Abstract
Insulin signalling begins with binding to its cell surface insulin receptor (IR), which is a tyrosine kinase. The insulin receptor kinase (IRK) is subsequently autophosphorylated and activated to tyrosine phosphorylate key cellular substrates that are essential for entraining the insulin response. Although IRK activation begins at the cell surface, it is maintained and augmented following internalization into the endosomal system (ENS). The peroxovanadium compounds (pVs) were discovered to activate the IRK in the absence of insulin and lead to a full insulin response. Thus, IRK activation is both necessary and sufficient for insulin signalling. Furthermore, this could be shown to occur with activation of only the endosomal IRK. The mechanism of pV action was shown to be the inhibition of IRK-associated phosphotyrosine phosphatases (PTPs). Our studies showed that the duration and intensity of insulin signalling are modulated within ENS by the recruitment of cellular substrates to ENS; intra-endosomal acidification, which promotes dissociation of insulin from the IRK; an endosomal acidic insulinase, which degrades intra-endosomal insulin; and IRK-associated PTPs, which dephosphorylate and, hence, deactivate the IRK. Therefore, the internalization of IRKs is central to insulin signalling and its regulation.
Keywords: endosomal acidic insulinase, endosomes, insulin receptor, phosphorylation, phosphotyrosine phosphatase, tyrosine kinase
Mots clés: insulinase endosomique acide, endosomes, récepteur de l’insuline, phosphorylation, phosphotyrosine phosphatase, tyrosine kinase
RÉSUMÉ
La signalisation de l’insuline commence par la liaison de l’insuline à son récepteur (IR) situé à la surface des cellules, soit la tyrosine kinase. La kinase du récepteur de l’insuline (KRI) s’est subséquemment autophosphorylée et activée vers les principaux substrats cellulaires de la tyrosine phosphorylée qui sont essentiels au déclenchement de la réponse à l’insuline. Bien que l’activation de la KRI commence à la surface des cellules, elle est maintenue et augmentée à la suite de l’internalisation du système endosomal (SEN). Il a été découvert que les composés de peroxovanadium (pV) activent la KRI en l’absence d’insuline et mènent à une réponse insulinique complète. Par conséquent, l’activation de la KRI est nécessaire et suffisante à la signalisation de l’insuline. De plus, il pourrait être démontré qu’elle apparaît avec l’activation de la KRI endosomale seulement. Il a été démontré que le mécanisme de l’activité du pV est l’inhibition des phosphotyrosines phosphatases (PTP) associées à la KRI. Nos études ont montré que la durée et l’intensité de la signalisation de l’insuline sont modulées au sein du SEN par le recrutement de substrats cellulaires du SEN; l’acidification intra-endosomale, qui favorise la dissociation de l’insuline de la KRI; une insulinase endosomique acide, qui dégrade l’insuline dans les endosomes; les PTP associées à la KRI, qui déphosphorylent et, donc, désactivent la KRI. Par conséquent, l’internalisation des KRI est essentielle au déclenchement de la signalisation de l’insuline et à sa régulation.
Introduction
Type 2 diabetes mellitus is characterized by both resistance to the action of insulin and defects in insulin secretion. The former has been an important motivating factor in the exploration of insulin action on its target tissues. By 1970, the notion that insulin and other peptide hormones interacted with specific cell surface receptors was established. Thus, incubating cells with 125I-insulin and increasing quantities of unlabelled insulin defined receptors for insulin as cell surface binding sites of high affinity (~10−9 M) and specificity (1). Shortly thereafter, my colleagues and I recognized that the demonstration of specific binding sites (i.e. receptors) in any tissue was a new way of defining hormone target tissues. We demonstrated insulin receptors in the classic target tissues (liver, fat and muscle) but also in a range of other tissues not previously regarded as insulin targets (e.g. placenta and brain) (2).
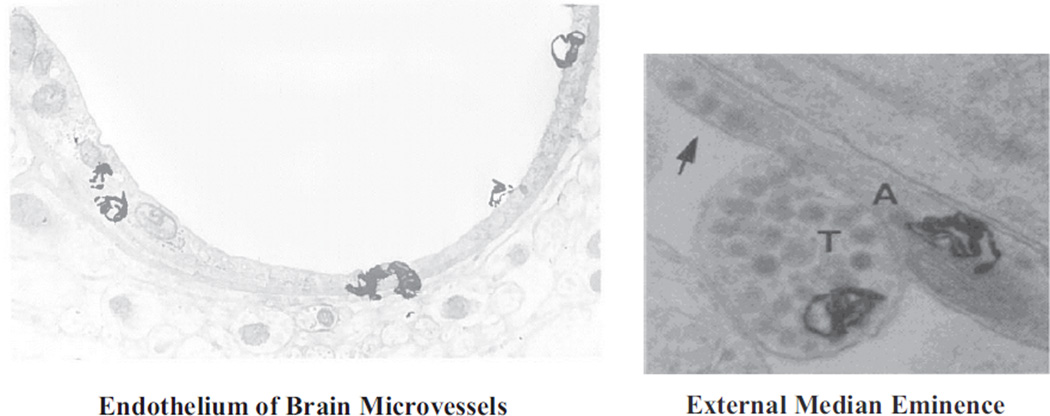
We subsequently developed an in vivo radioautographic method that permitted more precise cellular localization of peptide hormone receptors in a given tissue (3). Thus, it was possible to demonstrate insulin receptors on the endothelium of capillaries (4) and on nerve terminals in the median eminence of the hypothalamus (5) (Figure 1) as well as on other circumventricular organs of the brain where fenestrated capillaries permit access of circulating molecules directly to neuronal elements (6). The latter finding was extended to a range of peptide hormones, each with a specific pattern of neuronal interaction in the circumventricular organs (7). These findings provided a structural basis by which circulating insulin and other peptides could directly influence functions of the central nervous system.
