Abstract
Objective:
To investigate the effect of endovascular treatment on cognitive function as a prespecified secondary analysis of the REVASCAT (Endovascular Revascularization With Solitaire Device Versus Best Medical Therapy in Anterior Circulation Stroke Within 8 Hours) trial.
Methods:
REVASCAT randomized 206 patients with anterior circulation proximal arterial occlusion stroke to Solitaire thrombectomy or best medical treatment alone. Patients with established dementia were excluded from enrollment. Cognitive function was assessed in person with Trail Making Test (TMT) Parts A and B at 3 months and 1 year after randomization by an investigator masked to treatment allocation. Test completion within 5 minutes, time of completion (seconds), and number of errors were recorded.
Results:
From November 2012 to December 2014, 206 patients were enrolled in REVASCAT. TMT was assessed in 82 of 84 patients undergoing thrombectomy and 86 of 87 control patients alive at 3 months and in 71 of 79 patients undergoing thrombectomy and 72 of 78 control patients alive at 1 year. Rates of timely TMT-A completion were similar in both treatment arms, although patients undergoing thrombectomy required less time for TMT-A completion and had higher rates of error-free TMT-A performance. Thrombectomy was also associated with a higher probability of timely TMT-B completion (adjusted odds ratio 3.17, 95% confidence interval 1.51–6.66 at 3 months; and adjusted ratio 3.66, 95% confidence interval 1.60–8.35 at 1 year) and shorter time for TMT-B completion. Differences in TMT completion times between treatment arms were significant in patients with good functional outcome but not in those who were functionally dependent (modified Rankin Scale score >2). Poorer cognitive outcomes were significantly associated with larger infarct volume, higher modified Rankin Scale scores, and worse quality of life.
Conclusions:
Thrombectomy improves TMT performance after stroke, especially among patients who reach good functional recovery.
ClinicalTrials.gov identifier:
Classification of evidence:
This study provides Class I evidence that for patients with stroke from acute anterior circulation proximal arterial occlusion, thrombectomy improves performance on the TMT at 3 months.
Cognitive impairment is a common consequence of stroke,1–3 even in survivors with successful functional recovery,4 and it is closely related to disability and dependency.2 Cognitive impairment and dementia after stroke may involve multiple domains, attention and executive functioning being particularly affected.1,3 Altered executive functioning early after stroke has been reported as a predictor of long-term cognitive impairment.5
Because cognitive outcome has traditionally not been considered an outcome measure in randomized trials investigating the benefit of intravenous tissue plasminogen activator in acute stroke, little knowledge exists with regard to any potential benefit that intravenous tissue plasminogen activator may have on cognition. In a recent post hoc analysis based on pooled data from the VISTA (Virtual International Stroke Trials Archive) trials, the Cog-4 scale (based on 4 items of NIH Stroke Scale) was used as an indicator of cognitive function at 3 months after stroke. It was noted that the distribution of Cog-4 scores was better in patients who received intravenous recombinant tissue plasminogen activator compared to those who were not thrombolysed, although this scale did not provide additional information beyond the modified Rankin Scale (mRS) assessment.6
Endovascular treatment has recently demonstrated benefit in physical disability and functional outcome after acute stroke,7 but the effect on cognitive outcomes has not been established yet. REVASCAT (Endovascular Revascularization With Solitaire Device Versus Best Medical Therapy in Anterior Circulation Stroke Within 8 Hours) randomized acute stroke patients either to medical therapy plus endovascular treatment thrombectomy or to medical treatment alone. Analysis of the primary outcome consisting of ordinal mRS score analysis at the third month revealed that thrombectomy was beneficial.8
A prespecified secondary outcome of REVASCAT9 was to evaluate the effect of endovascular treatment on cognitive functioning at 3 months and 1 year after stroke as measured by the Trail Making Test (TMT). We also aimed to study the relationship among cognitive outcomes and other stroke-relevant outcomes (infarct volume, mRS score, and health-related quality of life). To assess whether the influence of endovascular treatment on TMT performance was relevant beyond physical disability, as a post hoc analysis, cognitive outcome was evaluated separately in patients with good/poor functional outcome (mRS score ≤2/>2).
