other organ sites was ST6GALNAC5, which encodes a molecule that mediates adhesion specifically to the brain vasculature [56]. Following the adhesion to the vascular walls, the first brain-specific challenge that cancer cells encounter is the BBB. An intact physiological BBB prevents the entry of cells into the brain; cells extravasating into the brain would therefore need to pass through the endothelial cell layer either para-cellularly (between the cells, through the tight junctions) or transcellularly (through the endothelial cells themselves). While transcellular migration has been observed in immune cell extravasation, only paracellular migration has been detected in brain metastasis [57]. Because tight junctions are composed of a variety of proteins, cancer cells need to express several proteases in order to penetrate intact tight junctions [58]. Alternatively, these junctions can be destabilized by a variety of cytokines, chemokines, and inflammatory mediators frequently expressed by cancer cells, including VEGF, basic fibroblast growth factor (bFGF), transforming growth factor–β (TGF-β), interleukin-1β (IL-1β), TNF-α, interferon-γ (IFN-γ ), CCL2, CXCL8, and prostaglandin-endoperoxide synthase 2 (COX2) [56, 59–61]; cancer cells can secrete MMPs and other proteases to disrupt the basement membrane (BM), as occurs in metastasis to other sites [59, 62]. Recently, cancer cells have been shown to actively induce endothelial cell death by heterotypic signaling [63]. When amyloid precursor protein (APP), expressed by cancer cells, interacts with its cognate receptor, death receptor 6 (DR6), expressed by endothelial cells, necroptosis is triggered in the endothelial cells, enhancing cancer extravasation and metastasis. Therefore, in addition to the paracellular extravasation employed by immune cells, cancer cells may actively extravasate through the killing of endothelial cells forming endothelial barriers.
After bypassing the BBB, cancer cells still have to cope with the unique nutrient environment of the brain (see 2.2 for more details). Part of this challenge involves obtaining sufficient oxygen in a tissue with a low pO2, where there is a high degree of competition from neurons. Neurons maintain sufficient oxygen for mitochondrial respiration by never being farther than 40μm away from the nearest capillary [64]; cancer cells in the brain converge upon this phenotype as well, as imaging studies on experimental brain metastasis showed that cancer cells stay in contact with blood vessels even following extravasation. This contact continues until either VEGF-A-mediated neo-angiogenesis occurs or, more frequently in brain metastasis, the vasculature is remodeled and co-opted by the cancer cells [65]. Notably, these neoangiogenesis and vascular co-option have been observed to be dependent upon the type of primary cancer from which the brain metastases are derived, with lung cancer demonstrating the neoangiogenesis phenotype and breast and melanoma showing the vascular co-option [65]. An ongoing controversy in the field of brain metastasis is the degree to which the BBB is disrupted following brain metastasis outgrowth. While some studies have detected a heterogeneous permeability to chemotherapeutic agents by brain metastases [66], others have shown that effects of brain metastasis on the leakiness of the BBB are cell line-dependent, with examples of both intact [67] or disrupted [68] BBB in different cancer cell contexts. In either case, this distinction has clinical implications; if the BBB were fully disintegrated around brain metastases, it might allow for delivery of efficacious doses of therapeutics to tumors in the brain. Frustratingly, even in advanced brain metastases in which a loss of functional integrity of the BBB would be expected, especially when metastases have implemented vascular remodeling or neo-angiogenesis [65, 69], the accumulation of the majority of chemotherapeutic and small molecule targeted therapies still appears at sub-optimal levels [70].
A better explanation for therapeutic resistance of brain metastasis may lie in the co-option of astrocytes, the brain’s essential support cells. Astrocytes in the physiological brain serve homeostatic functions in support of the BBB [71, 72] as well as signaling and delivering nutrients directly to neurons [73]. Astrocytes’ homeostatic functions include prevention of cancer cell invasion of the brain parenchyma; cancer cells breaching the BBB are soon met by astrocytes that release Fas ligand (FasL) to trigger cancer cell apoptosis [74]. Successful brain metastases win this molecular arms race by expressing serpins, which prevent astrocytic release of FasL. After evading elimination by astrocytes, cancer cells can take advantage of the protective benefits that astrocytes usually afford neurons by a variety of mechanisms. Through gap junction communication, astrocytes upregulate survival genes in brain metastatic cells, promoting chemoresistance [75]. This effect involves the engagement and upregulation of the endothelin axis [76]; antagonism of the endothelin axis chemosensitized tumor cells and significantly extended survival in mouse models of experimental brain metastasis of lung and breast cancer, leading to unprecedented complete responses even in aggressive brain metastasis models [77]. In addition to gap junction communication, brain metastases can activate the pro-growth/survival effects of astrocytes by secretion of IL-1β [78] or CCL2 [79], and have even been shown to enhance the differentiation of neural progenitor cells into astrocytes [80]. Clearly, the dynamic and reciprocal crosstalk between brain metastatic cells and the astrocytes fosters outgrowth and enhances therapeutic resistance of cancer cells in the brain.
The healthy brain, while not usually patrolled directly by the adaptive immune system, is protected by a specialized cell type known as microglia [81]; microglia are bone marrow-derived innate immune cells that serve to protect the brain and maintain homeostasis [82, 83]. While microglia are normally a specialized brain-resident macrophage population [83], under pathological conditions such as inflammation [84], chronic stress [85], and radiation [86], bone marrow-derived myeloid progenitors can infiltrate the brain and differentiate into microglia-like cells. As specialized macrophages, microglia can perform both cytotoxic and tissue repair functions based on their activation state [87]. Accordingly, brain metastatic cancer cells must be able to avoid detection and elimination by microglia in order to successfully colonize the brain. Early in the process of metastatic colonization, microglia have been observed to interact with metastatic tumor cells and become activated, as indicated by their TNF-α and inducible nitric oxide synthase (iNOS) expression [88, 89]. In vitro, microglia were shown to be capable of killing cancer cells in a nitric oxide-dependent manner [90], suggesting that successful brain metastases have to avoid detection and killing by microglia. On the other hand, in keeping with their physiological functions of performing both immune surveillance and anti-inflammatory tissue repair, microglia can be exploited by brain metastases to support their outgrowth. Those cancer cells that evade detection and elimination by microglia can take advantage of the supportive functions of microglia, thereby enhancing their own proliferation and survival by a variety of mechanisms [79, 91, 92] . Undoubtedly, the interaction of microglia and brain metastatic cancer cells is a complex one that is especially difficult to model in vitro. However, the potential of microglia to eliminate cancer cells at the early stages of brain metastasis demands further attention, as identification of molecular mechanisms that mediate microglial detection and killing of brain metastatic cells could represent a novel means of therapeutic targeting.
