Abstract
Severe stress in early life, such as childhood abuse and neglect, constitutes a major risk factor in the etiology of psychiatric disorders and somatic diseases. Importantly, these long-term effects may impact the next generation. The intergenerational transmission of maternal early life stress (ELS) may occur via pre-and postnatal pathways, such as alterations in maternal-fetal-placental stress physiology, maternal depression during pregnancy and postpartum, as well as impaired mother-offspring interactions. The neuropeptide oxytocin (OT) has gained considerable attention for its role in modulating all of these assumed transmission pathways. Moreover, central and peripheral OT signaling pathways are highly sensitive to environmental exposures and may be compromised by ELS with implications for these putative transmission mechanisms. Together, these data suggest that OT pathways play an important role in the intergenerational transmission of maternal ELS in humans. By integrating recent studies on gene-environment interactions and epigenetic modifications in OT pathway genes, the present review aims to develop a conceptual framework of intergenerational transmission of maternal ELS that emphasizes the role of OT.
Keywords: Development, Depression, Early life stress, Epigenetics, Gene-environment interactions, Intergenerational transmission, Mother-child, Oxytocin, Parenting behavior
1. Introduction
It is well-established that the exposure to one or multiple forms of early-life stress (ELS), like childhood abuse and neglect, constitutes a major risk factor in the etiology of a wide range of somatic and/or psychiatric diseases (Anda et al., 2006; Felitti et al., 1998; Heim and Binder, 2012). However, it becomes increasingly apparent that these adverse long-term consequences are not restricted to the exposed individual alone but might be transmitted to the next generation (Collishaw et al., 2007), who also are at increased risk for psychiatric and somatic disorders (Roberts et al., 2014; Roberts et al., 2013) – a phenomenon referred to as intergenerational transmission (Bowers and Yehuda, 2016). Most studies discuss postnatal transmission pathways, such as non-optimal parenting behavior and psychopathology in ELS-exposed parents (Miranda et al., 2013) and offspring victimization (Plant et al., 2013). It is important to acknowledge however, that postnatal behavioral pathways, like postpartum depression and/or altered maternal behavior, are already primed during pregnancy (Feldman et al., 2007; Skrundz et al., 2011). Moreover, initial empirical evidence reveals ELS-associated alterations in maternal-fetal-placental (MPF) stress physiology that have been shown to alter fetal developmental trajectories (Moog et al., 2015). We therefore propose a continuous intergenerational transmission of maternal ELS that likely occurs during both the pre- and postnatal period via ELS-associated alterations in stress-sensitive biological systems, which may affect fetal development as well as the quality of postnatal dyadic mother-child interactions.
Within this context, we want to emphasize the role of the neuropeptide oxytocin (OT) as a key neuroendocrine factor modulating these candidate pre- and postnatal transmission pathways of maternal ELS. OT has gained considerable attention in studies of human social behaviors (Lee et al., 2009), including parenting (Bakermans-Kranenburg and van Ijzendoorn, 2008; Gordon et al., 2010; Lee et al., 2009; Meyer-Lindenberg et al., 2011) and attachment formation (Levine et al., 2007). Interestingly, there is evidence that ELS is associated with lower OT concentrations in the cerebrospinal fluid (CSF) of adult women (Heim et al., 2009) and non-human primates (Winslow et al., 2003). This ELS-induced reduction in CSF-OT was linked to pronounced deficiencies in social behavior in the study by Winslow et al. (2003). This suggests that central availability and functioning of the OT system may be susceptible to environmental factors such as ELS, which persist into adulthood and may impact functional integrity of the “parental brain”. It is also suggested that OT may play an important role in the development of depression (McQuaid et al., 2014), which is a highly prevalent clinical consequence of ELS and a condition that may interfere with optimal parenting behavior (Beck, 1995; Feldman, 2015b; Field, 2010). Finally, OT modulates the activity of stress-sensitive biological systems, such as the HPA-axis (Cardoso et al., 2014) and the immune system (Wang et al., 2015), which have been previously proposed to affect fetal development (Entringer et al., 2015). Taken together, these findings suggest OT signaling to be an exquisite target for pre- and postnatal pathways of intergenerational transmission of maternal ELS, which is further substantiated by findings in rodents (Champagne, 2008).
For the identification of putative OT pathways in the intergenerational transmission of maternal ELS in humans, it is important to gain a better understanding of 1) the function of OT in central and peripheral mechanisms that act as likely transmission pathways (e.g., altered MPF stress physiology, depression, parenting behavior), 2) the mechanisms that explain how ELS exposure affects OT signaling and thereby influences physiological stress regulation, depression risk and parenting behavior, 3) individual (genetic) differences that confer higher risk or resilience to the effects of ELS on OT-dependent phenotypes, and 4) when and how the effects of maternal ELS are transmitted to the next generation to alter offspring developmental trajectories. Recent studies are beginning to address some of these questions by focusing on epigenetic modifications and common genetic variants in OT-pathway genes (i.e., genes that either code for the peptide and/or its receptor) that may interact with environmental factors (e.g., ELS) to account for phenotypic variability, such as risk for emotional dysregulation, insecure attachment, depression, or anxiety symptoms (Bradley et al., 2011; McQuaid et al., 2013; Thompson et al., 2011). In particular, sequence variations and epigenetic modifications in the oxytocin receptor gene have emerged as promising candidates in numerous studies and will therefore receive of particular attention in this review. Finally, the aim of the present work is to integrate relevant findings in order to elaborate a theoretical framework of oxytocin pathways in the intergenerational transmission of maternal ELS, which is shown in Figure 1.
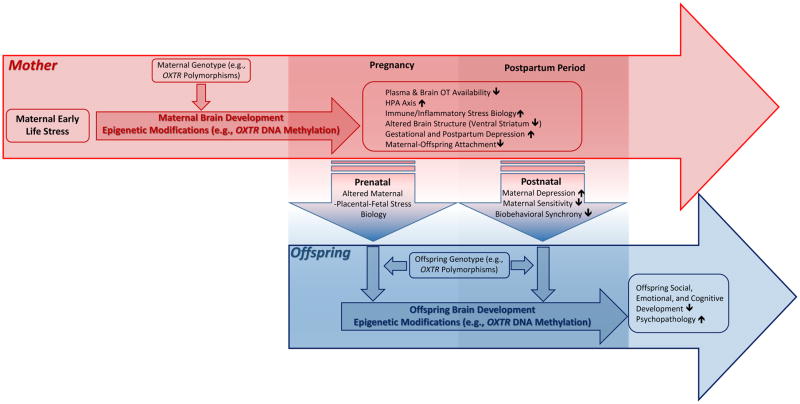
FIGURE 1.
Oxytocin Pathways of Intergenerational Transmission of Maternal Early Life Stress (ELS) – A Conceptual Framework ELS, such as childhood abuse and neglect, is a major risk factor in the etiology of psychiatric disorders (e.g., depression) and impaired parenting. These maladaptive long-term effects may also impact the next generation via multiple, partly overlapping pre- and postnatal pathways, such as maternal depression during pregnancy and postpartum and impaired parent-offspring interactions. Central oxytocin (OT) pathways, which can be altered by ELS, are known to be involved in the modulation of parenting behaviors, attachment formation, pre- and postnatal depressive symptoms, as well as MPF stress physiology. Thus, OT pathways may play an important role in the intergenerational transmission of ELS in humans. Gene-environment interactions and epigenetic modifications in OT pathways genes may moderate or mediate the assumed pre- and postnatal transmission pathways.