METHODS
Primary research question.
Does thrombectomy improve TMT performance at 3 months after acute ischemic stroke due to an anterior large vessel occlusion?
Classification of evidence.
This randomized interventional study provides Class I evidence that for patients with stroke from acute anterior circulation proximal arterial occlusion, thrombectomy improves performance on the TMT at 3 months compared to best medical treatment alone. From November 2012 to December 2014, REVASCAT enrolled 206 patients with stroke of the anterior circulation within 8 hours from onset who were randomized to receive thrombectomy (with a Solitaire device, Medtronic, Minneapolis, MN) or best medical treatment (both including intravenous recombinant tissue plasminogen activator). The trial was conducted in 4 comprehensive stroke centers in Catalonia, Spain. Patients with established dementia were excluded from enrollment. Detailed protocol and main results have previously been published.8,9 The trial was registered at Clinicaltrials.gov (NCT01692379) and funded by an unrestricted grant from the manufacturer of the device (Covidien, now Medtronic). The primary outcome of REVASCAT was distribution of functional outcome expressed as mRS score at 90 days. A prespecified secondary objective was to test thrombectomy effects on cognitive function at 3 months and 1 year after randomization.9
Cognitive function was assessed in person by an investigator masked to treatment arm using TMT Parts A and B. TMT measures attention, processing speed, working memory, visuospatial ability, and set shifting.10 TMT-A requires the patient to draw lines sequentially connecting 25 encircled numbers distributed on a sheet of paper. In TMT-B, the patient must alternate between numbers and letters (e.g., 1-A-2-B-3-C…L-13). Before each test trial, a practice trial of 6 items was administered to ensure task understanding. Tests must be completed in a maximum of 5 minutes. During performance, each error was immediately corrected by the examiner, and the patient was asked to continue the task. For TMT-A and TMT-B, cognitive outcome was evaluated by percentage of patients who completed the tests in the requested time (5 minutes), time of completion of each test (in seconds), and number of errors made (none vs one or more errors).
Infarct volumes (milliliters) at 24 hours on CT or MRI were adjudicated by investigators at an independent imaging core laboratory who were blinded to clinical data.8 Functional ability and health-related quality of life were evaluated at 3 months and 1 year after enrollment by masked certified assessors using mRS and EuroQoL Group 5-Dimension Self-Report Questionnaire (EQ-5D) 3L, respectively. EQ-5D was analyzed with a utility index adapted to the Spanish population (range −0.3 to 1) and visual analog scale (range 0–100), with higher values corresponding to better quality of life. Language impairment and right upper limb paresis were evaluated with the specific NIH Stroke Scale items at 3 months after stroke. This specific information was not available at 1 year.
Standard protocol approvals, registrations, and patient consents.
The REVASCAT trial received approval from the ethics committees on human experimentation at the 4 recruiting centers. Written informed consent was obtained from all patients (or relatives) participating in the study. The REVASCAT trial was registered at Clinicaltrials.gov (NCT01692379).
Statistical analysis.
Main analyses were performed in the complete case population. The primary objective was to evaluate the effect of treatment arm on executive cognitive outcome at 3 months and 1 year after randomization. Differences in TMT completion on requested time (yes vs no) and presence of errors (0 vs ≥1) between treatment arms were assessed with logistic regression models, with the effect expressed as odds ratio and 95% confident interval. Difference in times of completion of TMT (seconds) between treatment arms was assessed with multivariate linear regression models, with the effect expressed as the mean difference (β coefficient and 95% confidence interval for β). To avoid missing data, maximum time of completion (300 seconds) was considered in those patients who could not complete the tests in the requested time. Multivariable analyses were adjusted by treatment arm, age, baseline stroke severity (NIHSS Stroke Scale), and side of stroke. Association of TMT performance (completion times) with other outcome measures (infarct volume, mRS, EQ-5D) was assessed with Spearman correlation coefficients.
