Abstract
1. Objective
To describe the ocular findings, visual impairment, and association of structural complications of uveitis with visual impairment in a cohort of Ebola virus disease (EVD) survivors in Monrovia, Liberia
2. Design
Retrospective, uncontrolled, cross-sectional study
3. Participants
EVD survivors evaluated in an ophthalmology clinic at Eternal Love Winning Africa Hospital in Monrovia, Liberia.
4. Methods
A cohort of EVD survivors who underwent baseline ophthalmic evaluation at ELWA Hospital were retrospectively reviewed for demographic information, length of Ebola treatment unit (ETU) stay, visual acuity (VA), and ophthalmic examination findings. For patients with uveitis, disease activity (active vs. inactive) and grade of inflammation were recording according to Standardization of Uveitis Nomenclature (SUN) criteria. The level of VA impairment was categorized according to World Health Organization classification for visual acuity impairment as follows: Normal/Mild – VA 20/70 or better; Moderate – VA 20/70–20/200; Severe VA 20/200–20/400; Blindness – VA < 20/400. VA, length of ETU stay, and structural complications were compared between EVD survivors with and without uveitis. Structural complications associated with moderate VA impairment or poorer were also analyzed.
5. Main Outcomes
Frequency of ocular complications including uveitis and optic neuropathy in EVD survivors, level of visual acuity impairment in EVD survivors with uveitis, structural complications associated with visual acuity impairment in EVD survivors.
6. Results
96 EVD survivors were examined. 21 patients developed an EVD-associated uveitis and 3 patients developed an EVD-associated optic neuropathy. VA was blind (VA>20/400) in 38.5% of eyes with uveitis. Anatomic subtypes of uveitis included anterior, posterior, and panuveitis in 2, 13 and 6 patients respectively. Exam findings associated with at least moderate visual impairment by WHO criteria (VA<20/70) included keratic precipitates (p<0.002), posterior synechiae (p<0.002), vitritis (p<0.005) and chorioretinal scars (p<0.02).
7. Conclusions
EVD survivors are at risk for uveitis, which may lead to secondary structural complications, visual impairment, and blindness. Eye care resources should be mobilized for EVD survivors in West Africa owing to the frequency of this spectrum of disease complication and its potential for severe VA impairment and blindness.
Precis
Uveitis is common amongst Ebola virus disease (EVD) survivors with potential interventions that may impact visual outcomes and quality-of-life. There is an urgent need to mobilize eye care resources for EVD survivors in West Africa.
Introduction
The international community has witnessed the largest Ebola virus disease (EVD) outbreak in history, predominantly in the highest transmission West African countries of Guinea, Liberia, and Sierra Leone. Over 28,600 confirmed, probable or suspected EVD cases were identified during this outbreak and approximately 37% of cases occurred in Liberia; specifically, there were over 10,000 cases of EVD resulting in 4,809 deaths.1 The magnitude of the outbreak has lent itself to the largest cohort of EVD survivors in history. As the acute EVD outbreak has subsided, increased attention has shifted to ongoing Ebola survivor care needs.
Prior to this outbreak, there have been 23 EVD outbreaks with 2,345 laboratory confirmed cases and 1,546 deaths documented throughout Africa. These previous outbreaks have provided limited information on the long-term sequelae after EVD. The post Ebola virus disease syndrome (PEVDS), which develops during EVD convalescence, consists of fatigue, arthralgias, myalgias, neurological complications, abdominal pain, sensorineural hearing loss, increased risk of miscarriage, psychosocial stressors, and vision loss.2–4 PEVDS has been reported with greater frequency given the historic magnitude of the recent Ebola outbreak.
Ocular complications in EVD patients have been observed during acute disease and convalescence. During active Ebola virus infection, a bilateral viral conjunctivitis with or without subconjunctival hemorrhage is typical and acute vision loss has been described in some patients, but the etiology of vision loss has not been fully characterized.5 Ocular manifestations in EVD survivors during convalescence were first reported in the 1995 Kikwit outbreak. In four EVD survivors, a spectrum of uveitis ranging from anterior to posterior disease developed 42 to 72 days after EVD onset.5 During the most recent outbreak, two repatriated physicians who contracted EVD while working in West Africa developed acute, sight-threatening uveitis after recovering from EVD.6–7
More detailed observations from the recent West African outbreak have provided additional information about ophthalmologic sequelae. Specifically, Mattia et al. described uveitis, arthralgias, and auditory symptoms in 18%, 26%, and 24% of survivors respectively, from the Port Loko District, Sierra Leone during convalescence. In this cohort, a low RT-PCR cycling threshold during acute disease was an independent predictor of the development of uveitis.8 Other surveys on EVD survivors report a wide range of symptoms such as headache, anorexia, abdominal pain, fatigue, short term memory loss, and blurred vision.9–10 In another cohort of EVD survivors in Freetown, Sierra Leone, 34% of patients were diagnosed with uveitis. Eye injection/redness during acute EVD was found to be a risk factor for uveitis development. Other common ocular diseases in these patients included conjunctivitis, cataract, and glaucoma.11
Eye complaints are frequent among survivors and EVD-associated uveitis is an urgent diagnosis with potential interventions that may ultimately impact visual outcome and quality-of-life in West Africa survivors. Herein we report the detailed ophthalmic manifestations, structural complications and associated visual acuity impairment in recovered EVD patients evaluated at the Eternal Love Winning Africa (ELWA) Hospital in Monrovia, Liberia.
Methods
Study Design and Population
A retrospective, uncontrolled, cross-sectional study was performed on EVD survivors who underwent ophthalmic examination at the ELWA Hospital EVD Survivor Clinic in Monrovia, Liberia. This study was approved by the Emory University Institutional Review Board and the University of Liberia National Institutional Review Board and follows the tenets set forth by the Declaration of Helsinki.
