Abstract
There is evidence that variation in the gene encoding a kainate receptor subunit contributes to alcohol dependence risk. Further, there is suggestive evidence that alcohol consumption is mediated, in part, by kainate receptors. In this study, we used a novel kainate receptor antagonist, LY466195, to examine the potential role of kainate receptors in alcohol drinking behavior using a rodent model of voluntary ethanol consumption. Male Sprague Dawley and Long Evans rats were given access to 20% ethanol using the intermittent two-bottle choice paradigm. Following 6 weeks of ethanol consumption, rats were pretreated with an acute dose of LY466195 (0, 4.0 and 10.0 mg/kg, i.p.) prior to a two-bottle choice test session. Acute administration of LY466195 did not significantly affect ethanol-drinking behavior in Sprague Dawley rats. In contrast, Long Evans rats pretreated with 10.0 mg/kg LY466195 showed a significant reduction in alcohol preference compared to vehicle-treated controls. Decreased alcohol preference in the Long Evans rats was associated with increased water intake and no change in the amount of ethanol consumed. Taken together, these results suggest that systemic administration of a selective kainate receptor antagonist reduces ethanol preference in rats, an effect that could be due to non-specific effects on overall drinking behavior.
Keywords: Alcohol addiction, kainate receptor, glutamate, drinking, ethanol
1. Introduction
Alcohol abuse disorder (AUD) is a significant public health concerns in the United States. A recent epidemiologic survey showed that the lifetime prevalence of AUD in the United States was 36.0% in men and 22.7% in women [10]. The frequency of heaving drinking is a key risk factor for developing an AUD [6]. In 2014, 24.7% of individuals over the age of 18 reported consuming five or more drinks on a single occasion during the past month. Additionally, 6.8% of individuals over the age of 18 reported consuming five or more drinks on at least five occasions during the past month [1]. Despite these high rates of heavy drinking and the associated health risks, only 7.8% of adults who fit the criteria for an AUD seek treatment [10]. Moreover, current medications to treat AUD have limited efficacy and can produce significant adverse effects [3, 19].
Based on preclinical studies showing that topiramate reduces ethanol consumption and reinforcement in rats [4, 18], recent clinical trials have examined the efficacy of topiramate in alcohol dependence [25]. In a recent study, 12 weeks of topiramate treatment was sufficient to reduce the number of heavy drinking days among heavy drinkers [15]. Unfortunately, topiramate’s adverse effects, which include paresthesia, anorexia (with weight loss), difficulties with memory or concentration, and mild-to-moderate taste disturbances, limit its clinical utility [20]. Topiramate has several pharmacological mechanisms of action including antagonism of AMPA/kainate receptors [9, 21]. A recent study showed that the efficacy of topiramate in reducing the number of heavy drinking days in human patients was moderated by a polymorphism in GRIK1, the gene that encodes the GluK1 kainate receptor subunit [15]. These results suggest that the efficacy of topiramate is mediated, in part, by pharmacological inhibition of GluK1 kainate receptors.
Kainate receptors are ionotropic glutamate receptors [17, 23] that show distinct expression patterns in the brain with high levels in the hippocampus and striatum, brain regions known to play important roles in alcohol reinforcement [7, 8]. Recently, a potent and selective kainate receptor antagonist was developed for in vivo studies [24]. Because previous studies of glutamate receptors in preclinical models of ethanol consumption used the nonselective AMPA/kainate receptor antagonist CNQX to reduce ethanol intake [2], the precise role of kainate receptors in ethanol drinking remains to be determined. Here, we used the novel antagonist LY466195, which is highly selective for kainate receptors containing the GluK1 receptor subunit [24], to investigate the role of kainate receptors in a rodent model of voluntary ethanol consumption. Our hypothesis was that pharmacological inhibition of GluK1-containing kainate receptors would decrease ethanol drinking and preference in rats.
2. Materials and Methods
2.1 Animals and Housing
Male Sprague Dawley rats and male Long Evans rats weighing 250–300 g were obtained from Taconic Laboratories (Germantown, NY, USA). Rats were individually housed with food and water available ad libitum. A 12-h reverse light/dark cycle (lights off 07:00, lights on 19:00) was used throughout the experiments. All procedures were consistent with the ethical guidelines of the U.S. National Institutes of Health and were approved by the University of Pennsylvania School of Medicine Institutional Animal Care and Use Committee.