To assess whether the influence of endovascular treatment on TMT performance was relevant beyond physical disability, differences in time of completion of TMT between treatment arms were evaluated separately in patients with mRS scores ≤2 and those with mRS scores >2. In this stratified analysis, differences were assessed with linear regression analysis.
RESULTS
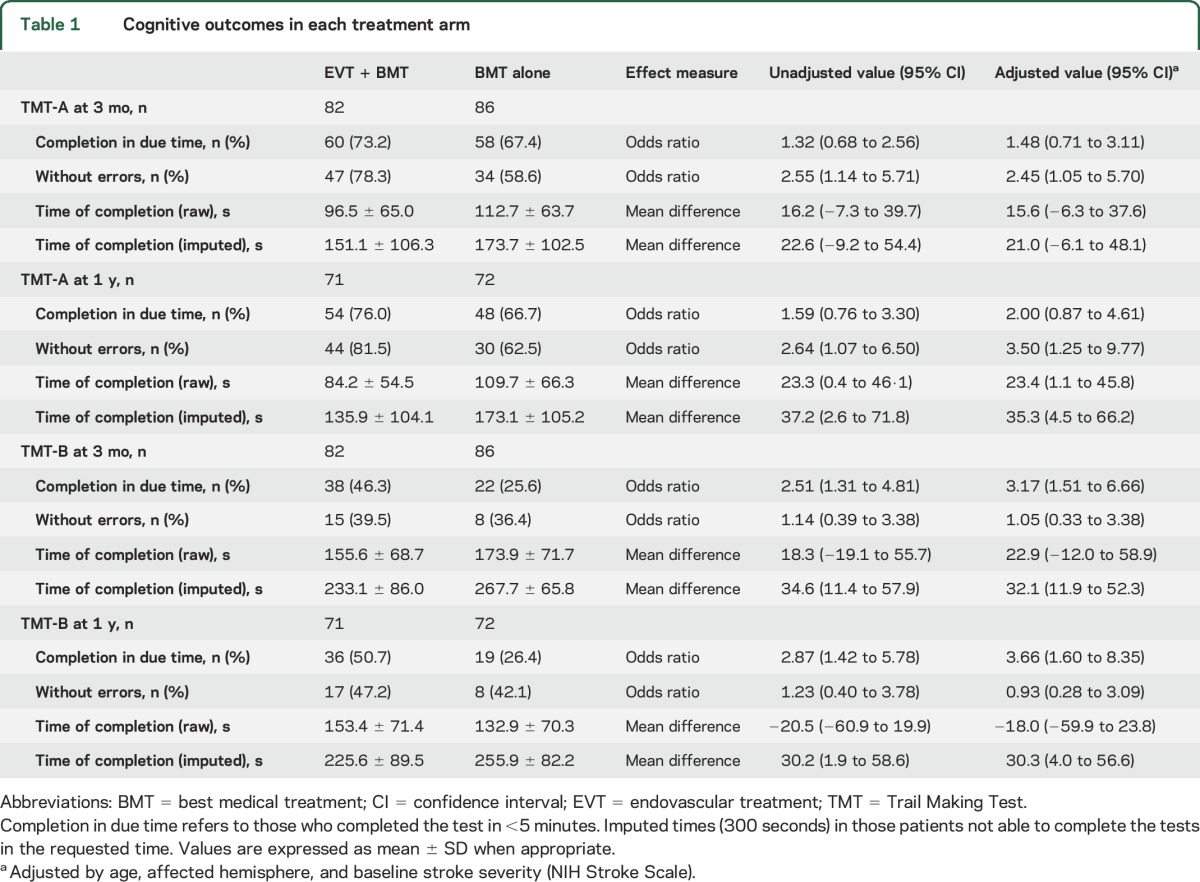
Executive cognitive function was assessed in 82 of 84 patients undergoing thrombectomy and 86 of 87 control patients alive at 3 months and in 71 of 79 patients undergoing thrombectomy and 72 of 78 control patients alive at 1 year (see flow diagram in figure 1). The percentage of patients who were able to complete TMT-A in due time (5 minutes) was similar between treatment arms, but thrombectomy increased ≈3-fold the odds of timely TMT-B completion at 3 months and 1 year after stroke (table 1). Furthermore, patients assigned to thrombectomy required less time for test completion and made fewer errors on TMT-A compared to those in the medical arm (table 1).
