Abstract
Recent studies of long-term anabolic-androgenic steroid (AAS) users reported amygdala structural and functional connectivity abnormalities. We assessed white matter microstructure in the inferior-fronto-occipital fasciculus (IFOF), a major associative bundle of the amygdala network. Diffusion weighted images acquired from 9 male long-term AAS users and 8 matched controls aged 36-51 years old were processed using a standardized pipeline (Tract-Based Spatial Statistics). Group differences were examined using linear regression with adjustment for age and current testosterone level. Compared to nonusers, AAS users exhibited significantly higher fractional anisotropy (FA) in the IFOF. Users showed markedly greater FA than nonusers on the left IFOF but only a modest, nonsignificant difference on the right IFOF. Moreover, FA was positively associated with lifetime cumulative AAS dose. Our results suggest that long-term AAS use alters IFOF white matter organization and integrity, which in turn might affect amygdala-related processes such as reward system function. Accordingly, further studies are needed to replicate findings in larger subject groups to determine the functional significance of the FA abnormality.
Keywords: diffusion tensor imaging (DTI), anabolic-androgenic steroids (AAS), inferior-fronto-occipital fasciculus (IFOF), tract-based spatial statistics (TBSS)
Introduction
The anabolic-androgenic steroids (AAS) are a group of hormones including testosterone and its synthetic derivatives such as nandrolone or stanozolol. These drugs are widely used by athlete and non-athlete weightlifters to improve performance or personal appearance (Pope et al., 2014a). Prior to the 1980s, AAS use was confined largely to elite athletes. However, in the last several decades, AAS use has spread widely into the general population (Kanayama et al., 2008). Today, there are nearly 4 million current or past users in the United States alone, virtually all male, of whom about 1 million have developed AAS dependence (Pope et al., 2014b). Human and animal studies have reported adverse effects of AAS use on the cardiovascular system (Achar et al., 2010; Baggish et al., 2010; Vanberg and Atar, 2010), the hypothalamic-pituitary-gonadal axis (Flanagan and Lehtihet, 2015; Kanayama et al., 2015b), and several other organ systems, including the hepatic (Martin et al., 2008; Solbach et al., 2015), urogenital (Harrington et al., 2011; Herlitz et al., 2010) and musculoskeletal systems (Kanayama et al., 2015a). It is also well recognized that AAS may cause acute psychiatric effects in some individuals, with hypomanic symptoms such as irritability, aggressiveness, and even violence during AAS exposure, and depressive symptoms during AAS withdrawal (Pope et al., 2014b). These psychiatric effects have been reported in numerous field studies of AAS users (Pope et al., 2014b), and have also been observed in blinded laboratory investigations administering supraphysiologic doses of AAS to normal volunteers (Pope et al., 2000; Su et al., 1993).
In addition to their acute psychiatric effects, AAS may also induce chronic neurotoxic effects. This possibility has been suggested by several recent laboratory studies showing apoptotic or other neurotoxic effects in mammalian or human neuronal cells exposed to supraphysiologic levels of testosterone or other AAS, at levels comparable to those that might plausibly be seen in human AAS users (Caraci et al., 2011; Cunningham et al., 2009; Estrada et al., 2006). Recently, several in vivo studies have also reported impairments following long term AAS use. Animal as well as human studies found deficits of visuospatial memory after AAS use (Magnusson et al., 2009; Pieretti et al., 2013; Tanehkar et al., 2013; Kanayama et al., 2013). Following up on these findings, our group recently compared AAS using and non-AAS-using weightlifters with structural and functional MRI (Kaufman et al., 2015). As compared to non-users, AAS users displayed increased amygdala volume and reduced resting-state functional MRI coupling of the amygdala with cognitive control and memory regions. These finding suggest that long-term AAS use may impair amygdala-related functional and structural brain networks. To assess whether these effects are accompanied by abnormalities in white matter structural connectivity, we performed an analysis of diffusion tensor imaging (DTI) data also acquired from a subset of study participants.
