Abstract
Purpose
Studies have found a variety of evidence regarding the association between residential segregation measures and health outcomes in the US. Some have focused on any individuals living in residentially segregated places, while others have examined whether persons of specific races or ethnicities living in places with high segregation of their own race or ethnicity have differential outcomes. This paper compares and contrasts these two approaches in the study of predictors of late-stage CRC diagnoses in a cross-national study. We argue that it is very important when interpreting results from studies like this to carefully consider the geographic scope of the analysis, which can significantly change the context and meaning of the results.
Methods
We use US Cancer Statistics Registry data from 40 states to identify late-stage diagnoses among over 500 thousand CRC cases diagnosed during 2004–2009. We pool data over the states and estimate a multilevel model with person, county, and state levels and a random intercepts specification to ensure robust effect estimates. The isolation index of residential segregation is defined for racial and ethnic groups at the county level using Census 2000 data. The association between isolation indices and late stage CRC diagnosis was measured by 1) anyone living in minority segregated areas (place-centered approach), and by 2) individuals living in areas segregated by one’s own racial or ethnic peers (person-centered approach).
Results
Findings from the place-centered approach suggest that living in a highly segregated African American community is associated with lower likelihood of late-stage CRC diagnosis, while the opposite is true for people living in highly segregated Asian communities, and living in highly segregated Hispanic communities has no significant association. Using the person-centered approach, we find that living in places segregated by one’s racial or ethnic peers is associated with lower likelihood of late-stage CRC diagnosis.
Conclusions
In a model that covers a large geographic area across the nation, the place-centered approach is most likely picking up geographic disparities that may be deepened by targeted interventions in minority communities. By contrast, the person-centered approach provides a national average estimate suggesting that residential isolation may confer community cohesion or support that is associated with better CRC prevention.
Introduction
Cancer is the second most common cause of death in the US (ACS, 2015; Siegel et al., 2013), and colorectal cancer (CRC) is second behind lung cancer in the number of people who died from it in the US in 2015 (ACS, 2015). The incidence rate for CRC is now fourth highest among all cancer types in the US (ACS, 2015; Jemal et al., 2009). CRC screening rates are lower than recommended, resulting in higher rates of late-staged cancers and higher morbidity and mortality rates (ACS, 2015; Henley et al., 2010; Richardson et al., 2011). Of policy importance, there are disparities across population racial or ethnic subgroups in the likelihood of cancer being diagnosed at late-stage (Henley et al, 2010).
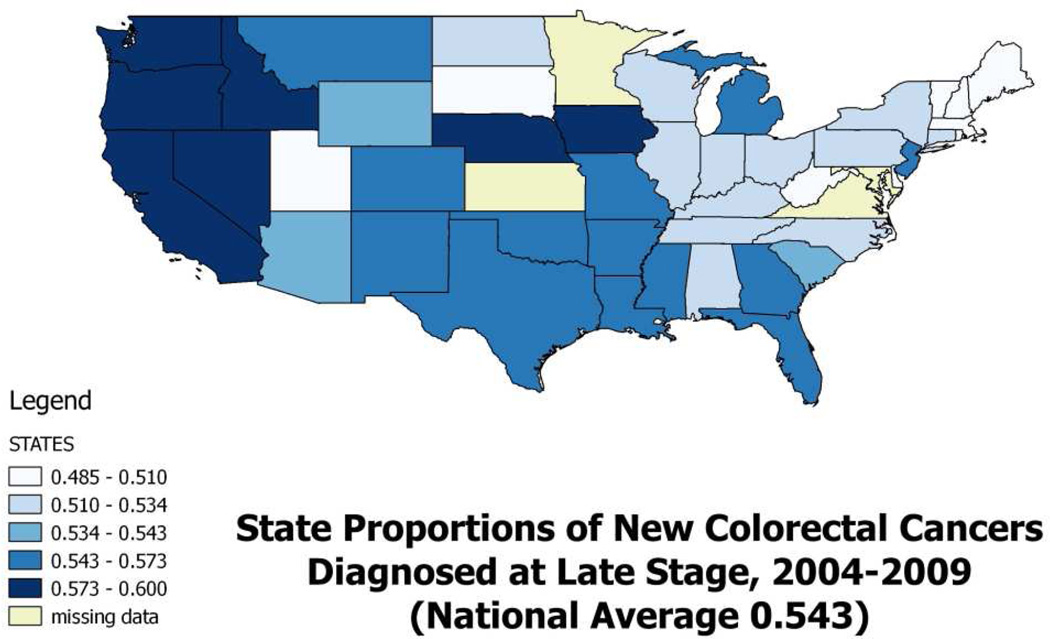
Using data from the United States Cancer Statistics (USCS) database, which is a population-based surveillance system of cancer registries with data representing 96% of the U.S. population (CDC, 2015), we examined all newly diagnosed CRC cases during 2004–2009. The overall rates of late-stage diagnoses for CRC vary considerably across the states (Figure 1), where states with proportions above the national average (54.3%) are shaded as the darkest two colors. The highest proportions are in the West and Pacific Northwest states.
Figure 1.
Proportions of CRC cases diagnosed at late-stage in the US, 2004–2009
A large literature has examined the role that social forces may play in shaping health outcomes such as these, where in addition to availability of services and financial means, personal information and motivation are required to enable timely access to preventive cancer screenings. We focus here on the role that residential segregation may play in providing this sort of support for colorectal cancer screening using endoscopy (colonoscopy and sigmoidoscopy).
Literature on Residential Segregation and Health
Williams and Collins (2001) were some of the first social scientists to argue that residential segregation caused racial or ethnic disparities in health outcomes, because it helped determine access to education and employment opportunities that can lead to differences in socioeconomic status, which is a fundamental cause of health disparities. Subsequently, many researchers have studied this phenomenon, using various different measures of residential segregation, citing the seminal work by Massey and Denton (1988) who rigorously defined several measures as a multidimensional phenomenon. Dimensions varied along five distinct axes of measurement: evenness, exposure, concentration, centralization, and clustering. Examples of these dimensions are found in measures such as the Diversity Index (evenness), Isolation Index (exposure), Interaction Index (exposure), Index of Spatial Proximity (clustering), and White's Clustering Measure (clustering).
Kramer and Hogue (2009) reviewed 39 studies of ecological factors and social outcomes to determine which of Massey and Denton’s segregation measures had been used in research, and by whom. They found that isolation, clustering, and dissimilarity indices had been used most often. In this study we chose to use the isolation index to measure residential segregation.
The isolation index used here is a minority-weighted average across census tracts of each county, using the formula defined as follows (Massey and Denton, 1988; Iceland et al, 2002):
where xi is the number of a minority group at tract i; X is the sum of all members of that minority group across all tracts; ti is the total number of people of all races or ethnicities in tract i; N is the number of tracts within each county. The county isolation index defined for a particular minority group reflects the extent to which the minority group comes into contact with others of this minority group within the county. The index ranges in value from 0 to 1, and a higher index value reflects the higher probability of contact among members of the minority group.