Patients were determined to be EVD survivors by providing the examiners with an Ebola survivor certificate that is given to each patient upon discharge from an Ebola Treatment Unit (ETU). Survivors were treated in the following ETU’s in Liberia: Foya, ELWA 2, ELWA 3, Medecins Sans Frontieres (MSF), Bong County, US Public Health Service, Ministry of Defense, Modi, John F. Kennedy, Gbanga, Congo, MSF B, Bomi, Island Clinic, and Firestone.
An ophthalmology clinic was established in partnership with Emory Eye Center health care providers and the ELWA Hospital in April 2015. Patients were evaluated, treated, and/or referred as needed by examining ophthalmologists.
Infection Control/Personal Protective Equipment
All patients underwent an initial general medical health screening by health care professionals wearing personal protective equipment (PPE) that included a face shield, gown, gloves, and rubber boots. Patients were screened for fever with infrared thermometers and a questionnaire on symptoms of Ebola. Any patient with active EVD symptoms (diarrhea, vomiting, headache, abdominal pain) or elevated temperature greater than 100.4 degrees Fahrenheit (38.0 degrees Celsius) failed screening and were not examined that day by the ophthalmic providers until a medical evaluation had been conducted. In the outpatient eye clinic, providers wore fluid impervious gowns and gloves when providing patient care. All equipment was cleaned with alcohol swabs between each exam.
Data Collection
The medical records of 96 EVD survivors evaluated in April 2015 were retrospectively reviewed. Data collected included past medical and ocular history, ETU admit and discharge dates, ocular and systemic complaints, ophthalmic exam consisting of corrected visual acuity or pinhole visual acuity (Snellen visual acuity or tumbling ‘E’ chart), pupil exam, extraocular motility, confrontational visual fields, slit lamp examination, IOP (Reichert Technologies, Depew, New York) measurement, and dilated fundus examination with indirect ophthalmoscopy.
Demographic data recorded included ethnicity, age, and gender. Ocular complaints recorded were eye pain, tearing, redness, difficulty with near vision, light sensitivity, floaters, and blurred vision. A full review of symptoms was performed, specifically documenting the presence of patient self-reported fatigue, joint pain, hearing loss, and hair loss.
Uveitis was classified based on the anatomic location of inflammation following the standardization of uveitis nomenclature (SUN) guidelines.12 Active uveitis was defined as the presence of inflammation (cell/flare) in the anterior chamber (i.e. trace cell or greater) and/or vitreous haze with/without keratic precipitates (KP), corneal infiltrates, vascular sheathing, and retinal or choroidal infiltrates.
Inactive disease was characterized by signs of previous inflammation including pigmented KP, corneal scars, posterior synechiae (PS), condensed vitreous opacities, and chorioretinal scars.
Ebola-associated eye disease was defined as eye disease/ findings with vision loss or symptoms that occurred during acute Ebolavirus infection or after discharge from an ETU that was not related to trauma, prior documented illness/infection, or congenital disease. Ocular hypertension was defined as an IOP above 21 mmHg and hypotony as an IOP below 5 mmHg.
Main Outcome Measures
The primary outcome measured was the level of visual impairment in EVD survivors. Secondary outcomes evaluated included anatomic diagnoses leading to visual impairment in EVD survivors, structural complications identified in EVD survivors, and the association of specific anatomic features and structural complications with vision loss.
Statistical Analysis
Statistical analysis was performed with R 3.2.4. Descriptive data was summarized including demographic data and ocular and systemic symptoms. An unpaired t-test was used to compare the number of days in an ETU and baseline visual acuity in patients with and without uveitis. Fisher’s exact test was used for categorical variable comparisons. Visual impairment was categorized by the World Health Organization’s classification: normal or mild visual impairment: 20/70 or better, moderate visual impairment: 20/70–20/200, severe visual impairment: 20/200–20/400, or blindness: > 20/400).13 Visual acuities were converted to LogMAR for continuous data analysis. Statistical significance was defined as p<0.05.
Results
Characteristics of Study Population
Ninety-six EVD survivors (191 eyes) were examined in April 2015. All patients were Liberian and 46% were male with a median age of 38.6 years (interquartile range 29.0–47.5). The demographic information, ocular symptoms and signs, and medical histories are summarized in Table 1. EVD survivors reported a mean of 18 days spent (interquartile range [IQR]: 11–20 days) in an ETU setting. Ocular symptoms of survivors in decreasing order of frequency were blurry vision, photophobia, tearing, pain, floaters, eye redness, and loss of near vision (Table 2). Systemic symptoms noted in descending order of frequency included joint pain, hair loss, fatigue, and hearing loss (Table 2).
Table 1.