DTI provides information about the extent and direction of water diffusion within tissues (Basser et al., 1996; Mori and Zhang, 2006; Pierpaoli and Basser, 1996). Since water preferentially diffuses along the axis of white matter axonal fiber bundles, DTI can detect pathologies in white matter organization (Tournier et al., 2011). Several measures have been proposed for quantification of water diffusion. Due to the small sample size of our study (N = 17 participants) we followed a strict a priori driven approach to analyze our data. We limited our analysis to a single metric, fractional anisotropy (FA), which is the most common diffusion imaging metric and is often used as an index of overall white mater organization. Additionally, we focused our analyses on a single white matter tract, the inferior-fronto-occipital fasciculus (IFOF), one of the largest associative bundles in the brain (Caverzasi et al., 2014; Makris and Pandya, 2009). IFOF connects major amygdala projection regions, namely the orbitofrontal and ventromedial frontal cortex, the middle and inferior frontal gyri, and the ventral temporal and occipital regions (Makris et al., 1999). Moreover, IFOF is involved in visuospatial functioning (Chechlacz et al., 2015; Peters et al., 2014), which we have shown in prior studies to be the neuropsychological domain most affected by chronic AAS exposure (Kanayama et al., 2013; Kaufman et al., 2015). Accordingly, we hypothesized that we would detect FA abnormalities in the IFOF in long-term AAS users.
Methods
Participants
Participants were 9 male AAS users with at least 2 years of lifetime AAS exposure and 8 non-AAS-using male weightlifters. We limit our studies to male participants because virtually all AAS users are male (Pope et al., 2014b). Study participants represent all individuals reported on in our previous paper (Kaufman et al., 2015) with evaluable diffusion images. Full details of the recruitment and screening procedures for these participants are presented in this earlier publication (Kaufman et al., 2015). Briefly, participants provided histories of alcohol and substance use, including AAS and other performance-enhancing drug use, medical and psychiatric histories, and drug testing of urine and hair samples. On the day of evaluation, participants were screened for recent alcohol (Alco-Sensor IV; Intoximeters, Inc., St. Louis, MO) and other illicit drug use (AmediCheck 12-Panel Drug Test Cups; Amedica Biotech, Hayward, CA). All participants provided informed consent for the study, which was approved by the McLean Hospital Institutional Review Board.
Image acquisition and processing
Diffusion-weighted images were acquired on a Siemens TIM Trio 3T scanner (Erlangen, Germany) with a 32-channel head coil using a double spin-echo EPI sequence with the following image parameters (TR = 9120ms, TE = 112ms, flip angle = 90°, FOV = 160×160, slices = 42, in plane = 1.4×1.4 mm, slice thickness = 3.5 mm, 64 gradient directions with b = 1000 mm/s2, baseline scans with b = 0).
Images were visually inspected and manually aligned to the A-P axis. Head motion and eddy current distortion correction were performed using an affine registration of each gradient weighted image to the b0 image with FLIRT (FSL, Oxford; http://fsl.fmrib.ox.ac.uk/fsl (Jenkinson et al., 2002)). Images were then processed through the Tract-Based Spatial Statistics (TBSS) pipeline (Smith et al., 2006) that was adapted by the Enhanced Neuroimaging Genetic by Meta-Analysis (ENIGMA) DTI Working Group at the University of Southern California (http://enigma.ini.usc.edu/protocols/dti-protocols). TBSS is a method in which the brain of each subject is registered to a white matter skeleton template that is parcellated into white matter regions (here provided by the ENIGMA consortium) in order to extract tracts of interest (Bach et al., 2014). We specifically focused on extracting average FA from the left and right IFOF.
Statistical analyses
Statistical Analysis was performed with Prism (GraphPadSoftware, 2014), the Statistical Package for Social Sciences (SPSS) version 22.0 (IBMCorp, 2013) and Stata 12.1 (Stata Corporation, College Station, Texas). We assessed the differences between the AAS users and non-users on demographic indices using the t-test, two-tailed, for continuous variables and Fisher's exact test, two-tailed for ordinal variables. We used linear regression to assess differences in FA between users and non-users in the IFOF and, within the user group, to assess the association between FA and cumulative lifetime AAS dose. Given the effects of age and blood hormone levels on white matter diffusion imaging metrics (Lebel et al., 2008; Voineskos et al., 2012), all analyses were adjusted for age and current serum testosterone levels. As our previous study reported group differences in visuospatial memory performance (Kanayama, 2013) we conducted a partial correlation analyses (correcting for age and current testosterone level) to assess potential associations between structural brain abnormalities and visuospatial memory performance. Subjects underwent cognitive tests from the CANTAB battery (Cambridge Cognition, Cambridge, UK), details of which were described previously (Kanayama, et al., 2013). We used the Pattern Recognition Memory test (serial visual memory testing) administered in immediate and delayed recall forms and the Paired Associates Learning test (visuospatial memory and new learning).
Finally, to determine anatomical specificity of our amygdala finding, we analyzed FA of the corpus callosum as a “control tract” in which we did not expect group differences based on the literature. We conducted a post-hoc analysis using ANCOVA, with adjustment for age and serum testosterone levels, to assess the effect of group (AAS users versus non-users) on corpus callosum FA.