2.2 Drugs
Eli Lilly and Co. (Indianapolis, IN, USA) donated LY466195. The drug was dissolved in bacteriostatic 0.9% saline (Midwest Veterinary Supply, Norristown, PA) for administration. The doses and time course of LY466195 pretreatment were based on preliminary studies demonstrating in vivo effects of LY466195 in behavioral models of addiction (data not shown) as well as rodent and human studies that identified efficacious and selective doses of LY466195 in models of migraine [14, 24]. Doses of LY466195 as high as 100 mg/kg were shown to maintain selectivity for GluK1-containing kainate receptors and do not produce changes in locomotor activity [24].
2.3 Intermittent-2-bottle choice
The intermittent-2-bottle choice paradigm was used to assess ethanol consumption in rats as described previously [5]. Briefly, rats were acclimated to having two water bottles present in their home cage continuously for one week prior to the first ethanol exposure. Every Monday, Wednesday and Friday, two hours after the onset of the dark cycle (09:00), one water bottle was replaced with a bottle containing an ethanol solution and rats were allowed to drink freely. After two hours of exposure, the ethanol bottle and water bottle were taken out, weighed, and put back into the cage. Following 24 hours of exposure, the ethanol bottle and water bottle were weighed a second time and the ethanol bottle was replaced with a bottle containing normal drinking water. During the first week, rats were exposed to progressively greater concentrations of ethanol (one day each of 3%, 6% and 10% ethanol (vol/vol)). For subsequent weeks, rats received 20% ethanol (vol/vol) on Mondays, Wednesdays and Fridays. A total of 20 acquisition sessions of 24-hour ethanol exposure were conducted prior to testing. To prevent the development of a side preference or bias, water and ethanol bottle positions were counterbalanced across days. An empty cage containing one water bottle and one ethanol bottle was used to measure evaporation and bottle leakage. Rats were weighed weekly, including prior to acute LY466195 administration, to provide the denominator for calculating ethanol consumption (g/kg). Ethanol preference was calculated as the total amount of ethanol consumed/(total ethanol + water consumption) * 100. All measurements were made immediately before the introduction of the ethanol bottle on test days.
2.4 Acute Administration of LY466195
For the final three of 20 total sessions involving 24-hour ethanol exposure, all rats were injected with saline (1 ml/kg, i.p) prior to the introduction of the ethanol bottle in order to habituate them to the injection procedures. On subsequent test days, rats received 0, 4 and 10 mg/kg LY466195 (i.p.) 10 min before a 24-hour two-bottle choice exposure. A within-subjects design was used with doses of LY466195 counterbalanced across the three test sessions.
2.5 Data Analyses
The effect of LY466195 on ethanol consumption, ethanol preference, and total fluid intake were analyzed using separate one-way ANOVAs. Following a significant main effect of treatment, post hoc analyses were conducted using Tukey’s HSD.
3. Results
3.1 Acquisition of ethanol drinking and ethanol preference in male Sprague Dawley and male Long Evans rats
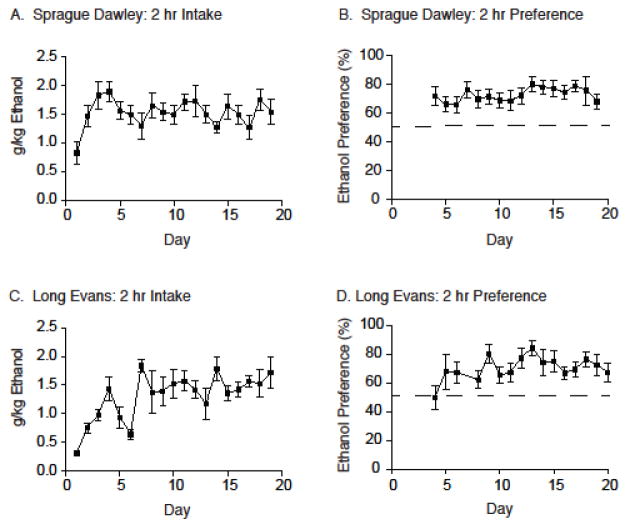
The intermittent two-bottle choice paradigm was used to study ethanol drinking and ethanol preference in the two strains of rats. Total ethanol intake and ethanol preference (mean +/− S.E.M.) at the two-hour time point for 19 days of intermittent ethanol exposure are displayed in Figure 1. Male Sprague Dawley rats consumed 1.60 +/− 0.27 g/kg of ethanol (Figure 1A) and displayed an ethanol preference of 68.04 +/− 5.46% (Figure 1B) prior to the testing phase (i.e., Day 19). Male Long Evans rats consumed 1.75 +/− 0.35 g/kg of ethanol (Figure 1C) and displayed an ethanol preference of 75.32 +/− 7.87% (Figure 1D) following 19 days of intermittent ethanol exposure (i.e., Day 19).