Abbreviations: DNA: Desoxyribonucleic Acid; HPA-axis: Hypothalamic-Pituitary-Adrenal axis; MPF: Maternal-Placental-Fetal; OT: Oxytocin; Oxtr: Oxytocin Receptor Coding Gene.
2. OT and Early-Life Stress (ELS) – Role in Shaping Neural Circuits That Underlie Parenting Behavior and Depression Risk
2.1 Oxytocin (OT) and the Oxytocin Receptor (OTR)
OT is a small nonapeptide which is highly conserved among mammalian species (Donaldson and Young, 2008). The OT gene, which first codes for a preprohormone that is then processed to OT and its carrier protein neurophysin 1, contains three exonic and two intronic regions and is located on chromosomal region 20p13 in humans (Gopal Rao et al., 1992). Magnocellular neurons of the hypothalamic paraventricular nucleus (PVN) and the supraoptic nucleus, the primary sources of OT synthesis, project to the posterior pituitary, where OT is stored in large secretory vesicles, so-called large dense-core vesicles. In response to calcium influx as well as intracellular calcium release from the endoplasmic reticulum, OT is released into systemic circulation (Meyer-Lindenberg et al., 2011) from axonal terminals within the neurohypophysis (Ludwig and Leng, 2006; Ludwig et al., 2002). The most prominent peripheral OT effects via the classical hypothalamic-neurohypophyseal pathway are the induction of parturition through increased contractibility of the uterine smooth muscles and milk ejection from the mammary gland in response to suckling stimuli in lactating females (Lee et al., 2009). In the central nervous system (CNS), however, OT’s communication pathways are more complex (Knobloch et al., 2012; Landgraf and Neumann, 2004) and still subject to investigation. There are two proposed mechanisms through which OT neurons in the hypothalamus communicate with extrahypothalamic neurons and brain structures. First, it has been suggested that there is a slow, “unwired”, and global transmission of OT that is released mainly from neuronal dendrites, but also from axons and soma in the hypothalamus to reach extrahypothalamic brain structures, such as the amygdala or the cingulate cortex (Boccia et al., 2013). This diffuse mode of communication, referred to as volume transmission (Landgraf and Neumann, 2004; Ludwig and Leng, 2006), enables OT to act as a neuromodulator within the brain and implies a slow enzymatic degradation of OT, which in turn permits OT to travel long distances (Landgraf and Neumann, 2004). Second, it has been shown that in the rodent (Knobloch et al., 2012) and human brain (Boccia et al., 2013) there is a variety of “hard-wired” oxytocinergic nerve fibers from the hypothalamus to limbic, mesencephalic, and cortical brain regions that allow fine-tuned and fast modulation of target structures (Knobloch et al., 2012; Landgraf and Neumann, 2004; Stoop, 2012; Strathearn, 2011). Effects of OT in the CNS and in the periphery (e.g., heart and cardiovascular system, kidney, reproductive organs) are critically dependent on presence of OT receptors (OTR) (Gimpl and Fahrenholz, 2001). The OTR is a 389-amino acid polypeptide and belongs to the G-protein coupled receptor superfamily (Gimpl and Fahrenholz, 2001; Kimura et al., 1992). The OTR expressing gene (OXTR) is located on chromosomal region 3p25–3p26.2, spans 17 kb, and consists of 3 introns and 4 exons (Gimpl and Fahrenholz, 2001). Studies on distribution patterns of OTRs in human post mortem brains found high OTR expression in amygadaloid nuclei, cingulate cortex, the hypothalamic medial preoptic area (MPOA), PVN, ventromedial nucleus, the solitary nucleus, and the spinal trigeminal nucleus (Boccia et al., 2013; Freeman et al., 2016). Given this wide spatial distribution of OTRs in the brain, OT is able to modulate an array of CNS-dependent processes such as social cognition, motivated behavior, and emotion regulation. Nevertheless, due to a lack of a radioligand for the OTR, there is a need for future in vivo studies on OTR distribution patterns in the living human brain (Insel, 2016).
2.2 OT Brain Circuits Relevant for Parenting Behavior
OT has been shown to be a pivotal neuromodulator of social cognition, empathy, emotion regulation, and motivation within an extensive neural network that is involved in human and non-human mammalian parenting and caregiving (Feldman, 2015a; Rilling, 2013; Rilling and Young, 2014; Swain et al., 2014). This network is comprised of hypothalamic nuclei, importantly the MPOA, the mesocorticolimbic system, which encompasses the amygdala, the hippocampus, the nucleus accumbens (nAcc), the ventral tegmental area (VTA), the pre-frontal cortical regions, the ventral pallidum (Love, 2014; Skuse and Gallagher, 2009; Strathearn, 2011), as well as the dopaminergic nigrostriatal pathway (Feldman, 2015a). Extensive research in rodents indicates that OTRs are abundantly expressed within this network rendering it sensitive to the effects of OT (Love, 2014; Numan and Stolzenberg, 2009). Already during the gestational period, the MPOA, which is thought to constitute the core of this network, is hormonally primed, likely through an estrogen (E)-induced upregulation of OTR expression (Bealer et al., 2006; Champagne et al., 2001; Meddle et al., 2007; Numan and Stolzenberg, 2009). This E-primed network is then “triggered” by pup stimuli after parturition to result in immediate onset of maternal behavior, which is then maintained in the postpartum period (Numan and Stolzenberg, 2009; Pedersen, 1997). Infusion of OT into the MPOA and VTA facilitates postpartum onset of maternal behavior and infusion of an OT antagonist into these regions can block pup retrieval and nursing (Pedersen et al., 1994). In rodents, it was demonstrated that dams characterized by high amounts of pup licking and grooming (high LG) as compared to low LG dams showed increased OT expression in the MPOA and the PVN, as well as stronger oxytocinergic projections from the MPOA and PVN to the VTA (Shahrokh et al., 2010). Infusion of OT into the VTA increased dopamine (DA) turnover in the nAcc, and naturally high LG mothers but not low LG mothers showed elevated DA signaling in the nAcc during episodes of interactions with their offspring. Interestingly and similar to the finding by Pedersen et al. (1994), infusion of an OT antagonist into the VTA eliminated these differences.