The isolation index has been interpreted as enhancing social cohesion or support (Warner and Gomez, 2010; Kuo et al., 2011; Haas et al., 2008; Mobley et al., 2006, 2008a, 2008b, 2009, 2010, 2012). However, some studies argue that residential isolation reflects an adverse environment (Dai, 2010; Williams and Collins, 2001; Schulz et al., 2002, 2005; Landrine and Corral, 2009; Morello-Frosch and Jesdale, 2006; Hao, et al., 2011). Others argue that segregation might be positively enhanced by a high degree of clustering into enclaves which increase political empowerment (Bell et al., 2006; Laveist, 1992, 1993). This political empowerment interpretation may be valid for the isolation index defined at larger geopolitical units such as metropolitan areas or states (rather than smaller neighborhoods or counties), because a higher-valued index at a larger scale indicates a greater degree of spatial clustering (Bell et al., 2006). It can be argued that the region may reflect broader factors such as political influence or community solidarity among minorities in the geopolitical units. Contradictory associations found within the same study contrasting models using different-sized areal units to define communities demonstrates that findings may be sensitive the areal unit size over which the isolation index is constructed (Mobley et al., 2008a).
We extend this argument here and posit that the geographic scope of the analysis may also impact the interpretation of the segregation effects. A study that is examining residential isolation effects within a metropolitan area or state may reflect something quite different than a cross-national study that pools data across 40 states, which is what we do in this paper. To date, there is no consensus in the health outcomes literature regarding whether residential isolation is a beneficial, or a harmful effect. We argue that the differences in signs and significance of associations within and across studies is likely due to a variety of factors: whether the isolation is defined specific to the individual or to the place, differences in size of areal units used to define neighborhood isolation, and differences in the geographic location or geographic scope of studies.
In several empirical cancer outcome studies, segregation indices for more than one minority were included in a regression model by linking the isolation indices to the multiracial/multiethnic study sample based only on place of residence (Hao et al., 2011; Dai, 2010; Mobley et al., 2008a, 2009, 2010; Mobley and Kuo, 2015). For example, all people living in county X were assigned the same isolation index values for each of the separate isolation indices included in the model. In these studies, the residential isolation indices included in the model were not explicitly matched to the study population’s race or ethnicity, thus the estimated isolation effect refers to anyone living in such places for each of the minority isolation indices included in the model. What this sort of modeling does not do is to capture the effect of living in a segregated place of one’s own race or ethnicity, which is a different construct altogether, as it centers the isolation index on the personal context.
In studies that include multiple races or ethnicities in the same empirical specification of the model, interacting the isolation index - defined for the race or ethnicity of each subject in their county of residence - with the person’s binary indicator of race or ethnicity is one method used to center the isolation index on the personal context, and to estimate a separate effect for each race or ethnicity included among the study subjects (Mobley et al, 2006; Mobley et al, 2008b). One study explicitly created a single composite person-centered isolation construct, as we do in this study, which provides an average effect across the races or ethnicities of the study subjects (Mobley et al., 2012). For example, an African American person living in county X is assigned the isolation index value for African Americans in that county, whereas a Hispanic person living in the same county is assigned the isolation index for Hispanics in that county. Other studies have centered the index on the person by estimating separate models for each race or ethnicity, and including only the single isolation index that pertains to the race or ethnicity of the subjects in the model (Warner and Gomez, 2010; Kuo et al., 2011; Haas et al., 2008).
Several studies examining late-stage cancer outcomes or cancer screening behavior have used the isolation index (Warner and Gomez, 2010; Kuo et al., 2011; Dai, 2010; Haas et al., 2008; Mobley et al., 2008a, 2008b, 2009, 2010, 2012, 2015). Three of these matched the person’s race or ethnicity to the isolation index used in the modeling (Haas et al., 2008; Kuo et al, 2011; Mobley et al, 2012). The first two studies analyzed different racial or ethnic subgroups in separate models, including only the own-race isolation index. The third study combined persons of all races and ethnicities together in a single model and included a composite race-matched isolation construct. All three studies interacted the isolation measure with higher-level constructs (such as state level insurance mandate or area level poverty rate), so the independent effect of the isolation measure is less clear. However, the effect seems beneficial among the SEER Registry subjects (Haas et al., 2008; Mobley et al, 2012) and beneficial for California women who are Hispanic or African American and living in the poorest communities (Kuo et al, 2011). A fourth study (Warner and Gomez, 2010) examined the impact of neighborhood racial composition (i.e. percent of African Americans in block group) and five different dimensions of segregation indices together – in an attempt to capture hyper-segregation - on late stage BC diagnosis and survival. Separate models were estimated for African American and white samples. Although beneficial effects of neighborhood composition and segregation measures were found for African Americans, the impact of the isolation index was jointly estimated with racial composition and 4 other segregation indices, so the independent effect of isolation could not be determined.
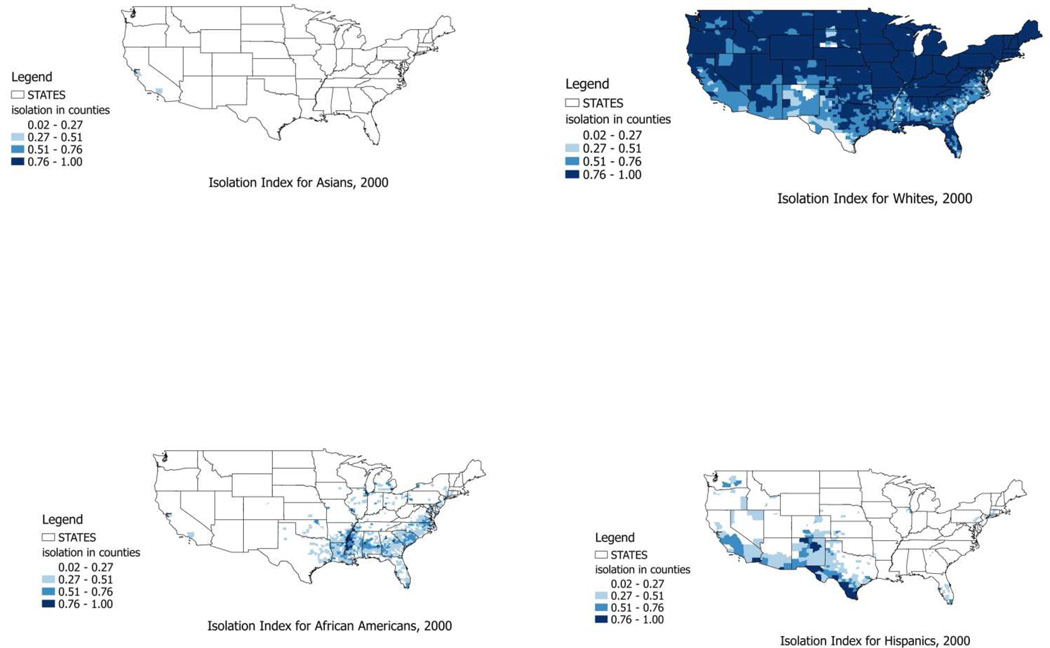
Figure 2 maps the isolation indices for the four dominant races or ethnicities, and demonstrates that highly isolated places exhibit distinct geospatial patterns by race or ethnicity in the US. Wilkes and Iceland (2004) argue that hyper-segregated places (i.e., the places that are highly segregated in one or more segregation dimensions) are rather atypical in the US, and more prevalent for African Americans and Hispanics. This is consistent with the somewhat regionalized geographic patterns of high isolation for these two groups evident in Figure 2. The quantiles of the distribution of the segregation index for whites were chosen as the cutpoints for different colors in the map, and these same cutpoints are consistently applied across the four maps to enhance comparability of the isolation indices across the maps.