Results
Demographic measures
Subject groups were well-matched on most demographic variables (Table 1). The age range of study subjects was 36 to 51 years old. AAS users reported a mean of 9.6 ± 3.7 years of cumulative lifetime AAS exposure, totaling 0.67 ± 0.56 kg. AAS use typically involved both injectable AAS (e.g., testosterone, boldenone) and oral preparations (e.g., methandienone, stanozolol). No subject had a diagnosis of current alcohol dependence. No subject tested positive for breath alcohol on the scan day. One AAS and one non-AAS group subject had a past history of alcohol dependence. Several participants were currently taking psychoactive medications (Table 1) including 2 AAS users taking prescription opioids (buprenorphine and oxycodone, respectively). One AAS user had taken cocaine and one nonuser had taken marijuana on the day prior to the evaluation day. Overall, 4 AAS users and 3 nonusers had taken at least one licit or illicit psychoactive substance within the past 24 hours.
Table 1. Attributes and Measures of AAS Users vs. Non-AAS-Using Weightlifters.
| Attribute/measure a | AAS users N = 9 | AAS Nonusers N = 8 | p b |
|---|---|---|---|
| Age, years | 42.4 (SD=4.4) | 44.1 (SD=6.5) | 0.54 |
| Age range, years | 36-51 | 36-51 | |
| Race/ethnicity | |||
| Non-Hispanic White | 8 (89%) | 8 (100%) | 1.0 |
| Hispanic White | 1 (11%) | ||
| Four-year college graduate | 4 (44%) | 6 (75%) | 0.33 |
| Lifetime years of regular weightlifting | 19.7 (SD=6.8) | 24.0 (SD=10.4) | 0.32 |
| Age at first AAS use, years | 23.1 (SD=4.8) | - | |
| Cumulative lifetime AAS use, weeks | 498 (SD=192) | - | |
| Cumulative lifetime AAS dose, grams c | 667 (SD=555) | - | |
| Time since last AAS use | |||
| Current use | 4 (44%) | - | |
| 2-4 months | 2 (22%) | - | |
| > 20 months | 3 (33%) | - | |
| Current psychoactive medication used | |||
| Opioids | 2 | 0 | |
| Selective serotonin reuptake inhibitors | 2 | 2 | |
| Other psychoactive medications | 3 | 0 | |
| Illicit drug use in past 24 h | 1 | 1 | |
| Cocaine | 1 | 0 | |
| Cannabis | 0 | 1 | |
| Current use of any psychoactive substance | 4 | 3 |
Demographic attributes shown as mean (SD) for continuous variables and N (%) for o rdinal variables.
By t-test for continuous variables and Fisher's exact test for ordinal variables.
Expressed as grams of testosterone equivalent, calculated as in previous studies (Pope & Katz, 1994)
Note that participants could be counted in more than one category (e.g., a participant could be using both opioids and other psychoactive medications).
Imaging findings
AAS users exhibited a significantly higher mean FA value in the IFOF than nonusers (estimated mean difference 0.035 [95% confidence interval 0.002, 0.069]; P = 0.038; η 2p=29). Within the AAS-user group, lifetime dose of AAS was positively associated with FA (regression coefficient B= 0.045 [0.016, 0.073]; P = 0.005, rpar=65; Figure 1). One AAS subject value was determined to be an outlier by leverage boxplot analysis (Figure 1). Accordingly, we conducted an additional regression analysis excluding this subject and found that the correlation persisted (regression coefficient B=0.038 [0.011, 0.066]; P = 0.009, rpar=63). A post-hoc laterality analysis showed that AAS users exhibited markedly greater FA than nonusers on the left IFOF (estimated mean difference 0.049 [0.013, 0.086]; P = 0.011; η 2p=40) but only a modest and nonsignificant difference on the right IFOF (0.024; [-0.020, 0.068]; P = 0.27; η 2p=094) (Figure 2). Correlation analyses with visuospatial memory tests did not show any significant effects (Supplement 1).
Figure 1.