Figure 1.
Electron microscope radioautographs illustrating the localization of 125I-insulin-specific binding sites (silver grains). Left, insulin binding sites on the endothelium of rat brain microvessels. Right, insulin binding sites over a free nerve terminal (T) and a preterminal axon (A) containing dense core vesicles (arrow) in the external median eminence of the rat hypothalamus.
Intracellular Receptors and Internalization
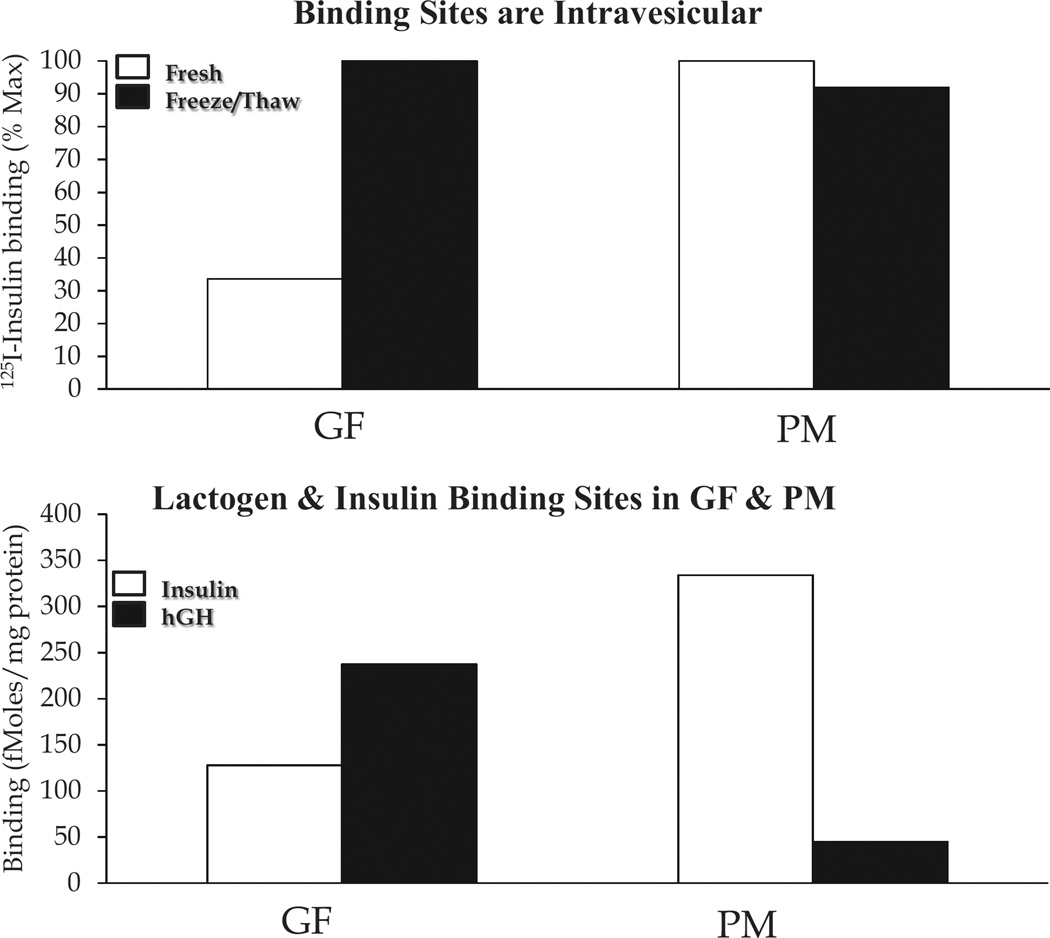
The localization of peptide hormone receptors was pursued at the subcellular level. Insulin binding sites had been observed in isolated hepatic Golgi fractions (GFs). However, it was unclear whether these sites existed on GF vesicles or on possible contaminating plasma membrane (PM). Insulin binding was examined in GFs and PM both before and after subjecting the cell fractions to freeze-thawing, a technique that would fracture vesicular structures (GFs) but not PM. As seen in Figure 2 (top), freeze-thawing markedly augmented insulin binding to GFs but not PM-indicating receptors in GF vesicles as well as on the cell surface. This was consolidated by demonstrating the different pattern of human growth hormone (hGH) binding to GFs and PM. As with other primate growth hormones, hGH binds to both lactogen and GH receptors. In the case of female rat liver, lactogen receptors predominate and are especially concentrated in GFs rather than in PM (Figure 2, bottom). In addition, we used electron microscope radioautography to demonstrate 125I-insulin binding to GF vesicular elements (8).