Figure 2.
Residential Isolation Among Asians, Whites, African Americans, and Hispanics in US Counties, 2000
The contribution of this paper is to compare and contrast models of late-stage CRC diagnosis outcomes using two different approaches to modeling segregation (isolation) in large geographic areas across the nation. Both approaches pool together all states and all races and ethnicities into a single model covering the same geographic footprint over 40 states. The first approach, which we shall call ‘place-centered’, includes isolation indices in the model defined for the major minority groups (African American, Hispanic, Asian) matched by county of residence for each person in the CRC population. Thus three place-centered isolation index measures are matched to each person by county of residence. The second approach, which we shall call ‘person-centered’, uses the county level isolation index specific to the person’s race or ethnicity, and includes only this index for each person, in a composite race-matched isolation construct. Using estimates from the first, place-centered approach one can examine associations between anyone living in a particular minority enclave and their health outcome. Using the second, person-centered approach one can examine whether living in an enclave of one’s own race or ethnicity (whether it be a minority or majority race) has beneficial effects, suggestive of social support. Because we focus here on how the geographic scope should be considered when interpreting the isolation effect estimates, we pool the data so that the same large geographic scope or footprint is included in both approaches. Pooling across the 40 states, these estimates reflect national average effects.
Population Data and Methods
We examined CRC cases diagnosed during 2004–2009 from the United States Cancer Statistics (USCS) database, which is a population-based surveillance system of cancer registries with data representing 96% of the U.S. population (CDC, 2015). All but three states (Kansas, Maryland, Minnesota) participate in the USCS registry data system, but four states do not allow use of county of residence information (Illinois, Michigan, Missouri, Ohio). We excluded these 7 states and an additional state, Virginia, because data were not available until 2007. We also excluded Hawaii and Alaska because of missing contextual data, leaving a total of 40 states included in the analysis.
The database includes information on demographics (age, gender, race, ethnicity), tumor characteristics, and geographic location (state, county) at time of diagnosis. We restricted the sample to all persons having a first CRC diagnosis and excluded records when CRC was not the primary cancer, records with unknown cancer stage or unstaged cancer, or when diagnosis was by autopsy or death certificate (< 1% of all cases). These restrictions resulted in 553,629 individuals with CRC residing in the 40 states studied.
County level data describing contextual characteristics of communities derive from numerous sources. Data description and brief rationale for inclusion of each covariate are provided in Table 1. Sample statistics are also provided in Table 1. County statistics are based on the county level of observation.
Table 1.
Multilevel Model Variables: Description, Rationale, and Sample Statistics
| Variable (units of measure) |
Rationale for Inclusion | ||
|---|---|---|---|
|
Outcome whether cancer patient was diagnosed at a late stage (regional or distant =1, else=0) |
Late stage diagnosis is indicative of lack of knowledge regarding personal cancer risk, or the importance or availability of screening; lack of timely or proximate access to services, lack of funds to pay for, and cultural or other barriers related to utilization of timely cancer screening. |
Proport ion* |
Sdev |
| 0.543 | 0.498 | ||
| Person-level predictors (categorical variables coded into binary variables) | |||
| female | Both male and female are included in the CRC study, with male designated as the reference group. |
0.487 | 0.500 |
| African American | The national statistics cite African Americans as a disadvantaged group, with higher likelihood of late-stage CRC than whites, the reference group. |
0.112 | 0.315 |
| Hispanic | The national statistics cite Hispanics as a disadvantaged group, with higher likelihood of late-stage CRC than whites, the reference group. |
0.080 | 0.271 |
| Asian | The national statistics cite Asians as a disadvantaged group, with higher likelihood of late-stage CRC than whites, the reference group. |
0.031 | 0.172 |
| White (ref) | The reference group | 0.765 | 0.424 |
| Race all others | This group includes American Indians, Pacific Islanders, and others not defined above. We grouped them into a single indicator for the regressions. |
0.013 | 0.113 |
| age < 50 | CRC screening protocols recommend to start screening at age <50 for higher risk individuals |
0.111 | 0.275 |
| age 50–64 | CRC screening protocols recommend to start screening at age 50 for average risk individuals; this is the prime age bracket for screening |
0.314 | 0.464 |
| age 65–74 | Medicare insurance coverage begins at age 65 for people who are eligible for Social Security benefits |
0.250 | 0.433 |
| age 75+ (ref) | Screening is not needed or recommended as often for older individuals who have had regular screening at younger ages |
0.325 | 0.468 |
| County-level predictors (n = 2,471 counties in 40 states) | mean | sdev | min | max | |
|---|---|---|---|---|---|
| Isolation white | This index reflects the degree to which whites are proximate to other whites in their county of residence |
0.825 | 0.17 | 0.02 | 1.00 |
| Isolation African American |
This index reflects the degree to which African Americans are proximate to other African Americans in the county |
0.141 | 0.19 | 0.00 | 0.88 |
| Isolation Hispanic | This index reflects the degree to which Hispanics are proximate to other Hispanics in the county |
0.094 | 0.151 | 0.00 | 0.98 |
| Isolation Asian | This index reflects the degree to which Asians are proximate to other Asians in the county |
0.016 | 0.031 | 0.00 | 0.43 |
| Isolation Native American |
This index reflects the degree to which Native Americans are proximate to other Native Americans in the county (statistics exclude AK, not included in regressions) |
0.034 | 0.112 | 0.00 | 0.94 |
| Isolation Pacific Islander |
This index reflects the degree to which Pacific Islanders are proximate to other Pacific Islanders in the county (statistics exclude HI, not included in regressions) |
0.002 | 0.004 | 0.00 | 0.58 |
| Person-centered isolation construct |
This variable is constructed by retaining, for each individual, the specific isolation index (above) that reflects their race or ethnicity. This construct reflects the degree to which people are proximate to others of their same race or ethnicity in their county of residence. |
0.719 | 0.227 | 0.00 | 0.94 |
| Managed care penetration (%) |
Managed care has transformed the way medicine is practiced in highly-penetrated markets, with preventive care services more prevalent/utilized more intensively (2005). |
4.83 | 8.83 | 0.00 | 53.49 |
| Distance (miles) | Calculated as the average distance (miles) over all ZIP codes with centroid in the county to closest provider ZIP code. Greater distance to provider of CRC (endoscopy) screening suggests impeded access to preventive care services. Based on 100% FFS Medicare utilization of CRC screening endoscopy services (2006). |
12.10 | 9.74 | 0.00 | 72.45 |
| Screening rate (%) | Percent of the 100% FFS Medicare population residing in the county and alive all year that utilized CRC screening by endoscopy in 2006. |
10.32 | 1.78 | 1.15 | 18.18 |
| Percent uninsured | % of the under-age-65 population with no health insurance (2005) | 18.92 | 6.21 | 7.10 | 46.80 |
| Percent Rural | % of county population living in rural area (2005) | 59.88 | 30.59 | 0.00 | 100 |
| Percent Poverty | % of county population living in poverty (2005) | 15.96 | 6.71 | 2.50 | 51.00 |
(* mean of binary variable)
To construct the person-centered isolation construct, indices for the following races or ethnicities were matched to the person’s race or ethnicity: white, African American, Hispanic, Asian, American Indian, and Pacific Islander. One group of individuals classified as all ‘others’, representing less than ½ of one percent of the cancer registry population, had no isolation index defined that could be matched to them and they were widely dispersed among the 40 states. For this very small number of individuals, we used the average value of all the existing isolation indices in their county of residence to define an isolation index to match with them, so that they could be included in the analysis. Results were not sensitive to whether or not these individuals were dropped from the regressions.