Figure 1. CONSORT flow diagram of patients included in REVASCAT trial.
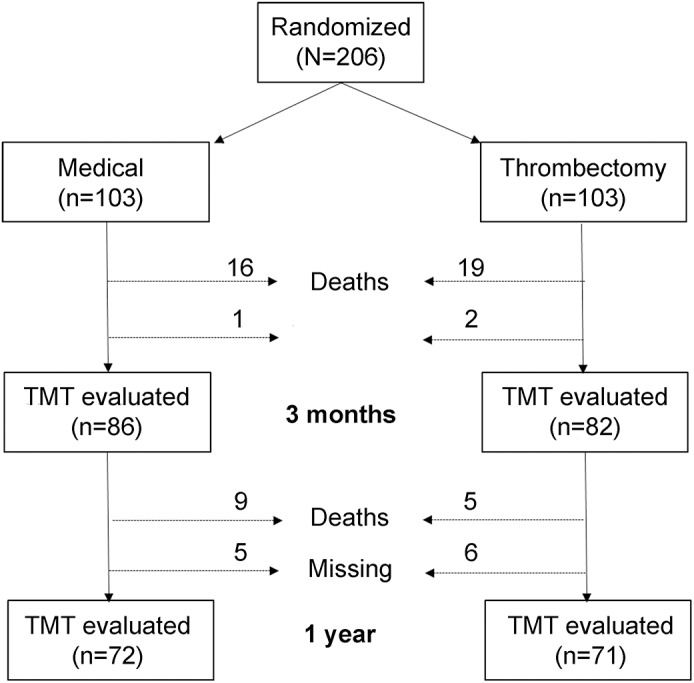
The diagram shows patient allocation, deaths, and individuals missing data for cognitive evaluation in each treatment arm and at both times of follow-up (2 and 12 months after randomization). CONSORT = Consolidated Standards of Reporting Trials; REVASCAT = Endovascular Revascularization With Solitaire Device Versus Best Medical Therapy in Anterior Circulation Stroke Within 8 Hours. TMT = Trail Making Test.
Table 1.
Cognitive outcomes in each treatment arm
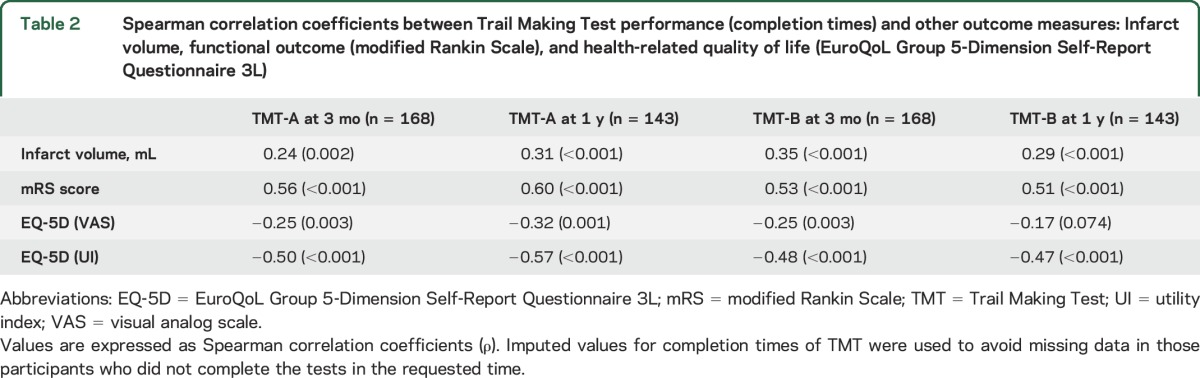
Longer times to complete TMT were significantly associated with larger infarct volume, higher mRS scores, and worse quality of life (table 2).