Figure 2.
Insulin binding sites in Golgi fractions (GFs) and PM from rat liver. Top, binding of 125I-insulin to GF and PM before and after freeze-thawing of the cell fractions. Bottom, binding of 125I-insulin vs. 125I-hGH to GF and PM after freeze-thawing. hGH, like all primate growth hormones, binds to both prolactin and GH binding sites. The former receptors predominate in rat liver. These figures were derived from the data in reference (8).
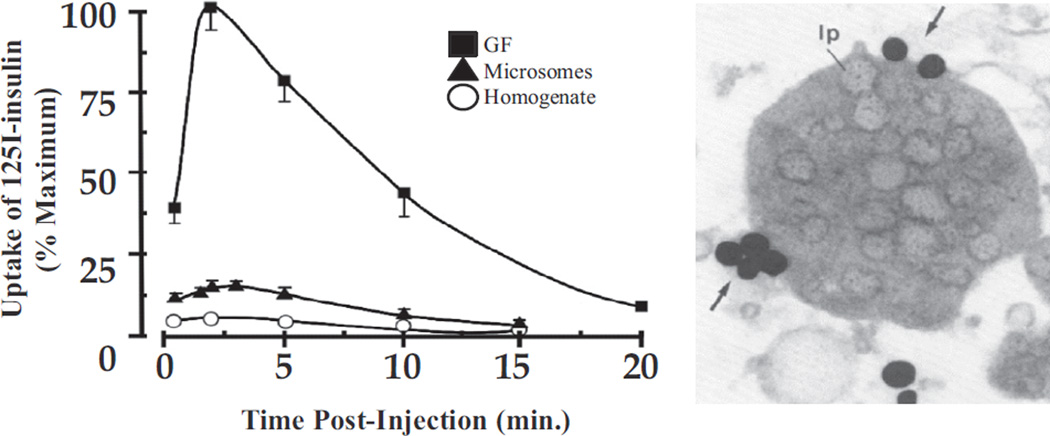
The question then arose as to whether these were newly synthesized receptors transiting the Golgi apparatus on their way to the cell surface or were receptors internalized from the cell surface. This was first explored by injecting 125I-insulin in vivo and following its association with different cell fractions with time. As seen in Figure 3 (left), 125I-insulin was most highly concentrated in the GF fraction and, as demonstrated by electron microscope-radioautography, especially in lipoprotein-filled vesicles of this fraction (Figure 3, right). Coinjection of unlabelled insulin competitively inhibited the binding of 125I-insulin to these elements (9). It was subsequently shown that the entities involved in insulin uptake could be resolved from true Golgi elements (10) and, hence, constituted a new organelle, which came to be known as the endosomal system or endosomes (ENS).
Figure 3.
Time course of 125I-insulin uptake into subcellular fractions. Left, concentration over time of injected 125I-insulin in homogenate, microsomes and GF. Results are expressed as a per cent of maximum concentration in GF. Right, EM radioautograph of GF fraction at 2 min post-125I-insulin injection. Silver grains are largely found over the periphery (arrows) of GF vesicles with their characteristic lipoprotein (lp) content. The data are from reference (9).
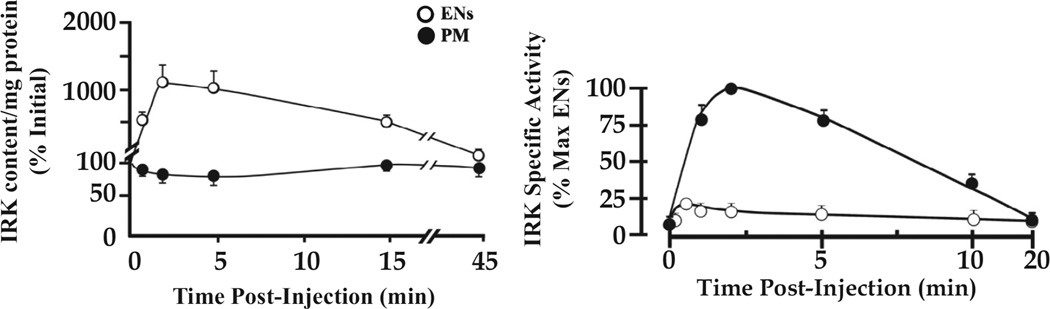
The cloning of the insulin receptor in the mid-1980s revealed it to be a tyrosine kinase capable of autophosphorylation and activation against external substrates. Furthermore, insulin receptor kinase (IRK) activity was shown to be necessary for insulin action to occur (11). Indeed, activation of the IRK in the absence of insulin was sufficient to realize the full range of insulin’s effects (12). The development of antibodies to both the IR and phosphotyrosine enabled direct analysis of IRK fate and activation state. As seen in Figure 4 (left), the injection of insulin in vivo resulted in a large increase in the receptor content of ENS and a corresponding decrease in IR content of PM, as expected for insulin-dependent internalization of IRKs. As seen in Figure 4 (right), the internalized IRK was highly activated, both in effecting autophosphorylation and the tyrosine phosphorylation of downstream substrates (13). The much greater specific activity of the IRK in ENS vs. PM is consistent with an important role for the internalized receptor in determining the insulin response.