Statistical Methods
We specified a three-level random intercepts logistic regression model for the late-stage diagnosis with patients nested in counties which were nested in states. We used a multilevel modeling framework because we wanted to fit the regression to individuals while accounting statistically for systematic, unexplained variation among counties and states. Omitted state-level factors include insurance regulations and mandates adopted by the states, who have autonomy to regulate the insurance practices within their states. Ignoring the county and state level effects, when they are important, is tantamount to having omitted variables in the model, which can bias the included coefficients’ estimates (Oakes, 2004). In addition, when the higher-level covariates (such as the isolation indices) have estimates that are of particular interest, failing to account for their structural similarity across individuals within their level (county) can increase their apparent statistical significance (Gelman and Hill, 2007). To avoid these empirical problems, we estimated the multilevel models using the Generalized Linear Latent and Mixed Model (GLLAMM) procedure (Rabe-Hesketh et al., 2004; 2008) in Stata (StataCorp, 2011). We estimated one model including three place-centered isolation indices (African American, Hispanic, Asian) and another model including the single person-centered isolation construct. Both of these models are cross-sectional, thus no reliable causal inferences can be made.
Results
The results from statistical modeling are presented in Table 2, and only statistically significant estimates are discussed. Person-level effects are consistent across the two models of residential isolation. Females are more likely than males to be diagnosed at late-stage for CRC. African Americans, Hispanics, and Asians are more likely to be diagnosed at late-stage than whites. The ‘other’ races and ethnicities are less likely than whites to be diagnosed at late stage. Younger people (less than age 50) are more likely than older people to be diagnosed at late stage, and this is the largest person-level effect estimate.
Table 2.
National Model Specified Using Two Different Approaches to Model Residential Isolation as a Predictor of Late-Stage Diagnosed CRC
| Model Using Several Place- Specific Isolation Measures |
Model Using Single Person- Centered Isolation Measure |
|||||
|---|---|---|---|---|---|---|
| Odds ratio | Lower CI | Upper CI | Odds ratio |
Lower CI |
Upper CI |
|
| female | 1.04 | 1.03 | 1.05 | 1.04 | 1.03 | 1.05 |
| African American | 1.09 | 1.07 | 1.11 | 1.07 | 1.04 | 1.09 |
| Hispanic | 1.10 | 1.07 | 1.12 | 1.08 | 1.06 | 1.11 |
| Asian | 1.10 | 1.06 | 1.14 | 1.07 | 1.03 | 1.12 |
| other | 0.51 | 0.49 | 0.54 | 0.50 | 0.47 | 0.53 |
| age <50 | 1.46 | 1.43 | 1.49 | 1.46 | 1.43 | 1.49 |
| age 50–64 | 1.02 | 1.01 | 1.04 | 1.02 | 1.01 | 1.04 |
| age 65–74 | 0.95 | 0.93 | 0.96 | 0.95 | 0.93 | 0.96 |
| Place-centered Isolation African American |
0.90 | 0.83 | 0.98 | |||
| Place-centered Isolation Asian | 1.37 | 1.01 | 1.86 | |||
| Place-centered Isolation Hispanic |
1.02 | 0.90 | 1.14 | |||
| person-centered isolation | 0.95 | 0.90 | 0.99 | |||
| managed care penetration | 1.04 | 0.92 | 1.18 | 1.07 | 0.95 | 1.19 |
| distance to closest provider | 1.00 | 1.00 | 1.01 | 1.00 | 1.00 | 1.00 |
| distance squared | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| CRC endoscopy screening rate | 0.97 | 0.96 | 0.98 | 0.97 | 0.96 | 0.97 |
| Percent uninsured | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.01 |
| Percent rural residence | 1.05 | 0.99 | 1.12 | 1.06 | 1.00 | 1.12 |
| Percent poverty | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Likelihood Ratio Test | 3172.64 (p<0.00001) | 3170.64 (p<0.00001) | ||||
| AIC | 758,066.02 | 758,065.94 | ||||
| Variance Components | ||||||
| Level 1 * (individual) | 3.2899 | 3.2899 | ||||
| Level 2 (county) | 0.02242 | 0.02260 | ||||
| Level 3 (state) | 0.00853 | 0.00821 | ||||
For logistic multilevel models, the variance for level one is assumed to be π2/3.
Shaded cells represent rows with that predictor not included in the model.
Among the county predictors, a higher countywide endoscopic CRC screening rate is associated with lower likelihood of late-stage diagnosis. (Other covariates including percent uninsured, percent living in poverty, managed care insurance penetration, and average distance to closest CRC screening provider are not significant predictors). Rural aspect of county of residence is a significant predictor in the person-centered isolation model, but is not significant in the place-centered model.
The county-level isolation measures are the main focus of this study. The place-centered model reflects the change in likelihood of late stage CRC cancer diagnosis with a one-unit change in the isolation index for anyone’s living in places with minority racial or ethnic enclaves. The results showed a lower likelihood of late-stage CRC diagnosis for anyone living in a higher segregated African American community (odds ratio= 0.90; 95% CI= 0.83 – 0.98), while living in a higher segregated Asian community (mainly the bay areas of California, Figure 2) is associated with higher likelihood of late-stage CRC diagnosis (odds ratio=1.37; 95% CI= 1.01 – 1.86). By contrast, the person-centered isolation index reflects the change in likelihood of late stage CRC cancer with a one-unit change in the isolation index for someone living in communities among one’s own race or ethnicity. We found that people living in more residentially isolated communities of their same race or ethnicity have lower likelihood of late-stage CRC diagnoses (odds ratio= 0.95; 95% CI= 0.90 – 0.99).