Figure 1.
The intermittent two-bottle choice paradigm was sufficient in establishing ethanol drinking and preference in both Sprague Dawley and Long Evans rats. (A) Total ethanol consumed (g/kg) is reported over the course of the 19-day acquisition period at the 2-hour time point for Sprague Dawley rats (N=19). (B) Ethanol preference (%) is reported over the course of the 19-day acquisition period at the 2-hour time point for Sprague Dawley rats (N=19). (C) Total ethanol consumed (g/kg) is reported over the course of the 19-day acquisition period at the 2-hour time point for Long Evans rats (N=6). (D) Ethanol preference (%) is reported over the course of the 19-day acquisition period at the 2-hour time point for Long Evans rats (N=6).
3.2 Acute systemic administration of LY466195 had no effect on ethanol intake and ethanol preference in male Sprague Dawley rats
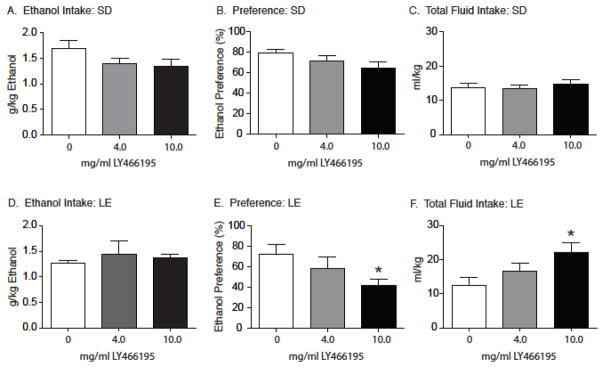
Following 20 days of intermittent ethanol access, rats were pretreated with the selective GluK1 kainate receptor antagonist LY466195 (0, 4 and 10 mg/ml, i.p.) 10 min prior to a two-hour test session in which total ethanol intake, ethanol preference and total fluid intake (mean +/− SEM) were measured. Separate one-way ANOVAs revealed no statistically significant effects of LY466195 pretreatment on ethanol intake (F2, 18=2.33, P=0.11; Figure 2A), ethanol preference (F2, 18=1.67, P=0.09; Figure 2B), and total fluid intake (F2, 18=0.34, P=0.72; Figure 2C) in male Sprague Dawley rats. There was a non-significant trend towards reduced ethanol preference (P=0.09) in these rats following pretreatment with 10 mg/ml LY466195.
Figure 2.