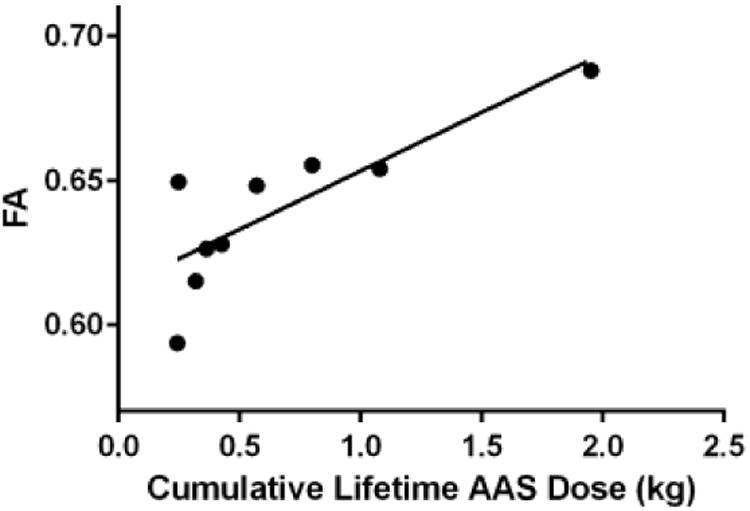
Association between left inferior fronto-occipital fasciculus (IFOF) fractional anisotropy (FA) and lifetime anabolic-androgenic steroid (AAS) dose (kg).
Figure 2.
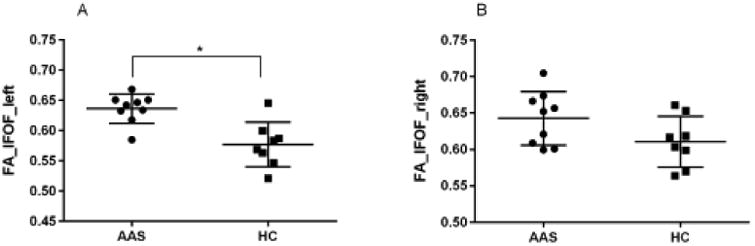
(A) Levels of fractional anisotropy (FA) of the left inferior fronto-occipital fasciculus (IFOF) in 9 long-term anabolic-androgenic steroid users (AAS) and 8 healthy controls (HC). (B) Levels of FA of the right IFOF in AAS and HC.
In a post hoc analyses, we assessed FA in a “control” region in which we did not expect to see any effect of AAS use, the corpus callosum. We found no group difference in corpus callosum FA, suggesting anatomical specificity of our IFOF finding (F=.013, df=1, p=.91).
Discussion
We used DTI to compare the coherence and organization of the IFOF in long-term AAS users versus non-users. AAS users exhibited increased FA relative to nonusers in the IFOF, and FA magnitude was strongly associated with AAS lifetime dose. The IFOF effect was lateralized to the left IFOF. These findings constitute the first in vivo report of white matter microstructural abnormalities in long-term AAS users.
Our previous studies of long-term AAS users have detected visuospatial memory impairments, right amygdala structural changes, and functional connectivity abnormalities (Kanayama et al., 2013; Kaufman et al., 2015). Since the right IFOF has been associated with visuospatial memory (Chechlacz et al., 2015; Peters et al., 2014), our FA finding could be related to visuospatial impairments in AAS users. However, the FA abnormality we detected was left-lateralized and was not significantly associated with visuospatial performance by study subjects. Further, although some studies demonstrate higher FA in pathological states (e.g., Williams syndrome (Haas et al., 2014)), neurodegenerative pathologies affecting white matter generally are associated with lower rather than higher FA values (Fu et al., 2012; Meng et al., 2012; Zhang et al., 2011). Thus, it is plausible that the left IFOF effect we detected either is unrelated to right amygdala abnormalities in AAS users (Kaufman et al., 2015) or could constitute some form of left hemispheric compensation for right-lateralized structural or functional connectivity abnormalities. While such an effect has yet to be demonstrated in the IFOF, white matter FA increases in the hemisphere contralateral to an ischemic stroke have been associated with motor recovery (Liu et al., 2015).
Long-term exposure to AAS also could promote white matter microstructural changes. In this regard, animal studies have reported an enhancing effect of testosterone on myelination during brain development as well as later in life (Patel et al., 2013; Stocker et al., 1994). Human developmental studies also have documented a relationship between higher testosterone levels and white matter volume increases in male adolescents (Perrin et al., 2008), suggesting that myelin growth is enhanced by androgens. Further, long-term testosterone supplementation in adult female to male transsexuals increased FA in two white matter tracts (Rametti et al., 2012). Accordingly, the FA increase we observed, which was strongly correlated with cumulative AAS exposure, could be an effect of androgenic stimulation of myelin growth in mature subjects.