To determine the importance of information available in the random intercept terms, it is customary to look at the variance components estimated as model parameters. The variances for the null model, which includes random intercepts but no other predictors, are 0.02393 for the county level and 0.01067 for the state level (data not shown). The unexplained variance (null model) is only reduced slightly after including covariates in the models (bottom Table 2). However, as noted in previous statistical studies, the small residual variance components at the higher levels of the fitted model suggest that the model fit is good. Gumpertz et al. (2006) used a random intercepts formulation for the multilevel model with person and area-level covariates to model advanced-stage breast cancer incidence in Los Angeles county. As described there and in Oakes (2004), a small residual variance estimate for the area-level random effect indicates that the contextual factors included in the model do a good job accounting for geographic heterogeneity in the explanatory factors. A more traditional approach to measuring goodness-of-fit is the Likelihood Ratio Test. The Likelihood Ratio Test statistics and p-values are shown in Table 2. Both suggest that the explanatory variables contribute significantly to explaining the variance in person-level late-stage cancer diagnosis incidence. The AIC statistics, also presented there, are almost identical for the two models, suggesting that their fit is approximately equal.
Discussion
CRC screening that detects and removes pre-cancerous lesions, thus preventing late-stage cancers, is done using endoscopic procedures (colonoscopy, sigmoidoscopy) that are both expensive and somewhat risky. Out-of-pocket costs during this period included copayments, deductibles, and facility costs which amounted to hundreds of dollars per procedure. Also, the preparation is a lengthy ordeal and the procedure itself carries a small but significant risk of serious complications (perforated bowel) and other risks (dehydration, hyperglycemia, low blood pressure, adverse reaction to sedatives)(Mobley and Kuo, 2015). Thus, considerable encouragement or motivation is likely required to convince someone (and some cultures: Beyer et al, 2011; Stimpson et al. 2012; Rosenwasser et al., 2013) that this sort of screening is necessary. To undergo the treatment likely requires social cohesion (who is an expert provider?) and support (help during the three day ordeal). The late-stage CRC diagnosis outcome is expected to be significantly associated with measures of social cohesion or support, and should be sensitive to the availability and prevalence of endoscopic CRC screening.
The main focus of the paper is the comparison of two different approaches to assess the associations between a measure of social cohesion/support (residential isolation) and health outcomes (late-stage CRC diagnosis). The place-centered isolation model shows a lower likelihood of late-stage CRC diagnosis for anyone living in a highly segregated African American community, while living in a highly segregated Asian community (mainly the bay areas of California, Figure 2) is associated with higher likelihood of late-stage CRC diagnosis. By contrast, the person-centered isolation model suggests that people living in more residentially isolated communities of their same race or ethnicity have lower likelihood of late-stage CRC diagnoses. We anticipated that this latter approach would reflect some sort of social cohesion or support that would perhaps motivate appropriate cancer screenings and result in lower incidence of late-stage CRC diagnoses. This expectation was met, where the person-centered effect estimate was significant and negative. Thus, the higher the segregation measured in the person-centered isolation index, the lower the probability of late stage CRC cancer incidence for people living in the areas.
The place-based isolation modeling approach has less clear-cut interpretation as evidence of social cohesion or support. The place-centered approach models the effects of anyone living in a place where certain races or ethnicities are most highly segregated. An immediate problem with this approach is apparent when one is conducting a cross-national study, as we do here. As shown in Figure 2, highly residentially isolated places for Asians, African Americans, and Hispanics exhibit distinct geospatial patterns in the US. Because of these distinctly clustered patterns of segregation (i.e. Southeast for African Americans, Southwest for Hispanics), the place-centered isolation index may well reflect geographic disparities rather than the racial or ethnic disparities imbedded in the residential isolation measures. For example, highly segregated African American neighborhoods may well reflect different conditions in the Southeast relative to the rest of the US, and the isolation index variable may not well-represent social cohesion or support as intended.
As noted in the geographic disparities literature, several factors confound the problem of separating racial from geographic disparities: there is considerable variation in health care utilization and outcomes across regions; minorities may use different providers than whites; and racial disparities may be higher in some areas (Chandra and Skinner, 2003). These factors may cause strong statistical interactions between geography and racial or ethnic identity that may lead researchers to falsely diagnose geographic variations as the determinant of racial disparities. For example, Coughlin et al. (2002) contrast Southern counties with other counties in the United States and find that racial disparities in cancer screening are wider across the two groups of counties than they are within them. Similarly, Mobley et al (2008b, person-centered, and 2010, place-centered) examine predictors of breast and colorectal cancer screening across 11 states, estimated in separate models for each state - and find that the effects of residential isolation indices vary from positive to negative to statistically insignificant when states are examined separately (assessing effects of isolation within, rather than across states). Studies of smaller geographic scope (states, metropolitan areas) are more likely to identify social cohesion or support associations from residential isolation variables than are larger multi-state or cross-national studies. This fact alone may explain some of the inconsistencies in racial disparities found in the residential isolation-health outcomes literature.
Another mitigating factor worth considering is the likely impact of CRC screening interventions, which have not been uniformly distributed across the US. Cancer control efforts are largely decentralized to the states, and funded interventions have largely promoted reducing adverse minority disparities in health outcomes. In a literature review of PubMed articles, we identified 37 interventions aimed at increasing CRC screening conducted 1999–2009. Over half were targeted to minorities, low income, or non-English speaking groups in urban areas. Only two targeted rural communities. Minority enclaves in urban areas were more likely to receive intervention than other groups, and only 19 states and Washington DC had any (published) interventions (AZ, CA, CO, CT, GA, HI, IL, MD, MA, MI, MN, NH, NY, NC, PA, SC, TX, UT, WA, DC). The states in this list highlighted in bold had interventions targeted to African Americans (summarized in Table 3).
Table 3.
