Abstract
Teenage binge drinking is a common practice that has been shown to increase the risk for developing mood disorders in adulthood. The hypothalamo-pituitary-adrenal (HPA) axis is often dysfunctional in mood disorder patients, and animal models of adolescent binge alcohol exposure similarly show disordered HPA axis function, even after long periods of alcohol abstinence. Here, we sought to investigate the anxiety-like behavioral consequences of binge alcohol exposure in a Wistar rat model. Male rats were administered alcohol in a binge pattern during peri-puberty, and one month later, anxiety-like behaviors were measured using the elevated plus maze. A subset of the rats then underwent 30 minutes of restraint stress, and the anxiety-like behaviors were measured again. We observed an increase in risk assessment behaviors due to both adolescent binge alcohol exposure and restraint stress, but no differences in canonical anxiety-like behaviors. We also repeated the observation that adolescent binge alcohol induces long-term changes in HPA axis sensitivity. Therefore, we concluded that a history of peri-pubertal binge alcohol exposure subtly alters the behavioral response to subsequent acute psychological stress during adulthood, which may over time contribute to the development of mood disorders. This relatively pragmatic animal model represents a more clinically relevant tool in understanding the molecular mechanisms underlying the long-term effects of adolescent binge drinking.
Keywords: Alcohol, Puberty, Stress, Behavior
1 Introduction
Teenage binge drinking is an increasing public health concern in the United States and many other developed countries worldwide. In the United States, the costs associated with underage drinking totaled $24.3 billion in 2010 (Sacks et al., 2015), and in 2015, 46.7% of high school seniors reported having been drunk at some point in their lives (Johnston et al., 2016). Because adolescence is a critical period of brain development, teenage binge alcohol consumption has the potential to produce long-lasting effects on health and behavior beyond the immediate effects of being acutely intoxicated. Teenagers who drink alcohol have region-specific cortical thinning and white matter disorganization (Luciana et al., 2013; Wilson et al., 2015). In addition to these anatomical changes, adolescent binge drinking increases the risk for developing alcohol dependence and other mental health disorders during adulthood (McCambridge et al., 2011; Rose et al., 2014; Viner and Taylor, 2007).
The hypothalamo-pituitary-adrenal (HPA) axis comprises the major neuroendocrine stress response system. Following exposure to an acute psychological or physical stressor, parvocellular neurons in the hypothalamic paraventricular nucleus (PVN) are activated, releasing corticotrophin releasing factor (CRF) and arginine vasopressin (AVP) into the portal system of the anterior pituitary gland. This stimulates the release of adrenocorticotrophic hormone (ACTH) into the systemic circulation, which acts on the adrenal cortex to release glucocorticoids into the blood. Glucocorticoids have a myriad of effects throughout the body, including the regulation of glucose homeostasis and immune function, as well as exerting negative feedback on the hypothalamus and pituitary gland to decrease further release of CRF, AVP, and ACTH (Smith and Vale, 2006).
The HPA axis undergoes important changes during puberty and the function of this axis can be permanently altered by exposure to a stressor during this developmental timeframe (Romeo et al., 2006; Sisk and Zehr, 2005). Heavy-drinking youth show greater volume decreases in the ventral diencephalon (the brain region which contains the hypothalamus) than age-matched controls (Squeglia et al., 2015, 2014). HPA axis dysfunction has also been reported among female college students who engage in problematic drinking (Wemm et al., 2013). Importantly, dysfunction of the HPA axis is associated with many psychiatric disorders, including anxiety (Naughton et al., 2014), raising the possibility that adolescent binge drinking might increase the risk of mental health disorders by altering the function of the HPA axis.
To examine the causal effects of teenage binge drinking on HPA axis dysfunction, our lab and others have established animal models of adolescent binge alcohol exposure (Allen et al., 2011; Przybycien-Szymanska et al., 2010). We have previously shown that our animal model of adolescent binge alcohol exposure (which utilizes male Wistar rats exposed to alcohol via oral gavage from post-natal day 37–44) results in long-term biochemical alterations of the HPA axis (Przybycien-Szymanska et al., 2011b). However, it was unknown if these biochemical changes translated to an altered behavioral phenotype. Here we tested the hypothesis that male Wistar rats exposed to our adolescent binge-pattern alcohol paradigm would exhibit increased anxiety-like behaviors in the elevated-plus maze after subsequent exposure to an acute psychological stressor during adulthood. Our data demonstrated that adolescent binge alcohol exposure increased risk assessment behaviors in adulthood when the animals were exposed to an acute mild psychological stressor; however there were no differences in baseline anxiety-like behavior in the absence of a stressor. Together, these data suggest that adolescent binge alcohol exposure could sensitize individuals to subsequent mild stressors and increase their risk of developing anxiety disorders as adults.
2 Methods
2.1 Ethics Statement
All animal protocols were approved by the Loyola University Medical Center Institutional Animal Care and Use Committee (IACUC) permit #2013034. All measures were taken to minimize animal numbers and suffering.
2.2 Animals
Male Wistar rats were purchased from Charles River Laboratories (Wilmington, MA) at weaning (post-natal day [PND] 25) and allowed to acclimate for 5 days after arrival. Animals were pair-housed on a 12:12 light/dark cycle with lights on at 7:00h. Food and water were available ad libitum.
2.3 Experimental Paradigm
2.3.1 Repeated binge alcohol exposure
After acclimation to the housing environment, beginning on PND 30, animals were handled for 5 min once per day for 7 days by the same individual, between 09:30 and 11:00 hrs. Pubertal binge ethanol (EtOH) treatments commenced on PND 37, which is defined as peri-puberty in this species (Ketelslegers et al., 1978; Södersten et al., 1977). Animals were randomly assigned to either 1) binge EtOH treated (n=20), or 2) water treated control (n=20) groups. The binge EtOH treated animals received 3g/kg ethanol (20% v/v in water) intragastrically (i.g.) via oral gavage once per day at 10:00h for 3 consecutive days, then an equivalent volume of water i.g. for 2 days, then an additional 3 days with EtOH. This once/day (total of 8-days) binge paradigm has been used previously to mimic the pattern of binge alcohol consumption in adolescents (Lauing et al., 2008; Przybycien-Szymanska et al., 2010). Our previous studies, and others, have demonstrated that this dose and method of EtOH delivery resulted in blood alcohol concentrations (BAC) between 150–180 mg/dL one hour after the last dose, and does not interfere with normal growth rates or feeding behavior (Prins et al., 2014; Przybycien-Szymanska et al., 2010; Walker and Ehlers, 2009). The water treated control group received 8 days of an equivalent volume of water i.g via oral gavage.