2.2 Metabolic environment of brain metastasis
The limited availability of nutrients in the brain is another significant evolutionary bottleneck in the pathogenesis of brain metastasis. As the BBB excludes most molecules from simple diffusive entry into the brain, specialized transporters are necessary for their import. Transporters exist for glucose and some amino acids; the high capacity of the endothelial cell glucose transporter GLUT-1 (at 1420 nmol/min x g tissue) ensures the continuous ingress of glucose into the brain [93]. The availability of glucose, inability of other metabolic substrates for entering the brain, and high energy demands of the brain together result in the brain’s utilization of 20% of the body’s glucose [94], which is evident by the intense background signal of the brain in 18F-deoxyglucose (18-FDG) positron emission tomography (PET) imaging [95]. Astrocytes and neurons can both utilize the glucose that gets into the brain, astrocytes through GLUT-1 and neurons through GLUT-3, which has a higher glucose affinity [96]. Once inside a cell, glucose can be used for either ATP synthesis or the generation of other biomolecules. In either event, the first step of glucose metabolism is the phosphorylation of glucose by hexokinase, generating glucose-6-phosphate [97]. At this point, the glucose-6-phosphate can either continue down the glycolysis pathway and be used for ATP generation, or be shunted into the pentose phosphate pathway in order to generate NADPH to be used for the production of other biomolecules [97]. Brain metastases have been observed to utilize both glycolysis and the pentose phosphate pathway. A study of human breast cancer brain metastases, which are still restricted by a functional BBB in their early stages of colonization, showed that the gene most highly upregulated relative to unmatched primary tumors was hexokinase 2 (HK2), demonstrating the importance of glycolysis for intracerebral tumor growth and survival [98]. Another study using experimental brain metastasis models showed enhanced utilization of glucose in both glycolytic and pentose phosphate pathways [99]. The similar findings of these independent studies demonstrate that the physiological constraints of limited nutrient availability imposed by the brain must be overcome for successful metastasis.
While it has been widely reported that due to the BBB, amino acids exist at lower levels in the brain than the plasma, quantification of this phenomenon are scarce. Further, the presence of the large neutral amino acid transporter 1 (LAT1) system enables the facilitated diffusion of some amino acids [100], so proclaiming that amino acids exist at lower levels in the brain is likely an oversimplification. Although to the best of our knowledge, a comprehensive set of quantification data on amino acids in the brain is not available, an Alzheimer’s disease study from 1990 did examine the cerebrospinal fluid (CSF) content of amino acids [101]. That study compared the amino acid content in plasma and CSF of healthy volunteers to Alzheimer’s patients; in the healthy control group, the only amino acid found at a similarly high level in the CSF as in the general circulation was glutamine, an important precursor of the neurotransmitters glutamate and γ-aminobutyric acid (GABA) [101]. This suggests that utilization of glutamine may be an important functional constraint on successful brain metastasis outgrowth. Intriguingly, a study in brain metastasis has shown that breast cancer brain metastases may display neuronal phenotypes, using GABA as an oncometabolite in the brain microenvironment [102].
In general, glutamine metabolism in the brain occurs in the form of a cycle rather than as a unidirectional catabolic process. This series of reactions is known as the glutamine-glutamate cycle, and it is responsible for maintaining the supply of the neurotransmitter glutamate while also preventing constitutive neuronal hyperpolarization. Since glutamate can be converted into glutamine, which is non-neuroactive, glutamine is the preferred form in the brain. The glutamine-glutamate cycle occurs when glutamate is released into a glutamatergic synapse by the pre-synaptic neuron. Some glutamate binds to its receptors on the post-synaptic neuron, and the excess synaptic glutamate is taken up by the surrounding astrocytes, which express high levels of excitatory amino acid transporters (EAATs). Inside the astrocytes, the glutamate is metabolized by glutamine synthetase, and processed into glutamine by the addition of an amino group. This glutamine is then released by the astrocytes and taken up by pre-synaptic neurons. It is within pre-synaptic neurons that glutaminolysis occurs, with glutaminase breaking down glutamine into its constituents glutamate and ammonia. The newly synthesized glutamate is then packaged into vesicles and released into the synapse, and the cycle proceeds again. Notably, cancers and cancer cell lines have exhibited the potential to participate in the glutamine-glutamate cycle, demonstrating a similar phenotype as neurons, with uptake of glutamine and release of glutamate [103–106].
Moreover, both brain metastasis and primary tumors have previously been reported to exhibit altered glutamine metabolism [107]. Specifically, they have both been shown to possess an enhanced ability to utilize glutamine as both an energy source and as a precursor to cellular building blocks, including purines, pyrimidines, and non-essential amino acids [108–110]. However, it remains unknown whether brain metastatic cells possess an intrinsic ability to better utilize glutamine, or if this phenotype arises out of adaptation to the brain environment. Importantly, a mechanistic understanding of the regulation and cause of this enhanced glutamine utilization is still lacking, so it cannot yet serve as a therapeutic target. Finally, as most isotopically labeled metabolic tracer studies have been performed in vitro, it will be crucial to test the importance of glutamine utilization in brain metastasis in vivo and clinically to determine the key glutamine fates [111].
3. Opportunities for breakthroughs
The diagnosis of brain metastasis currently represents a death sentence, and as treatment options have improved for primary and non-brain metastatic cancers, that fact has become increasingly conspicuous. Owing to a variety of factors, including the dearth of dedicated brain metastasis research funding as well as the difficulties of accurately modeling the disease, little progress has been made in understanding the biology of brain metastasis in order to make therapeutic breakthroughs.
However, all of that can change. Although in vivo modeling of cancer is technically challenging and expensive, awareness and acknowledgement that it is the best approach to understanding the nuanced relationship between brain metastatic cancer cells and the CNS microenvironment is an important step in designing and performing the proper experiments in the future. While none of the current models is ideal, the combination of human cancer cells xenografted into immuno-deficient mice and mouse cancer cells allografted into immunocompetent mice can serve complementary roles in surveying the specific interactions between brain and cancer cells that support or suppress cancer cell growth and modulate therapeutic resistance. Since cancer cells growing in the brain take advantage of specialized cell types such as astrocytes and microglia in order to gain a number of privileges normally afforded neurons (Fig. 2), the understanding of these interactions is crucial in order to engineer solutions. One such exciting possibility is Fidler’s work on targeting the endothelin axis to disrupt cancer cells’ reliance on astrocytes [77]; the resulting dramatic increase in mouse survival following brain metastasis is unprecedented and demands greater attention. Similar studies to reverse engineer the reliance of cancer cells on the unique features of the brain environment may bear fruit, turning cancer’s brain-specific advantages into brain-specific vulnerabilities. Finally, even though the physiological brain environment has its own specialized immune system, the pathological brain may be subject to T cell-mediated immunity [112–114]. As T cell checkpoint blockade-based immunotherapy has shown remarkable clinical efficacy in certain cancer types (reviewed in [115]), the prospect of using immunological approaches to treat brain metastasis is intriguing. To this end, clinical responses to both checkpoint blockade [116–118] and adoptive T cell transfer [119] have been observed in melanoma brain metastases; other immunotherapy-responsive cancers that metastasize to the brain, like non-small cell lung cancer (NSCLC), may be amenable to immune-oncological treatment approaches in the context of brain metastasis. When the clinical applications of immunotherapy improve even further, expanding beyond T cell-focused efforts, brain metastases from other cancer types may also be candidates for immunotherapy.