Specific demographic and clinical characteristics of patients with uveitis and neuro-ophthalmologic findings associated with Ebola virus disease
| Patient | Age/Gender | Eye | VA | Anatomic Location of uveitis |
Ocular Symptoms |
Ocular Findings |
PMH/POH | ROS |
|---|---|---|---|---|---|---|---|---|
| 1 | 12/M | OS | LP | Anterior | NR | PS, Cataract, LXT | Malaria | |
| 2 | 40/F | OD | 20/30 | Anterior | T, F, R, Bl | PS | JT, HL | |
| 3 | 36/F | OD | 20/25 | Anterior | Ph, T, Bl | PS | JT, HR | |
| OS | 20/20 | Anterior | Ph, T, Bl | PS | ||||
| 4 | 20/F | OD | 20/30 | Posterior | Pa, Ph, T, F, R, Bl |
CRS | Typhoid | FA, JT, HR |
| 5 | 42/F | OD | CF | Posterior | Pa, T | Vi, Macular CRS | ||
| 6 | 37/M | OD | 20/20 | Posterior | NVa, Pa, Ph, F, R, Bl, |
CRS | JT | |
| 7 | 38/M | OS | 20/70 | Posterior | Ph, T, R, Bl | CRS | FA, JT | |
| 8 | 44/F | OS | 20/20 | Posterior | T, Bl | CRS | JT, HR, HL | |
| 9 | OS | 20/40 | Posterior | Pa, Ph, T, R, Bl |
CRS | |||
| 10 | 28/M | OS | 20/20 | Posterior | Ph, F, Bl | CRS | ||
| 11 | 27/F | OD | 20/30 | Posterior | Pa, Ph, T, R, Bl |
CRS | FA, JT | |
| 12 | 42/F | OD | CF | Posterior | Pa, Ph, T, F, Bl |
CRS | Typhoid | HR |
| 13 | 31/M | OS | 20/50 | Posterior | Pa | CRS | JT | |
| 14 | 67/M | OD | 20/50 | Posterior | NVa, Ph, F, R, Bl |
CRS | FA, JT | |
| OS | 20/100 | Posterior | NVa, Ph, F, R, Bl |
CRS | ||||
| 15 | 55/M | OD | 20/20 | Posterior | R, Bl | CRS | JT | |
| 16 | 46/M | OS | LP | Panuveitis | Pa, Ph, F, R, Bl |
PS, Phthisis | Malaria | JT |
| 17 | 60/F | OD | CF | Panuveitis | Pa, Ph, R, Bl | Ac, KP, PS, Vi | FA, JT, HL | |
| 18 | 29/F | OS | CF | Panuveitis | Pa, Ph, T, R, Bl |
Ac, KP, PS, Vi | FA, JT, HL | |
| 19 | 28/F | OD | HM | Panuveitis | None | Ac, KP, Ked, PS, Vi, Engorged iris vessels |
JT | |
| OS | HM | Panuveitis | None | Ac, KP with blood, Ked, PS, Vi, Engorged iris vessels |
||||
| 20 | 36/F | OD | 20/20 | Posterior | Pa, Ph, T, R, Bl |
CRS | Corneal scar | |
| OS | CF | Panuveitis | Pa, Ph, T, R, Bl |
KP, PS, Macular CRS |
JT, HR | |||
| 21 | 25/F | OD | LP | Panuveitis | F, Bl | PS, Vi | FA, JT, HL | |
| OS | 20/25 | Posterior | F, Bl | CRS | ||||
| Neuro-ophthalmologic Findings and Disease Characteristics | ||||||||
| Patient | Age/Gender | Eye | VA |
Neuro- ophthalmologic Diagnosis |
Ocular Symptoms |
Neuro- ophthalmologic findings |
PMH/ POH | ROS |
| BD | 21/M | OD | 20/20 | Optic neuropathy, Argyll-Robertson pupil |
Pa, Ph, T, F, R, Bl |
Optic nerve pallor, Bilateral upgaze palsy |
FA, JT, HR | |
| OS | 20/20 | Optic neuropathy, Argyll-Robertson pupil, left internuclear ophthalmoplegia |
Pa, Ph, T, F, R, Bl |
Optic nerve pallor |
||||
| SM | 41/M | OS | HM | Optic neuropathy | Pa, T, F, Bl | Malaria | FA, JT | |
| HM | 42/F | OS | CF | Optic neuropathy | Pa, Ph, T, R, Bl |
Torsional nystagmus, optic nerve pallor |
JT, HR | |
Abbreviatons NVa – CF Counting fingers, HM Hand motions, LP Light perception, Near visual acuity, Pa Pain, Ph Photophobia, F Floaters, R Red, Blurred vision, Ac Anterior chamber cell, KP Keratic precipitates, Ked Corneal edema, Vi Vitritis, PS Posterior synechiae, LXT Left exotropia, CRS Chorioretinal scar, OA Optic atrophy, FA Fatigue, JT Joint pain, HR Hair loss, HL Hearing loss
Table 2.
Ocular and systemic symptoms in all Ebola virus disease survivors
| Ocular symptoms | Number of Patients (%) |
|---|---|
| Blurry vision | 73 (76) |
| Photophobia | 65 (68) |
| Tearing | 60 (62) |
| Pain | 54 (56) |
| Floaters | 45 (47) |
| Redness | 43 (45) |
| Loss of near vision | 1 (10) |
| Systemic Symptoms | |
| Joint Pain | 80 (83) |
| Hair Loss | 32 (33) |
| Fatigue | 20 (21) |
| Hearing Loss | 1 (10) |
EVD-Associated Uveitis
EVD-associated uveitis was diagnosed in 21 patients (22%) with five patients presenting with bilateral disease (26 eyes). The median age of this subset of patients was 36 years old (IQR: 28–42) and 38% were male. The patients with uveitis spent a median of 17 days in an ETU. Anterior uveitis, intermediate uveitis, posterior uveitis, and panuveitis were diagnosed in 3 (14%), 0, 12 (57%), and 6 (29%) patients respectively. Active disease was noted in six eyes, all in patients with a diagnosis of panuveitis. Exam findings indicative of uveitis are summarized in Table 3. Vision was normal or mildly impaired in 14 eyes (54%), moderately impaired in 2 eyes (8%), and 10 eyes (39%) were blind according to WHO classification.
Table 3.