2.3.2 Acute stress paradigm
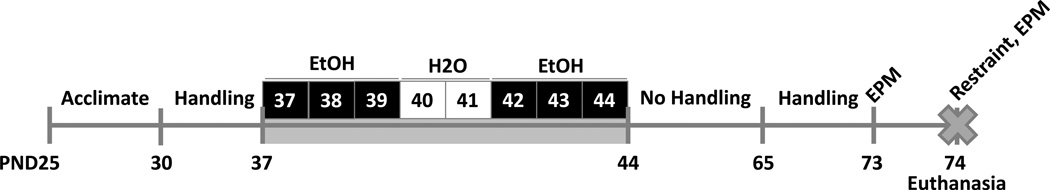
After pubertal binge alcohol treatments, both groups of animals were left undisturbed for 3 weeks (see Fig. 1). At the end of this 3-week period, the animals were again handled 5 min once per day for 7 days, as described above. At 10:00h on PND 73, prior to further manipulation, animals were given a trial test in the elevated plus maze (EPM) to establish a baseline level of anxiety-like behavior. Then, animals within each group were randomly assigned to either a) a restraint stress group (n=10 per group), or b) an unstressed control group (n=10 per group). Next, on PND 74 at 09:30h, animals in the restraint stress group were placed in a plastic rodent restraint tube (Stoelting Co. #51335) inside a fresh cage for 30 min, then 5 min after being removed from the restraint tube, the rats were tested in the EPM again, and 5 min after ending the EPM test, the animals were euthanized. It has been previously demonstrated that plasma corticosterone (CORT) levels reach a peak after 30 min inside a plastic restraint tube (Cole et al., 2000). Animals in the unstressed control group were placed singly in a fresh cage for 35 min, then tested in the EPM again, and 5 min after ending the EPM test, the animals were euthanized.
Figure 1. Experimental paradigm.
Beginning on PND 30, animals were handled for 5 min once per day for 7 days. Pubertal binge ethanol (EtOH) treatments commenced on PND 37. Animals were randomly assigned to either binge EtOH treated or water treated control groups. The binge EtOH treated animals received 3g/kg ethanol i.g. via oral gavage once per day for 3 consecutive days, then an equivalent volume of water i.g. for 2 days, then an additional 3 days with EtOH. The water treated control group received 8 days of an equivalent volume of water i.g. After pubertal treatments, both groups of animals were left undisturbed for 3 weeks, then handled 5 min once per day for 7 days. On PND 73, animals were tested in the elevated plus maze (EPM) to establish a baseline level of anxiety-like behavior. Then, animals within each group were randomly assigned to either a restraint stress group or an unstressed control group. On PND 74, animals in the restraint stress group were placed in a plastic restrainer tube for 30 min, then 5 min after being removed from the restrainer tube, the rats were tested in the EPM again, and 5 min after ending the EPM test, the animals were euthanized. Animals in the unstressed control group were placed singly in a fresh cage for 35 min, then tested in the EPM again, and 5 min after ending the EPM test, the animals were euthanized.
2.4 Elevated Plus Maze Testing
Elevated Plus Maze testing was conducted with the Rat Elevated Plus Maze apparatus (Stoelting Co. #60240) and recorded using a video camera and ANY-maze software (Stoelting Co.). Testing was conducted in a dimly lit room (~5 lux) with white noise generated by a HoMedics Sound Spa Relaxation machine (~70dB, equivalent to the white noise generated by the HVAC system in the animal housing room). Rats were placed singly in the center of the maze facing an open arm by a female experimenter, marking the beginning of the test period. The rat was then allowed 5 min to explore the maze freely, after which the recording stopped automatically, and then the rat was returned to its cage. Later, both spatiotemporal and ethological analyses were conducted using the recorded videos and ANY-maze.
Elevated plus maze analysis
All parameters were analyzed from the video recordings by an investigator blinded to the animal treatment paradigms. For the spatiotemporal analysis, the maze was divided into 3 zones, specified using the ANY-maze software: the open arms, the closed arms, and the intersection. In order to be considered in the open or closed arms, at least 80% of the rat’s body surface area had to be inside that zone (consistent with the “four-paw rule”). The rat was considered to be in the intersection if it was not considered to be in either the open or closed arms. The amount of time spent in any given zone was divided by the total test duration to calculate the percentage of time spent in the zone. The total distance travelled and average speed were also measured to consider differences in overall locomotor activity. For the ethological behavioral analyses, scoring of head dips, stretched attend postures, and rearing behaviors were manually recorded by a blinded trained observer. A head dip was defined as the rat extending its head over the edge of the maze and down toward the floor. A stretched attend posture was defined as the rat extending forward with its front paws, then retracting back to its original position. A rearing was defined as the rat sitting back on its hind paws and elevating its front paws, moving vertically.
2.5 Tissue Processing
Animals were euthanized humanely by rapid decapitation after anesthesia with inhaled isoflurane. Trunk blood was immediately collected in heparinized tubes on ice, centrifuged at 3000 rpm at 4°C for 10 min., then the plasma was stored at −20°C. Brains were rapidly dissected and flash frozen in isopentane chilled with dry ice, then stored at −80°C. Plasma CORT levels were measured using a Corticosterone ELISA Kit (Enzo Life Sciences #ADI-900-097), according to manufacturer instructions. Microdissection of the paraventricular nucleus of the hypothalamus (PVN) and ventral hippocampus (V.Hipp) was performed as previously described (Prins et al., 2014; Przybycien-Szymanska et al., 2011b). Briefly, brains were sectioned at 200µm using a Leica CM3050 S cryostat, then the specified brain regions were microdissected using a Palkovit’s brain punch tool (Stoelting Co.) and confirmed using The Rat Brain in Stereotaxic Coordinates, Fourth Edition Atlas (G. Paxinos and C. Watson). For the PVN, we microdissected 0.75 mm area on each side of the third ventricle between 0.8 mm and 2.12 mm posterior to Bregma, 8 mm below the top of the brain. For the V.Hipp, we microdissected between 3 mm and 6 mm lateral to the midline, between 4.16 mm and 6.05 mm posterior to Bregma, 3 mm below the top of the brain and 2 mm above the bottom of the brain. Brain tissue punches were stored at −80°C; later, genomic DNA, RNA, and protein were isolated using an AllPrep DNA/RNA/Protein Mini Kit (Qiagen #80004), according to manufacturer instructions. This kit first removes genomic DNA by column centrifugation before the separation of RNA and protein.