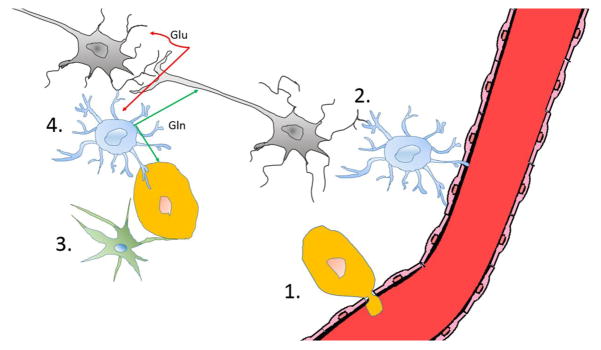
Figure 2.
Brain metastatic cancer cells may exploit a number of unique features (illustrated in 1 to 4) of the brain microenvironment. 1. Brain metastatic cancer cells first need to pass the BBB in order to enter the brain. Once they extravasate, they can benefit from the BBB’s exclusion of large molecules (i.e., therapeutic antibodies) and barring the active efflux of compounds. 2. Astrocytes serve homeostatic roles in supporting neuronal function and survival, but preventing brain colonization of cancer cells by expressing FasL. Cancer cells that evade FasL-mediated killing are able to benefit from astrocytes’ pro-survival functions, rendering brain metastases chemo-resistant. 3. Microglia serve as the resident immune cells of the brain. Microglia have the potential to recognize and eliminate brain metastatic cancer cells by TNF-α and inducible nitric oxide synthase (iNOS). Successful brain metastatic cancer cells must be able to avoid microglial detection. 4. The brain environment serves to support the optimal function of neurons, promoting their survival and preventing excitotoxicity. Glutamate, the essential neurotransmitter, cannot freely exist in the brain parenchyma. Following synaptic glutamate (Glu) release, astrocytes scavenge the excess glutamate and convert it into the non-neuroactive glutamine (Gln), which is then released to be taken up by neurons. Cancer cells may use this glutamine, which exists at relatively high concentrations in the brain, as an important biomolecular building block and energy source. Neurons-gray, astrocytes-blue, microglia-green, endothelial cells-red. Figure drawn in collaboration with Chia-Chi Chang.
In addition to cell type-specific interactions that allow brain metastatic tumors to enjoy survival advantages in the brain, it is important to consider how the unique nutrient environment of the brain imparts functional constraints and opportunities on metastatic cancer cells. As they are foreigners surviving in a hostile setting, metastatic cancer cells are forced to live by the rules of their metastatic organ setting, not by their tissue of origin. This implies that brain metastases of different cancer types may converge on similar pathways for survival and proliferation based on nutrient availability. In the case of the brain, there is a relatively high availability of glucose and glutamine and low abundance of oxygen. Brain metastases overcome these specific functional barriers by, for example, developing means to more efficiently metabolize glutamine into specific biomolecular building blocks. While this represents a competitive advantage for brain metastases, over-reliance on these functions can serve as a therapeutic Achilles’ heel. This concept, borrowed from ecology, can be thought of as an evolutionary double bind [120, 121]; cancer cells that have become adept at certain specific functions also make themselves vulnerable to complementary therapeutic targeting. By using the right models and asking the right questions, it will be possible to identify the pathways regulating such functional advantages and turn them into a new wave of brain metastasis-specific therapeutic targets.
4. Conclusions
Brain metastasis remains a daunting unmet medical challenge, as its incidence continues to rise while its treatments remain inadequate. Because of the brain’s function as the central processing unit of the body, it has evolved with a number of specialized features to protect this function, including neuronal support cells as well as the BBB to restrict the chemical and nutrient content of the CNS. These features represent both barriers to successful brain metastasis and sanctuary for cancer cells that are able to metastasize to the brain; brain metastases must adapt to survive in the highly competitive brain environment but can exploit cells like astrocytes, using them to develop therapeutic resistance.
Due to the complex nature of the interactions between brain microenvironment and metastatic cancer cells, in vitro modeling of brain metastasis is of limited value in terms of advancing knowledge of the field. In vivo models of brain metastasis, while more demanding in terms of resources and techniques, offer the ability to investigate how cancer cells interact with brain microenvironmental cells at different stages of the metastatic cascade. Additionally, the various in vivo experimental metastasis or secondary tumor growth assays allow investigators to focus on certain steps of the brain metastatic process.
By using the appropriate experimental tools available, researchers have the ability to change the currently dismal clinical reality of brain metastasis. Through identification of how brain metastatic tumor cells use the brain microenvironment to avoid elimination by classical therapeutic approaches, new vulnerabilities may be discovered. Brain metastases that become dependent upon certain brain-specific nutrients or cell types for growth and survival may reciprocally expose themselves to a new wave of therapeutic options, turning brain metastasis into a treatable disease.
Acknowledgments
This work was supported partially by Isaiah Fidler Fellowship in Cancer Metastasis Research (FJL), RO1-CA112567-06 (DY), R01CA184836 (DY), METAvivor Research Grant (DY), China Medical University Research Fund (DY). D. Yu is the Hubert L. & Olive Stringer Distinguished Chair in Basic Science at MDACC. Special thanks to Dr. Chia-Chi Chang for artistic support in generating Figure 2.