Demographic and clinical characteristics of patients with Ebola virus disease associated uveitis
| Patient specific characteristics | |
| Number of patients | 21 |
| Median age at diagnosis of uveitis (IQR) | 36 (28–42) |
| Average number of days in ETU (IQR) | 17 (12.5–21.5) |
| Gender, % male | 38 |
| Race (Liberian,%) | 100 |
| Bilateral uveitis, (%) | 5 (23.8) |
| Anterior uveitis, (%) | 3 (14.3) |
| Intermediate uveitis, (%) | 0 |
| Posterior uveitis, (%) | 12 (57.1) |
| Panuveitis, (%) | 6 (28.6) |
| Anatomic form of uveitis | |
| Number of eyes affected | 26 |
| Anterior uveitis, (%) | 4 (15.4) |
| Intermediate uveitis, (%) | 0 |
| Posterior uveitis, (%) | 15 (57.7) |
| Panuveitis, (%) | 7 (26.9) |
| Ocular findings | Number (%) |
| Corneal edema | 2 (7.7) |
| Keratic precipitates | 5 (19.2) |
| Anterior segment cell | 4 (15.4) |
| Posterior synechiae | 11 (42.3) |
| Vitritis | 5 (19.2) |
| Cataract | 1 (3.8) |
| Chorioretinal scar | 16 (61.5) |
| Phthisis Bulbi | 1 (3.8) |
| Afferent pupillary defect | 4 (15.4) |
| Ocular hypertension | 2 (7.7) |
| Exotropia | 2 (7.7) |
|
World Health Organization Classification of Vision Impairment |
Number of eyes (Percentage of total eyes) |
| Normal/mild visual impairment (better than 20/70) |
14 (53.8) |
| Moderate visual impairment (20/70–20/200) | 2 (7.7) |
| Severe visual impairment (20/200–20/400) | 0 |
| Blindness (worse than 20/400) | 10 (38.5) |
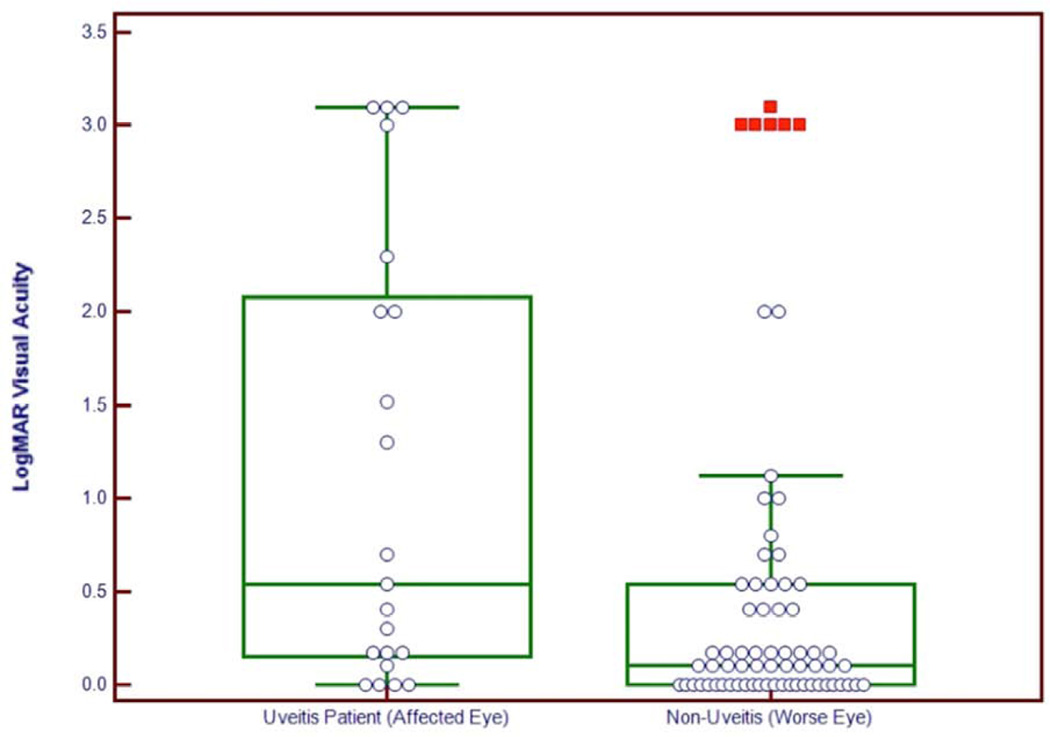
When comparing age, gender, systemic and ocular symptoms, and number of days in an ETU between survivors who developed uveitis and survivors who did not develop uveitis, there was no significant difference in these demographic and clinical characteristics (Table 4). Comparing the visual acuity in the affected eye of uveitis patients and the worse eye in patients without uveitis, patients with uveitis had worse vision (p=0.01) with uveitis patients presenting with worse vision (Figure 1). Exam findings including anterior chamber cells (p=0.01), keratic precipitates (p=0.01), posterior synechiae (p<0.001), and chorioretinal scars (p<0.001) were significantly more frequent in patients who developed uveitis (Table 4, Figures 2–4). The frequency of corneal edema was similar between patients who developed and those who did not develop uveitis (p=0.22).
Table 4.
Descriptive statistics in patients with and without Ebola virus disease associated uveitis
| Demographic, clinical feature or examination finding |
No Uveitis | EVD Associated Uveitis |
P value |
|---|---|---|---|
| Number of Patients | 75 | 21 | |
| Age (median) | 39 | 36 | 0.53 |
| Gender (% male) | 48 | 38 | 0.42 |
| Number of Days in ETU (median) | 14 | 17 | 0.27 |
| Symptoms (patients, %) | |||
| Blurry Vision | 56 (75) | 17 (81) | 0.55 |
| Photophobia | 52 (69) | 13 (62) | 0.52 |
| Pain | 43 (57) | 11 (52) | 0.69 |
| Tearing | 49 (65) | 11 (52) | 0.28 |
| Redness | 32 (43) | 11 (52) | 0.43 |
| Floaters | 37 (49) | 8 (38) | 0.32 |
| Joint Pain | 64 (85) | 16 (76) | 0.33 |
| Hair Loss | 24 (32) | 8 (38) | 0.60 |
| Fatigue | 13 (17) | 7 (33) | 0.13 |
| Hearing Loss | 8 (11) | 2 (10) | 1.00 |
|
Ocular Exam Findings (patients,%) |
|||
| Anterior Segment Cell | 0 | 3 (14) | 0.01* |
| Keratic Precipitates | 0 | 3 (14) | 0.01* |
| Corneal Edema | 0 | 1 (5) | 0.22 |
| Posterior Synechiae | 1 (1) | 8 (38) | <0.001* |
| Vitritis | 0 | 4 (19) | 0.002* |
| Chorioretinal Scars | 2 (3) | 13 (62) | <0.001* |
Statistically significant difference between EVD survivors with and without uveitis, P < 0.05 considered significant
Figure 1.