In addition, the IFOF has been associated with executive function (Kucukboyaci et al., 2012), language (Egger et al., 2015, Mohades et al., 2012), reading (Takeuchi et al., 2016), and mathematical (Li et al., 2013) skills. Given its anatomical connections with amygdala and ventromedial and orbitofrontal areas, the IFOF could also function as part of the cognitive control circuitry which, if impaired, could contribute to drug dependence. Moreover, the left IFOF could play a role in reward systems, given its connectivity and its possible association with appetitive learning (Wessa et al., 2015). Therefore, the FA increase we detected may also indicate an abnormality of reward circuits, possibly associated with vulnerability for drug dependence. Notably, in this regard, it is estimated that some 30% of AAS users eventually develop an AAS dependence syndrome (chronic AAS use despite adverse effects on physical, psychosocial, or occupational functioning (see details in Kanayama et al., 2009a and formal diagnostic criteria in Kanayama et al., 2009b), and AAS users frequently also display polydrug use or dependence (Pope et al., 2014b; Skarberg et al., 2010).
Limitations to this study include its small sample size together with the possibility that the participants may not have been representative of AAS users and non-using weightlifters in the general population. Further studies are needed to replicate findings in larger groups. Also, participants' AAS use histories were obtained by self-report and are subject to reporting errors. However, inaccurate self-reports would likely have induced random errors, which would have been expected to obscure, rather than exaggerate, associations between AAS use and imaging measures. Similarly, if our non-user group included an undetected surreptitious AAS user, this also would likely have caused us to underestimate the group difference between AAS users and nonusers. Several of our participants were using other drugs at the time of evaluation which may affect white matter microstructure (Kaag et al., in press). While these potential interaction effects need to be studied further, it is most likely that group differences were underestimated rather than overestimated due to sample inhomogeneity.
In summary, we report an FA increase in AAS users in the left IFOF, a tract of the amygdala network involved in several cognitive processes including executive function and reward. The significant association between FA and lifetime AAS exposure suggests the clinical relevance of our finding and underscores the importance of conducting further research on the effects of long-term AAS exposure on brain structure and function.
Supplementary Material
Highlights.
Anabolic-androgenic steroids (AAS) cause psychiatric and cognitive abnormalities
We performed the first Diffusion Tensor Imaging study of long-term AAS users
Fractional anisotropy (FA) was higher in AAS users in an amygdala network tract
Among AAS users, FA in this tract was positively associated with lifetime AAS dose
The FA abnormality is consistent with prior human and animal studies of AAS effects
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achar S, Rostamian A, Narayan SM. Cardiac and metabolic effects of anabolic-androgenic steroid abuse on lipids, blood pressure, left ventricular dimensions, and rhythm. Am J Cardiol. 2010;106:893–901. doi: 10.1016/j.amjcard.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach M, Laun FB, Leemans A, Tax CM, Biessels GJ, Stieltjes B, Maier-Hein KH. Methodological considerations on tract-based spatial statistics (TBSS) Neuroimage. 2014;100:358–369. doi: 10.1016/j.neuroimage.2014.06.021. [DOI] [PubMed] [Google Scholar]
- Baggish AL, Weiner RB, Kanayama G, Hudson JI, Picard MH, Hutter AM, Jr, Pope HG., Jr Long-term anabolic-androgenic steroid use is associated with left ventricular dysfunction. Circulation. 2010;3:472–476. doi: 10.1161/CIRCHEARTFAILURE.109.931063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser P, Matielo B, Bihan D. MR diffusion tensor spectroscopy and imaging. Biophysics. 1996;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraci F, Pistara V, Corsaro A, Tomasello F, Giuffrida ML, Sortino MA, Nicoletti F, Copani A. Neurotoxic properties of the anabolic androgenic steroids nandrolone and methandrostenolone in primary neuronal cultures. J Neurosci Res. 2011;89:592–600. doi: 10.1002/jnr.22578. [DOI] [PubMed] [Google Scholar]