Figure 4.
Time course of IRK concentration and activation following insulin administration. Left, IRK content per mg cell fraction protein was determined by immunoblotting with IRK-specific antibody. Results are expressed as a per cent of the basal levels in ENS and PM (100% at zero time). Right, IRK enzyme activity per mg cell fraction protein (specific activity) was expressed as a per cent of maximum measured in ENS. The data were derived from figures 6 and 9 of reference (13).
Insulin Degradation: An Endosomal Process
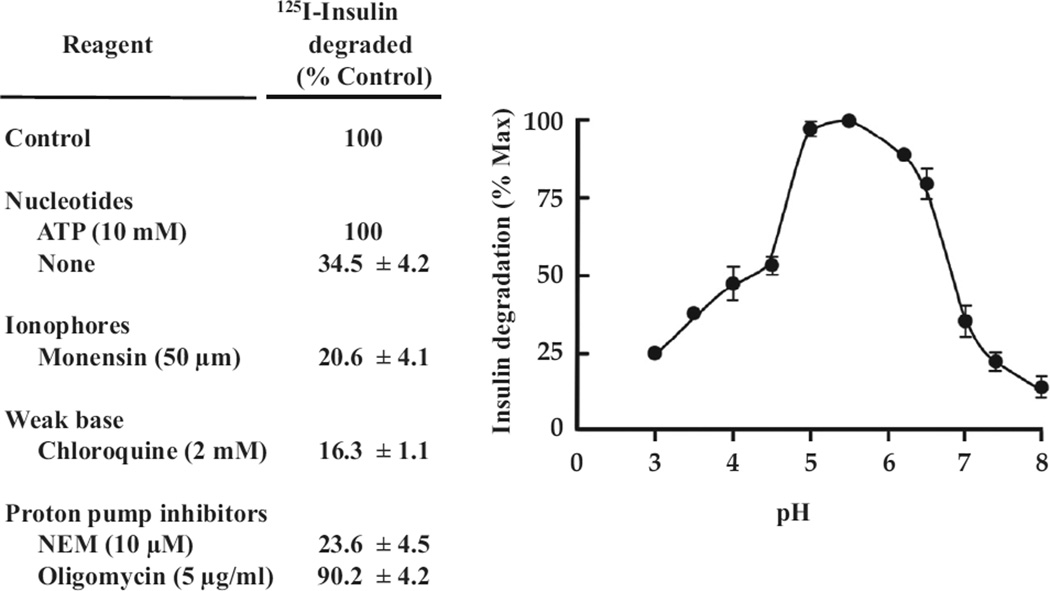
For some years prior, the degradation of insulin in tissues was attributed to an insulin-degrading enzyme, which was shown to have a pH optimum for insulin degradation of ~7.0, to be absent from ENS, present in the cytosol and especially enriched in peroxisomes, where it was found to be involved in the processing of peroxisomal proteins (14).
By 1988, it had become clear that degradation of insulin occurred subsequent to its internalization into ENS (15). Degradation of internalized insulin could be demonstrated in purified intact ENS (16) and by this means, characterized in some detail (Figure 5). It is noteworthy that there is a pH optimum of ~5.0. The requirement for adenosine triphosphate demonstrates the role of proton translocation into ENS to achieve the optimal intra-endosomal acidic pH. Thus, neutralizing the pH in ENS with either the proton ionophore, monensin, or the weak base, chloroquine, substantially inhibited insulin degradation. Also N-ethylmaleimide, an endosomal proton pump inhibitor, but not oligomycin, a mitochondrial proton pump inhibitor, inhibited insulin degradation, again emphasizing the importance of an acidic endosomal pH for this process (Figure 5).
Figure 5.
Degradation of endocytosed insulin in isolated ENS: requirement for acidic pH. At 5 min after 125I-insulin injection ENS were prepared and incubated in vitro under the noted conditions, and degradation of endosomal insulin was assayed as described in reference 16. Left, effect on insulin degradation of ATP and agents that alter endosomal proton accumulation. Right, effect of pH of incubation medium on endosomal insulin degradation. The data are from reference (16).