2.6 RT-qPCR
Total RNA (250–350 ng) was reverse transcribed using the First-Strand Synthesis SuperMix for RT-qPCR (Invitrogen). cDNA products were treated with RNase H (Promega). PCR was performed in triplicate using iTaq™ Universal SYBR® Green Supermix and the following primers: rat AVP forward 5’-GGGCAGGTAGTTCTCCTCCT-3’, rat AVP reverse 5’-CACCTCTGCCTGCTACTTCC-3’; rat cFos forward 5’-AGCATGGGCTCCCCTGTCA-3’, rat cFos reverse 5’-GAGACCAGAGTGGGCTGCA-3’; rat CRF forward 5’-GAGAAAGGGGAAAGGCAAAG-3’, rat CRF reverse 5’-ATCAGAATCGGCTGAGGTTG-3’; rat GR forward 5’-CACCCATGATCCTGTCAGTG-3’, rat GR reverse 5’-AAAGCCTCCCTCTGCTAACC-3’; rat HPRT forward 5’-GTTCTTTGCTGACCTGCTGGAT-3’, rat HPRT reverse 5’-CCAACACTTCGAGAGGTCCTTT-3’. All primer sets were intron-spanning, with the exception of the GR primer set. RT-negative control reactions were performed to ensure that there was no interfering genomic DNA contamination. All samples were normalized to the hypoxanthine guanine phosphoribosyl transferase 1 (HPRT) housekeeping gene, as it is not altered by EtOH treatment (Przybycien-Szymanska et al., 2010), and transcript fold changes were calculated using the ΔΔCt method (Livak and Schmittgen, 2001).
2.7 Statistical Analysis
Statistical analyses were performed using GraphPad Prism 7. The EPM pre-test data (baseline, trial one) were analyzed using unpaired t-tests (n=20 per group). The biochemical (ELISA, qRT-PCR) and EPM post-test (after acute stressor, trial 2) data were analyzed by two-way ANOVA, with Sidak’s multiple comparisons post-hoc tests (n=10 per group, except PVN cFos mRNA, where n=7–9 per group due to sample exhaustion). In all cases, p<0.05 was considered significant.
3 Results
3.1 Adolescent binge alcohol exposure did not alter baseline anxiety-like behaviors in adulthood
Our previous studies demonstrated that adolescent binge alcohol exposure caused significant long-term biochemical alterations in genes regulating the physiological stress response, leading to the hypothesis that animals exposed to alcohol during adolescence might exhibit altered baseline anxiety-like behaviors as adults (Przybycien-Szymanska et al., 2011b). The first behavioral trial was conducted on an elevated plus maze following 4 weeks of alcohol abstinence (see Fig. 1). The data showed that there were no significant differences in the time spent in the closed arms, open arms, or intersection of the arms on the elevated plus maze between rats that were exposed to binge alcohol (or water) as adolescents (see Table 1). Likewise, there were no significant differences between treatment groups on the ethological parameters: number of stretched attend postures (SAPs), head dips, or rearings (see Table 1). Total distance covered in the maze and average speed of exploration were also equivalent in the two treatment groups (see Table 1).
Table 1. Elevated plus maze trial 1 results.
Adolescent alcohol exposure did not significantly change any parameters measured in the elevated plus maze on PND73, prior to further manipulation. n=20 per group.
| Dependent variable (units) | H2O mean (SEM) | ETOH mean (SEM) | 95% CI |