Footnotes
Note: We apologize for not being able to cite all the relevant original research and review articles due to space limitation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Davis FG, Dolecek TA, McCarthy BJ, Villano JL. Toward determining the lifetime occurrence of metastatic brain tumors estimated from 2007 United States cancer incidence data. Neuro Oncol. 2012;14(9):1171–7. doi: 10.1093/neuonc/nos152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nathoo N, Chahlavi A, Barnett GH, Toms SA. Pathobiology of brain metastases. J Clin Pathol. 2005;58(3):237–42. doi: 10.1136/jcp.2003.013623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Cancer Society. Cancer Statistics Center. 2016 [cited 2016 March 23, 2016]; Available from: https://cancerstatisticscenter.cancer.org/
- 4.Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Curr Oncol Rep. 2012;14(1):48–54. doi: 10.1007/s11912-011-0203-y. [DOI] [PubMed] [Google Scholar]
- 5.Fink KR, Fink JR. Imaging of brain metastases. Surg Neurol Int. 2013;4(Suppl 4):S209–19. doi: 10.4103/2152-7806.111298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patchell RA. The management of brain metastases. Cancer Treat Rev. 2003;29(6):533–40. doi: 10.1016/s0305-7372(03)00105-1. [DOI] [PubMed] [Google Scholar]
- 7.Sen M, Demiral AS, Cetingoz R, Alanyali H, Akman F, Senturk D, Kinay M. Prognostic factors in lung cancer with brain metastasis. Radiother Oncol. 1998;46(1):33–8. doi: 10.1016/s0167-8140(97)00124-2. [DOI] [PubMed] [Google Scholar]
- 8.Qian M, Ma MW, Fleming NH, Lackaye DJ, Hernando E, Osman I, Shao Y. Clinicopathological characteristics at primary melanoma diagnosis as risk factors for brain metastasis. Melanoma Res. 2013;23(6):461–7. doi: 10.1097/CMR.0000000000000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bottoni U, Clerico R, Paolino G, Ambrifi M, Corsetti P, Calvieri S. Predictors and survival in patients with melanoma brain metastases. Med Oncol. 2013;30(1):466. doi: 10.1007/s12032-013-0466-2. [DOI] [PubMed] [Google Scholar]
- 10.Sperduto PW, Kased N, Roberge D, Chao ST, Shanley R, Luo X, Sneed PK, Suh J, Weil RJ, Jensen AW, Brown PD, Shih HA, Kirkpatrick J, Gaspar LE, Fiveash JB, Chiang V, Knisely JP, Sperduto CM, Lin N, Mehta M. The effect of tumor subtype on the time from primary diagnosis to development of brain metastases and survival in patients with breast cancer. J Neurooncol. 2013;112(3):467–72. doi: 10.1007/s11060-013-1083-9. [DOI] [PubMed] [Google Scholar]
- 11.Markesbery WR, Brooks WH, Gupta GD, Young AB. Treatment for patients with cerebral metastases. Arch Neurol. 1978;35(11):754–6. doi: 10.1001/archneur.1978.00500350058012. [DOI] [PubMed] [Google Scholar]
- 12.Freilich RJ, Seidman AD, DeAngelis LM. Central nervous system progression of metastatic breast cancer in patients treated with paclitaxel. Cancer. 1995;76(2):232–6. doi: 10.1002/1097-0142(19950715)76:2<232::aid-cncr2820760212>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 13.Sawaya R. Considerations in the diagnosis and management of brain metastases. Oncology (Williston Park) 2001;15(9):1144–54. 1157–8. discussion 1158, 1163–5. [PubMed] [Google Scholar]
- 14.Lassman AB, DeAngelis LM. Brain metastases. Neurol Clin. 2003;21(1):1–23. vii. doi: 10.1016/s0733-8619(02)00035-x. [DOI] [PubMed] [Google Scholar]
- 15.Patchell RA, Tibbs PA, Regine WF, Dempsey RJ, Mohiuddin M, Kryscio RJ, Markesbery WR, Foon KA, Young B. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280(17):1485–9. doi: 10.1001/jama.280.17.1485. [DOI] [PubMed] [Google Scholar]
- 16.Ammannagari N, Ahmed S, Patel A, Bravin EN. Radiological response of brain metastases to novel tyrosine kinase inhibitor lapatinib. QJM. 2013;106(9):869–70. doi: 10.1093/qjmed/hct090. [DOI] [PubMed] [Google Scholar]
- 17.Peak S, Abrey LE. Chemotherapy and the treatment of brain metastases. Hematol Oncol Clin North Am. 2006;20(6):1287–95. doi: 10.1016/j.hoc.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Fabi A, Felici A, Metro G, Mirri A, Bria E, Telera S, Moscetti L, Russillo M, Lanzetta G, Mansueto G, Pace A, Maschio M, Vidiri A, Sperduti I, Cognetti F, Carapella CM. Brain metastases from solid tumors: disease outcome according to type of treatment and therapeutic resources of the treating center. J Exp Clin Cancer Res. 2011;30:10. doi: 10.1186/1756-9966-30-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cancer Genome Atlas Research, N. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511(7511):543–50. doi: 10.1038/nature13385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, Ou SH, Dezube BJ, Janne PA, Costa DB, Varella-Garcia M, Kim WH, Lynch TJ, Fidias P, Stubbs H, Engelman JA, Sequist LV, Tan W, Gandhi L, Mino-Kenudson M, Wei GC, Shreeve SM, Ratain MJ, Settleman J, Christensen JG, Haber DA, Wilner K, Salgia R, Shapiro GI, Clark JW, Iafrate AJ. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363(18):1693–703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cancer Genome Atlas, N. Genomic Classification of Cutaneous Melanoma. Cell. 2015;161(7):1681–96. doi: 10.1016/j.cell.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cancer Genome Atlas, N. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin NU. Targeted therapies in brain metastases. Curr Treat Options Neurol. 2014;16(1):276. doi: 10.1007/s11940-013-0276-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fokas E, Steinbach JP, Rodel C. Biology of brain metastases and novel targeted therapies: time to translate the research. Biochim Biophys Acta. 2013;1835(1):61–75. doi: 10.1016/j.bbcan.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Bartsch R, Berghoff AS, Vogl U, Rudas M, Bergen E, Dubsky P, Dieckmann K, Pinker K, Bago-Horvath Z, Galid A, Oehler L, Zielinski CC, Gnant M, Steger GG, Preusser M. Activity of T-DM1 in Her2-positive breast cancer brain metastases. Clin Exp Metastasis. 2015;32(7):729–37. doi: 10.1007/s10585-015-9740-3. [DOI] [PubMed] [Google Scholar]
- 26.Krop I, Winer EP. Trastuzumab emtansine: a novel antibody-drug conjugate for HER2-positive breast cancer. Clin Cancer Res. 2014;20(1):15–20. doi: 10.1158/1078-0432.CCR-13-0541. [DOI] [PubMed] [Google Scholar]
- 27.Saunus JM, Quinn MC, Patch AM, Pearson JV, Bailey PJ, Nones K, McCart Reed AE, Miller D, Wilson PJ, Al-Ejeh F, Mariasegaram M, Lau Q, Withers T, Jeffree RL, Reid LE, Da Silva L, Matsika A, Niland CM, Cummings MC, Bruxner TJ, Christ AN, Harliwong I, Idrisoglu S, Manning S, Nourse C, Nourbakhsh E, Wani S, Anderson MJ, Fink JL, Holmes O, Kazakoff S, Leonard C, Newell F, Taylor D, Waddell N, Wood S, Xu Q, Kassahn KS, Narayanan V, Taib NA, Teo SH, Chow YP, Jat PS, Brandner S, Flanagan AM, Khanna KK, Chenevix-Trench G, Grimmond SM, Simpson PT, Waddell N, Lakhani SR kConFab. Integrated genomic and transcriptomic analysis of human brain metastases identifies alterations of potential clinical significance. J Pathol. 2015;237(3):363–78. doi: 10.1002/path.4583. [DOI] [PubMed] [Google Scholar]
- 28.Sleeman J, Steeg PS. Cancer metastasis as a therapeutic target. Eur J Cancer. 2010;46(7):1177–80. doi: 10.1016/j.ejca.2010.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Metastatic Breast Cancer Alliance. 2015 Metastatic Breast Cancer Alliance Full Report. 2015 MBCAlliance.org.