The endosomal degrading activity showed relative specificity for insulin compared to epidermal growth factor (EGF) or prolactin (16). Subsequent studies have identified the aspartic acid protease cathepsin D as the insulin protease catalyzing the inactivating cleavage of endosomal insulin (17).
Peroxovanadium Compounds and IRK-Associated Phosphotyrosine Phosphatase
As noted above, the IRK is a tyrosine kinase whose activity is crucial for entraining the insulin response. In early studies, we explored the impact of various known insulin mimetics (viz. vanadate and H2O2) on the relationship between insulin action on adipocytes and IRK activation. We discovered that adding vanadate and H2O2 individually had modest effects on these parameters, whereas combining them in the same incubation resulted in a markedly synergistic effect on both IRK activation and insulin action (18). This was shown to be due to the formation of peroxides of vanadate (pVs), which were the molecular species responsible for the augmented activation of the IRK (19).
In exploring the mechanism by which pVs activate the IRK, we recognized that subsequent to its activation, and on the withdrawal of insulin, the endosomal IRK underwent dephosphorylation (20). Indeed, we could show that the tyrosine-phosphorylated state of the endosomal IRK reflected the dynamic consequence of IRK autophosphorylation, on the one hand, and dephosphorylation, on the other (21). This indicated that the endosomal IRK was intimately associated with 1 or more PTPs, which function to restrain IRK autoactivation. Furthermore, pVs were shown to inhibit the IRK-associated PTPs (12,21). In the face of this inhibition, and even in the absence of insulin, the low intrinsic autophosphorylating activity of the IRK was sufficient to permit IRK autophosphorylation activation. This was shown for the EGF receptor as well, thus identifying an important mechanism regulating receptor tyrosine kinase activity.
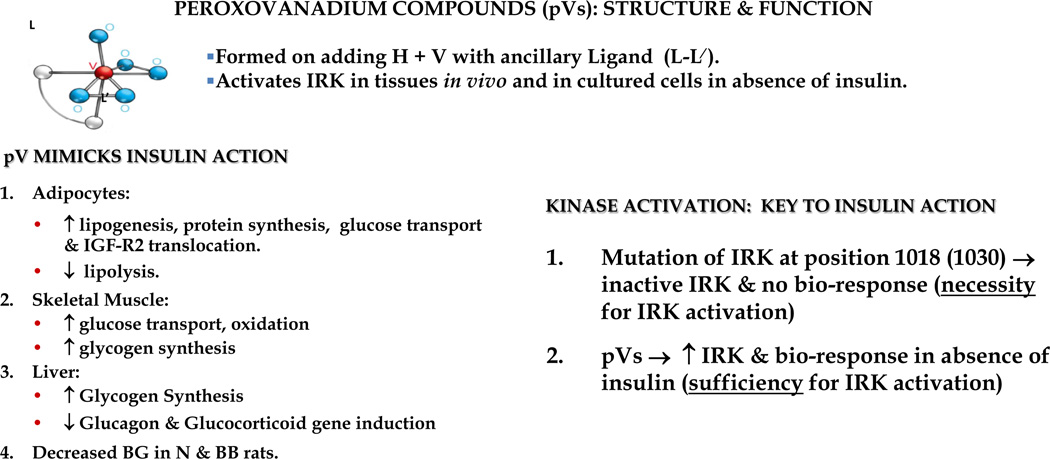
In order to facilitate the use of pVs in cell culture and in vivo, we collaborated with Alan Shaver in the Department of Chemistry to synthesize 12 different chemically stable pVs. Each contained 1 or 2 peroxo anions and a unique organic ligand in the inner coordination sphere of the vanadium atom (viz. bpV(phen) (Figure 6). Each compound activated the IRK in cultured cells and in tissues (viz. liver) in vivo, and displayed an excellent correlation between PTP inhibitory and IRK activating potencies (12) (Figure 6). The fact that pVs could promote insulin action in the absence of insulin confirmed the notion that not only was IRK activity necessary, but it was also sufficient for insulin action. Furthermore, it raised the possibility of employing such agents as alternatives to insulin in the treatment of type 2 diabetes. However, their inhibition of PTPs was nonspecific and probably contributed to cellular injury, thus precluding these agents as potential therapeutics.
Figure 6.
Characteristics of peroxovanadium compounds (pVs). Note that a given compound’s properties are significantly influenced by the nature of the ancillary ligand bound in the inner coordination sphere of vanadium. The major insulin mimetic effects are summarized (left); and the importance of pVs in establishing that IRK activation in the absence of insulin is sufficient for promoting a full insulin response (right).