Despite being less robust than the reported findings in rodents, studies on human parenting also suggest an important role of OT in the above described brain circuits. In observational studies, maternal plasma OT correlates with affectionate parenting, such as “motherese” vocalizations and affectionate touch (Gordon et al., 2010). Looking at functional changes in the CNS, intranasal OT administration led to an increased activation of the VTA after presentation of reproduction-associated stimuli, i.e., pictures containing sexual and infant stimuli in healthy women (Gregory et al., 2015). Furthermore, in response to infant cry sounds, women’s amygdala reactivity was diminished by intranasal OT (Riem et al., 2011). Simultaneously, intranasal OT application led to increased activity in the insula and inferior frontal gyrus after hearing infant crying. These findings indicate that OT may selectively attenuate reactivity in limbic structures involved in stress and anxiety (amygdala), while simultaneously enhancing activity of dopaminergic motivational circuits that underlie reward learning and parenting behaviors (VTA). In addition, OT may contribute to enhanced emotion recognition of salient infant social cues through increased activation of specific brain regions (insula and inferior frontal gyrus) that are part of the empathy related brain network (Shamay-Tsoory, 2011). It should be acknowledged, however, that imaging studies using intranasal OT challenge are controversial (Leng and Ludwig, 2015). For example, although administering supraphysiological doses of OT, only a very small fraction can be detected in the CSF (Leng and Ludwig, 2015) and it is questionable whether this has direct modulating effects on brain functioning and behavior. Furthermore, it is very likely that strong increases of peripheral OT concentrations after intranasal OT application have effects on peripheral organs (e.g., the heart, gastrointestinal tract, reproductive tract) – and might confound the direct effects of OT in the brain.
Despite these methodological drawbacks, OT in the brain seems to play a fundamental role in shaping maternal parenting behavior, which is substantially supported by animal data. Its effects are in part dependent upon regulation by other hormones (e.g., E), target brain structures (e.g., amygdala vs. VTA), or interactions with other neurotransmitter systems (e.g., DA). Drawing primarily from experimental work in rodents, it must be kept in mind that inferences to humans should be made with necessary precaution due to important cross-species differences in parenting behaviors. More research in humans is thus warranted to better understand how OT affects brain circuits that are involved in parent-offspring interactions and motivated maternal behavior.
2.2.1 Effects of ELS on OT Brain Circuits Relevant for Parenting Behavior and Implications for Intergenerational Transmission of Maternal ELS
It is now crucial to understand how these OT networks in the “parental brain” can be influenced by early experiences, such as increased ELS, to perpetuate a vicious circle of dysfunctional attachment formation. The effects of ELS on key regions of the neural circuits that underlie parenting have been most rigorously studied in animal models (Champagne, 2008; Champagne et al., 2001; Pena et al., 2014). In female offspring of rats, a low level of maternal care (LG) is associated with lower OTR expression in the MPOA, PVN, the central nucleus of the amygdala, and the lateral septum (Champagne et al., 2001). Moreover, downstream targets of hypothalamic OT projections in the mesolimbic system, i.e., the VTA and the nAcc, are equally affected by early rearing experiences. Offspring of low LG dams exhibit reduced dopaminergic projections from the VTA, as well as lower expression of dopamine receptors in the nAcc (Pena et al., 2014). These experience-induced differences in OT-DA neural circuits that underlie parenting correspond to observable parenting behaviors. Lower levels of OTR expression are associated with less maternal responsiveness towards pups (Champagne, 2008; Champagne et al., 2001). This intergenerational transmission of maternal behavior in rats is partly mediated by epigenetic modifications of the estrogen receptor α with downstream effects on OTR expression regulation in the brain, which will be further described below (see section 3.2.2).
An experience-dependent transmission of maternal care via OT neural pathways is also assumed in humans (Feldman et al., 2013; Herpertz and Bertsch, 2015; Rilling and Young, 2014), although empirical evidence is sparse. As indicated above, adult women who report forms of ELS, i.e., emotional abuse and physical abuse, exhibit decreased OT concentrations in CSF compared to non-abused women (Heim et al., 2009), suggesting that ELS has enduring effects on central OT activity. It is highly plausible that these decreased CSF OT concentrations that occur as a function of ELS have implications for maternal parenting behavior, but this has not been directly studied to date. Studies of mothers with observable differences in parenting behavior that can be a result of ELS (Dubowitz et al., 2001; Mielke et al., 2016) focusing on OT physiology and activity of OT brain circuits when processing infant cues would allow further insight into the ELS-associated biological underpinnings of the intergenerational transmission of maternal ELS. A study in primiparous women found that mothers who were classified as insecurely attached as opposed to mothers with a secure attachment style had lower peripheral OT concentrations after interaction with their infants. The maternal OT rise after interaction with their infants correlated positively with maternal brain activation in the ventral striatum after presentation of visual stimuli of their own child. Involvement of the ventral striatum, which is part of the reward-related dopaminergic nigrostriatal system, suggests that pictures of one’s own child may have a higher “incentive value” for securely attached mothers and hence facilitate maternal responsiveness and approach behaviors (Strathearn et al., 2009). On the other hand, adult women with an insecure attachment representation show increased activation of the amygdala in response to baby cries (Riem et al., 2012) and heightened amygdala reactivity may partly stem from insufficient OT signaling (Kirsch et al., 2005). One may speculate that mothers with an insecure attachment style, which can be a result of problematic parental care experiences in the mother’s own childhood, are more prone to perceive infant distress as aversive, which can lead to frustration or even abusive behavior towards the child (Reijneveld et al., 2004). In addition, hyper-reactivity of the amygdala in response to infant distress as has been shown in ELS-exposed mothers is a neural correlate for maternal intrusive parenting (Atzil et al., 2011), which in turn is a risk factor for higher infant anxious and depressive behaviors (Wagner et al., 2015). The relevance of these findings to the proposed intergenerational framework is immediately evident: they clearly suggest that early maternal attachment experiences (in her own childhood) or ELS may contribute to alterations in CNS OT signaling pathways, which underlie parenting and maternal responsivity. Below (see section 4.1.2), we will propose a mechanism through which these maternal oxytocinergic dysregulations might be transmitted to the next generation in dyadic mother-child interactions.
2.3 OT Brain Circuits Relevant for Depression
Exposure to ELS contributes to depression risk in adulthood and there is a wealth of evidence associating maternal depression with non-optimal parenting (Field, 2010; O’hara and McCabe, 2013) and reduced dyadic reciprocity in mother-child interactions (Righetti-Veltema et al., 2002). Depressed mothers are more likely to withdraw from interactions with their child, show lower amounts of sensitive parenting (Feldman et al., 2009), and perceive infant cues as more negative than non-depressed mothers (Forman et al., 2007). In addition, maternal postpartum depression (PPD) increases the likelihood of offspring internalizing and externalizing problems (Goodman et al., 2011), insecure attachment (Carter et al., 2001), increased stress reactivity (Halligan et al., 2004), and lower social engagement and empathy (Apter-Levy et al., 2014). Moreover, adult offspring of mothers with ELS-exposure have a higher risk of developing depressive symptoms themselves (Roberts et al., 2015). ELS-associated maternal depression can therefore be considered a main pathway of the intergenerational transmission of maternal ELS in humans. Given the co-occurrence of maternal depression and impaired parenting, one may speculate that they share common biological underpinnings, likely including OT neural circuits, which have recently been suggested to play an etiologically relevant role in depression (McQuaid et al., 2014). Therefore, the following section will highlight the role of OT pathways in modulation of biological processes that underlie major depression (MDD)/PPD, with a particular focus on the hypothalamic pituitary adrenal (HPA) axis and the immune system.