|---|---|---|---|
| Time in closed arms (%) | 38.53 (3.735) | 38.27 (2.577) | −9.446 to 8.926 |
| Time in open arms (%) | 35.33 (4.572) | 33.07 (3.463) | −13.87 to 9.347 |
| Time in intersection (%) | 25.4 (1.682) | 28.31 (1.648) | −1.861 to 7.673 |
| Stretched attend postures (count) | 19.2 (1.417) | 19.15 (1.022) | −3.587 to 3.487 |
| Head dips (count) | 33.6 (3.646) | 38.35 (3.75) | −5.838 to 15.34 |
| Rearing (count) | 23.3 (2.442) | 24.9 (1.304) | −4.004 to 7.204 |
| Total distance travelled (m) | 13.93 (1.148) | 14.66 (0.6127) | −1.911 to 3.358 |
| Average speed (m/s) | 0.049 (0.002967) | 0.04885 (0.002023) | −0.00742 to 0.00712 |
3.2 Adolescent binge alcohol exposure increased risk assessment behaviors in adulthood following an acute mild psychological stressor
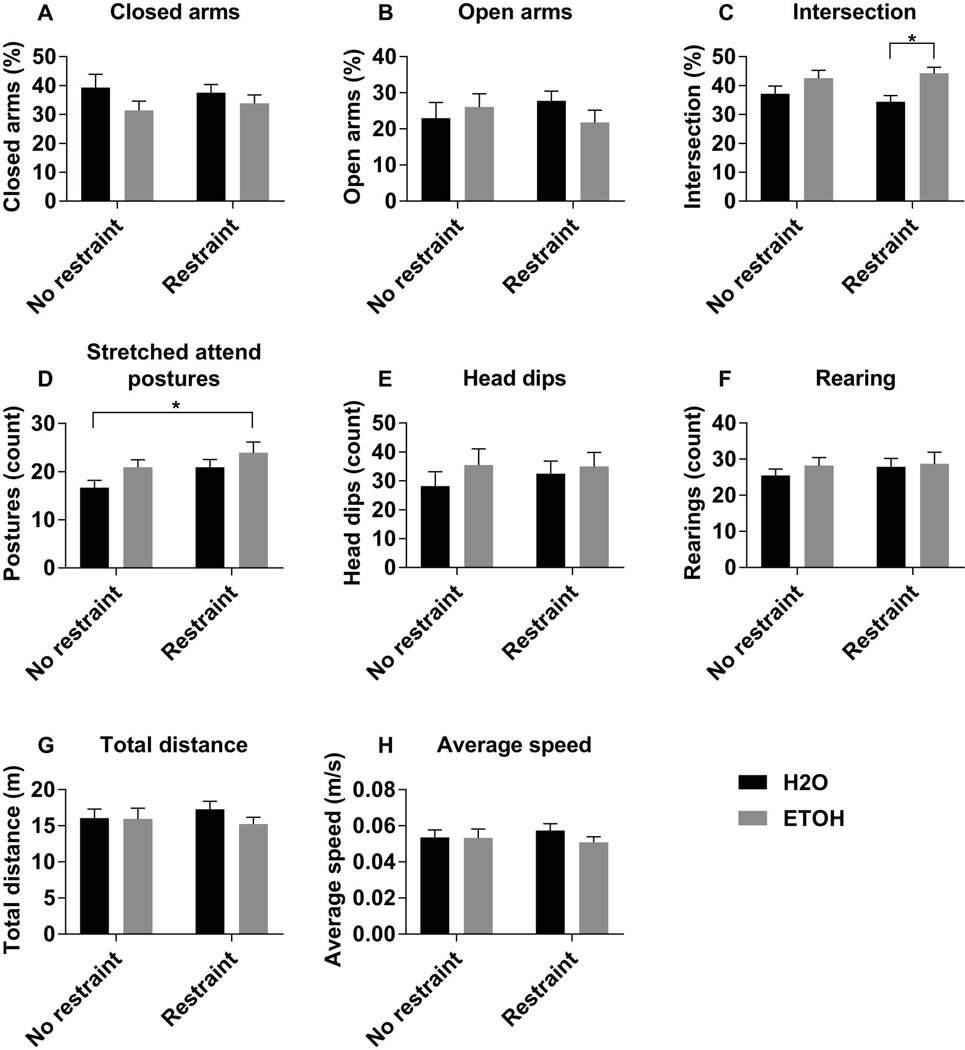
Next, we tested whether prior alcohol exposure altered the behavioral response to a mild psychological stressor (30 min. restraint) in adulthood. Similar to the previous experiment, there were no differences in spatiotemporal or ethological parameters between treatment groups in the unstressed condition (Fig. 2). However, animals subjected to adolescent alcohol exposure spent significantly more time in the intersection of the four arms following a mild psychological stressor (Fig. 2C, Table 2). Further, there were statistically significant main effects of both adolescent alcohol exposure and of acute restraint stress on the number of SAPs, such that the rats exposed to binge alcohol as adolescents and then subjected to restraint stress just prior to entering the maze exhibited the highest number of SAPs (Fig. 2D, Table 2). There were no statistically significant differences between treatment groups on the number of head dips or rearings (Fig. 2E, F). Importantly, there were no significant differences between treatment groups on the distance traveled and average speed (Fig. 2G, H), indicating that any significant differences were not simply due to an increase in general motor activity.
Figure 2. Anxiety-like behavior following restraint stress.
The percent of time in the closed arms (A), open arms (B), and intersection (C) of the elevated plus maze was calculated by dividing the amount of time spent in the given zone by the total duration of the test (300s). The number of stretched attend postures (D), head dips (E), and rearing behaviors (F), were scored by a blinded observer. The total distance travelled (G), and the average speed (H) were used as indicators of overall motor activity. Data are expressed as mean ± SEM, and were analyzed by two-way ANOVA with Sidak’s multiple comparisons post-hoc tests, in which p<0.05 was considered significant. There was a significant main effect of gavage on time spent in the intersection of the arms (C), and significant main effects of gavage and restraint on stretched attend postures (D). Asterisk (*) denotes significant pairwise comparisons between indicated groups.
Table 2. Statistical analysis for elevated plus maze trial 2 and biochemical parameters.
F and p-values from two-way ANOVA on EPM results at PND74, qRT-PCR, and ELISA.