Acute administration of LY466195 had no effect on total ethanol intake or ethanol preference in Sprague Dawley (SD) rats (N=19) at the 2-hour time point. Following acute pretreatment with LY466195, Sprague Dawley rats showed no significant changes in total ethanol consumed (A), ethanol preference (B) or total fluid consumed (C). Acute administration of LY466195 significantly reduced ethanol preference alongside an increase in total fluid intake in Long Evans (LE) rats (N=6) at the 2-hour time point. Acute LY466195 pretreatment did not alter total ethanol consumed (D) but did significantly reduce ethanol preference (E) in Long Evans rats. However, there was a significant increase in the total fluid consumed following pretreatment with LY466195 when compared to vehicle (F). * p < 0.05. SD=Sprague Dawley; LD=Long Evans
3.3 Acute systemic administration of LY466195 dose-dependently attenuated ethanol preference and increased total fluid intake in male Long Evans rats
Total ethanol preference, ethanol intake and total fluid intake (mean +/− SEM) in male Long Evans rats pretreated with the selective GluK1 kainate receptor antagonist LY466195 are shown in Figure 3. Total ethanol intake (Figure 2D) was analyzed with a one-way ANOVA, which did not reveal a significant main effect of pretreatment (F2, 5=0.25; P=0.66). In contrast, there was a significant main effect of treatment on ethanol preference (F2, 5=4.54, P=0.04; Figure 2E). A subsequent post-hoc analysis showed reduced ethanol preference following 10 mg/kg LY466195 pretreatment when compared to vehicle (Tukey’s HSD; P<0.05). A significant main effect of LY466195 pretreatment on total fluid intake was also observed (F2, 5=2.26, P=0.032; Figure 2F), with a significant increase noted in rats pretreated with 10 mg/kg LY466195 compared to vehicle (Tukey’s HSD; P<0.05).
4. Discussion
Here, we show for the first time that pharmacological inhibition of kainate receptors is sufficient to reduce ethanol preference in rats. Specifically, the present findings indicate that acute administration of the selective GluK1 kainate receptor antagonist LY466195 significantly attenuates ethanol preference in male Long Evans rats. While not statistically significant, there was also a trend towards reduced ethanol preference in male Sprague Dawley rats pretreated with LY466195. Further analyses of drinking behavior revealed that the LY466195-mediated decrease in ethanol preference in Long Evans rats could be due to an increase in total fluid intake. The trend toward reduced ethanol preference in male Sprague Dawley rats was not associated with a change in total water intake. While these results suggest that selective antagonists of kainate receptors could reduce ethanol preference, future studies are needed to define the behavioral mechanisms underlying the effects of LY466195 on drinking behaviors and to identify the role of central kainate receptors in alcohol consumption.
Glutamate transmission in the central nervous system plays an important role in the neurobiology of AUD [12]. Preclinical studies utilizing an intermittent access paradigm in mice showed that heavy ethanol drinking was associated with increased extracellular glutamate levels in the nucleus accumbens [11]. Consistent with these findings, administration of the AMPA/kainate receptor antagonist NBQX attenuated ethanol self-administration [22]. However, ethanol self-administration was not altered by the non-competitive AMPA receptor antagonist GYKI 52466, which suggests that kainate receptors, rather than AMPA receptors, play a crucial role in voluntary ethanol consumption [22]. Although these studies support an important role of ionotropic glutamate receptor signaling in ethanol drinking and reward, the exact contribution of kainate receptors to these behavioral responses has not been identified. Our findings add to this literature and indicate that selective pharmacological inhibition of kainate receptors may be sufficient to reduce ethanol preference in male rats.
In addition to identifying a novel role for kainate receptors in an animal model of alcohol drinking, we found a strain-dependent effect of LY466195 on ethanol preference and drinking behaviors. These strain-dependent effects may reflect an effect of genetic variation in mediating the efficacy of a GluK1 selective kainate receptor antagonist. This finding is particularly interesting when viewed in the context of findings from a recent study [15] showing that topiramate is more efficacious for reducing heavy drinking in humans with a particular genotype for a single nucleotide polymorphism in a non-coding region of GRIK1, which encodes the GluK1 kainate receptor subunit [16]. It has been hypothesized that this polymorphism, or another GRIK1 polymorphism that may be linked to it, reduces the transcription of GRIK1, thereby reducing GluK1 mRNA expression and the efficacy of topiramate treatment [15]. While topiramate has been shown to be effective in reducing heavy drinking, the adverse effects associated with topiramate can lead to non-adherence to the medication and an early discontinuation of treatment [13]. Because the efficacy of topiramate in heavy alcohol drinkers appears to rely on pharmacological inhibition of GluK1-containing kainate receptors, LY466195 and other potent GluK1 receptor antagonists may represent novel pharmacological treatments for AUD. Moreover, due to their greater selectivity, GluK1 receptor antagonists may be associated with fewer adverse effects than topiramate, which has activity in multiple neurotransmitter and enzyme systems.