Eleven Interventions to Increase African American Colorectal Cancer Screening
| CRC screening Intervention name |
Date and Place of intervention |
Which minorities were targeted? |
Was the intervention evaluated/successful in increasing minority CRC screening? |
Citation |
|---|---|---|---|---|
| 1.Wellness for African Americans through Churches (WATCH) |
2000, five rural eastern North Carolina counties |
African American adults |
TPV intervention achieved a 15% increase in fecal occult blood testing screening. Those who spoke with a lay health advisor were significantly more likely to get a non-invasive screening test. Among those 50 years and older who received the newsletter/video intervention, there was an increase in colorectal cancer screening. |
Campbell, M.K., James, A., Hudson MA,& et al.(2004). Improving multiple behaviors for colorectal cancer prevention among African American church members. Health Psychol,23(5):492–502 |
| 2.Provider Education Intervention to Improve Colorectal Cancer Screening Rates among African American Patients |
Prior to 2007, Washington D.C. |
Health care providers and their African American patients aged 50 and older |
There was no statistical difference in the rates at which rectal exams and fecal occult blood tests were conducted before and after the intervention. There was a statistically significant increase in the performance of endoscopic assessments performed from 26.7% pre-intervention to 59.1% post- intervention. |
Friedman, M., and Borum, M.L. (2007). Colorectal cancer screening of African Americans by internal medicine resident physicians can be improved with focused educational efforts. J Natl Med Assoc., 99(9):1010–1012. |
| 3.A Tailored Telephone Outreach Program to Increase Screening in Urban African Americans |
2000–2003, New York City Metropolitan Area |
African Americans 52 years and older |
CRC screening was documented in 61 of 226 (27.0%) intervention participants and in 14 of 230 (6.1%) controls (prevalence rate difference=20.9%; 95% CI = 14.34, 27.46). Compared with the control group, the intervention group was 4.4 times more likely to receive CRC screening within 6 months of randomization. |
Basch, C.E., Wolf, R.L., Brouse, C.H., et al. (2006). Telephone outreach to increase colorectal cancer screening in an urban minority population. Am J Public Health, 96(12):2246–2253. |
| 4.Continuous Quality Improvement in Federally Qualified Health Centers |
January2000– January2005, Chicago, IL |
Lower income African-American and Hispanic patients over 50 years old |
At 1-year follow-up, rates of screening completion had increased from 11.5% to 27.9 percent (p< .001), and physician recommendation had increased from 31.6% to 92.9% (p< .001). |
Khankari, K., Eder, M., Osborn, C.Y., & et al. (2007). Improving colorectal cancer screening among the medically underserved: a pilot study within a federally qualified health center. J Gen Intern Med,22(10):1410–1414. |
| 5.Increasing Colorectal Cancer Screening in an Urban Public Hospital by Reducing Health System Barriers |
May-June 2003, Bronx borough of New York City |
African-American and Hispanic adults aged 50 and older |
The likelihood of keeping the screening appointment after the patient navigators were hired increased by nearly three-fold and the amount of broken appointments decreased dramatically (67% to 3%). The proportion of uninsured persons and Medicaid-insured persons who received a screening increased significantly. The proportion of screenings that had a patient navigator associated with them increased significantly. |
Nash, D., Azeez, S., Vlahov, D., & Schori, M. (2006). Evaluation of an intervention to increase screening colonoscopy in an urban public hospital setting. J Urban Health, 83(2):231–243 |
| 6.Increasing Colorectal Cancer Screening in Rural African American Women |
Prior to 2004, a rural southeastern state |
African American women aged 50 and older |
Experimental Full Intervention(s) Group 61% (n = 33) participated in FOBT. Modified Intervention(s) Group 46% (n = 15) participated in FOBT Control Group 15% (n=5) participated in FOBT at 1 year follow up. Participants in the Cultural and Self-Empowerment Group and those with greater knowledge of colorectal cancer were more likely to participate in fecal occult blood testing at the end of the 12-month period. |
Powe, B.D., Ntekop, E., & Barron, M. (2004). An intervention study to increase colorectal cancer knowledge and screening among community elders. Public Health Nurs,21(5):435–442. |
| 7. Offering Colorectal Cancer Screening and Education to Uninsured Minorities |
July 2001–June 30 2003, Montgomery County, Maryland |
Lower income and uninsured African American, Hispanic and Asian adults, aged 50 and older |
Over half of the participants who were eligible for invasive screening scheduled appointments. Over half of the total registrants completed some type colorectal cancer screening, and of those, ninety percent were minorities. |
Sarfaty, M., and Feng, S. (2006). Choice of screening modality in a colorectal cancer education and screening program for the uninsured. J Cancer Educ,21(1):43–49. |
| 8.Colorectal Cancer Screening Intervention Trial (CCSIT) |
January 2003–April 2005. Atlanta GA |
African Americans | Among completers, there were significant increases in knowledge in both educational cohorts. By the 6 month follow- up, 17.7% (11/62) of control group members reported having undergone screening, as compared to 33.9% (22/65) of the group education cohort (p = 0.039). |
Blumenthal, D., Smith, S., & Alema- Mensah, E. (2010). A Trial of Three Interventions to Promote Colorectal Cancer Screening in African Americans. Cancer,116(4): 922–929. |
| 9.Training African American PCP to perform colonoscopy |
October 1999–July 2006, South Carolina |
African American patients of trained African American PCPs (study group) vs. untrained PCPs (comparison group) |
African American patients in the study group showed a >5-fold increase (8.9% pre training vs 52.8% post-training), with no change among whites (18.2% vs 25.0%). |
Xirasagar, S., Thomas, G., Burch, J., & et al. (2011). Colonoscopy screening rates among patients of colonoscopy-trained African American primary care physicians. Cancer,117(22): 5151–5160. |
| 10.A Randomized Controlled Trial of the Impact of Targeted and Tailored Interventions on Colorectal Cancer Screening |
2001–2002, Philadelphia PA |
Male and female patients of the Jefferson Family Medicine Associates, Serving a substantial African American population (58%) |
Screening rates in study groups were 33% in the control group, 46% in the SI group, 44% in the TI group, and 48% in the TIP group. Screening was found to be significantly higher in all 3 intervention groups compared with the control group (odds ratio [OR] of 1.7 [95% confidence interval (95% CI), 1.3–2.5], OR of 1.6 [95% CI, 1.2–2.1], and OR of 1.9 [95% CI, 1.4–2.6], respectively) |
Myers, R., Sifri, R., Hyslop, T., & et al. (2007). A randomized controlled trial of the impact of targeted and tailored interventions on colorectal cancer screening. Cancer,110:2083–2091. |
| 11.Prostate, Lung, and Colorectal Cancer Screening Trial |
1999–2002, Detroit, Michigan |
African American men ≤ 55 years |
No statistically significant differences in adherence rates for flexible sigmoidoscopy screening for colorectal cancer. |
Ford, M., Havstad, S., Vernon, S., & et al. (2006). Enhancing adherence among older African American men enrolled in a longitudinal cancer screening trial. Gerontologist,46:545–550. |
The finding in this paper that living in a place with highly segregated African Americans is associated with lower rates of late-stage CRC diagnoses perhaps suggests that cancer control interventions to increase CRC screening which have targeted highly segregated African American communities have been quite effective. Between the years 1999–2009, eleven colorectal screening interventions were identified involving African American populations in the United States (summarized in Table 3). Several of these studies not only aimed to and succeeded in increasing CRC screening rates but also in enhancing social support/cohesion among minority populations. Several interventions were aimed at Hispanics/Latinos, but a much greater number were targeted to African Americans and some were targeted to both groups. In a rather stark contrast, no CRC screening interventions were found to specifically target Asian Americans, which is consistent with the finding that living in a highly segregated Asian American community is associated with higher rates of late-stage CRC diagnoses. Intervention among Asian Americans regarding the importance of CRC screening launched in the California bay areas may be warranted.
The place-centered isolation measures may reflect but do not specifically measure the importance (in terms of social cohesion or support) of living among people of one’s own race or ethnicity. The person-centered isolation measure in this study includes the effects of all races or ethnicities together and provides an association estimate for the overall average effect of living in more segregated communities of one’s racial or ethnic peers. This association is negative (reduces the odds of a late-stage diagnosis), and may reflect the fact that greater social cohesion or support is needed to promote the use of endoscopic CRC screening, which is quite invasive, moderately risky, unpleasant, and costly to undergo.