2.3.1 OT and the HPA Axis
Dysregulated activity of the HPA axis is the most robust neuroendocrine finding in MDD (Heim et al., 2008), and may also be etiologically relevant to PPD (Brummelte and Galea, 2015; Glynn et al., 2013; Jolley et al., 2007). Given the expression of OTRs within key stress-regulatory regions (e.g., amygdala, PVN), stress-buffering effects of OT (Cardoso et al., 2014; Heinrichs et al., 2003), and OT-induced downregulation of amygdala reactivity (Kirsch et al., 2005), OT might serve a protective role against the development of stress-related disorders such as MDD or PPD. In a recent meta-analysis, OT was shown to not attenuate HPA axis reactivity after stress induction per se but does so after laboratory stress protocols that robustly activate the HPA axis (Cardoso et al., 2014). In healthy postpartum women, breastfeeding, which often serves as a proxy for OT activity, was associated with lower cortisol secretion after a psychosocial stressor (Heinrichs et al., 2001). Interestingly, at 2 months postpartum, mothers with high depression scores had lower peripheral OT concentrations during breastfeeding (Stuebe et al., 2013). Thus, one might speculate that insufficient OT release in breastfeeding women with PPD may have consequences for dysregulated HPA-axis activity. Another study suggests a slightly different view (Cox et al., 2015). Breastfeeding was generally associated with attenuated HPA-axis responsiveness after the Trier Social Stress Test and women with high depressive symptoms expectedly showed lower OT concentrations during breastfeeding and higher cortisol concentrations during both breastfeeding and the social stressor. However, in women with severe PPD symptoms, OT and cortisol were positively correlated (Cox et al., 2015). This may indicate insufficient OT signaling, possibly via altered OTR sensitivity in brain areas relevant to regulation of the HPA axis (e.g., the PVN or amygdala). Combined with data indicating that lower peripheral OT concentrations in late pregnancy predict PPD symptoms (Skrundz et al., 2011) and that women with PPD are more likely to discontinue breastfeeding earlier than non-depressed women (McLearn et al., 2006), these findings imply that OT signaling pathways may play an etiologically relevant role in PPD. Studies in rodents partly revealed the underlying biological mechanisms that link OT signaling pathways to regulation of the HPA-axis. In female prairie voles subjected to an immobilization stressor, intracerebroventricular (i.c.v.) injection of an OTR-antagonist into the PVN blocked the stress buffering effects of social support. Administration of the OTR-antagonist had both behavioral (i.e., more anxiety-like behavior) and neuroendocrine (i.e., more corticosterone) effects (Smith and Wang, 2014). In mice, stimulation of OTRs in the central nucleus of the amygdala activates a GABAergic inhibitory network of neurons within the amygdala, which is consistent with anxiolytic OT effects (Huber et al., 2005). OT also increases activity of inhibitory GABAergic neurons in the PVN (Smith et al., 2016). Concurrent treatment with a GABAA-receptor antagonist eliminated OT’s capacity to reduce secretion of corticotropin releasing hormone (CRH) in the PVN and corticosterone in female prairie voles, a finding that has also been shown in rats (Bülbül et al., 2011). Looking at intracellular signaling pathways, i.c.v. infusion of OT significantly delayed CRH transcription in a restraint stress paradigm in male mice (Jurek et al., 2015). This OT effect was mediated through attenuation of nuclear translocation of CREB-regulated transcription coactivator 3, resulting in less CRH-gene transcription, which plausibly would lead to a less pronounced glucocorticoid secretion rate in the adrenal cortex. Taken together, these animal studies provide important insights into biological mechanisms that underlie oxytocinergic modulation of neuroendocrine stress responses and support the widely held view that OT is protective against overshooting responsiveness of the HPA-axis. It remains to be elucidated whether these mechanisms apply to humans as well. If replicated, these findings may have important implications for pharmacological treatment of affective disorders, such as PPD.
Effects of ELS on OT-HPA interactions
As outlined above, OT has an important role in the regulation of the endocrine stress response. The experience of sensitive parental care is indispensable for children’s stress-regulation in the first years of life (Gunnar and Donzella, 2002), likely involving actions of OT. Moreover, ELS is associated with higher infant cortisol reactivity, more negative emotionality (Feldman et al., 2009), and deficient OT signaling in social interactions (Wismer Fries et al., 2005). Importantly, effects of ELS-exposure may be long lasting and are reliable predictors for later life MDD (Heim and Binder, 2012), PPD (Meltzer-Brody et al., 2013), dysregulated HPA axis reactivity (Heim et al., 2008), as well as lower CSF (Heim et al., 2009) and plasma (Opacka-Juffry and Mohiyeddini, 2012) OT concentrations. This co-occurrence of ELS-dependent alterations begs the question of whether abnormalities in bi-directional OT-HPA-axis communication may critically shape the relationship between ELS and psychiatric risk in adulthood. Animal models could show that exposure to ELS is associated with lower OTR mRNA expression in the adult amygdala (Hill et al., 2014), rendering it less sensitive to the putative stress protective effects of OT. Human studies show similar ELS-effects. In healthy men with a history of ELS, OT has a diminished capacity to downregulate cortisol under basal conditions (Meinlschmidt and Heim, 2007). The stress-dampening effects of intranasal OT on amygdala reactivity may even be reversed in individuals with ELS-exposure; thus, intranasal OT increased cortisol reactivity and hippocampal deactivation after exposure to acute psychosocial stress only in individuals with ELS-exposure (Grimm et al., 2014). This seemingly paradoxical finding may be explained by the social salience hypothesis (Shamay-Tsoory and Abu-Akel, 2015), which postulates that OT increases the salience of social stimuli. In a situation characterized by social evaluative threat, administration of OT could contribute to increased stress reactivity in individuals that may already have an attentional bias towards threatening cues (i.e., ELS-survivors). The observed effects in humans, although speculative at this point, may be attributable to ELS-associated reductions in OTR sensitivity and/or reduced OTR expression in key nodes of the central stress response.
2.3.2 OT and the Immune System
Excess activity of pro-inflammatory cytokines are assumed to play an etiologically relevant role in MDD (Hodes et al., 2015; Maes et al., 2011; Raison et al., 2006) and also PPD (Corwin et al., 2008; Garfield et al., 2015; Yim et al., 2015). Although not fully understood, CNS inflammatory pathways may interfere with serotonin metabolism, augment glutamatergic activity, and potentiate the activity of the HPA-axis, thereby contributing to depression risk (Miller et al., 2009; Raison et al., 2006). Presence of OTRs on human immune cells suggests that OT plays a role in the neuroendocrine regulation of immunity with mainly anti-inflammatory effects (Wang et al., 2015). For example, administration of OT significantly attenuates lipopolysaccharide-induced production of pro-inflammatory cytokines tumor necrosis factor alpha (TNF-α) and interleukin 6 (IL-6) in serum of healthy human subjects (Clodi et al., 2008) and microglial production of TNF-α and interleukin 1 beta (IL-1β) in mice (Yuan et al., 2016). Considering its anti-inflammatory effects and growing evidence that inflammatory cytokines are involved in the etiology of MDD and PPD, it can be hypothesized that insufficient OT signaling may contribute to depression risk via this pathway (McQuaid et al., 2014). Consistent with this view, socially isolated mice that were treated with OT showed reduced levels of depressive-like behavior and lower expression of IL-1β in the frontal cortex after induction of a nerve injury compared to isolated animals without OT treatment (Norman et al., 2010). Likewise, an OTR antagonist blocked the antidepressant and anti-inflammatory effects of social housing in non-isolated animals. To our knowledge, no study has yet investigated interactions of OT and inflammatory markers in humans in the context of MDD or PPD directly but this may be a pathway worth further investigating in future studies (Garfield et al., 2015; McQuaid et al., 2014).