Box plot shows visual logarithm of minimum angle of resolution (logMAR) visual acuity of Ebola virus disease survivors with uveitis compared to those without uveitis (P < 0.0088).
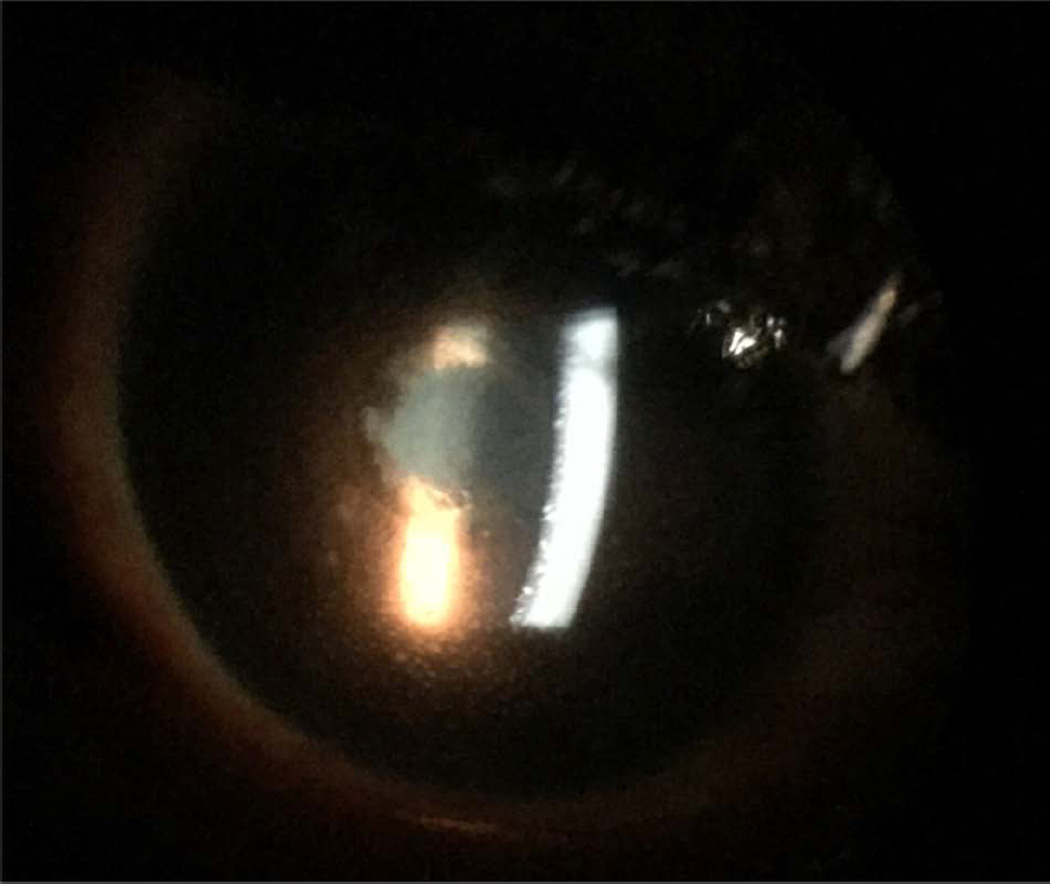
Figure 2.
Slit lamp photograph of an Ebola virus disease survivor with keratic precipitates, posterior synechiae and cataract in an eye with active, panuveitis.
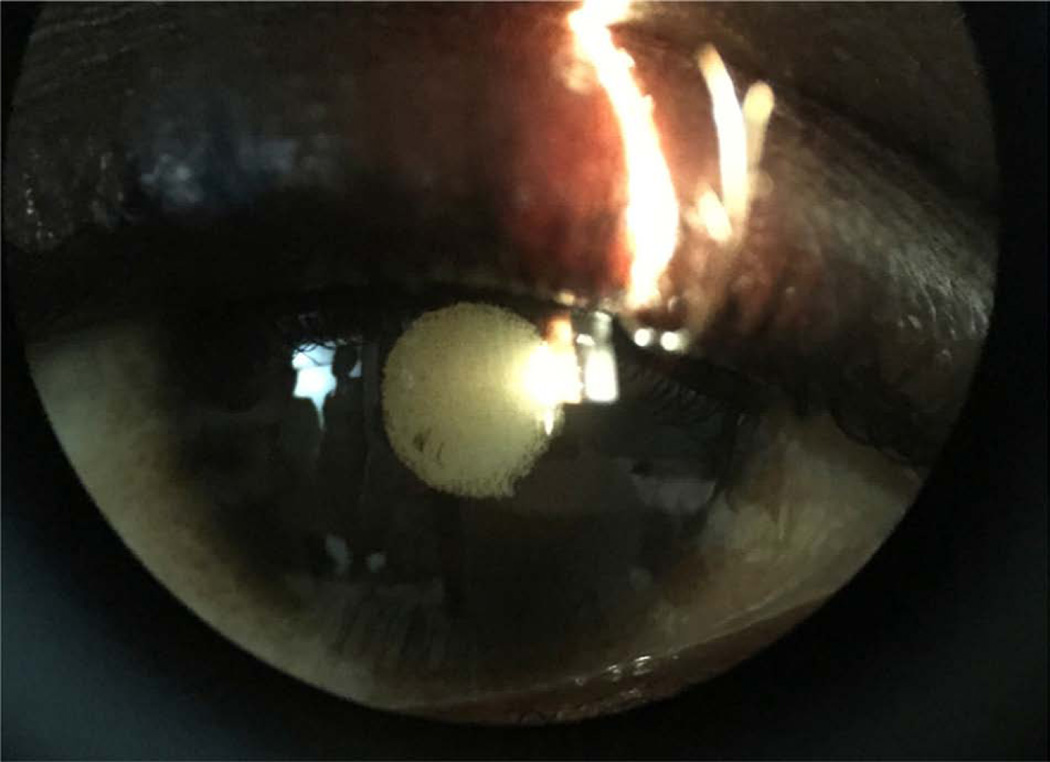
Figure 4.