- 30.Mehlen P, Puisieux A. Metastasis: a question of life or death. Nat Rev Cancer. 2006;6(6):449–58. doi: 10.1038/nrc1886. [DOI] [PubMed] [Google Scholar]
- 31.Lakdawalla DN, Sun EC, Jena AB, Reyes CM, Goldman DP, Philipson TJ. An economic evaluation of the war on cancer. J Health Econ. 2010;29(3):333–46. doi: 10.1016/j.jhealeco.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 32.Talmadge JE, I, Fidler J. AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Res. 2010;70(14):5649–69. doi: 10.1158/0008-5472.CAN-10-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lawson DA, Bhakta NR, Kessenbrock K, Prummel KD, Yu Y, Takai K, Zhou A, Eyob H, Balakrishnan S, Wang CY, Yaswen P, Goga A, Werb Z. Single-cell analysis reveals a stem-cell program in human metastatic breast cancer cells. Nature. 2015;526(7571):131–5. doi: 10.1038/nature15260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Powell E, Shao J, Yuan Y, Chen HC, Cai S, Echeverria GV, Mistry N, Decker KF, Schlosberg C, Do KA, Edwards JR, Liang H, Piwnica-Worms D, Piwnica-Worms H. p53 deficiency linked to B cell translocation gene 2 (BTG2) loss enhances metastatic potential by promoting tumor growth in primary and metastatic sites in patient-derived xenograft (PDX) models of triple-negative breast cancer. Breast Cancer Res. 2016;18(1):13. doi: 10.1186/s13058-016-0673-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fidler IJ. The relationship of embolic homogeneity, number, size and viability to the incidence of experimental metastasis. Eur J Cancer. 1973;9(3):223–7. doi: 10.1016/s0014-2964(73)80022-2. [DOI] [PubMed] [Google Scholar]
- 36.Warren S, Gates O. The Fate of Intravenously Injected Tumor Cells. The American Journal of Cancer. 1936;27(3):485–492. [Google Scholar]
- 37.Arguello F, Baggs RB, Frantz CN. A Murine Model of Experimental Metastasis to Bone and Bone Marrow. Cancer Research. 1988;48(23):6876–6881. [PubMed] [Google Scholar]
- 38.Schackert G, I, Fidler J. Development of in vivo models for studies of brain metastasis. Int J Cancer. 1988;41(4):589–94. doi: 10.1002/ijc.2910410419. [DOI] [PubMed] [Google Scholar]
- 39.Schackert G, Price JE, Bucana CD, Fidler IJ. Unique patterns of brain metastasis produced by different human carcinomas in athymic nude mice. Int J Cancer. 1989;44(5):892–7. doi: 10.1002/ijc.2910440524. [DOI] [PubMed] [Google Scholar]
- 40.Shapiro WR, Basler GA, Chernik NL, Posner JB. Human brain tumor transplantation into nude mice. J Natl Cancer Inst. 1979;62(3):447–53. doi: 10.1093/jnci/62.3.447. [DOI] [PubMed] [Google Scholar]
- 41.Ni J, Ramkissoon SH, Xie S, Goel S, Stover DG, Guo H, Luu V, Marco E, Ramkissoon LA, Kang YJ, Hayashi M, Nguyen QD, Ligon AH, Du R, Claus EB, Alexander BM, Yuan GC, Wang ZC, Iglehart JD, Krop IE, Roberts TM, Winer EP, Lin NU, Ligon KL, Zhao JJ. Combination inhibition of PI3K and mTORC1 yields durable remissions in mice bearing orthotopic patient-derived xenografts of HER2-positive breast cancer brain metastases. Nat Med. 2016;22(7):723–6. doi: 10.1038/nm.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Purves D, Williams SM. Neuroscience. 2. Sunderland, Mass: Sinauer Associates; 2001. p. xviii.p. 681.p. 16.p. 3.p. 25. [Google Scholar]
- 43.Vanderah TW, Gould DJ, Nolte J. Nolte's The human brain : an introduction to its functional anatomy. 7. Philadelphia, PA: Elsevier; 2016. p. xi.p. 703. [Google Scholar]
- 44.Tata M, Ruhrberg C, Fantin A. Vascularisation of the central nervous system. Mech Dev. 2015;138(Pt 1):26–36. doi: 10.1016/j.mod.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tian L, Ma L, Kaarela T, Li Z. Neuroimmune crosstalk in the central nervous system and its significance for neurological diseases. J Neuroinflammation. 2012;9:155. doi: 10.1186/1742-2094-9-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523(7560):337–41. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ndubuizu O, LaManna JC. Brain tissue oxygen concentration measurements. Antioxid Redox Signal. 2007;9(8):1207–19. doi: 10.1089/ars.2007.1634. [DOI] [PubMed] [Google Scholar]
- 48.Steeg PS, Camphausen KA, Smith QR. Brain metastases as preventive and therapeutic targets. Nat Rev Cancer. 2011;11(5):352–63. doi: 10.1038/nrc3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abbott NJ. Dynamics of CNS barriers: evolution, differentiation, and modulation. Cell Mol Neurobiol. 2005;25(1):5–23. doi: 10.1007/s10571-004-1374-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wolburg H, Lippoldt A. Tight junctions of the blood-brain barrier: development, composition and regulation. Vascul Pharmacol. 2002;38(6):323–37. doi: 10.1016/s1537-1891(02)00200-8. [DOI] [PubMed] [Google Scholar]
- 51.Butt AM, Jones HC, Abbott NJ. Electrical resistance across the blood-brain barrier in anaesthetized rats: a developmental study. J Physiol. 1990;429:47–62. doi: 10.1113/jphysiol.1990.sp018243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fakhrejahani E, Toi M. Tumor angiogenesis: pericytes and maturation are not to be ignored. J Oncol. 2012;2012:261750. doi: 10.1155/2012/261750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cabezas R, Avila M, Gonzalez J, El-Bacha RS, Baez E, Garcia-Segura LM, Jurado Coronel JC, Capani F, Cardona-Gomez GP, Barreto GE. Astrocytic modulation of blood brain barrier: perspectives on Parkinson's disease. Front Cell Neurosci. 2014;8:211. doi: 10.3389/fncel.2014.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carreau A, El Hafny-Rahbi B, Matejuk A, Grillon C, Kieda C. Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J Cell Mol Med. 2011;15(6):1239–53. doi: 10.1111/j.1582-4934.2011.01258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith QR. Transport of glutamate and other amino acids at the blood-brain barrier. J Nutr. 2000;130(4S Suppl):1016S–22S. doi: 10.1093/jn/130.4.1016S. [DOI] [PubMed] [Google Scholar]