Effects of ELS on OT-immune system interactions
The notion that ELS “programs” a persistent inflammatory phenotype and thereby potentially increases risk for MDD and PPD as well as somatic disorders in adulthood has gained much support (Danese et al., 2008; Garfield et al., 2015). For example, depressed adults who had experienced early maltreatment showed increased markers of inflammation (C-reactive protein) compared to individuals who were not maltreated (Danese et al., 2008). In older subjects, chronic caregiving stress was related to both heightened IL-6 levels and shorter telomere length only in survivors of ELS (Kiecolt-Glaser et al., 2011). Moreover, ELS is a significant predictor of lipopolysaccharide-stimulated IL-6, decreased glucocorticoid sensitivity (Miller and Chen, 2010), and increased DNA binding of the main inflammatory transcription factor nuclear factor κB in patients who suffer from ELS-associated posttraumatic stress disorder or MDD (Pace et al., 2006; Pace et al., 2012). Whether alterations in the OT-immune cross-talk are involved in the ELS-depression relationship has yet to be established, but certainly yields potential for new insights into the etiology of ELS-associated depression. ELS is associated with both decreased OT signaling (Heim et al., 2009; Opacka-Juffry and Mohiyeddini, 2012) and an increased inflammatory phenotype (Miller et al., 2011) in adults. ELS-related attenuation of OT/OTR mediated inhibition of inflammatory pathways may result in an inflammatory excess to culminate in higher risk of MDD in ELS-affected individuals. On the other hand, an ELS-associated inflammatory milieu may compromise efficient OT signaling, as it has been shown that the inflammatory cytokines IL-1β and IL-6 downregulate OTR mRNA expression in humans (Schmid et al., 2001).
3. Gene-Environment Interactions and Epigenetic Modifications in Oxytocin Pathway Genes – Relevance for Parenting Behaviors, Psychiatric Risk, and the Intergenerational Transmission of Maternal ELS
In recent years, common sequence variations in OT pathway genes have attracted considerable attention in research on inter-individual differences in parenting behavior, social cognition, and psychopathologies that are characterized by impairments in social functioning, such as autism spectrum disorders (LoParo and Waldman, 2015) or schizophrenia (Montag et al., 2013). For the OXTR gene, most data exist for 2 single nucleotide polymorphisms (SNPs), rs53576 and rs2254298, which are located in intron 3 of the gene. This intron contains enhancer regions, but neither of the SNPs is located directly within these regions, suggesting that they only tag potential functional variants. While these two SNPs are only 2.1 kb apart, their linkage disequilibrium is low with r-squared of 0.028 in European population, indicating that they represent independent genetic information.
A common problem of this candidate gene approach is small effect sizes and poor replicability of genetic main effects. Nevertheless, a finding that showed higher amygdala reactivity after presentation of emotional faces in homozygous G allele carriers of the OXTR rs53576 SNP compared to A allele carriers (Tost et al., 2010) could be directly replicated in an independent sample (Dannlowski et al., 2015), showing the merit of combining functional imaging and genetic techniques. With regard to the scope of the present work, i.e. elaboration of the role of the endogenous OT system as a pathway of intergenerational transmission of maternal ELS, it is important to acknowledge that individual genetic differences in OT pathway genes may modify the association between ELS experience and OT-associated phenotypes, such as parenting behavior or psychiatric risk. Therefore, the focus here will be on gene-environment (G*E) interaction studies; an overview of genetic main effects that are relevant to the proposed theoretical framework is provided in supplemental Table 1 (Table S1). In addition to gene-environment interactions, epigenetic modifications in OT pathway genes and genes that interfere with OTR mRNA expression may represent a possible mechanism of how ELS effects may endure over years to impact psychosocial functioning in adults. The following section therefore provides a summary of studies that show a) G*E interactions (focusing on ELS as the environmental variation) and b) epigenetic modifications in OT pathway genes that are relevant for the understanding of maternal ELS-transmission.
3.1 Gene-Environment Interactions in OT Pathway Genes Relevant for the Intergenerational Transmission of Maternal ELS
Rs53576
First evidence for a G*E interaction including OT pathway genes was found in a large sample of low SES, African American participants (Bradley et al., 2011). OXTR rs53576 GG homozygous subjects who experienced three or more types of severe ELS were at higher risk for adult emotional dysregulation and a disorganized attachment style than A allele carriers, indicating a higher susceptibility to ELS in G allele homozygotes. This finding was partly replicated in a sample of university students (McQuaid et al., 2013). Only rs53576 G allele carriers (AG/GG) who experienced high levels of ELS reported more depressive symptoms than AA homozygous subjects. Among G allele carriers this association was mediated by subjective ratings of interpersonal distrust. Support for this higher sensitivity to a dysfunctional or stressful social environment in rs53576 G allele carriers was further substantiated by both observational (Burkhouse et al., 2015; Dannlowski et al., 2015; Hostinar et al., 2014; Smearman et al., 2015; Sturge-Apple et al., 2012; Windle and Mrug, 2015) and experimental studies (Chen et al., 2011; McQuaid et al., 2015; Norman et al., 2012). As examples of observational studies, it could be shown that rs53576 GG homozygous subjects with ELS exposure have a smaller bilateral volume of the ventral striatum, a key structure of the dopaminergic nigrostriatal pathway (Dannlowski et al., 2015) which is implicated in both depression risk (Nestler and Carlezon, 2006) and parenting behavior (Strathearn et al., 2009). Moreover, GG homozygous adolescents with ELS reported significantly lower social support as well as higher internalizing problems compared to A allele carriers (Hostinar et al., 2014). Similarly, GG homozygous adolescent girls as opposed to A allele carrying girls reported substantially more depressive symptoms after the experience of parental divorce (Windle and Mrug, 2015). Experimental studies applying standardized laboratory stress protocols also support the assumed higher sensitivity to the social environment in G allele carriers, suggesting that the rs53576 G allele may be thought of as a plasticity allele. Men carrying the G allele were more likely to exhibit an attenuated cortisol response to a public speaking challenge when receiving social support (Chen et al., 2011), whereas A allele homozygotes appeared to be resistant to the stress-buffering effect of social support. Finally, in an experimental manipulation of social exclusion, only G allele homozygous women reported reduced self-esteem compared to participants who were not excluded, indicating a higher rejection sensitivity in GG women (McQuaid et al., 2015). Concordantly, excluded GG carriers’ systolic blood pressure remained elevated during the game and 30 minutes thereafter, and excluded GG carriers also had higher cortisol levels than included GG individuals.
A very informative G*E interaction in the prediction of maternal parenting found that rs53576 GG mothers exhibit differential maternal sensitivity towards their 2-year old children, varying as a function of inter-parental conflict (Sturge-Apple et al., 2012). Specifically, G allele homozygous women under high marital strain were less sensitive in interaction with their offspring than A allele mothers, whereas under conditions of low marital conflict this pattern reversed – GG allele mothers were more sensitive than their A allele counterparts, a finding that is consistent with the concept of differential susceptibility to the environment (Belsky and Pluess, 2009).