Conclusions
The United States is a very heterogeneous collection of states and counties. How to best represent factors that capture aspects of social cohesion or support is an ongoing enterprise. We have presented results which contrast a person-centered measure with a more general place-centered approach, and demonstrated that the effect estimates have different interpretations. The place-centered approach may also have different interpretations when employed across broad geographic regions, versus within specific regions, and must be carefully interpreted. The modeling approach used here is one that limits bias from omitted variables in model specifications (Oakes, 2004), and is robust to differences in population sizes across areas (Gelman and Hill, 2007). The modeling also captures quite well the heterogeneity in factors across the landscape of the 40 states studied. The correctly interpreted findings are therefore quite robust, however there are several limitations to this study and more research is needed to fully understand the implications.
We sought to examine and contrast two approaches for the study of residential isolation as a predictor of late-stage colorectal cancer diagnoses. We argue that social cohesion or support is perhaps better captured by the person-centered than the place-centered approach. However, the study has several limitations and leaves several important directions for future research. First, by including all racial and ethnic groups in one model that pooled data across all 40 states, we ensured that both approaches were using the same data from the same geographic footprint, making the findings more comparable. However, we also forced the effects of the person-centered isolation index to be the same for all racial and ethnic groups – providing a national average estimate of the beneficial effects of social cohesion or support. With a study population that consists of a vast majority of whites, this effect may reflect the dominant segregation effect of the white population. If this finding does indeed largely reflect the isolation effect for the white population, this may actually be a contribution because we have found no studies in the health literature focusing on white segregation effects. A similar limitation that pertains to the place-centered segregation model is that by pooling across the states, we forced the effects of the place-based isolation indices to be the same across all states. Thus these models produce estimates that are national averages, and do not reflect subsets of the data over geography or race. Another limitation of this study is that the segregation measures are modeled as a linear relationship with late stage CRC incidence. It is possible that the association is more complicated than this. Future areas of possibly fruitful research include examining the effect of race-specific or place-specific subsets of the data, or nonlinear specifications of the isolation variable. Fruitful analyses beyond the scope of the present paper might also include using various different logistic regression approaches (Merlo at al., 2006).
Barely any research has been done using the rich USCS database, which represents a collaboration among the US cancer registries and (at present) must be conducted inside secure Federal Research Data Centers, which are limited to about 15 locations nationwide. Alternatively, researchers can pay hefty fees to instruct NCHS programmers to conduct the analyses for them. All analyses must obtain prior approval by the NCHS staff and results of analyses are carefully scrutinized before release, to prevent invasion of privacy or disclosure of sensitive information. The NCHS and CDC are presently working together to develop infrastructure to make these data more broadly available. More work is definitely needed in controlling CRC, which exhibits high rates of late-stage diagnosis that could perhaps be reduced with more strategic screening intervention. Our findings will perhaps stimulate further research in this area.
Acknowledgments
This study was funded by a National Cancer Institute grant (2R01CA126858). CDC’s National Program of Cancer Registries contributed funds to cover the standard RDC fees for researchers conducting analyses under approved research projects. The content is solely the responsibility of the authors and does not necessarily represent the official views of Georgia State University, the University of North Carolina, the National Center for Health Statistics, the National Cancer Institute, or the National Institutes of Health.
List of abbreviations
- ACS
American Cancer Society
- CDC
Centers for Disease Control and Prevention
- CRC
colorectal cancer
- GLLAMM
generalized linear latent and mixed models
- NCSL
National Conference of State Legislators
- RDC
research data center
- USCS
US Cancer Statistics
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Lee R. Mobley, School of Public Health and Andrew Young School of Policy Studies, Georgia State University, 1 Park Place, Atlanta, Georgia, 30304, USA, lmobley@gsu.edu, 404-413-2338.
Lia Scott, School of Public Health, Georgia State University, Atlanta, Georgia, USA.
Yamisha Rutherford, School of Public Health, Georgia State University, Atlanta, Georgia, USA.
Tzy-Mey Kuo, Lineberger Cancer Center, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA.
References
- American Cancer Society (ACS) Cancer Facts and Figures 2015. Atlanta, GA: American Cancer Society; 2015. Available online August 2015: http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2015/index. [Google Scholar]
- Bell JF, Zimmerman FJ, Almgren GR, Mayer JD, Huebner CE. Birth outcomes among urban African-American women: a multilevel analysis of the role of racial residential segregation. Social science & medicine. 2006;63(12):3030–3045. doi: 10.1016/j.socscimed.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Beyer K, Comstock S, Seagren S, Rushton G. Explaining place-based colorectal cancer health disparities: Evidence from a rural context. Social Science & Medicine. 2011;72:373–382. doi: 10.1016/j.socscimed.2010.09.017. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) National Program of Cancer Registries, US Cancer Statistics. 2015 Available online October 2016: http://www.cdc.gov/rdc/b1datatype/dt131.htm.