Intracellular Signalling is Sufficient for Insulin Action
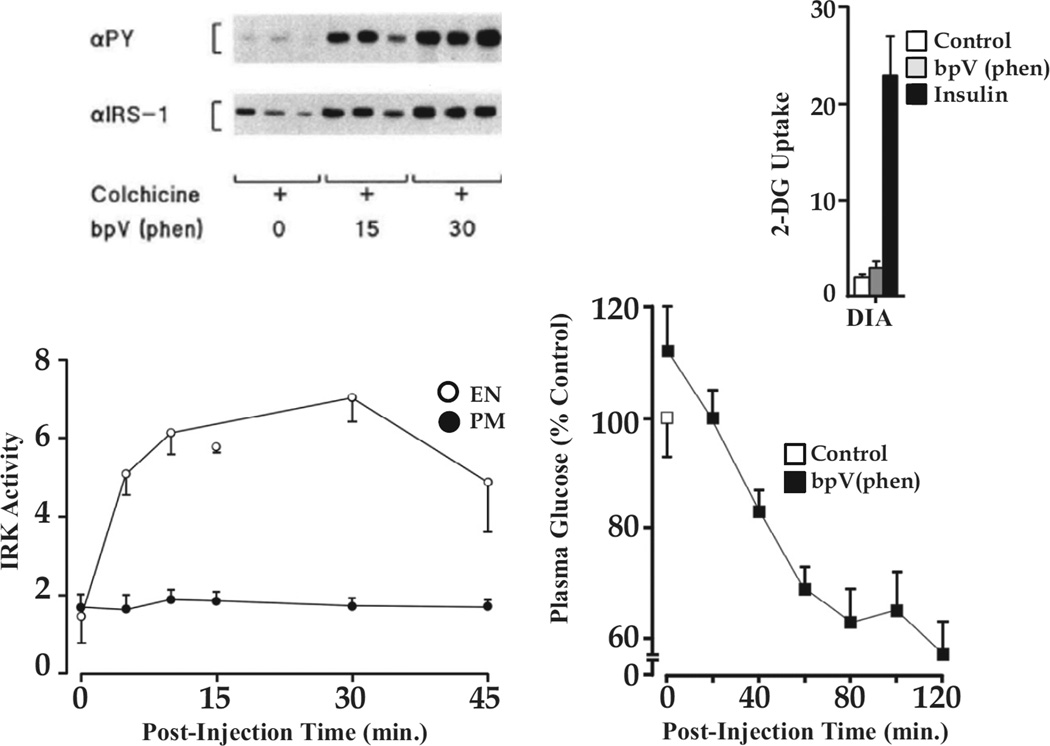
We subsequently examined the time course of IRK activation in PM and ENS following bpV(phen) administration to rats in vivo and found that activation of endosomal IRK preceded and exceeded that of PM (22). When receptor recycling was blocked with colchicine, activation of the IRK was observed only in ENS along with tyrosine phosphorylation of IRS-1, a key substrate of the IRK (Figure 7, left). Thus, this early step in insulin action could be effected by activation of the endosomal IRK alone.
Figure 7.
Activation of the endosomal IRK is sufficient to promote insulin signalling. Left, (bottom) IRK activity in ENS and PM following bpV(phen) administration to rats pretreated with colchicine to block recycling of IRK from ENS to PM. IRK activation is seen exclusively in the ENS; (top) IRS-1 phosphorylation is seen when only endosomal IRK is activated. Right, (bottom) bpV(phen) lowers blood glucose levels; (top) in the absence of any effect to increase glucose uptake as measured by 2-deoxyglucose (2-DG) accumulation in skeletal muscle exemplified by diaphragm (DIA). (bottom) Lowering of blood glucose levels by bpV(phen) in colchicine-treated rats. For details see reference (22).
We chose bpV(phen) because it was unable to stimulate glucose uptake into skeletal muscle as shown in Figure 7, right. Nevertheless bpV(phen), in the presence of colchicine, significantly lowered blood glucose levels which, in the absence of increased glucose uptake into skeletal muscle, pointed to a hepatic effect on glucose output. Thus, activation of the hepatic endosomal IRK is sufficient to effect signalling. This has also been shown for the EGF receptor (23).