- 56.Bos PD, Zhang XH, Nadal C, Shu W, Gomis RR, Nguyen DX, Minn AJ, van de Vijver MJ, Gerald WL, Foekens JA, Massague J. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459(7249):1005–9. doi: 10.1038/nature08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Strell C, Entschladen F. Extravasation of leukocytes in comparison to tumor cells. Cell Commun Signal. 2008;6:10. doi: 10.1186/1478-811X-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Feng S, Cen J, Huang Y, Shen H, Yao L, Wang Y, Chen Z. Matrix metalloproteinase-2 and -9 secreted by leukemic cells increase the permeability of blood-brain barrier by disrupting tight junction proteins. PLoS One. 2011;6(8):e20599. doi: 10.1371/journal.pone.0020599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boecke A, Carstens AC, Neacsu CD, Baschuk N, Haubert D, Kashkar H, Utermohlen O, Pongratz C, Kronke M. TNF-receptor-1 adaptor protein FAN mediates TNF-induced B16 melanoma motility and invasion. Br J Cancer. 2013;109(2):422–32. doi: 10.1038/bjc.2013.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lucas JT, Jr, Salimath BP, Slomiany MG, Rosenzweig SA. Regulation of invasive behavior by vascular endothelial growth factor is HEF1-dependent. Oncogene. 2010;29(31):4449–59. doi: 10.1038/onc.2010.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stamatovic SM, Keep RF, Andjelkovic AV. Brain endothelial cell-cell junctions: how to "open" the blood brain barrier. Curr Neuropharmacol. 2008;6(3):179–92. doi: 10.2174/157015908785777210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sevenich L, Joyce JA. Pericellular proteolysis in cancer. Genes Dev. 2014;28(21):2331–47. doi: 10.1101/gad.250647.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Strilic B, Yang L, Albarran-Juarez J, Wachsmuth L, Han K, Muller UC, Pasparakis M, Offermanns S. Tumour-cell-induced endothelial cell necroptosis via death receptor 6 promotes metastasis. Nature. 2016;536(7615):215–8. doi: 10.1038/nature19076. [DOI] [PubMed] [Google Scholar]
- 64.Huchzermeyer C, Berndt N, Holzhutter HG, Kann O. Oxygen consumption rates during three different neuronal activity states in the hippocampal CA3 network. J Cereb Blood Flow Metab. 2013;33(2):263–71. doi: 10.1038/jcbfm.2012.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kienast Y, von Baumgarten L, Fuhrmann M, Klinkert WE, Goldbrunner R, Herms J, Winkler F. Real-time imaging reveals the single steps of brain metastasis formation. Nat Med. 2010;16(1):116–22. doi: 10.1038/nm.2072. [DOI] [PubMed] [Google Scholar]
- 66.Lockman PR, Mittapalli RK, Taskar KS, Rudraraju V, Gril B, Bohn KA, Adkins CE, Roberts A, Thorsheim HR, Gaasch JA, Huang S, Palmieri D, Steeg PS, Smith QR. Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin Cancer Res. 2010;16(23):5664–78. doi: 10.1158/1078-0432.CCR-10-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schackert G, Simmons RD, Buzbee TM, Hume DA, Fidler IJ. Macrophage infiltration into experimental brain metastases: occurrence through an intact blood-brain barrier. J Natl Cancer Inst. 1988;80(13):1027–34. doi: 10.1093/jnci/80.13.1027. [DOI] [PubMed] [Google Scholar]
- 68.Zhang RD, Price JE, Fujimaki T, Bucana CD, Fidler IJ. Differential permeability of the blood-brain barrier in experimental brain metastases produced by human neoplasms implanted into nude mice. Am J Pathol. 1992;141(5):1115–24. [PMC free article] [PubMed] [Google Scholar]
- 69.Carbonell WS, Ansorge O, Sibson N, Muschel R. The vascular basement membrane as "soil" in brain metastasis. PLoS One. 2009;4(6):e5857. doi: 10.1371/journal.pone.0005857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jacus MO, V, Daryani M, Harstead KE, Patel YT, Throm SL, Stewart CF. Pharmacokinetic Properties of Anticancer Agents for the Treatment of Central Nervous System Tumors: Update of the Literature. Clin Pharmacokinet. 2016;55(3):297–311. doi: 10.1007/s40262-015-0319-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gee JR, Keller JN. Astrocytes: regulation of brain homeostasis via apolipoprotein E. Int J Biochem Cell Biol. 2005;37(6):1145–50. doi: 10.1016/j.biocel.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 72.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7(1):41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 73.Pellerin L, Pellegri G, Bittar PG, Charnay Y, Bouras C, Martin JL, Stella N, Magistretti PJ. Evidence supporting the existence of an activity-dependent astrocyte-neuron lactate shuttle. Dev Neurosci. 1998;20(4–5):291–9. doi: 10.1159/000017324. [DOI] [PubMed] [Google Scholar]
- 74.Valiente M, Obenauf AC, Jin X, Chen Q, Zhang XH, Lee DJ, Chaft JE, Kris MG, Huse JT, Brogi E, Massague J. Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell. 2014;156(5):1002–16. doi: 10.1016/j.cell.2014.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim SJ, Kim JS, Park ES, Lee JS, Lin Q, Langley RR, Maya M, He J, Kim SW, Weihua Z, Balasubramanian K, Fan D, Mills GB, Hung MC, Fidler IJ. Astrocytes upregulate survival genes in tumor cells and induce protection from chemotherapy. Neoplasia. 2011;13(3):286–98. doi: 10.1593/neo.11112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim SW, Choi HJ, Lee HJ, He J, Wu Q, Langley RR, Fidler IJ, Kim SJ. Role of the endothelin axis in astrocyte- and endothelial cell-mediated chemoprotection of cancer cells. Neuro Oncol. 2014;16(12):1585–98. doi: 10.1093/neuonc/nou128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee HJ, Hanibuchi M, Kim SJ, Yu H, Kim MS, He J, Langley RR, Lehembre F, Regenass U, Fidler IJ. Treatment of experimental human breast cancer and lung cancer brain metastases in mice by macitentan, a dual antagonist of endothelin receptors, combined with paclitaxel. Neuro Oncol. 