Another study investigated the role of children’s rs53576 genotype in detecting emotional facial expressions (Burkhouse et al., 2015). A significant G*E interaction emerged in the way that GG homozygous children whose mothers had had at least one episode of MDD during the child’s lifetime were better at detecting sad faces and worse in detecting happy faces. Importantly, this result remained significant after controlling for children’s own affective symptoms. One may speculate that this genotype-dependent attentional bias towards emotional cues that are congruent with maternal depression may make these children more prone to develop depressive symptoms themselves.
Together, these studies suggest a higher sensitivity to the effects of ELS exposure and current psychosocial stress, especially in homozygous G allele carriers of OXTR rs53576. Sometimes, however, the presence of at least one copy of the G allele is sufficient to demonstrate this higher environmental sensitivity. Replication in larger samples is thus warranted to test whether these allele load dependent effects can vary across different exposure conditions and contexts. Overall, this SNP may represent a genetic component that can contribute to the intergenerational transmission of the effects of stressful experiences.
It must be acknowledged however that not all findings support the notion of OXTR rs53576 G allele carriers being more sensitive to the social environment (Hammen et al., 2015; McInnis et al., 2015; Thompson et al., 2014). For instance, OXTR rs53576 moderated the relationship between exposure to maternal depression in childhood and depressive symptoms at age 15 years (Thompson et al., 2014). Presence of at least one A allele conferred higher risk to depressive symptoms in individuals whose mothers reported at least one episode of MDD during the child’s lifetime. There is also evidence for a differential susceptibility to develop symptoms of Borderline personality disorder (BPD) at age 20 in response to family quality in adolescence (Hammen et al., 2015). Under conditions of low familial constraint, presence of at least one A allele was associated with lower BPD symptoms compared to G allele homozygous participants, whereas under high family discord A allele carriers were more likely to develop more severe BPD symptoms than G allele homozygotes. These two studies (Hammen et al., 2015; Thompson et al., 2014) that found A allele carriers of the rs53576 SNP to be more susceptible to environmental variation do not discuss inconsistencies with studies in support of G allele carriers being more susceptible, which impedes the formulation of general conclusions. Together, the majority of findings clearly supports the G allele being associated with higher environmental sensitivity and consequently the G allele of the rs53576 SNP may be more accurately described as a plasticity allele. To address the reported inconsistencies, replication of findings with this polymorphism in larger samples is needed in future investigations.
Rs2254298
A study investigating the role of the OXTR rs2254298 genotype in the etiology of anxiety and depression in adolescent girls revealed evidence for a G*E interaction within an intergenerational transmission perspective (Thompson et al., 2011). Compared to GG homozygous girls, girls carrying the heterozygous A/G genotype were more susceptible to developing symptoms of depression and anxiety if their mothers had a history of recurrent MDD. Recently, an fMRI study in juveniles provided evidence for possible biological underpinnings that confer an increased risk in A allele carriers after ELS (Marusak et al., 2015). At a structural and functional level of analysis, it could be shown that A allele carriers had higher bilateral amygdala gray matter volume and higher amygdala activation when viewing pictures of emotional faces. Interestingly, there was a positive correlation between number of stressful life events and amygdala reactivity in A allele carriers only. Similar to the rs53576 G allele carriers, rs2254298 A allele carriers may be more attuned to selectively focus on socially relevant cues in their environment, which could place them at higher risk for the development of psychopathology, consistent with common diathesis-stress concepts. It has been proposed, however, that it may be more accurate to conceptualize the rs225498 A allele as a plasticity allele, which means that A allele carriers might be more susceptible to both favorable as well as adverse social environments, for better or for worse (Brüne, 2012). This hypothesis needs further support from future investigations.
Other SNPs
Despite the prominent focus on these two candidate gene variants of the OXTR (rs53576 and rs225498) (Kumsta and Heinrichs, 2013), there is also evidence for the moderating effects of other SNPs in OT pathway genes that may help to better understand how environmental exposures might affect individuals differently. A sequence variation in the OXT gene (rs2740210, A/C) has been shown to interact with maternal ELS to predict postpartum depression (PPD) and breastfeeding duration in two independent samples (Jonas et al., 2013). Mothers homozygous for the C allele tended to discontinue breastfeeding much earlier when they were exposed to ELS than women who carried the protective A allele. Likewise, CC homozygous mothers were more vulnerable to the long-term effects of ELS with regard to development of PPD symptoms at six months postpartum. Moreover, only in CC homozygous individuals, the association of ELS and breastfeeding duration was mediated by maternal depression. Another study could indirectly replicate and extend this finding (Mileva-Seitz et al., 2013), as maternal ELS in interaction with rs2740210, but also with OXT rs4813627 (A/G), predicted maternal PPD. CC (rs2740210) homozygous and GG (rs4813627) homozygous mothers exposed to ELS reported significantly more depressive symptoms at 6 months postpartum compared to A allele carriers of the respective SNPs. Somewhat counterintuitively, there was also a negative association between maternal ELS and maternal instrumental care in mothers homozygous for these genotypes; i.e., homozygous mothers (CC, GG) with ELS-exposure displayed more instrumental care towards their children compared to women exposed to low ELS. In this study, the mother-child interaction had been designed to minimize instrumental care behaviors (e.g., cleaning the child) and elicit spontaneous free play. This may indicate that women with a more susceptible genotype and adverse early experiences may have more difficulties engaging in spontaneous free play and rely on more structured and instrumental caregiving. Additionally, a significant genetic main effect of both SNPs on maternal behavior emerged - CC (rs2740210) and GG (rs4813627) mothers vocalized significantly longer towards their children than A allele carrying women. Both SNPs showing association in the OXT gene are located in the 5′ upstream region, either in the potential promoter region or an upstream enhancer. Variation in another rare OXTR SNP (rs139832701, G/T) has been shown to be associated with increased risk for anxiety, stress, and depression in individuals with a history of ELS exposure (Myers et al., 2014) but risk alleles have not been specified. This SNP, like rs53576 and rs2254298, is located in close proximity to the other two SNPs in intron 3, but also only has low linkage disequilibrium with them (r-squared below 0.06 for both). Finally, a study by Wade et al. (Wade et al., 2015) revealed a significant interaction between children’s OXTR rs11131149 (A/G) genotype and maternal sensitivity in predicting social-cognitive abilities (Theory of Mind; ToM) in 4.5 years old children. More precisely, children carrying one or two copies of the G allele (AG or GG) performed significantly better in the ToM test with increasing amounts of maternal sensitivity. Of note, the interaction of maternal sensitivity and child genotype accounted for 26% of variance in ToM scores among children, which compared to other studies is a remarkably large effect.