| Dependent variable | Interaction: Gavage × Restraint |
Main effect: Gavage |
Main effect: Restraint |
|---|---|---|---|
| Time in closed arms | NS | NS | NS |
| Time in open arms | NS | NS | NS |
| Time in intersection | NS | F(1,36)=9.47, p=0.0040 | NS |
| Stretched attend postures | NS | F(1,36)=4.193, p=0.0479 | F(1,36)=4.193, p=0.0479 |
| Head dips | NS | NS | NS |
| Rearing | NS | NS | NS |
| Total distance travelled | NS | NS | NS |
| Average speed | NS | NS | NS |
| V. hipp CRF | NS | NS | NS |
| V. hipp cFos | NS | NS | F(1,36)=6.059, p=0.0188 |
| PVN CRF | NS | NS | F(1,36)=6.102, p=0.0184 |
| PVN cFos | NS | NS | F(1,28)=36.21, p<0.0001 |
| PVN AVP | NS | NS, F(1,36)=3.999, p=0.0531 | NS |
| CORT | NS | F(1,36)=5.223, p=0.0283 | F(1,36)=70.98, p<0.0001 |
| V. hipp GR | NS | NS | F(1,36)=5.234, p=0.0281 |
| PVN GR | NS | NS | NS |
3.3 Adolescent alcohol exposure altered neuroendocrine regulators of the HPA axis in adulthood
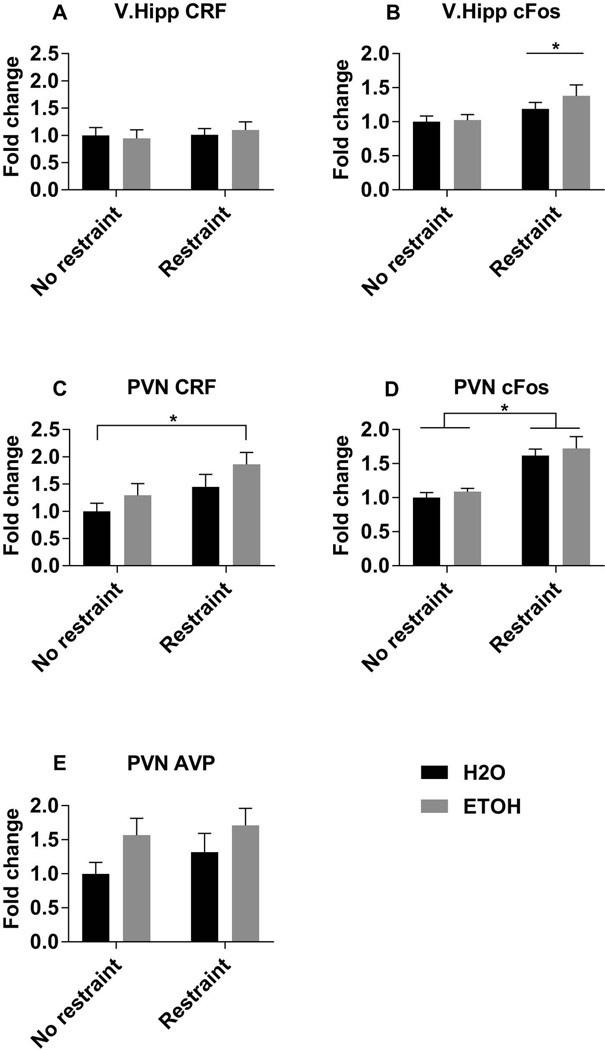
We next tested whether prior adolescent alcohol exposure altered key neuroendocrine regulators of the HPA axis following an acute stressor in adulthood. The ventral hippocampus (V.Hipp) has afferent projections to the paraventricular nucleus (PVN), which is the main hypothalamic nuclei containing CRF and AVP-expressing neurons that project to the median eminence (Smith and Vale, 2006). In addition to measuring CRF and AVP levels, we also measured cFos transcript levels as a proxy for general neuronal activity in these brain areas. In the V.Hipp, neither prior adolescent alcohol exposure nor restraint stress altered CRF mRNA (Fig. 3A). Restraint stress did significantly increase CRF mRNA levels in the PVN of both alcohol exposed and control animals, as expected, but there was not a statistically significant effect of adolescent alcohol exposure (Fig. 3C, Table 2). Restraint stress also significantly increased cFos expression in both the V.Hipp and the PVN, but again alcohol did not significantly affect cFos expression in either of these brain regions (Fig. 3 B,D; Table 2). Interestingly, adolescent alcohol exposure (but not restraint stress) increased AVP mRNA in the PVN (Fig. 3E, Table 2).
Figure 3. Regional levels of CRF, AVP, and cFos transcripts.
mRNA levels of corticotropin releasing factor (A and C), cFos (B and D), and arginine vasopressin (E), in the ventral hippocampus (A and B) and paraventricular nucleus of the hypothalamus (C–E), measured by RT-qPCR relative to the unrestrained H2O group. Data are expressed as mean ± SEM, and were analyzed by two-way ANOVA with Sidak’s multiple comparisons post-hoc tests, in which p<0.05 was considered significant. There were significant main effects of restraint on V.hipp cFos (B), PVN CRF (C), and PVN cFos (D) levels. Asterisk (*) denotes significant main effect of restraint in panel B and significant pairwise comparisons between indicated groups in panels C and D.
3.4 Adolescent alcohol exposure altered circulating glucocorticoid levels, but not glucocorticoid receptor transcript levels
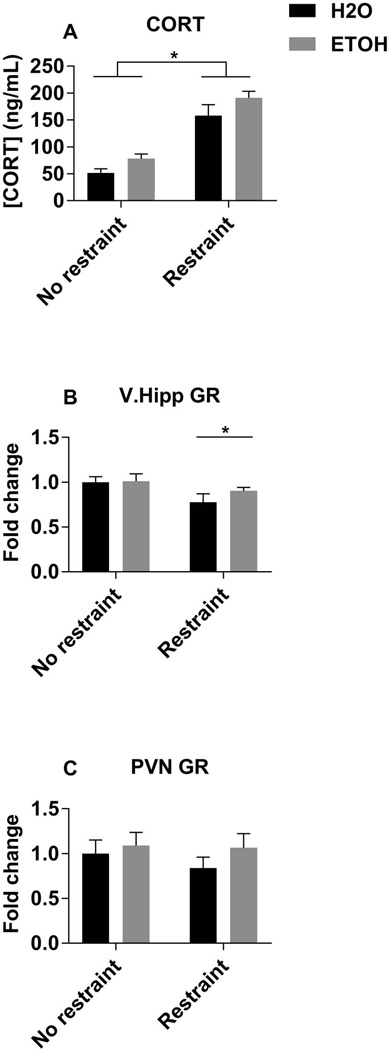
Finally, we measured plasma corticosterone (CORT) levels, as well as glucocorticoid receptor (GR) levels in the V.Hipp and PVN, since it is known that CORT can downregulate GR expression (Yuan et al., 2016). Plasma CORT levels were significantly higher following an acute stressor in both groups, such that alcohol and acute restraint stress increased CORT levels (Fig 4A, Table 2). Restraint stress significantly decreased GR mRNA levels in the V.Hipp; however, the animals with adolescent alcohol exposure exhibited a smaller decrease compared to unrestrained controls than the animals exposed to water gavage during adolescence (Fig. 4B, Table 2). There were no statistically significant differences in GR mRNA in the PVN (Fig. 4C).
Figure 4. Plasma CORT and brain region expression of GR.
Circulating plasma levels of corticosterone (A), measured by ELISA. mRNA levels of glucocorticoid receptor in the ventral hippocampus (B), and paraventricular nucleus of the hypothalamus (C), measured by RT-qPCR relative to the unrestrained H2O group. Data are expressed as mean ± SEM, and were analyzed by two-way ANOVA with Sidak’s multiple comparisons post-hoc tests, in which p<0.05 was considered significant. There were significant main effects of gavage and restraint on plasma CORT (A), and a significant main effect of restraint on V. hipp GR mRNA (B). Asterisk (*) denotes significant pairwise comparisons between indicated groups in panel A and significant main effect of restrain in panel B.