Slit lamp photograph shows posterior synechiae and cataract in an Ebola virus disease survivor who had signs of inactive anterior uveitis.
Factors associated with at least moderate visual impairment by WHO criteria (VA<20/70) were also analyzed. Exam findings associated with at least moderate visual impairment included keratic precipitates (p<0.002), posterior synechiae (p<0.002), vitritis (p<0.005) and chorioretinal scars (p<0.02).
EVD-Associated Optic Neuropathy
EVD-associated optic neuropathy was observed in three patients (3%) with bilateral disease in 1 patient. In this subcategory, two patients were male who were 21 and 42 years old. Visual acuities ranged from 20/20 to hand motions in these three patients. Torsional nystagmus was observed in association with an optic neuropathy in one patient. Another patient with bilateral optic nerve pallor had an associated unilateral internuclear ophthalmoplegia, an Argyll Robertson pupil (i.e. reactive to accommodation but poor light reactivity in both eyes), and stutter in speech, which was reported to develop following his ETU discharge.
Cataracts in EVD survivors with and without uveitis
Because of the public health implications of potential Ebola viral persistence within ocular fluids, the frequency of cataract in EVD survivors with and without uveitis was reviewed Cataract was observed in ten of 96 EVD survivors evaluated (10%). Specifically, cataract was observed in nine of 75 EVD survivors without uveitis (12%) and one of 21 survivors with uveitis (5%). There was no significant difference in cataract prevalence in EVD survivors with or without uveitis (p > 0.05, two-tailed Fishers).
Other Ophthalmic Disease
In 35 patients (36.5%), normal ocular exams were observed. Other non-EVD associated eye diseases were diagnosed in this cohort that include senile nuclear sclerotic cataracts, refractive error, dry eyes, keratoconus, megalocornea, glaucoma, retinal detachment, congenital toxoplasmosis, and optic neuropathy.
Discussion
Our single-center retrospective, uncontrolled series of a EVD survivors who underwent an ophthalmic examination at ELWA Hospital in Monrovia, Liberia showed an 22% prevalence of uveitis, comparable to prior series from Sierra Leone, in which uveitis prevalence varied from 18% to 34% in EVD survivor clinics (Table 5).8,11 We identified a greater proportion of patients with posterior and panuveitis according to Standardization of Uveitis Nomenclature classification when compared to other series in the literature.
Table 5.
Prior case series and reports of eye disease in Ebola virus disease survivors
| Author | Site / Organization |
Key Findings |
|---|---|---|
| Kibadi et al5 |
Kikwit General Hospital, DRC |
Four survivors of 1995 Ebola outbreak of DRC developed ocular symptoms of ocular pain, lacrimation and decreased visual acuity. Anterior and posterior uveitis documented 42–72 days following acute diagnosis, all of which improved with steroids and 1% atropine |
| Mattia et al8 |
Lunsar Baptist Eye Hospital, Port Loko, Sierra Leone |
Uveitis was observed in 50 of 277 survivors (18%)Higher Ebola virus load associated with uveitis and new ocular symptoms or diagnosis |
| Tiffany et al11 |
MSF, Kissy UMC Hospital, Freetown, Sierra Leone |
Ocular disease observed in 94/ 166 survivors; Uveitis was documented in 57 (34%) patients. Survivors were 10- times more likely to develop uveitis if they presented with red/injected eyes during acute phase |
| Scott et al9 | Military Hospital 34, Freetown, Sierra Leone |
Six of 44 survivors with ophthalmic problems; Symptoms included eye pain, discharge, red eyes, and blurred vision within two weeks after discharge |
| Varkey et al7 |
Emory University (Sierra Leone) |
Case report of HCW who developed anterior uveitis progressing to severe panuveitis. Live Ebola virus identified in aqueous humor 100 days after acute EVD |
| Chancellor et al6 |
University of Massachusetts (Liberia) |
Case report of a HCW with anterior and intermediate uveitis with cystoid macular edema that responded to topical corticosteroids (anterior uveitis) and systemic prednisone (intermediate uveitis), also with elevated serum markers of inflammation |
| Chertow et al15 |
National Institutes of Health (Sierra Leone) |
Case report of a 34-year-old HCW evacuated from Sierra Leone with critical illness requiring intubation; bilateral anterior and posterior uveitis observed at 33 days post EVD diagnosis |
MSF Medecins sans frontieres
UMC United Methodist Church
DRC Democratic Republic of the Congo
EVD Ebola virus disease
HCW Health care worker
These proportions of inflammatory eye disease with posterior segment involvement likely impact visual acuity measures given that 38% showed poorer than 20/400 visual acuity at baseline. Vision impairment to this degree is most commonly associated with posterior uveitis14; however, untreated disease may also be associated with structural complications including cataract and vitreous opacity. Cataract and vitreous opacities were identifiable causes of vision loss in our patient cohort, and have implications from medical and surgical perspectives, particularly given our prior finding of live Ebola virus in the ocular fluids of a recovered Ebola survivor.7
Risk factors for the development of uveitis remain under investigation but one prior study by Mattia et al showed that lower RT-PCR cycling threshold representing higher Ebola viral load at the time of acute EVD infection was associated with uveitis and new ocular symptoms or new ocular diagnosis. Specifically, every five-point decrease in cycling threshold was associated with a three-fold or greater risk of uveitis and new ocular symptoms or diagnosis.8 A more recent study by Tiffany et al demonstrated that EVD survivors were 10-times more likely to develop uveitis if they showed red/injected eyes at the time of their acute EVD diagnosis; however, the precise etiology of the red/injected eyes is unknown (i.e. subconjunctival hemorrhage, conjunctivitis, uveitis or other cause) is not known because detailed ophthalmic examination was unavailable for these patients.11
The EVD survivors evaluated in our series were referred from a number of ETUs throughout Liberia. The length of ETU stay, sometimes as a surrogate for acute EVD severity, was not found to be associated with the development of uveitis. Systemic symptoms including fatigue, arthritis/arthralgias, hearing loss and hair loss were also not found to be associated with uveitis development. However, the prevalence of these findings was high in both uveitis and non-uveitis patients. In addition, other demographic factors (age, sex) were unassociated with uveitis development.