- Caverzasi E, Papinutto N, Amirbekian B, Berger MS, Henry RG. Q-ball of inferior fronto-occipital fasciculus and beyond. PLoS One. 2014;9:e100274. doi: 10.1371/journal.pone.0100274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chechlacz M, Gillebert CR, Vangkilde SA, Petersen A, Humphreys GW. Structural variability within frontoparietal networks and individual differences in attentional functions: an approach using the theory of visual attention. J Neurosci. 2015;35:10647–10658. doi: 10.1523/JNEUROSCI.0210-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham RL, Giuffrida A, Roberts JL. Androgens induce dopaminergic neurotoxicity via caspase-3-dependent activation of protein kinase C delta. Endocrinology. 2009;150:5539–5548. doi: 10.1210/en.2009-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger K, Yang S, Reisert M, Kaller C, Mader I, Beume L, Weiller C, Urbach H. Tractography of Association Fibers Associated with Language Processing. Clin Neuroradiol. 2015;25(2):231–236. doi: 10.1007/s00062-015-0447-2. [DOI] [PubMed] [Google Scholar]
- Estrada M, Varshney A, Ehrlich BE. Elevated testosterone induces apoptosis in neuronal cells. J Biol Chem. 2006;281:25492–25501. doi: 10.1074/jbc.M603193200. [DOI] [PubMed] [Google Scholar]
- Flanagan JN, Lehtihet M. The Response to Gonadotropin-Releasing Hormone and hCG in Men with Prior Chronic Androgen Steroid Abuse and Clinical Hypogonadism. Horm Metab Res. 2015;47:668–673. doi: 10.1055/s-0034-1398492. [DOI] [PubMed] [Google Scholar]
- Fu JL, Zhang T, Chang C, Zhang YZ, Li WB. The value of diffusion tensor imaging in the differential diagnosis of subcortical ischemic vascular dementia and Alzheimer's disease in patients with only mild white matter alterations on T2-weighted images. Acta Radiol. 2012;53:312–317. doi: 10.1258/ar.2011.110272. [DOI] [PubMed] [Google Scholar]
- Haas BW, Barnea-Goraly N, Sheau KE, Yamagata B, Ullas S, Reiss AL. Altered microstructure within social-cognitive brain networks during childhood in Williams syndrome. Cereb Cortex. 2014;24:2796–2806. doi: 10.1093/cercor/bht135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington P, Ali G, Chan A. The development of focal segmental glomerulosclerosis secondary to anabolic steroid abuse. BMJ Case Rep. 2011;2011 doi: 10.1136/bcr.07.2011.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlitz LC, Markowitz GS, Farris AB, Schwimmer JA, Stokes MB, Kunis C, Colvin RB, D'Agati VD. Development of focal segmental glomerulosclerosis after anabolic steroid abuse. J Am Soc Nephrol. 2010;21:163–172. doi: 10.1681/ASN.2009040450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBMCorp. IBM SPSS statistics for Windows, Version 22.0. Armonk, NY: IBMCorp; 2013. [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Kaag AM, van Wingen GA, Caan MW, Homberg JR, van den Brink W, Reneman L. White matter alterations in cocaine users are negatively related to the number of additionally (ab)used substances. Addict Biol. doi: 10.1111/adb.12375. in press. [DOI] [PubMed] [Google Scholar]
- Kanayama G, DeLuca J, Meehan WP, 3rd, Hudson JI, Isaacs S, Baggish A, Weiner R, Micheli L, Pope HG., Jr Ruptured tendons in anabolic androgenic steroid users: a cross-sectional cohort study. The American Journal of Sports Medicine. 2015a;43:2638–2644. doi: 10.1177/0363546515602010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama G, Hudson JI, DeLuca J, Isaacs S, Baggish A, Weiner R, Bhasin S, Pope HG., Jr Prolonged hypogonadism in males following withdrawal from anabolic-androgenic steroids: an under-recognized problem. Addiction. 2015b;110:823–831. doi: 10.1111/add.12850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama G, Brower KJ, Wood RI, Hudson JI, Pope HG., Jr Anabolic-androgenic steroid dependence: an emerging disorder. Addiction. 2009a;104(12):1966–78. doi: 10.1111/j.1360-0443.2009.02734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama G, Brower KJ, Wood RI, Hudson JI, Pope HG., Jr Issues for DSM-V: clarifying the diagnostic criteria for anabolic-androgenic steroid dependence. Am J Psychiatry. 2009b;166:642–5. doi: 10.1176/appi.ajp.2009.08111699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama G, Hudson JI, Pope HG., Jr Long-term psychiatric and medical consequences of anabolic-androgenic steroid abuse: a looming public health concern? Drug Alcohol Depend. 2008;98:1–12. doi: 10.1016/j.drugalcdep.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama G, Kean J, Hudson JI, Pope HG., Jr Cognitive deficits in long-term anabolic-androgenic steroid users. Drug Alcohol Depend. 