Table 2.
Spearman correlation coefficients between Trail Making Test performance (completion times) and other outcome measures: Infarct volume, functional outcome (modified Rankin Scale), and health-related quality of life (EuroQoL Group 5-Dimension Self-Report Questionnaire 3L)
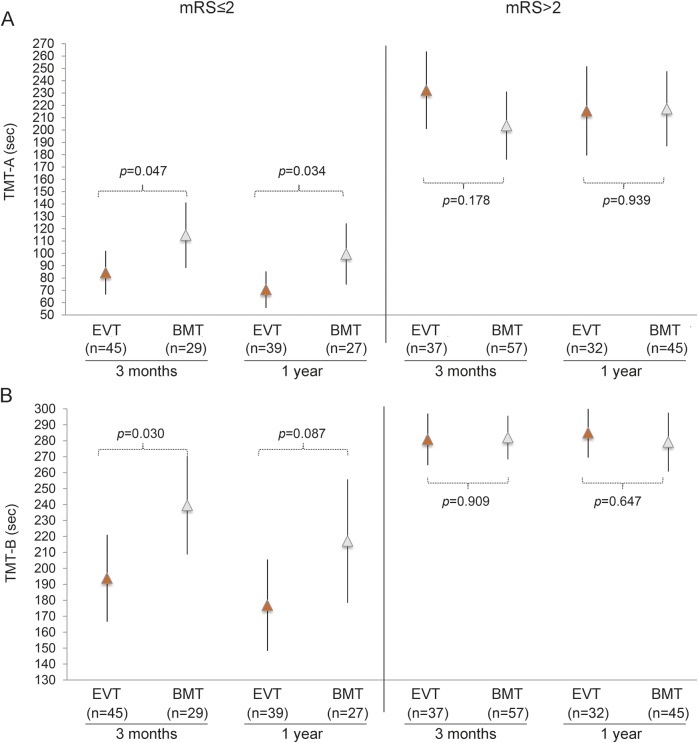
Among those patients who reached functional independency at 3 months (mRS score ≤2), all except one completed TMT-A in the requested time in both treatment arms. TMT-B was completed in due time by ≈69% of patients in the thrombectomy arm and by only 48% of patients in the best medical treatment arm (table 3). Differences in TMT completion times in favor of endovascular treatment were significant only in those patients who achieved a good functional outcome (mRS score ≤2) (table 3 and figure 2) and remained significant after adjustment for aphasic symptoms and paresis of the right upper limb (among patients with an mRS score ≤2 at 3 months, only 9 had aphasic symptoms and only one patient had right hand paresis).
Table 3.