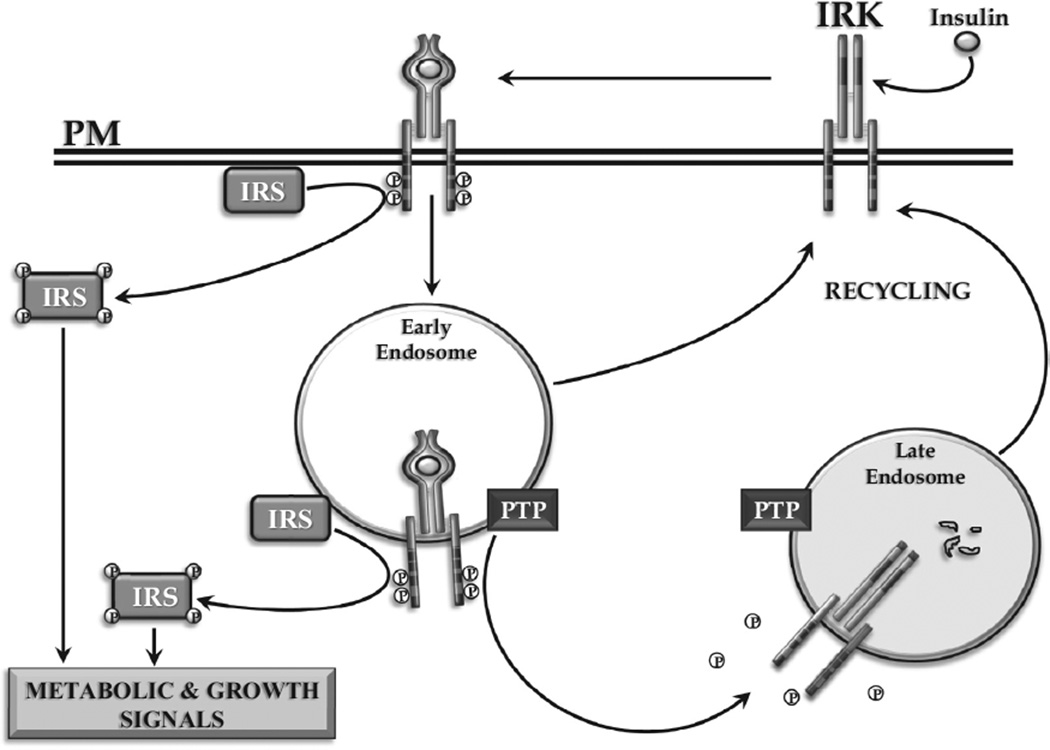
Endosomal Signalling and Its Regulation
Insulin signalling is regulated by autophosphorylation of the IRK and the concentration of activated IRK in endosomes, wherein the intensity and duration of action is limited by the degradation of insulin and the dephosphorylation of activated IRK by associated PTPs (Figure 8). The importance of the endosomal system in regulating signalling for a range of peptide hormones and growth factors, including G-protein coupled receptors, such as glucagon, has now been firmly established (24).
Figure 8.
Internalization of the activated insulin-IRK complex. Signalling from within ENS of the activated IRK is depicted. The role of IRK-associated PTP and endosomal insulin degradation in terminating intracellular insulin signalling is shown.
Acknowledgments
I express my appreciation to my collaborators, especially Dr. John J.M. Bergeron and Dr. Alan Shaver, whose efforts were essential to the accomplishment of this work. I am indebted to my teachers at the University of Manitoba, the Massachusetts Institute of Technology, the Tufts New England Medical Center, and the Heart and Lung Institute of the National Institutes of Health. I thank my many colleagues in the Polypeptide Hormone Laboratory of McGill University for their unfailing support and want to express my appreciation for the excellent laboratory work done by Gerardo Baquiran, Victor Dumas and Mary Lapenna over the years. This manuscript is based on the lecture given as the 2015 recipient of the Lifetime Achievement Award of the Canadian Diabetes Association. The work herein reported was supported by grants from the Canadian Institutes of Health Research, the NIH and the Canadian Diabetes Association.
References
- 1.Freychet P, Roth J, Neville DM., Jr Insulin receptors in the liver: Specific binding of (125 I) insulin to the plasma membrane and its relation to insulin bioactivity. Proc Natl Acad Sci U S A. 1971;68:1833–1837. doi: 10.1073/pnas.68.8.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Posner BI, Kelly PA, Shiu RP, Friesen HG. Studies of insulin, growth hormone and prolactin binding: Tissue distribution, species variation and characterization. Endocrinology. 1974;95:521–531. doi: 10.1210/endo-95-2-521. [DOI] [PubMed] [Google Scholar]
- 3.Bergeron JJ, Levine G, Sikstrom R, et al. Polypeptide hormone binding sites in vivo: Initial localization of 125I-labeled insulin to hepatocyte plasmalemma as visualized by electron microscope radioautography. Proc Natl Acad Sci U S A. 1977;74:5051–5055. doi: 10.1073/pnas.74.11.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Houten M, Posner BI. Insulin binds to brain blood vessels in vivo. Nature. 1979;282:623–625. doi: 10.1038/282623a0. [DOI] [PubMed] [Google Scholar]
- 5.Van Houten M, Posner BI, Kopriwa BM, Brawer JR. Insulin binding sites localized to nerve terminals in rat median eminence and arcuate nucleus. Science. 1980;207:1081–1083. doi: 10.1126/science.6986652. [DOI] [PubMed] [Google Scholar]
- 6.Van Houten M, Posner BI, Kopriwa BM, Brawer JR. Insulin-binding sites in the rat brain: In vivo localization to the circumventricular organs by quantitative radioautography. Endocrinology. 1979;105:666–673. doi: 10.1210/endo-105-3-666. [DOI] [PubMed] [Google Scholar]
- 7.Van Houten M, Posner BI. Circumventricular organs: Receptors and mediators of direct peptide hormone action on brain. Adv Metab Disord. 1983;10:269–289. doi: 10.1016/b978-0-12-027310-2.50015-3. [DOI] [PubMed] [Google Scholar]
- 8.Bergeron JJ, Posner BI, Josefsberg Z, Sikstrom R. Intracellular polypeptide hormone receptors: The demonstration of specific binding sites for insulin and human growth hormone in Golgi fractions isolated from the liver of female rats. J Biol Chem. 1978;253:4058–4066. [PubMed] [Google Scholar]