2016;18(4):486–96. doi: 10.1093/neuonc/now037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xing F, Kobayashi A, Okuda H, Watabe M, Pai SK, Pandey PR, Hirota S, Wilber A, Mo YY, Moore BE, Liu W, Fukuda K, Iiizumi M, Sharma S, Liu Y, Wu K, Peralta E, Watabe K. Reactive astrocytes promote the metastatic growth of breast cancer stem-like cells by activating Notch signalling in brain. EMBO Mol Med. 2013;5(3):384–96. doi: 10.1002/emmm.201201623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang L, Zhang S, Yao J, Lowery FJ, Zhang Q, Huang WC, Li P, Li M, Wang X, Zhang C, Wang H, Ellis K, Cheerathodi M, McCarty JH, Palmieri D, Saunus J, Lakhani S, Huang S, Sahin AA, Aldape KD, Steeg PS, Yu D. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature. 2015;527(7576):100–4. doi: 10.1038/nature15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Neman J, Choy C, Kowolik CM, Anderson A, Duenas VJ, Waliany S, Chen BT, Chen MY, Jandial R. Co-evolution of breast-to-brain metastasis and neural progenitor cells. Clin Exp Metastasis. 2013;30(6):753–68. doi: 10.1007/s10585-013-9576-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Solomos AC, Rall GF. Get It through Your Thick Head: Emerging Principles in Neuroimmunology and Neurovirology Redefine Central Nervous System "Immune Privilege". ACS Chem Neurosci. 2016;7(4):435–41. doi: 10.1021/acschemneuro.5b00336. [DOI] [PubMed] [Google Scholar]
- 82.Perry VH, Teeling J. Microglia and macrophages of the central nervous system: the contribution of microglia priming and systemic inflammation to chronic neurodegeneration. Semin Immunopathol. 2013;35(5):601–12. doi: 10.1007/s00281-013-0382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, Hume DA, Perlman H, Malissen B, Zelzer E, Jung S. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38(1):79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kokovay E, Cunningham LA. Bone marrow-derived microglia contribute to the neuroinflammatory response and express iNOS in the MPTP mouse model of Parkinson's disease. Neurobiol Dis. 2005;19(3):471–8. doi: 10.1016/j.nbd.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 85.Ataka K, Asakawa A, Nagaishi K, Kaimoto K, Sawada A, Hayakawa Y, Tatezawa R, Inui A, Fujimiya M. Bone marrow-derived microglia infiltrate into the paraventricular nucleus of chronic psychological stress-loaded mice. PLoS One. 2013;8(11):e81744. doi: 10.1371/journal.pone.0081744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Okonogi N, Nakamura K, Suzuki Y, Suto N, Suzue K, Kaminuma T, Nakano T, Hirai H. Cranial irradiation induces bone marrow-derived microglia in adult mouse brain tissue. J Radiat Res. 2014;55(4):713–9. doi: 10.1093/jrr/rru015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mills CD. M1 and M2 Macrophages: Oracles of Health and Disease. Crit Rev Immunol. 2012;32(6):463–88. doi: 10.1615/critrevimmunol.v32.i6.10. [DOI] [PubMed] [Google Scholar]
- 88.He BP, Wang JJ, Zhang X, Wu Y, Wang M, Bay BH, Chang AY. Differential reactions of microglia to brain metastasis of lung cancer. Mol Med. 2006;12(7–8):161–70. doi: 10.2119/2006-00033.He. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lorger M, Felding-Habermann B. Capturing changes in the brain microenvironment during initial steps of breast cancer brain metastasis. Am J Pathol. 2010;176(6):2958–71. doi: 10.2353/ajpath.2010.090838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brantley EC, Guo L, Zhang C, Lin Q, Yokoi K, Langley RR, Kruzel E, Maya M, Kim SW, Kim SJ, Fan D, Fidler IJ. Nitric oxide-mediated tumoricidal activity of murine microglial cells. Transl Oncol. 2010;3(6):380–8. doi: 10.1593/tlo.10208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pukrop T, Dehghani F, Chuang HN, Lohaus R, Bayanga K, Heermann S, Regen T, Van Rossum D, Klemm F, Schulz M, Siam L, Hoffmann A, Trumper L, Stadelmann C, Bechmann I, Hanisch UK, Binder C. Microglia promote colonization of brain tissue by breast cancer cells in a Wnt-dependent way. Glia. 2010;58(12):1477–89. doi: 10.1002/glia.21022. [DOI] [PubMed] [Google Scholar]
- 92.Fitzgerald DP, Palmieri D, Hua E, Hargrave E, Herring JM, Qian Y, Vega-Valle E, Weil RJ, Stark AM, Vortmeyer AO, Steeg PS. Reactive glia are recruited by highly proliferative brain metastases of breast cancer and promote tumor cell colonization. Clin Exp Metastasis. 2008;25(7):799–810. doi: 10.1007/s10585-008-9193-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pardridge WM. Brain metabolism: a perspective from the blood-brain barrier. Physiol Rev. 1983;63(4):1481–535. doi: 10.1152/physrev.1983.63.4.1481. [DOI] [PubMed] [Google Scholar]
- 94.Raichle ME, Gusnard DA. Appraising the brain's energy budget. Proc Natl Acad Sci U S A. 2002;99(16):10237–9. doi: 10.1073/pnas.172399499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Huang Z, Zuo C, Guan Y, Zhang Z, Liu P, Xue F, Lin X. Misdiagnoses of 11C-choline combined with 18F-FDG PET imaging in brain tumours. Nucl Med Commun. 2008;29(4):354–8. doi: 10.1097/MNM.0b013e3282f4a21e. [DOI] [PubMed] [Google Scholar]
- 96.Simpson IA, Carruthers A, Vannucci SJ. Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cereb Blood Flow Metab. 2007;27(11):1766–91. doi: 10.1038/sj.jcbfm.9600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mergenthaler P, Lindauer U, Dienel GA, Meisel A. Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends Neurosci. 2013;36(10):587–97. doi: 10.1016/j.tins.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Palmieri D, Fitzgerald D, Shreeve SM, Hua E, Bronder JL, Weil RJ, Davis S, Stark AM, Merino MJ, Kurek R, Mehdorn HM, Davis G, Steinberg SM, Meltzer PS, Aldape K, Steeg PS. Analyses of resected human brain metastases of breast cancer reveal the association between up-regulation of hexokinase 2 and poor prognosis. Mol Cancer Res. 2009;7(9):1438–45. doi: 10.1158/1541-7786.MCR-09-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen EI, Hewel J, Krueger JS, Tiraby C, Weber MR, Kralli A, Becker K, Yates JR, 3rd, Felding-Habermann B. Adaptation of energy metabolism in breast cancer brain metastases. Cancer Res. 2007;67(4):1472–86. doi: 10.1158/0008-5472.CAN-06-3137. [DOI] [PubMed] [Google Scholar]