4 Discussion
Binge drinking is a common behavior among teenagers and alcohol exposure during this developmental period is associated with long-lasting structural and functional changes within the brain (Johnston et al., 2016; Luciana et al., 2013; Wemm et al., 2013). Here, we have demonstrated that early adolescent alcohol exposure can alter risk assessment behaviors following a relatively mild psychological stressor in adulthood. Our paradigm is novel in that the animals were kept and tested in extremely low stress conditions (i.e. dim light, frequent handling by the same individual, soothing white noise), thereby eliminating a robust stress response when the animals were first exposed to the maze. Interestingly, prior adolescent alcohol exposure did not alter the EPM behavior or neuroendocrine parameters in these unstressed conditions, which is consistent with the idea that adolescent binge alcohol exposure does not necessarily cause all individuals to develop mental health disorders. Rather, what we have demonstrated is that adolescent alcohol exposure causes subtle changes in the way that mild stressors are perceived. Moreover, these changes correlated with an overall heightened (at baseline and after stress) HPA neuroendocrine profile. Taken together, these data suggest that adolescent alcohol exposure can predispose individuals to the development of anxiety disorders in adulthood by changing the way mild stressors are perceived and assessed.
Our data demonstrated that both prior adolescent binge alcohol exposure and acute restraint stress in adulthood increased stretched attend postures (SAPs), a risk assessment behavior, in the elevated plus maze immediately following restraint. SAPs differ from conventional spatiotemporal measures of anxiety-like behaviors in both their sensitivity to one-trial tolerance and pharmacological modulation by benzodiazepines (Albrechet-Souza et al., 2007). SAPs have been shown to be positively correlated with circulating glucocorticoid levels, increase with administration of exogenous corticosterone, and decrease after a pharmacological blockade of glucocorticoid synthesis with metyrapone (Mikics et al., 2005; Rodgers et al., 1999). Consistent with those studies, our data revealed that adolescent alcohol exposure and restraint stress increased plasma CORT levels. Therefore, it is likely to predict that the hormonal abnormalities induced by adolescent binge alcohol exposure underlie the behavioral changes we observed.
As expected, restraint stress elevated the levels of many HPA axis effectors, including CRF transcript levels in the PVN and plasma CORT levels, as well as cFos transcript levels in the V.Hipp and PVN, which indicates a higher level of neuronal activity in these areas. Interestingly, although a history of adolescent alcohol exposure did not increase CRF or cFos transcript levels in either brain region studied, it did increase the levels of AVP transcript in the PVN. Because AVP released from the parvocellular neurons of the PVN acts to potentiate the effects of CRF on ACTH secretion (Smith and Vale, 2006), it is possible that alcohol’s effects on AVP contribute to the increase in plasma CORT observed due to adolescent alcohol exposure. In this study, we cannot exclude the possibility that alcohol increases AVP expression in the magnocellular PVN neurons, but previous work from our lab has demonstrated that AVP expression in the supraoptic nucleus of the hypothalamus (SON) is not changed by this binge alcohol administration paradigm, suggesting that the increase in AVP observed in the PVN is contributing more to the neuroendocrine stress response than to osmoregulation (Przybycien-Szymanska et al., 2010).
Furthermore, although both adolescent alcohol exposure and restraint stress increased plasma CORT levels, we observed no change in the levels of GR transcript in the PVN, and only observed an effect of restraint (but not adolescent alcohol) on GR transcript in the V.Hipp, suggesting that the negative feedback of glucocorticoids on the stress axis is impaired by adolescent alcohol exposure. This is consistent with our previous observations in vivo (Przybycien-Szymanska et al., 2011b), and in vitro, in which we observed that alcohol inhibits GR binding to the CRF promoter in IVB cells, which are derived from rat PVN (Przybycien-Szymanska et al., 2011a).
One unexpected observation we made was that rats exposed to binge pattern alcohol as adolescents spent more time in the intersection of the EPM arms when they were tested a second time in the maze, but not upon the first exposure to the maze. To our knowledge, this is the first publication to report a change in the amount of time spent in the intersection of the EPM without a significant change in open or closed arm time, so we can only speculate about the relevance of this observation. Because the adolescent alcohol exposure also increased SAPs, a known risk assessment behavior, it is possible that this increased amount of time spent in the intersection of the maze is also a type of risk assessment. In regards to this phenomenon only occurring upon the second exposure to the maze, it has been shown that repeated EPM trials can produce different behavioral observations with different susceptibilities to pharmacological treatments, likely due to learning effects or an emotional shift in the animal upon re-exposure to the maze (Albrechet-Souza et al., 2007). Therefore, an alternative explanation for this behavior is that the rats exposed to alcohol as adolescents did not remember their prior exposure to the EPM. Indeed, adolescent intermittent alcohol exposure has been shown to induce learning deficits in a conditional discrimination task (Pascual et al., 2007), as well as deficits in spatial working memory (Schulteis et al., 2008). Further behavioral studies are required to determine whether the observed differences in the amount of time spent in the intersection of the maze between rats exposed to alcohol as adolescents versus controls on repeated trials in the EPM are due to changes in learning abilities or the emotional state of the animal.To better examine the causal effects of adolescent binge alcohol consumption on the HPA axis and anxiety behavior, many different animal studies have been conducted with varying results. Our lab and others have established animal models demonstrating that adolescent binge alcohol exposure does indeed induce long-term effects on the regulation of the HPA axis (Allen et al., 2011; Przybycien-Szymanska et al., 2011a). However, observations that adolescent binge alcohol exposure induces changes in anxiety-like behaviors seem to vary based on the specific animal model used. For example, adolescent Sprague-Dawley rats that are trained to self-administer alcohol exhibit increased immobility in the open field test, which is indicative of anxiety-like behavior (Briones and Woods, 2013). Likewise, Sprague-Dawley rats that were given 2g/kg of ethanol i.p. from PND28–41 in a 2-day on, 2-day off fashion demonstrated increased anxiety-like behaviors in both the light-dark box and EPM tasks as adults (Pandey et al., 2015). Conversely, C57BL/6 mice that were given 5 g/kg ethanol i.p. for 10 days during adolescence (PND28–37) did not exhibit increased anxiety-like behaviors in the EPM or open field test (Coleman et al., 2011). These studies highlight that the effects of adolescent alcohol exposure on anxiety behavior might not be the same across strains and species. Furthermore, the timing, dosage, and route of alcohol administration and the conditions under which the anxiety behaviors are tested are also important considerations in interpreting the results.