Trail Making Test performance in each treatment arm stratified by functional outcome (modified Rankin Scale score ≤2 and >2)
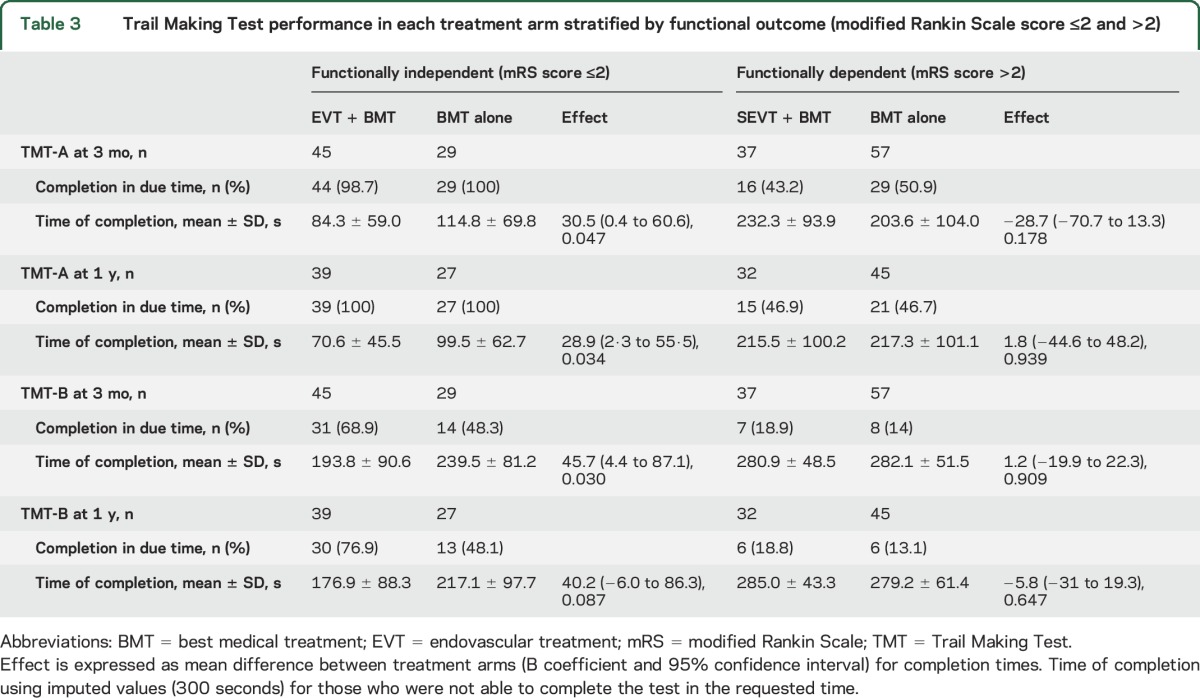
Figure 2. TMT-A (A) and TMT-B (B) completion times stratified by functional outcome (mRS score ≤2 and >2) in both treatment arms.
Vertical axis represents completion times (seconds). Arrows and lines represent mean and 95% confidence interval of the mean. Gray arrows indicate endovascular treatment (EVT); white arrows indicate best medical treatment (BMT). p Value represents unadjusted mean difference (linear regression analysis) between treatment arms. mRS = modified Rankin Scale. TMT = Trail Making Test.
DISCUSSION
Our study demonstrates that in patients with acute stroke due to a proximal large vessel occlusion, treatment with thrombectomy improves TMT performance, a measure of cognitive functioning, at 3 months and 1 year after stroke. These findings are important because they demonstrate that the benefit derived from thrombectomy, shown so far to affect only disability and health-related quality of life, also encompasses the cognitive domain.
Because executive dysfunction is frequently found after stroke,1 we decided to evaluate this specific cognitive domain in REVASCAT. The TMT is one of the most commonly used neuropsychological tests. It is easy and fast to administer and measures multiple executive functions simultaneously: attention, processing speed, set shifting, visuospatial ability, and working memory.10 We found that the proportion of patients able to complete Part A was similar between treatment arms, although patients treated with thrombectomy were less prone to make errors than control patients in this specific test and required shorter times for completion. These findings suggest less pronounced differences in processing speed and more substantial differences in attention and visuospatial abilities between patients undergoing thrombectomy and patients assigned to medical treatment. Furthermore, thrombectomy improves the completion of Part B in the requested time, a more complex task related to cognitive flexibility.11 Regarding time used to complete the tests, linear regression analyses revealed significant differences in favor of thrombectomy in both tests. It is important to note that in our study, the percentage of completion of Part B in the requested time was low, so a high proportion of patients received the maximum time score (300 seconds). Although this is a usual practice in administering TMT, it masks performance variability among severely impaired patients who cannot complete the task. Therefore, some authors have tried to find scores that take into account not only time but also errors and correct moves.12 Because errors and moves were not collected in those patients who did not complete the test in the required time, we were not able to investigate this aspect of cognitive performance, which should be incorporated into future trials assessing the effect of reperfusion on the TMT.