- 100.O'Kane RL, Hawkins RA. Na+-dependent transport of large neutral amino acids occurs at the abluminal membrane of the blood-brain barrier. Am J Physiol Endocrinol Metab. 2003;285(6):E1167–73. doi: 10.1152/ajpendo.00193.2003. [DOI] [PubMed] [Google Scholar]
- 101.Basun H, Forssell LG, Almkvist O, Cowburn RF, Eklof R, Winblad B, Wetterberg L. Amino-Acid-Concentrations in Cerebrospinal-Fluid and Plasma in Alzheimers-Disease and Healthy Control Subjects. Journal of Neural Transmission-Parkinsons Disease and Dementia Section. 1990;2(4):295–304. doi: 10.1007/BF02252924. [DOI] [PubMed] [Google Scholar]
- 102.Neman J, Termini J, Wilczynski S, Vaidehi N, Choy C, Kowolik CM, Li H, Hambrecht AC, Roberts E, Jandial R. Human breast cancer metastases to the brain display GABAergic properties in the neural niche. Proc Natl Acad Sci U S A. 2014;111(3):984–9. doi: 10.1073/pnas.1322098111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Seidlitz EP, Sharma MK, Saikali Z, Ghert M, Singh G. Cancer cell lines release glutamate into the extracellular environment. Clin Exp Metastasis. 2009;26(7):781–7. doi: 10.1007/s10585-009-9277-4. [DOI] [PubMed] [Google Scholar]
- 104.Carrascosa JM, Martinez P, Nunez de Castro I. Nitrogen movement between host and tumor in mice inoculated with Ehrlich ascitic tumor cells. Cancer Res. 1984;44(9):3831–5. [PubMed] [Google Scholar]
- 105.Takano T, Lin JH, Arcuino G, Gao Q, Yang J, Nedergaard M. Glutamate release promotes growth of malignant gliomas. Nat Med. 2001;7(9):1010–5. doi: 10.1038/nm0901-1010. [DOI] [PubMed] [Google Scholar]
- 106.Ye ZC, Sontheimer H. Glioma cells release excitotoxic concentrations of glutamate. Cancer Res. 1999;59(17):4383–91. [PubMed] [Google Scholar]
- 107.Venneti S, Dunphy MP, Zhang H, Pitter KL, Zanzonico P, Campos C, Carlin SD, La Rocca G, Lyashchenko S, Ploessl K, Rohle D, Omuro AM, Cross JR, Brennan CW, Weber WA, Holland EC, Mellinghoff IK, Kung HF, Lewis JS, Thompson CB. Glutamine-based PET imaging facilitates enhanced metabolic evaluation of gliomas in vivo. Sci Transl Med. 2015;7(274):274ra17. doi: 10.1126/scitranslmed.aaa1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen J, Lee HJ, Wu X, Huo L, Kim SJ, Xu L, Wang Y, He J, Bollu LR, Gao G, Su F, Briggs J, Liu X, Melman T, Asara JM, Fidler IJ, Cantley LC, Locasale JW, Weihua Z. Gain of glucose-independent growth upon metastasis of breast cancer cells to the brain. Cancer Res. 2015;75(3):554–65. doi: 10.1158/0008-5472.CAN-14-2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wise DR, Thompson CB. Glutamine addiction: a new therapeutic target in cancer. Trends Biochem Sci. 2010;35(8):427–33. doi: 10.1016/j.tibs.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tardito S, Oudin A, Ahmed SU, Fack F, Keunen O, Zheng L, Miletic H, Sakariassen PO, Weinstock A, Wagner A, Lindsay SL, Hock AK, Barnett SC, Ruppin E, Morkve SH, Lund-Johansen M, Chalmers AJ, Bjerkvig R, Niclou SP, Gottlieb E. Glutamine synthetase activity fuels nucleotide biosynthesis and supports growth of glutamine-restricted glioblastoma. Nat Cell Biol. 2015;17(12):1556–68. doi: 10.1038/ncb3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mayers JR, Vander Heiden MG. Famine versus feast: understanding the metabolism of tumors in vivo. Trends Biochem Sci. 2015;40(3):130–40. doi: 10.1016/j.tibs.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wilson EH, Weninger W, Hunter CA. Trafficking of immune cells in the central nervous system. J Clin Invest. 2010;120(5):1368–79. doi: 10.1172/JCI41911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Engelhardt B, Ransohoff RM. Capture, crawl, cross: the T cell code to breach the blood-brain barriers. Trends Immunol. 2012;33(12):579–89. doi: 10.1016/j.it.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 114.Larochelle C, Alvarez JI, Prat A. How do immune cells overcome the blood-brain barrier in multiple sclerosis? FEBS Lett. 2011;585(23):3770–80. doi: 10.1016/j.febslet.2011.04.066. [DOI] [PubMed] [Google Scholar]
- 115.Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol. 2015;33(17):1974–82. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Margolin KA, Di Giacomo AM, Maio M. Brain metastasis in melanoma: clinical activity of CTLA-4 antibody therapy. Semin Oncol. 2010;37(5):468–72. doi: 10.1053/j.seminoncol.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 117.Hodi FS, Oble DA, Drappatz J, Velazquez EF, Ramaiya N, Ramakrishna N, Day AL, Kruse A, Mac Rae S, Hoos A, Mihm M. CTLA-4 blockade with ipilimumab induces significant clinical benefit in a female with melanoma metastases to the CNS. Nat Clin Pract Oncol. 2008;5(9):557–61. doi: 10.1038/ncponc1183. [DOI] [PubMed] [Google Scholar]
- 118.Schartz NE, Farges C, Madelaine I, Bruzzoni H, Calvo F, Hoos A, Lebbe C. Complete regression of a previously untreated melanoma brain metastasis with ipilimumab. Melanoma Res. 2010;20(3):247–50. doi: 10.1097/CMR.0b013e3283364a37. [DOI] [PubMed] [Google Scholar]
- 119.Hong JJ, Rosenberg SA, Dudley ME, Yang JC, White DE, Butman JA, Sherry RM. Successful treatment of melanoma brain metastases with adoptive cell therapy. Clin Cancer Res. 2010;16(19):4892–8. doi: 10.1158/1078-0432.CCR-10-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gatenby RA, Brown J, Vincent T. Lessons from applied ecology: cancer control using an evolutionary double bind. Cancer Res. 2009;69(19):7499–502. doi: 10.1158/0008-5472.CAN-09-1354. [DOI] [PubMed] [Google Scholar]
- 121.Enriquez-Navas PM, Wojtkowiak JW, Gatenby RA. Application of Evolutionary Principles to Cancer Therapy. Cancer Res. 2015;75(22):4675–80. doi: 10.1158/0008-5472.CAN-15-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]