It is therefore interesting and important that our model of adolescent binge alcohol exposure results in clear biological changes to the HPA axis, but a subtler display of increased risk assessment behaviors, as opposed to a more obvious anxiety-like phenotype. Indeed, in humans, risk assessment in the form of rumination is highly associated with anxiety (Blanchard et al., 2011), and HPA axis dysfunction is observed across many mood disorders (Naughton et al., 2014), underpinning the relevance of our observations in this animal model. Because the rats in this study were exposed to alcohol during a shorter, distinct developmental time period (peri-puberty) and were tested under less stressful conditions (i.e. dimly lit, in the presence of familiar experimenters), we believe this paradigm is applicable to a wider proportion of the human population, who may not display clinical symptoms of anxiety or depression, but are at a higher risk for developing a mood disorder under further psychosocial pressure.
Overall, this study contributes vital information regarding the impact of adolescent binge alcohol exposure on subsequent behavioral responses to a stressful stimulus in a physiologically relevant rat model. While our data did not reveal a canonical anxiety-like phenotype in the young adult rats that had been previously exposed to binge alcohol as adolescents, we did observe increased risk assessment behaviors and HPA axis hypersensitivity among those rats. We believe this is reflective of the observation among human patients suggesting that a history of teenage binge drinking contributes to an increased risk for mood disorders (McCambridge et al., 2011; Rose et al., 2014; Viner and Taylor, 2007). Therefore, we will continue to utilize this animal model as a tool to study the molecular mechanisms by which adolescent alcohol exposure contributes to the development of psychiatric disease.
Highlights.
Adolescent binge alcohol and restraint stress increased risk assessment behaviors
History of binge alcohol did not increase canonical anxiety-like behaviors
Both history of binge alcohol and restraint stress increased plasma CORT
Acknowledgments
We thank Dr. James Sinacore for assistance with statistical analysis.
This study was funded by the National Institutes of Health, National Institute on Alcohol Abuse and Alcoholism (NIH RO1 AA021517 and NIH T32 AA013527).
Role of Funding Source
The funding agency had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions
Audrey Torcaso: designed experiments, performed procedures, analyzed data, wrote manuscript.
AnnaDorothea Asimes: performed procedures, edited manuscript
Margaret Meagher: analyzed data, edited manuscript
Toni R. Pak: designed experiments, analyzed data, wrote and edited manuscript
Conflict of Interest
The authors AT, AA, MM, and TP have no conflicts of interest.
References
- Albrechet-Souza L, Cristina de Carvalho M, Rodrigues Franci C, Brandão ML. Increases in plasma corticosterone and stretched-attend postures in rats naive and previously exposed to the elevated plus-maze are sensitive to the anxiolytic-like effects of midazolam. Horm. Behav. 2007;52:267–273. doi: 10.1016/j.yhbeh.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Allen CD, Lee S, Koob GF, Rivier C. Immediate and prolonged effects of alcohol exposure on the activity of the hypothalamic-pituitary-adrenal axis in adult and adolescent rats. Brain. Behav. Immun. 2011;25(Suppl 1):S50–S60. doi: 10.1016/j.bbi.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard DC, Griebel G, Pobbe R, Blanchard RJ. Risk assessment as an evolved threat detection and analysis process. Neurosci. Biobehav. Rev. 2011;35:991–998. doi: 10.1016/j.neubiorev.2010.10.016. [DOI] [PubMed] [Google Scholar]
- Briones TL, Woods J. Chronic binge-like alcohol consumption in adolescence causes depression-like symptoms possibly mediated by the effects of BDNF on neurogenesis. Neuroscience. 2013;254:324–334. doi: 10.1016/j.neuroscience.2013.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MA, Kalman BA, Pace TW, Topczewski F, Lowrey MJ, Spencer RL. Selective blockade of the mineralocorticoid receptor impairs hypothalamic-pituitary-adrenal axis expression of habituation. J. Neuroendocrinol. 2000;12:1034–1042. doi: 10.1046/j.1365-2826.2000.00555.x. [DOI] [PubMed] [Google Scholar]
- Coleman LG, He J, Lee J, Styner M, Crews FT. Adolescent binge drinking alters adult brain neurotransmitter gene expression, behavior, brain regional volumes, and neurochemistry in mice. Alcohol. Clin. Exp. Res. 2011;35:671–688. doi: 10.1111/j.1530-0277.2010.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Miech RA, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use, 1975–2015: Overview, key findings on adolescent drug use. 2016 [Google Scholar]
- Ketelslegers JM, Hetzel WD, Sherins RJ, Catt KJ. Developmental changes in testicular gonadotropin receptors: plasma gonadotropins and plasma testosterone in the rat. Endocrinology. 1978;103:212–222. doi: 10.1210/endo-103-1-212. [DOI] [PubMed] [Google Scholar]