5. Conclusions
Using the intermittent two-bottle choice paradigm, we found that LY466195 pretreatment produced a significant reduction in ethanol preference in male Long Evans rats and a trend towards reduced ethanol preference in male Sprague Dawley rats. Because reduced ethanol preference was accompanied by increased water intake in Long Evans rats, but not Sprague Dawley rats, further studies are needed to replicate these findings and more clearly define the effects of LY499195 on drinking behaviors. This is the first preclinical study to indicate an important role for GluK1 kainate receptors in voluntary ethanol consumption. Consistent with recent clinical findings [15], these results suggest that selective kainate receptor antagonists may be useful in treating AUD in humans.
Highlights.
We examined the effects of a novel kainate receptor antagonist on ethanol drinking and preference in rats.
Administration of LY466195 significantly reduced ethanol preference in Long Evans rats.
Administration of LY466195 produced a non-significant trend towards decreased ethanol preference in Sprague Dawley rats.
Further studies are needed to more fully characterize the role of kainate receptors in drinking behaviors.
Acknowledgments
This work was supported by the following grants from the National Institutes of Health (NIH): AA023192 (HRK); DA15214 (RCP); DA037897 and DA039393 (HDS). Eli Lilly and Company donated supplies of LY499195 for the conduct of the research.
Footnotes
Potential Conflicts of Interest
Dr. Kranzler has been an advisory board member, consultant, or CME speaker for Indivior, Lundbeck, and Otsuka. He is also a member of the American Society of Clinical Psychopharmacology’s Alcohol Clinical Trials Initiative, which was supported in the last three years by AbbVie, Alkermes, Ethypharm, Indivior, Lilly, Lundbeck, Otsuka, Pfizer, and XenoPort.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.U.D.o.H.a.H. Services, editor. National Survey on Drug Use and Health. 2014. [Google Scholar]
- 2.Backstrom P, Hyytia P. Ionotropic glutamate receptor antagonists modulate cue-induced reinstatement of ethanol-seeking behavior. Alcohol Clin Exp Res. 2004;28:558–565. doi: 10.1097/01.alc.0000122101.13164.21. [DOI] [PubMed] [Google Scholar]
- 3.Blodgett JC, Del Re AC, Maisel NC, Finney JW. A meta-analysis of topiramate’s effects for individuals with alcohol use disorders. Alcohol Clin Exp Res. 2014;38:1481–1488. doi: 10.1111/acer.12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breslin FJ, Johnson BA, Lynch WJ. Effect of topiramate treatment on ethanol consumption in rats. Psychopharmacology (Berl) 2010;207:529–534. doi: 10.1007/s00213-009-1683-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carnicella S, Ron D, Barak S. Intermittent ethanol access schedule in rats as a preclinical model of alcohol abuse. Alcohol. 2014;48:243–252. doi: 10.1016/j.alcohol.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esser MB, Hedden SL, Kanny D, Brewer RD, Gfroerer JC, Naimi TS. Prevalence of alcohol dependence among US adult drinkers, 2009–2011. Prev Chronic Dis. 2014;11:E206. doi: 10.5888/pcd11.140329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 8.Foster AC, Mena EE, Monaghan DT, Cotman CW. Synaptic localization of kainic acid binding sites. Nature. 1981;289:73–75. doi: 10.1038/289073a0. [DOI] [PubMed] [Google Scholar]
- 9.Gibbs JW, 3rd, Sombati S, DeLorenzo RJ, Coulter DA. Cellular actions of topiramate: blockade of kainate-evoked inward currents in cultured hippocampal neurons. Epilepsia. 2000;41(Suppl 1):S10–16. doi: 10.1111/j.1528-1157.2000.tb02164.x. [DOI] [PubMed] [Google Scholar]