2013;130:208–214. doi: 10.1016/j.drugalcdep.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman MJ, Janes AC, Hudson JI, Brennan BP, Kanayama G, Kerrigan AR, Jensen JE, Pope HG., Jr Brain and cognition abnormalities in long-term anabolic-androgenic steroid users. Drug Alcohol Depend. 2015;152:47–56. doi: 10.1016/j.drugalcdep.2015.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucukboyaci NE, Girard HM, Hagler DJ, Jr, Kuperman J, Tecoma ES, Iragui VJ, Halgren E, McDonald CR. Role of frontotemporal fiber tract integrity in task-switching performance of healthy controls and patients with temporal lobe epilepsy. J Int Neuropsychol Soc. 2012;18:57–67. doi: 10.1017/S1355617711001391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;40:1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Li Y, Hu Y, Wang Y, Weng J, Chen F. Individual structural differences in left inferior parietal area are associated with schoolchildrens' arithmetic scores. Front Hum Neurosci. 2013;7:844. doi: 10.3389/fnhum.2013.00844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Dang C, Chen X, Xing S, Dani K, Xie C, Peng K, Zhang J, Li J, Zhang J, Chen L, Pei Z, Zeng J. Structural remodeling of white matter in the contralesional hemisphere is correlated with early motor recovery in patients with subcortical infarction. Restor Neurol Neurosci. 2015;33:309–19. doi: 10.3233/RNN-140442. [DOI] [PubMed] [Google Scholar]
- Magnusson K, Hanell A, Bazov I, Clausen F, Zhou Q, Nyberg F. Nandrolone decanoate administration elevates hippocampal prodynorphin mRNA expression and impairs Morris water maze performance in male rats. Neurosci Lett. 2009;467:189–193. doi: 10.1016/j.neulet.2009.09.041. [DOI] [PubMed] [Google Scholar]
- Makris N, Meyer JW, Bates JF, Yeterian EH, Kennedy DN, Caviness VS. MRI-Based topographic parcellation of human cerebral white matter and nuclei II. Rationale and applications with systematics of cerebral connectivity. Neuroimage. 1999;9:18–45. doi: 10.1006/nimg.1998.0384. [DOI] [PubMed] [Google Scholar]
- Makris N, Pandya DN. The extreme capsule in humans and rethinking of the language circuitry. Brain Struct Funct. 2009;213:343–358. doi: 10.1007/s00429-008-0199-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin NM, Abu Dayyeh BK, Chung RT. Anabolic steroid abuse causing recurrent hepatic adenomas and hemorrhage. World J Gastroenterol. 2008;14:4573–4575. doi: 10.3748/wjg.14.4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng JZ, Guo LW, Cheng H, Chen YJ, Fang L, Qi M, Jia ZY, Mohammed W, Hong XN. Correlation between cognitive function and the association fibers in patients with Alzheimer's disease using diffusion tensor imaging. J Clin Neurosci. 2012;19:1659–1663. doi: 10.1016/j.jocn.2011.12.031. [DOI] [PubMed] [Google Scholar]
- Mohades SG, Struys E, Van Schuerbeek P, Mondt K, Van De Craen P, Luypaert R. DTI reveals structural differences in white matter tracts between bilingual andmonolingual children. Brain Res. 2012;1435:72–80. doi: 10.1016/j.brainres.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Mori S, Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006;51:527–539. doi: 10.1016/j.neuron.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Patel R, Moore S, Crawford DK, Hannsun G, Sasidhar MV, Tan K, Molaie D, Tiwari-Woodruff SK. Attenuation of corpus callosum axon myelination and remyelination in the absence of circulating sex hormones. Brain Pathol. 2013;23:462–475. doi: 10.1111/bpa.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin JS, Herve PY, Leonard G, Perron M, Pike GB, Pitiot A, Richer L, Veillette S, Pausova Z, Paus T. Growth of white matter in the adolescent brain: role of testosterone and androgen receptor. J Neurosci. 2008;28:9519–9524. doi: 10.1523/JNEUROSCI.1212-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters BD, Ikuta T, DeRosse P, John M, Burdick KE, Gruner P, Prendergast DM, Szeszko PR, Malhotra AK. Age-related differences in white matter tract microstructure are associated with cognitive performance from childhood to adulthood. Biol Psychiatry. 2014;75:248–256. doi: 10.1016/j.biopsych.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieretti S, Mastriota M, Tucci P, Battaglia G, Trabace L, Nicoletti F, Scaccianoce S. Brain nerve growth factor unbalance induced by anabolic androgenic steroids in rats. Med Sci Sports Exerc. 2013;45:29–35. doi: 10.1249/MSS.0b013e31826c60ea. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med. 1996;36:893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Kanayama G, Athey A, Ryan E, Hudson JI, Baggish A. The lifetime prevalence of anabolic-androgenic steroid use and dependence in Americans: current best estimates. The American journal on addictions / American Academy of Psychiatrists in Alcoholism and Addictions. 2014a;23:371–377. doi: 10.1111/j.1521-0391.2013.12118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope HG, Jr, Wood RI, Rogol A, Nyberg F, Bowers L, Bhasin S. Adverse health consequences of performance-enhancing drugs: an Endocrine Society scientific statement. Endocr Rev. 2014b;35:341–375. doi: 10.1210/er.2013-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope HG, Kouri EM, Hudson JI. Effects of supraphysiologic doses of testosterone on mood and aggression in normal men: a randomized controlled trial. Arch Gen Psychiatry. 2000;57:133–140. doi: 10.1001/archpsyc.57.2.133. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Katz DL. Psychiatric and medical effects of anabolic-androgenic steroid use. A controlled study of 160 athletes. Arch Gen Psychiatry. 1994;51:375–382. doi: 10.1001/archpsyc.1994.03950050035004. [DOI] [PubMed] [Google Scholar]
- Rametti G, Carrillo B, Gómez-Gil E, Junque C, Zubiaurre-Elorza L, Segovia S, Gomez A, Karadi K, Guillamon A. Effects of androgenization on the white mattermicrostructure of female-to-male transsexuals. A diffusion tensor imaging study. Psychoneuroendocrinology. 2012;37:1261–9. doi: 10.1016/j.psyneuen.2011.12.019. [DOI] [PubMed] [Google Scholar]
- Skarberg K, Nyberg F, Engstrom I. Is there an association between the use of anabolic-androgenic steroids and criminality? Eur Addict Res. 2010;16:213–219. doi: 10.1159/000320286. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Solbach P, Potthoff A, Raatschen HJ, Soudah B, Lehmann U, Schneider A, Gebel MJ, Manns MP, Vogel A. Testosterone-receptor positive hepatocellular carcinoma in a 29-year old bodybuilder with a history of anabolic androgenic steroid abuse: a case report. BMC Gastroenterol. 2015;15:60. doi: 10.1186/s12876-015-0288-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker S, Guttinger HR, Herth G. Exogenous testosterone differentially affects myelination and neurone soma sizes in the brain of canaries. Neuroreport. 1994;5:1449–1452. doi: 10.1097/00001756-199407000-00010. [DOI] [PubMed] [Google Scholar]
- Su TP, Pagliaro M, Schmidt PJ, Pickar D, Wolkowitz O, Rubinow DR. Neuropsychiatric effects of anabolic steroids in male normal volunteers. JAMA. 1993;269:2760–2764. [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Hashizume H, Asano K, Asano M, Sassa Y, Yokota S, Kotozaki Y, Nouchi R, Kawashima R. Impact of reading habit on white matter structure: Cross-sectional and longitudinal analyses. Neuroimage. 2016;133:378–389. doi: 10.1016/j.neuroimage.2016.03.037. [DOI] [PubMed] [Google Scholar]
- Tanehkar F, Rashidy-Pour A, Vafaei AA, Sameni HR, Haghighi S, Miladi-Gorji H, Motamedi F, Akhavan MM, Bavarsad K. Voluntary exercise does not ameliorate spatial learning and memory deficits induced by chronic administration of nandrolone decanoate in rats. Horm Behav. 2013;63:158–165. doi: 10.1016/j.yhbeh.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Tournier JD, Mori S, Leemans A. Diffusion tensor imaging and beyond. Magn Reson Med. 2011;65:1532–1556. doi: 10.1002/mrm.22924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanberg P, Atar D. Androgenic anabolic steroid abuse and the cardiovascular system. Handb Exp Pharmacol. 2010:411–457. doi: 10.1007/978-3-540-79088-4_18. [DOI] [PubMed] [Google Scholar]
- Voineskos AN, Rajji TK, Miranda D, Shenton M, Kennedy JL, Pollock BG, Mulsant BH. Age-related decline in white matter tract integrity and cognitive performance: A DTI tractography and structural equation modeling study. Neurobiol Aging. 2012;33:21–34. doi: 10.1016/j.neurobiolaging.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessa M, King AV, Meyer P, Frolich L, Flor H, Poupon C, Hoppstadter M, Linke J. Impaired and preserved aspects of feedback learning in aMCI: contributions of structural connectivity. Brain Struct Funct. 2016;221:2831–2846. doi: 10.1007/s00429-015-1075-y. [DOI] [PubMed] [Google Scholar]
- Zhang YZ, Chang C, Wei XE, Fu JL, Li WB. Comparison of diffusion tensor image study in association fiber tracts among normal, amnestic mild cognitive impairment, and Alzheimer's patients. Neurol India. 2011;59:168–173. doi: 10.4103/0028-3886.79129. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