- Chandra A, Skinner J. Geography and racial health disparities. NBER Working Paper. 2003 No.W9513. Available online October 2016: http://www.nber.org/papers/w9513. [Google Scholar]
- Coughlin S, Thompson T, Seeff L, Richards T, Stallings F. Breast, cervical, and colorectal carcinoma screening in a demographically defined region of the southern U.S. Cancer. 2002;95:2211–2222. doi: 10.1002/cncr.10933. [DOI] [PubMed] [Google Scholar]
- Dai D. Black residential segregation, disparities in spatial access to health care facilities, and late-stage breast cancer diagnosis in metropolitan Detroit. Health Place. 2010;16(5):1038–1052. doi: 10.1016/j.healthplace.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Gelman A, Hill J. Data analysis using regression and multilevel/hierarchical models. Cambridge University Press; 2007. [Google Scholar]
- Gumpertz ML, Pickle LW, Miller BA, Bell BS. Geographic patterns of advanced breast cancer in Los Angeles: associations with biological and socio- demographic factors (United States) Cancer Causes and Control. 2006;17:325–339. doi: 10.1007/s10552-005-0513-1. [DOI] [PubMed] [Google Scholar]
- Haas JS, Earle CC, Orav JE, Brawarsky P, Keohane M, Neville BA, Williams DR. Racial segregation and disparities in breast cancer care and mortality. Cancer. 2008b;113(8):2166–2172. doi: 10.1002/cncr.23828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y, Landrine H, Smith T, Kaw C, Corral I, Stein K. Residential segregation and disparities in health-related quality of life among Black and White cancer survivors. Health Psychology. 2011;30(2):137–144. doi: 10.1037/a0022096. [DOI] [PubMed] [Google Scholar]
- Henley SJ, King JB, German RR, Richardson LC, Plescia M. Surveillance of screening-detected cancers (colon and rectum, breast, and cervix)-United States, 2004–2006. Morbidity and mortality weekly report. Surveillance summaries (Washington, DC: 2002) 2010;59(9):1–25. [PubMed] [Google Scholar]
- Iceland J, Weinberg DH, Steinmetz E. U.S. Census Bureau, Series CENSR-3. Washington, DC: U.S Government Printing Office; 2002. Racial and Ethnic Residential Segregation in the United States: 1980–2000. 2002. [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA: a cancer journal for clinicians. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- Kramer MR, Hogue CR. Is segregation bad for your health? Epidemiologic reviews. 2009;31(1):178–194. doi: 10.1093/epirev/mxp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo TM, Mobley LR, Anselin L. Geographic disparities in late-stage breast cancer diagnosis in California. Health & place. 2011;17(1):327–334. doi: 10.1016/j.healthplace.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrine H, Corral I. Separate and unequal: residential segregation and black health disparities. Ethnicity & disease. 2009;19(2):179. [PubMed] [Google Scholar]
- LaVeist TA. The political empowerment and health status of African-Americans: mapping a new territory. American Journal of Sociology. 1992:1080–1095. [Google Scholar]
- LaVeist TA. Segregation, poverty, and empowerment: health consequences for African Americans. The Milbank Quarterly. 1993:41–64. [PubMed] [Google Scholar]
- Massey DS, Denton NA. The Dimensions of Residential Segregation. Social Forces. 1988;67(2):281–315. Available online October 2016: http://doi.org/10.2307/2579183. [Google Scholar]
- Merlo J, Chaix B, Ohlsson H, Beckman A, Johnell K, Hjerpe P, Råstam L, Larsen K. A brief conceptual tutorial of multilevel analysis in social epidemiology: using measures of clustering in multilevel logistic regression to investigate contextual phenomena. J Epidemiol Community Health. 2006 Apr;60(4):290–297. doi: 10.1136/jech.2004.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley LR, Root ED, Finkelstein EA, Khavjou O, Farris RP, Will JC. Environment, obesity, and cardiovascular disease risk in low-income women. American Journal of Preventive Medicine. 2006;30(4):327–332. doi: 10.1016/j.amepre.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Mobley LR, Kuo T, Andrews LS. How Sensitive are Multilevel Regression Findings to Defined Area of Context? A Case Study of Mammography Use in California. Medical Care Research and Review. 2008a Jun 1;65:315–337. doi: 10.1177/1077558707312501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley L, Kuo T, Driscoll D, Clayton L, Anselin L. Heterogeneity in mammography use across the nation: Separating evidence of disparities from the disproportionate effects of geography. International Journal of Health Geographics. 2008b;7:32. doi: 10.1186/1476-072X-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley L, Kuo M, Clayton L, Evans D. Mammography facilities are accessible, so why is utilization so low? Cancer Causes and Control. 2009;20(6):1017–1028. doi: 10.1007/s10552-009-9295-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley L, Kuo M, Urato M, Boos J, Lozano-Gracia N, Anselin L. Predictors of Endoscopic Colorectal Cancer Screening over Time in 11 States. Cancer Causes and Control. 2010;21(3):445–461. doi: 10.1007/s10552-009-9476-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley LR, Kuo TM, Watson L, Brown G. Geographic disparities in late-stage cancer diagnosis: multilevel factors and spatial interactions. Health & place. 2012;18(5):978–990. doi: 10.1016/j.healthplace.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley L, Kuo T. United States Health Policies and Late-Stage Breast and Colorectal Cancer Diagnosis: Why Such Disparities by Age? Health Economics Review. 2015 Jul 15;5:20. doi: 10.1186/s13561-015-0058-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morello-Frosch R, Jesdale BM. Separate and Unequal: Residential Segregation and Estimated Cancer Risks Associated with Ambient Air Toxics in U.S. Metropolitan Areas. Environmental Health Perspectives. 2006;114(3):386–393. doi: 10.1289/ehp.8500. Available online October 2016: http://www.jstor.org/stable/3436682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes JM. The (mis)estimation of neighborhood effects: causal inference for a practicable social epidemiology. Social Science & Medicine. 2004;58:1929–1952. doi: 10.1016/j.socscimed.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Rabe-Hesketh S, Skrondal A, Pickles A. GLLAMM Manual. U.C. Berkeley Division of Biostatistics Working Paper Series. 2004 Oct; Working Paper 160. Available online October 2016: http://www.bepress.com/ucbbiostat/paper160. [Google Scholar]
- Rabe-Hesketh S, Skrondal A. Multilevel and Longitudinal Modeling Using Stata. Second. College Station, TX: Stata Press; 2008. [Google Scholar]
- Richardson L, Tai E, Rim S, Joseph D, Plescia M. Vital Signs: Colorectal Cancer Screening, Incidence, and Mortality—United States, 2002–2010. Morbidity and Mortality Weekly Report. 2011;60(26):884–889. [PubMed] [Google Scholar]
- Rosenwasser LA, McCall-Hosenfeld JS, Weisman CS, Hillemeier MM, Perry AN, Chuang CH. Barriers to colorectal cancer screening among women in rural central Pennsylvania: Primary care physicians' perspective. Rural and Remote Health. 2013;13:2504. [PMC free article] [PubMed] [Google Scholar]
- Palloni A, Arias E. Paradox lost: explaining the Hispanic adult mortality advantage. Demography. 2004;41(3):385–415. doi: 10.1353/dem.2004.0024. [DOI] [PubMed] [Google Scholar]
- Schulz AJ, Kannan S, Dvonch JT, Israel BA, Allen A, III, James SA, House JS, Lepkowski J. Social and physical environments and disparities in risk for cardiovascular disease: the healthy environments partnership conceptual model. Environ Health Perspect. 2005;113(12):1817–1825. doi: 10.1289/ehp.7913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz AJ, Williams DR, Israel BA, Lempert LB. Racial and spatial relations as fundamental determinants of health in Detroit. Milbank Q. 2002;80(4):677–707. iv. doi: 10.1111/1468-0009.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R, Naishadham D, Jemal A. Cancer Statistics, 2013. CA: A Cancer Journal for Clinicians. 2013 Jan-Feb;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- StataCorp. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP; 2011. [Google Scholar]
- Stimpson JP, Pagán JA, Chen LW. Reducing racial and ethnic disparities in colorectal cancer screening is likely to require more than access to care. Health Affairs. 2012;31:2747–2754. doi: 10.1377/hlthaff.2011.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner ET, Gomez SL. Impact of Neighborhood Racial Composition and Metropolitan Residential Segregation on Disparities in Breast Cancer Stage at Diagnosis and Survival Between Black and White Women in California. Journal of Community Health. 2010;35(4):398–408. doi: 10.1007/s10900-010-9265-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkes R, Iceland J. Hypersegregation in the twenty-first century. Demography. 2004;41(1):23–36. doi: 10.1353/dem.2004.0009. [DOI] [PubMed] [Google Scholar]
- Williams DR, Collins C. Racial residential segregation: a fundamental cause of racial disparities in health. Public health reports. 2001;116(5):404. doi: 10.1093/phr/116.5.404. [DOI] [PMC free article] [PubMed] [Google Scholar]