The reported timing of vision loss by patients varied but some patients reported vision loss while in the ETU setting. While it is unknown whether an acute anterior, posterior, panuveitis, or optic neuropathy developed during acute EVD, possibilities include optic neuritis with optic disc edema, posterior scleritis or retinochoroiditis. Besides uveitis related to Ebola, other potential causes of the scarring patterns observed include toxoplasmosis and other causes of uveitis endemic to the region.
Besides anterior, intermediate, posterior, and panuveitis, optic nerve disease including optic nerve pallor was identified in 3% of patients with 4 affected eyes. These patients had visual acuity impairment varying from 20/20 to HM and underscore the breadth of pathology that may be observed post EVD, and potentially acute EVD setting.
Neuro-ophthalmologic findings suggestive of central nervous system disease included nystagmus, ocular motility disorders, and internuclear ophthalmoplegia. Notably, severe meningoencephalitis has been observed in a health care worker with acute EVD and multiorgan failure. Both peripheral and central nervous system involvement were observed, as well as ocular deviation, cerebellar signs, and bilateral anterior and posterior uveitis. Magnetic resonance imaging showed evidence of microvascular ischemia and occlusion in this patient who eventually recovered from critical illness.15
Recently, World Health Organization officials put forth interim guidelines for the care of Ebola survivors in West Africa.16 Given the magnitude of the West African Ebola outbreak and historic number of EVD survivors, the interim guidelines have provided a starting point for longitudinal medical care of EVD survivors with chronic conditions including uveitis, musculoskeletal disease, and mental health syndromes, amongst a constellation of post EVD sequelae. Guidelines related to invasive ophthalmic procedures are currently being developed; however, given the potential for EBOV viral persistence in ocular fluid7, further studies into EBOV persistence in ocular fluids are needed. Improved understanding of the pathogenesis of this spectrum of inflammatory eye disease should include an assessment of the prevalence and timing of Ebola virus persistence in ocular fluids and concomitant biomarkers of ocular and systemic inflammation. This will help to inform appropriate medical therapy, as well as the infection control precautions necessary for the protection of eye care providers, patients and families related to invasive ophthalmic procedures.
Limitations of our study include the retrospective nature of data collection and the potential for recall bias. The study population was referred to the eye clinic, particularly if they complained of any ocular symptoms, introducing potential referral bias. Therefore, these findings may not be translatable to all EVD survivors. It is notable, however, that the presence or absence of ocular symptoms was not associated with uveitis diagnosis in this cohort of EVD survivors. Moreover, this study was not controlled and the baseline eye disease including prevalence of uveitis in this Liberian population is unknown. Future controlled studies related to the natural history of this disease entity and its response to therapy would be extremely helpful to affect the significant visual morbidity observed in this series.
Despite these limitations, our findings confirm recently reported findings from EVD survivor cohorts in Sierra Leone and importantly add the first detailed characterization of a uveitis phenotype and its association with visual acuity impairment. The lack of correlation between ophthalmic symptoms and the diagnosis of uveitis highlight the ongoing need for timely ophthalmic screening examination in EVD survivors, as well as the need for improved understanding of this spectrum of eye disease. Moreover, the finding of a high prevalence of sequelae of untreated ocular inflammation highlights the tremendous need for infusion of resources and training for eye care needs where expensive ophthalmic capital equipment and medications including corticosteroids, topical ocular hypotensives, and cycloplegics are required for the treatment of acute uveitis and related sequelae.
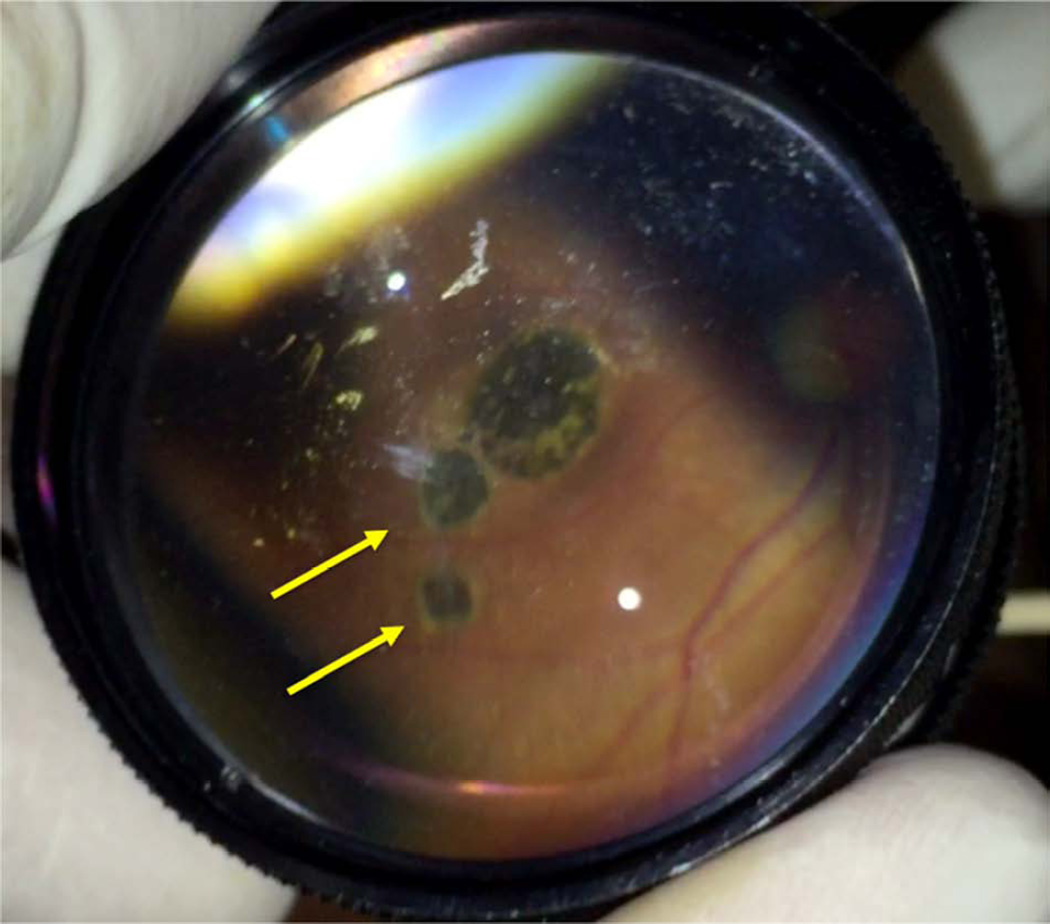
Figure 3.