In line with previous reports, we found that infarct volume was negatively correlated with executive functioning.13 Other relevant stroke outcome measures such as health-related quality of life and disability status were significantly and directly correlated with poor cognitive status.
In the stratified analysis by functional independence status, differences in TMT performance between treatment arms were significant only in functionally independent patients (mRS score ≤2). These findings may therefore justify the evaluation of cognitive outcomes in future stroke trials because they seem to provide relevant information beyond the widely used mRS.
There are several limitations to this study. First, REVASCAT was not sized to guarantee power for secondary objectives, and the present study may be underpowered. Although established dementia was an exclusion criterion for enrollment in REVASCAT (because patients enrolled had to score 0 to 1 on the mRS), we do not have precise information on prestroke cognitive functioning of included patients; therefore, we cannot ensure that the only factor influencing TMT performance was stroke itself. Analyses were adjusted for other covariates that may influence understanding and ability to perform cognitive tasks (e.g., age, stroke severity, and side of stroke). However, data on education level, a factor known to significantly affect cognitive function, were not collected. We do not believe that this limitation may alter conclusions because education level accounted only for 3% and 6% of the variance of TMT-A and TMT-B in a sample of 911 healthy volunteers.14 In addition, symptoms of depression/anxiety were collected only indirectly by EQ-5D and were not included in multivariable models. Although we cannot ensure the correct balance of prestroke cognitive status, educational level, and depressive symptoms between treatment arms, the randomized nature of the REVASCAT trial makes it at least possible. Finally, cognitive outcome was evaluated with only 2 cognitive tests focused on executive functioning; therefore, we cannot draw conclusions on other cognitive domains. We chose the TMT to evaluate cognition in REVASCAT because of its simplicity in administration and its established correlation with multiple cognitive dimensions. Furthermore, consensus on optimal measurement tools for poststroke cognitive impairment is lacking. Further studies assessing the validity and reliability of different tools in the evaluation of poststroke cognitive impairment should be performed. Major strengths of this study include its prospective nature, the randomized cohort of patients studied, and the high rate of patients available for follow-up.
GLOSSARY
- EQ-5D
EuroQoL Group 5-Dimension Self-Report Questionnaire
- mRS
modified Rankin Scale
- REVASCAT
Endovascular Revascularization With Solitaire Device Versus Best Medical Therapy in Anterior Circulation Stroke Within 8 Hours
- TMT
Trail Making Test
AUTHOR CONTRIBUTIONS
E. López-Cancio wrote the first draft of the manuscript. N. Cerdá performed and E. Cobo revised the statistical analyses. C. Cáceres contributed to the interpretation of neuropsychological results. M. Jiménez, M. Gomis, M. Hernández-Pérez, P. Cardona, B. Lara, L. Llull, A. Renú, S. Boned, and M. Muchada contributed to patient enrollment, data collection, and outcome assessments. A. Dávalos and T.G Jovin are the principal investigators of REVASCAT trial and reviewed the present manuscript for main intellectual content.