In summary, these findings hold the promise to better understand how genetic polymorphisms in OT pathway genes may interact with environmental factors (e.g., ELS; maternal sensitivity) to explain individual differences in a wide array of phenotypic outcomes that could potentially impact parent-offspring interactions for better or worse. At the molecular level, however, there is still a lack of knowledge on the functional significance of genetic variation of the OXTR. Thus, elucidation of the molecular mechanisms linked to specific gene variants would meaningfully extend our current understanding of genotype-phenotype associations. Importantly, when generating hypotheses about OT-associated intergenerational transmission pathways, it must be kept in mind that both parental and offspring characteristics (e.g., genotype) have to be considered. Moreover, there is ample evidence for gene-environment interactions, polygenic interactions (epistasis), or epigenetic mechanisms in other target genes that would be relevant for the proposed framework, but could not be considered separately because this would exceed the scope of this review. A recent review on intergenerational transmission of stress in humans summarizes some other relevant target genes, e.g., NR3CI or SLC6A4 (Bowers and Yehuda, 2016) and another study (Beaver and Belsky, 2012) exemplifies how a combination of multiple genes (DAT1, DRD2, DRD4, and 5HTTLPR) in interaction with early experiences predicts parenting behaviors in adulthood.
3.2 Epigenetic Modifications in OT Pathway Genes Relevant for the Intergenerational Transmission of Maternal ELS
In addition to sequence variations in OT pathway genes, epigenetic modifications of the OXTR gene have recently attracted considerable attention in clinical, behavioral, and cognitive neurosciences (Kumsta et al., 2013) and have been associated with different phenotypes including maternal PPD (Bell et al., 2015; Kimmel et al., 2016), amygdala reactivity (Puglia et al., 2015), autism (Gregory et al., 2009), and social anxiety disorder (Ziegler et al., 2015) amongst others. Briefly, epigenetics encompasses a set of biochemical modifications of genome function (e.g., histone modifications, DNA methylation, or the effects of small non-coding RNAs, e.g., micro RNAs) that interfere with transcriptional or translational events and can therefore regulate gene expression. These epigenetic modifications occur without sequential variation. Epigenetic control of gene expression is highly responsive to environmental influences such as ELS, especially during developmentally sensitive periods in early life, as has been shown in rodents (Champagne, 2008; Meaney, 2001; Weaver et al., 2004) and humans (McGowan et al., 2009). DNA methylation, i.e., the attachment of methyl groups (CH3) to the 5-carbon position of cytosine, which typically occurs at so-called CpG dinucleotides where a cytosine base is followed by a guanine base, generally results in gene silencing and is the only epigenetic modification of the human OXTR gene studied to date. Animal studies revealed differential DNA methylation patterns within specific genes that are implied in stress-regulation (e.g., the GR coding gene) as a result of natural variations in early maternal care. These modifications appear to be highly persistent throughout the life span but are potentially reversible (Meaney, 2010; Weaver et al., 2004). Despite these well-established long-term effects of ELS on epigenetic regulation of specific genes, evidence for the stability of ELS-associated DNA methylation patterns across different developmental stages is lacking. This is a critical limitation in the current literature, especially considering the evidence for dynamic epigenetic variation that occurs for example during episodes of acute psychosocial stress in human subjects (Unternaehrer et al., 2012). Future studies with serial assessments of epigenetic modifications in association with environmental variation will need to provide further insight into the stability of epigenetic changes.
In animal models of non-genetic intergenerational transmission of maternal parenting behaviors (Champagne, 2008), experience-dependent differential methylation of the estrogen receptor α gene has been shown to be associated with observable differences in maternal parenting. The E-activated ERα acts as a transcription factor at an estrogen-response element in the rat Oxtr gene, thereby upregulating OTR mRNA expression in brain regions that are critically involved in maternal behavior such as the MPOA. Low maternal care in early life results in higher methylation of the ERα gene with downstream silencing effects on Oxtr gene transcription in adult animals. This effect was also found in the first generation of female offspring that has not been directly exposed (F2 generation), i.e., before conception of the F0 dam. This transmission therefore represents a true transgenerational transmission, as opposed to an intergenerational transmission, where the subsequent generation is directly exposed (see: Klengel, Dias, & Ressler, 2015 for review). An interesting study by Beery et al. (2015), directly investigated Oxtr methylation in that animal model. The authors compared DNA methylation of that locus in peripheral blood cells and the hippocampus, striatum, and hypothalamus in off-spring of high vs. low licking mothers. They observed Oxtr gene methylation differences depending on maternal care in peripheral blood but not any of the brain tissues and suggest that inferences across tissues are not supported for individual variation in Oxtr methylation. These findings need to be kept in mind when interpreting human studies investigating peripheral tissues.
Initial evidence of OXTR gene DNA methylation and its implications for gene expression in humans was obtained in hepatic cell cultures (Kusui et al., 2001). Hypermethylation of the OXTR promoter including a CpG rich region (CpG island spanning from −2860 to +1342 bases relative to the transcription start site) reduced OTR mRNA expression by approximately 70%. Methylation-induced downregulation of the OTR was also confirmed in post mortem cortical brain tissue of male patients with autism spectrum disorders (Gregory et al., 2009). Two recent studies revealed a role for OXTR DNA methylation in peripheral blood cells to predict risk for PPD (Bell et al., 2015; Kimmel et al., 2016). GG homozygous (rs53576) women, who were not depressed during pregnancy had an almost 3-fold risk (adjusted OR: 2.63) to develop PPD for every 10% increase of DNA methylation at a CpG dinucleotide located on intron 1, 934 basepairs upstream of the translation start site (CpG −934) compared to A allele carriers. Hypermethylation of this specific CpG has been shown to be associated with reduced OTR expression in the cortex of deceased autism patients (Gregory et al., 2009), suggesting that hypermethylation measured in peripheral tissue of this specific CpG may partly reflect OTR expression regulation in brain tissue (Bell et al., 2015). In contrast, another study found lower OXTR DNA methylation in an intronic region (cg 12695586) in women with high depressive symptoms both pre- and postpartum compared to women with PPD symptoms only (Kimmel et al., 2016). However, this study has important methodological weaknesses including very small sample sizes, and therefore replication of these findings is warranted to further clarify the role of OXTR DNA methylation in the etiology of PPD. In another sample of women, DNA methylation of OXTR exon 1 obtained from leukocytes was lower in non-pregnant women with depression compared to healthy control subjects, but only in individuals homozygous for the rs53576 G allele (Reiner et al., 2015). Moreover, there was a significant main effect of rs53576 genotype on OXTR exon 2 methylation – women carrying the A allele had significantly higher methylation status than GG homozygous individuals. Importantly, there is evidence that early maternal care is associated with differential methylation of regions in the OXTR gene of adults (Unternaehrer et al., 2015). In one target sequence positioned at exon 3 of the OXTR gene, there was a negative association between maternal care and blood-derived OXTR DNA-methylation levels. Effects of maternal care were small, however, and phenotypic differences (i.e., higher depression risk) that could link these differentially methylated regions to variation in observable behavior were not assessed. It has been recently shown that increased peripheral blood cell OXTR promoter methylation at CpG −934 is correlated with higher amygdala reactivity and also with lower functional connectivity between right amygdala and brain areas involved in emotion regulation (cingulate and orbitofrontal cortex), suggesting an inhibition of top-down regulation of the amygdala (Puglia et al., 2015). This finding provides first evidence of the predictive value of peripheral OXTR methylation in explaining variability of functional alterations in brain areas that are key in modulation of affective states.