- 10.Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B, Hasin DS. Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757–766. doi: 10.1001/jamapsychiatry.2015.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griffin WC, 3rd, Haun HL, Hazelbaker CL, Ramachandra VS, Becker HC. Increased extracellular glutamate in the nucleus accumbens promotes excessive ethanol drinking in ethanol dependent mice. Neuropsychopharmacology. 2014;39:707–717. doi: 10.1038/npp.2013.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holmes A, Spanagel R, Krystal JH. Glutamatergic targets for new alcohol medications. Psychopharmacology (Berl) 2013;229:539–554. doi: 10.1007/s00213-013-3226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson BA, Rosenthal N, Capece JA, Wiegand F, Mao L, Beyers K, McKay A, Ait-Daoud N, Anton RF, Ciraulo DA, Kranzler HR, Mann K, O’Malley SS, Swift RM B. Topiramate for Alcoholism Advisory, G. Topiramate for Alcoholism Study. Topiramate for treating alcohol dependence: a randomized controlled trial. JAMA. 2007;298:1641–1651. doi: 10.1001/jama.298.14.1641. [DOI] [PubMed] [Google Scholar]
- 14.NE, Johnson KW, Johnson MP, Dieckman DK, Clemens-Smith A, Siuda ER, Dell CP, Dehlinger V, Hudziak KJ, Filla SA, Ornstein PL, Ramadan NM, Bleakman D. Innovative drug development for headache disorders: glutamate. Vol. 16. Oxford University Press; Oxford: 2008. [Google Scholar]
- 15.Kranzler HR, Covault J, Feinn R, Armeli S, Tennen H, Arias AJ, Gelernter J, Pond T, Oncken C, Kampman KM. Topiramate treatment for heavy drinkers: moderation by a GRIK1 polymorphism. Am J Psychiatry. 2014;171:445–452. doi: 10.1176/appi.ajp.2013.13081014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kranzler HR, Gelernter J, Anton RF, Arias AJ, Herman A, Zhao H, Burian L, Covault J. Association of markers in the 3′ region of the GluR5 kainate receptor subunit gene to alcohol dependence. Alcohol Clin Exp Res. 2009;33:925–930. doi: 10.1111/j.1530-0277.2009.00913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lerma J. Kainate receptor physiology. Curr Opin Pharmacol. 2006;6:89–97. doi: 10.1016/j.coph.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Lynch WJ, Bond C, Breslin FJ, Johnson BA. Severity of drinking as a predictor of efficacy of the combination of ondansetron and topiramate in rat models of ethanol consumption and relapse. Psychopharmacology (Berl) 2011;217:3–12. doi: 10.1007/s00213-011-2253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maisel NC, Blodgett JC, Wilbourne PL, Humphreys K, Finney JW. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108:275–293. doi: 10.1111/j.1360-0443.2012.04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Markind JE. Topiramate: a new antiepileptic drug. Am J Health Syst Pharm. 1998;55:554–562. doi: 10.1093/ajhp/55.6.554. [DOI] [PubMed] [Google Scholar]
- 21.MacDonald MRR. Cellular effects of antiepileptic drugs. In: Engel TAPJ, editor. Epilepsy: A Comprehensive Textbook. Vol. 2. Williams & Wilkins; Philadelphia: 2008. pp. 1433–1446. [Google Scholar]
- 22.Stephens DN, Brown G. Disruption of operant oral self-administration of ethanol, sucrose, and saccharin by the AMPA/kainate antagonist, NBQX, but not the AMPA antagonist, GYKI 52466. Alcohol Clin Exp Res. 1999;23:1914–1920. [PubMed] [Google Scholar]
- 23.Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiss B, Alt A, Ogden AM, Gates M, Dieckman DK, Clemens-Smith A, Ho KH, Jarvie K, Rizkalla G, Wright RA, Calligaro DO, Schoepp D, Mattiuz EL, Stratford RE, Johnson B, Salhoff C, Katofiasc M, Phebus LA, Schenck K, Cohen M, Filla SA, Ornstein PL, Johnson KW, Bleakman D. Pharmacological characterization of the competitive GLUK5 receptor antagonist decahydroisoquinoline LY466195 in vitro and in vivo. J Pharmacol Exp Ther. 2006;318:772–781. doi: 10.1124/jpet.106.101428. [DOI] [PubMed] [Google Scholar]
- 25.Zindel LR, Kranzler HR. Pharmacotherapy of alcohol use disorders: seventy-five years of progress. J Stud Alcohol Drugs Suppl. 2014;75(Suppl 17):79–88. doi: 10.15288/jsads.2014.s17.79. [DOI] [PMC free article] [PubMed] [Google Scholar]