STUDY FUNDING
REVASCAT was funded by a local independent Catalan institution (Fundació Ictus Malaltia Vascular, www.fundacioictus.com/es) by means of an unrestricted grant from the manufacturer of the device (Covidien, now Medtronic), which was not involved in the conduct, analysis, or report of the trial. This project has been partially supported by a grant from the Spanish Ministry of Health cofinanced by FEDER (Fondo Europeo de Desarrollo Regional) (Instituto de Salud Carlos III, RETICS [Red Temática de Investigación en Salud]-INVICTUS [Investigación en ictus] RD 12/0014/008) and a grant from the Generalitat de Catalunya (SGR 464/2014) to the GRBIO (Group de Recerca en Bioestadística i Bioinformàtica) group. The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
DISCLOSURE
E. López-Cancio reports no disclosures relevant to the manuscript. T.G. Jovin received a grant, nonfinancial, from Fundació Ictus Malaltia Vascular and modest honoraria from Silk Road (consultant), Medtronic and Stryker Neurovascular (consultant/advisory board), and J&J and Neuravi (consultant). E. Cobo received a nonfinancial research grant from Generalitat de Catalunya (research group GRBIO) and modest honoraria from Fundació Ictus Malaltia Vascular. Institutional conflict of interest: Barcelona-Tech received a grant for statistical sequential design of the REVASCAT trial. N. Cerdá, M. Jiménez, M. Gomis, M. Hernández-Pérez, C. Cáceres, P. Cardona, B. Lara, A. Renú, L. Llull, S. Boned, and M. Muchada report no disclosures relevant to the manuscript. A. Dávalos received significant research grant from Covidien and modest honoraria from Covidien (lectures). Go to Neurology.org for full disclosures.
REFERENCES
- 1.Weinstein G, Preis SR, Beiser AS, et al. Cognitive performance after stroke: the Framingham Heart Study. Int J Stroke 2014;9(suppl A100):48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel MD, Coshall C, Rudd AG, Wolfe CD. Cognitive impairment after stroke: clinical determinants and its associations with long-term stroke outcomes. J Am Geriatr Soc 2002;50:700–706. [DOI] [PubMed] [Google Scholar]
- 3.Knopman DS, Roberts RO, Geda YE, et al. Association of prior stroke with cognitive function and cognitive impairment: a population-based study. Arch Neurol 2009;66:614–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jokinen H, Melkas S, Ylikoski R, et al. Post-stroke cognitive impairment is common even after successful clinical recovery. Eur J Neurol 2015;22:1288–1294. [DOI] [PubMed] [Google Scholar]
- 5.Nys GM, van Zandvoort MJ, de Kort PL, et al. The prognostic value of domain-specific cognitive abilities in acute first-ever stroke. Neurology 2005;64:821–827. [DOI] [PubMed] [Google Scholar]
- 6.Hajjar K, Fulton RL, Diener HC, Lees KR; VISTA Collaborators. Does the cognitive measure Cog-4 show improvement among patients treated with thrombolysis after acute stroke? Int J Stroke 2013;8:652–656. [DOI] [PubMed] [Google Scholar]
- 7.Chen CJ, Ding D, Starke RM, et al. Endovascular vs medical management of acute ischemic stroke. Neurology 2015;85:1980–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015;372:2296–2306. [DOI] [PubMed] [Google Scholar]
- 9.Molina CA, Chamorro A, Rovira À, et al. REVASCAT: a randomized trial of revascularization with SOLITAIRE FR device vs. best medical therapy in the treatment of acute stroke due to anterior circulation large vessel occlusion presenting within eight-hours of symptom onset. Int J Stroke 2015;10:619–626. [DOI] [PubMed] [Google Scholar]
- 10.Sánchez-Cubillo I, Periáñez JA, Adrover-Roig D, et al. Construct validity of the Trail Making Test: role of task-switching, working memory, inhibition/interference control, and visuomotor abilities. J Int Neuropsychol Soc 2009;15:438–450. [DOI] [PubMed] [Google Scholar]
- 11.Kortte KB, Horner MD, Windham WK. The Trail Making Test, Part B: cognitive flexibility or ability to maintain set? Appl Neuropsychol 2002;9:106–109. [DOI] [PubMed] [Google Scholar]
- 12.Correia S, Ahern DC, Rabinowitz AR, et al. Lowering the floor on Trail Making Test Part B: psychometric evidence for a new scoring metric. Arch Clin Neuropsychol 2015;30:643–656. [DOI] [PubMed] [Google Scholar]
- 13.Muir RT, Lam B, Honjo K, et al. Trail Making Test elucidates neural substrates of specific poststroke executive dysfunctions. Stroke 2015;46:2755–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol 2004;19:203–214. [DOI] [PubMed] [Google Scholar]