- 9.Posner BI, Patel B, Verma AK, Bergeron JJ. Uptake of insulin by plasmalemma and Golgi subcellular fractions of rat liver. J Biol Chem. 1980;255:735–741. [PubMed] [Google Scholar]
- 10.Kay DG, Khan MN, Posner BI, Bergeron JJ. In vivo uptake of insulin into hepatic Golgi fractions: Application of the diaminobenzidine-shift protocol. Biochem Biophys Res Commun. 1984;123:1144–1148. doi: 10.1016/s0006-291x(84)80252-1. [DOI] [PubMed] [Google Scholar]
- 11.Ebina Y, Araki E, Taira M, et al. Replacement of lysine residue 1030 in the putative ATP-binding region of the insulin receptor abolishes insulin- and antibody-stimulated glucose uptake and receptor kinase activity. Proc Natl Acad Sci U S A. 1987;84:704–708. doi: 10.1073/pnas.84.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Posner BI, Faure R, Burgess JW, et al. Peroxovanadium compounds: A new class of potent phosphotyrosine phosphatase inhibitors which are insulin mimetics. J Biol Chem. 1994;269:4596–4604. [PubMed] [Google Scholar]
- 13.Khan MN, Baquiran G, Brule C, et al. Internalization and activation of the rat liver insulin receptor kinase in vivo. J Biol Chem. 1989;264:12931–12940. [PubMed] [Google Scholar]
- 14.Authier F, Bergeron JJ, Ou WJ, et al. Degradation of the cleaved leader peptide of thiolase by a peroxisomal proteinase. Proc Natl Acad Sci U S A. 1995;92:3859–3863. doi: 10.1073/pnas.92.9.3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamel FG, Posner BI, Bergeron JJ, et al. Isolation of insulin degradation products from endosomes derived from intact rat liver. J Biol Chem. 1988;263:6703–6708. [PubMed] [Google Scholar]
- 16.Doherty JJ, 2nd, Kay DG, Lai WH, et al. Selective degradation of insulin within rat liver endosomes. J Cell Biol. 1990;110:35–42. doi: 10.1083/jcb.110.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Authier F, Metioui M, Fabrega S, et al. Endosomal proteolysis of internalized insulin at the C-terminal region of the B chain by cathepsin D. J Biol Chem. 2002;277:9437–9446. doi: 10.1074/jbc.M110188200. [DOI] [PubMed] [Google Scholar]
- 18.Kadota S, Fantus IG, Deragon G, et al. Stimulation of insulin-like growth factor II receptor binding and insulin receptor kinase activity in rat adipocytes: Effects of vanadate and H2O2. J Biol Chem. 1987;262:8252–8256. [PubMed] [Google Scholar]
- 19.Kadota S, Fantus IG, Deragon G, et al. Peroxide(s) of vanadium: A novel and potent insulin-mimetic agent which activates the insulin receptor kinase. Biochem Biophys Res Commun. 1987;147:259–266. doi: 10.1016/s0006-291x(87)80115-8. [DOI] [PubMed] [Google Scholar]
- 20.Fantus IG, Kadota S, Deragon G, et al. Pervanadate (peroxide(s) of vanadate) mimics insulin action in rat adipocytes via activation of the insulin receptor tyrosine kinase. Biochemistry. 1989;28:8864–8871. doi: 10.1021/bi00448a027. [DOI] [PubMed] [Google Scholar]
- 21.Faure R, Baquiran G, Bergeron JJ, Posner BI. The dephosphorylation of insulin and epidermal growth factor receptors: Role of endosome-associated phosphotyrosine phosphatase(s) J Biol Chem. 1992;267:11215–11221. [PubMed] [Google Scholar]
- 22.Bevan AP, Burgess JW, Drake PG, et al. Selective activation of the rat hepatic endosomal insulin receptor kinase: Role for the endosome in insulin signaling. J Biol Chem. 1995;270:10784–10791. doi: 10.1074/jbc.270.18.10784. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Pennock S, Chen X, Wang Z. Internalization of inactive EGF receptor into endosomes and the subsequent activation of endosome-associated EGF receptors: Epidermal growth factor. Sci STKE. 2002;2002:l17. doi: 10.1126/stke.2002.161.pl17. [DOI] [PubMed] [Google Scholar]
- 24.Bergeron JJ, Di Guglielmo GM, Dahan S, et al. Spatial and temporal regulation of receptor tyrosine kinase activation and intracellular signal transduction. Annu Rev Biochem. 2016;85:573–597. doi: 10.1146/annurev-biochem-060815-014659. [DOI] [PubMed] [Google Scholar]