- Lauing K, Himes R, Rachwalski M, Strotman P, Callaci JJ. Binge alcohol treatment of adolescent rats followed by alcohol abstinence is associated with site-specific differences in bone loss and incomplete recovery of bone mass and strength. Alcohol. 2008;42:649–656. doi: 10.1016/j.alcohol.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Luciana M, Collins PF, Muetzel RL, Lim KO. Effects of alcohol use initiation on brain structure in typically developing adolescents. Am. J. Drug Alcohol Abuse. 2013;39:345–355. doi: 10.3109/00952990.2013.837057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCambridge J, McAlaney J, Rowe R. Adult consequences of late adolescent alcohol consumption: a systematic review of cohort studies. PLoS Med. 2011;8:e1000413. doi: 10.1371/journal.pmed.1000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikics E, Barsy B, Barsvári B, Haller J. Behavioral specificity of non-genomic glucocorticoid effects in rats: effects on risk assessment in the elevated plus-maze and the open-field. Horm. Behav. 2005;48:152–162. doi: 10.1016/j.yhbeh.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Naughton M, Dinan TG, Scott LV. Corticotropin-releasing hormone and the hypothalamic-pituitary-adrenal axis in psychiatric disease. Handb. Clin. Neurol. 2014;124:69–91. doi: 10.1016/B978-0-444-59602-4.00005-8. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Sakharkar AJ, Tang L, Zhang H. Potential role of adolescent alcohol exposure-induced amygdaloid histone modifications in anxiety and alcohol intake during adulthood. Neurobiol. Dis. 2015 doi: 10.1016/j.nbd.2015.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Blanco AM, Cauli O, Miñarro J, Guerri C. Intermittent ethanol exposure induces inflammatory brain damage and causes long-term behavioural alterations in adolescent rats. Eur. J. Neurosci. 2007;25:541–550. doi: 10.1111/j.1460-9568.2006.05298.x. [DOI] [PubMed] [Google Scholar]
- Prins SA, Przybycien-Szymanska MM, Rao YS, Pak TR. Long-term effects of peripubertal binge EtOH exposure on hippocampal microRNA expression in the rat. PLoS One. 2014;9:e83166. doi: 10.1371/journal.pone.0083166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybycien-Szymanska MM, Mott NN, Pak TR. Alcohol dysregulates corticotropin-releasing-hormone (CRH) promoter activity by interfering with the negative glucocorticoid response element (nGRE) PLoS One. 2011a;6:e26647. doi: 10.1371/journal.pone.0026647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybycien-Szymanska MM, Mott NN, Paul CR, Gillespie RA, Pak TR. Binge-pattern alcohol exposure during puberty induces long-term changes in HPA axis reactivity. PLoS One. 2011b;6:e18350. doi: 10.1371/journal.pone.0018350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybycien-Szymanska MM, Rao YS, Pak TR. Binge-pattern alcohol exposure during puberty induces sexually dimorphic changes in genes regulating the HPA axis. Am. J. Physiol. Endocrinol. Metab. 2010;298:E320–E328. doi: 10.1152/ajpendo.00615.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers RJ, Haller J, Holmes A, Halasz J, Walton TJ, Brain PF. Corticosterone response to the plus-maze: High correlation with risk assessment in rats and mice. Physiol. Behav. 1999;68:47–53. doi: 10.1016/s0031-9384(99)00140-7. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Bellani R, Karatsoreos IN, Chhua N, Vernov M, Conrad CD, McEwen BS. Stress history and pubertal development interact to shape hypothalamic-pituitary-adrenal axis plasticity. Endocrinology. 2006;147:1664–1674. doi: 10.1210/en.2005-1432. [DOI] [PubMed] [Google Scholar]
- Rose RJ, Winter T, Viken RJ, Kaprio J. Adolescent alcohol abuse and adverse adult outcomes: evaluating confounds with drinking-discordant twins. Alcohol. Clin. Exp. Res. 2014;38:2314–2321. doi: 10.1111/acer.12491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks JJ, Gonzales KR, Bouchery EE, Tomedi LE, Brewer RD. 2010 National and State Costs of Excessive Alcohol Consumption. Am. J. Prev. Med. 2015;49:e73–e79. doi: 10.1016/j.amepre.2015.05.031. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Archer C, Tapert SF, Frank LR. Intermittent binge alcohol exposure during the periadolescent period induces spatial working memory deficits in young adult rats. Alcohol. 2008;42:459–467. doi: 10.1016/j.alcohol.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Front. Neuroendocrinol. 2005 doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 2006;8:383–395. doi: 10.31887/DCNS.2006.8.4/ssmith. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Södersten P, Damassa DA, Smith ER. Sexual behavior in developing male rats. Horm. Behav. 1977;8:320–341. doi: 10.1016/0018-506x(77)90006-x. [DOI] [PubMed] [Google Scholar]
- Squeglia LM, Rinker DA, Bartsch H, Castro N, Chung Y, Dale AM, Jernigan TL, Tapert SF. Brain volume reductions in adolescent heavy drinkers. Dev. Cogn. Neurosci. 2014;9:117–125. doi: 10.1016/j.dcn.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Tapert SF, Sullivan EV, Jacobus J, Meloy MJ, Rohlfing T, Pfefferbaum A. Brain development in heavy-drinking adolescents. Am. J. Psychiatry. 2015;172:531–542. doi: 10.1176/appi.ajp.2015.14101249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viner RM, Taylor B. Adult outcomes of binge drinking in adolescence: findings from a UK national birth cohort. J. Epidemiol. Community Health. 2007;61:902–907. doi: 10.1136/jech.2005.038117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Ehlers CL. Age-related differences in the blood alcohol levels of Wistar rats. Pharmacol. Biochem. Behav. 2009;91:560–565. doi: 10.1016/j.pbb.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wemm S, Fanean A, Baker A, Blough ER, Mewaldt S, Bardi M. Problematic drinking and physiological responses among female college students. Alcohol. 2013;47:149–157. doi: 10.1016/j.alcohol.2012.12.006. [DOI] [PubMed] [Google Scholar]
- Wilson S, Malone SM, Thomas KM, Iacono WG. Adolescent drinking and brain morphometry: A co-twin control analysis. Dev. Cogn. Neurosci. 2015;16:130–138. doi: 10.1016/j.dcn.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S-Y, Liu J, Zhou J, Lu W, Zhou H-Y, Long L-H, Hu Z-L, Ni L, Wang Y, Chen J-G, Wang F. AMPK Mediates Glucocorticoids Stress-Induced Downregulation of the Glucocorticoid Receptor in Cultured Rat Prefrontal Cortical Astrocytes. PLoS One. 2016;11:e0159513. doi: 10.1371/journal.pone.0159513. [DOI] [PMC free article] [PubMed] [Google Scholar]