Fundus photograph using 28-diopter condensing lens and iPhone shows chorioretinal scarring with characteristic hyperpigmented scars with hypopigmented halo in an Ebola virus disease survivor with posterior uveitis. Similar lesions were observed in the retinal periphery.
Acknowledgments
Financial Support
SIM/ELWA Hospital. The funding organization had no role in the design or conduct of research.
This project was supported by an unrestricted departmental grant from Research to Prevent Blindness, Inc. (New York, NY) and by NIH/NEI core grant P30-EY06360 (Department of Ophthalmology, Emory University School of Medicine), an Emory University School of Medicine University Research Committee Grant (Dr. Yeh), an unrestricted research grant from the Alcon Research Institute (Dr. Yeh), the Heed Ophthalmic Foundation (Dr. Shantha), the Alcon Foundation, and an unrestricted research grant from Santen, Incorporated.
Acronyms/Abbreviations
- EVD
Ebola Virus Disease
- ELWA
Eternal Love Winning Africa
- VA
Visual Acuity
- ETU
Ebola Treatment Unit
- SUN
Standardization of Uveitis Nomenclature
- PEVDS
Post Ebola Virus Disease Syndrome
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meeting Presentations
Association for Research in Vision and Ophthalmology Annual Meeting, Seattle, WA, May 2016 American Academy of Ophthalmology Annual Meeting, Chicago, IL, October 2016
Conflicts of Interest
None declared
References
- 1.World Health Organization: Ebola Situation Report. 2015 Dec 16;
- 2.Clark DV, Kibuuka H, Millard M, et al. Long-term sequelae after Ebola virus disease in Bundibugyo, Uganda: aretrospective cohort study. Lancet Infect Dis. 2015;15:905–912. doi: 10.1016/S1473-3099(15)70152-0. [DOI] [PubMed] [Google Scholar]
- 3.Qureshi AI, Chughtai M, Loua TO, et al. Study of Ebola Virus Disease Survivors in Guinea. Clin Infect Dis. 2015;61:1035–1042. doi: 10.1093/cid/civ453. [DOI] [PubMed] [Google Scholar]
- 4.Goeijenbier M, van Kampen JJ, Reusken CB, et al. Ebola virus disease: a review on epidemiology, symptoms, treatment and pathogenesis. Neth. J. Med. 2014;72:442–448. [PubMed] [Google Scholar]
- 5.Kibadi K, Mupapa K, Kuvula K, et al. Late ophthalmologic manifestations in survivors of the 1995 Ebola virus epidemic in Kikwit, Democratic Republic of the Congo. J. Infect. Dis. 1999;179(Suppl 1):S13–S14. doi: 10.1086/514288. [DOI] [PubMed] [Google Scholar]
- 6.Chancellor JR, Padmanabhan SP, Greenough TC, et al. Uveitis and Systemic Inflammatory Markers in Convalescent Phase of Ebola Virus Disease. Emerg Infect Dis. 2016;22:295–297. doi: 10.3201/eid2202.151416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varkey JB, Shantha JG, Crozier I, et al. Persistence of Ebola Virus in Ocular Fluid during Convalescence. The New England journal of medicine. 2015;372:2423–2427. doi: 10.1056/NEJMoa1500306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mattia JG, Vandy MJ, Chang JC, et al. Early clinical sequelae of Ebola virus disease in Sierra Leone: a cross-sectional study. Lancet Infect Dis. 2016;16:331–338. doi: 10.1016/S1473-3099(15)00489-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scott JT, Sesay FR, Massaquoi TA, et al. Emerg Infect Dis. 2016;22:641–646. doi: 10.3201/eid2204.151302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carod-Artal FJ. Post-Ebolavirus disease syndrome: what do we know? Expert Rev Anti Infect Ther. 2015;13:1185–1187. doi: 10.1586/14787210.2015.1079128. [DOI] [PubMed] [Google Scholar]
- 11.Tiffany A, Vetter P, Mattia J, et al. Ebola virus disease complications as experienced by survivors in Sierra Leone. Clin Infect Dis. 2016;62:1360–1366. doi: 10.1093/cid/ciw158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jabs DA, Nussenblatt RB, Rosenbaum JT Standardization of Uveitis Nomenclature(SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140:509–516. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. http://www.who.int/blindness/Change%20the%20Definition%20of%20Blindness.pdf.
- 14.Tomkins-Netzer O, Talat L, Bar A, et al. Long-term clinical outcome and causes of vision loss in patients with uveitis. Ophthalmology. 2014;121:2387–2392. doi: 10.1016/j.ophtha.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Chertow DS, Nath A, Suffredini AF, et al. Severe Meningoencephalitis in a Case of Ebola Virus Disease: A Case Report. Ann Intern Med. 2016;165:301–304. doi: 10.7326/M15-3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reference – Interim Guidelines on www.who.org. http://www.who.int/csr/resources/publications/ebola/guidance-survivors/en.