In conclusion, these studies suggest that epigenetic modifications of the OXTR gene may have etiological relevance in PPD, MDD, ASD, or brain activity patterns underlying higher threat salience, stress reactivity, or intrusive parenting (increased amygdala activity). DNA methylation of the OXTR gene may be moderated by environmental contingencies, like poor maternal care in childhood (Beery et al., 2015; Unternaehrer et al., 2015), and genotype (Bell et al., 2015). However, comparability between studies is difficult because they differ greatly in target sequences and partly show effects that are not in the expected direction. Moreover, with one exception (Gregory et al., 2009), these studies did not quantify OTR mRNA expression levels varying as a function of methylation status; thus the biological significance of the observed methylation changes is not addressed. Another important limitation of this body of literature is that it has exclusively focused on DNA methylation of the OXTR gene. Other epigenetic mechanisms, such as histone modifications or epigenetic modifications of other target genes including the protein-coding OT gene, are thus needed to further our understanding of epigenetic regulation in the OT/OTR system. Lastly, the unresolved issue of how meaningful peripherally derived methylation patterns are with respect to brain activity and consequently behavior and psychiatric risk remains controversial. Epigenetic changes are highly tissue specific and only a subset of DNA methylation changes show cross-tissue correlation between peripheral tissues and the brain (Davies et al., 2012; Farré et al., 2015; Hannon et al., 2015). Taken together, it would be premature to draw general conclusions from the reported findings. Nevertheless, based on the exciting initial empirical evidence (Bell et al., 2015; Kimmel et al., 2016; Puglia et al., 2015), epigenetic modifications of the OXTR gene clearly deserve further attention when considering the role of OT-related intergenerational transmission pathways in the future.
4. OT Pathways in the Intergenerational Transmission of Maternal ELS During Pregnancy and Postnatal Life
Above, an overview has been provided of how ELS may have sustained effects on brain OT networks that constitute neural substrates for depression and parenting. Furthermore, empirical evidence was summarized in support of the contributing role of individual genetic variations and epigenetic modifications in OT pathway genes to the intergenerational transmission of maternal ELS. Building on this empirical evidence, a framework will be presented that intends to integrate these findings to help explain how alterations in the maternal OT system, which is susceptible to her early life experience, contribute to the intergenerational transmission of maternal ELS during sensitive developmental periods in the next generation’s lifetime. As a powerful modulator of social interactions and mother-infant bonding, it appears logical to primarily comprehend OT pathways of intergenerational transmission of maternal ELS as mainly occurring in the early postnatal period of life. However, we will argue that OT-related transmission processes of maternal ELS may already occur during the time of pregnancy. ELS-associated decreases in peripheral and central OT may have an effect on mediators of MPF stress biology (e.g., HPA axis and immune system) and could therefore affect fetal development in utero. Also, OT is able to pass the placenta (Malek et al., 1996) and may thus serve as a signal to the fetus of (early) maternal experiences and the quality of the postnatal environment. There is also evidence suggesting that parenting behavior and risk for PPD is already prepared for during pregnancy through alterations in OT pathways (Feldman et al., 2007; Skrundz et al., 2011), and it is plausible to assume that gestational oxytocinergic adaptations mediate the link between ELS and postnatal maternal behavior. Although some of these assumptions may appear speculative at this point due to a lack of empirical evidence, these tentative prenatal OT pathways may partly determine aspects of the postnatal mother-child interaction and should therefore be considered in future research.
4.1 Transmission of Maternal ELS during the Prenatal Period and the Role of OT
4.1.1 OT modulation of MPF stress physiology, Trans-Placental OT Signaling, and Programming the fetal OT system
The prenatal period represents a particularly sensitive developmental period, during which plasticity to environmental conditions is high (Gluckman and Hanson, 2004). Accumulating evidence suggests that exposure to maternal stress during intrauterine life may result in an increased propensity of the offspring to develop behavioral or emotional problems (Buss et al., 2012), as well as cognitive developmental delays (Bergman et al., 2007; Buss et al., 2011; Glover, 2015). Importantly, these prenatally programmed offspring phenotypes may also influence the mothers’ reaction to the child, an aspect of the reciprocal mother-offspring relationship that is oftentimes underestimated. The mechanisms through which information about pre-conceptional and gestational stressors are conveyed to the fetus likely include MPF stress biology (Entringer et al., 2015). Given the fact that ELS-exposed individuals show marked alterations in stress responsive systems such as the HPA axis (Heim et al., 2008) and the immune system (Pace et al., 2012), it is highly plausible that these conditions may extend to the gestational period and provide cues about maternal ELS to her unborn child and shape its developmental trajectory. Some preliminary evidence supports the notion that MPF endocrine stress biology is altered in the context of maternal ELS. It has been shown that ELS-exposure predicts alterations of placental CRH (Moog et al., 2015) and cortisol concentrations in saliva (Bublitz and Stroud, 2012) and hair (Schreier et al., 2015) during pregnancy. As discussed earlier, OT has the potential to down-regulate HPA-axis mediators and an ELS-associated lower OT activity and/or reduced OTR sensitivity may partly explain the elevated cortisol levels in pregnant women. Moreover, considering the anti-inflammatory properties of OT, ELS-associated deficiencies in OT signaling could favor a pro-inflammatory phenotype in pregnant women. Evidence from animal studies and epidemiological data suggest that in utero exposure to higher levels of pro-inflammatory cytokines may alter fetal brain development and is associated with higher risk for neurodevelopmental disorders such as autism or schizophrenia (Estes and McAllister, 2016).
Importantly, OT itself was shown to pass the placental barrier by means of passive diffusion in vitro (Malek et al., 1996) to potentially participate in fetal brain development and could therefore constitute a possible mother-to-fetus signaling pathway conveying information to the fetus about (early) maternal experiences and the quality of the postnatal environment. However, others could not confirm OT passage across the placenta in vivo (Patient et al., 1999). Moreover, in addition to the placenta, OT degrading placental leucine aminopeptidase and the fetal blood-brain barrier may only permit a small fraction of maternal OT to reach the fetal brain. Thus, more research on the mechanisms, determinants (e.g., maternal ELS, placental enzyme activity), and effects (e.g., fetal brain development) of trans-placental OT signaling is needed to establish a role for this possible intergenerational maternal-to-fetal communication pathway.
Bearing in mind the pronounced counter-regulatory interactions of the HPA-axis mediators with OT neural circuits, one could also hypothesize that prenatal exposure to high concentrations of glucocorticoids or pro-inflammatory cytokines, as a correlate of maternal ELS, may alter the developmental trajectory of the fetal brain, including the fetal oxytocinergic system and may consequently affect postnatal attachment. Not taking into account maternal ELS, there is preliminary evidence that supports the programming effects of maternal stress on the newborn OT system. For example, higher prenatal risk (e.g., maternal psychopathology, drug use) has been shown to be associated with higher OXTR exon 2 methylation at birth in a subset of individuals with low internalizing symptoms (Cecil et al., 2014). This higher DNA methylation at birth in turn was positively correlated with callous unemotional traits at age 13 years, a condition that is characterized by strong impairments in psychosocial functioning such as low empathy and a lack of guilt. Another recent report revealed that higher maternal psychological (depressive symptoms) and biological (cortisol) stress during pregnancy was negatively associated with OXTR exon 3 methylation in fetal cord blood (Unternaehrer et al., 2016). Assuming that this lower methylation implies higher OTR expression, this would suggest a counter-regulatory epigenetic mechanism in newborns exposed to high levels of intrauterine stress. Despite the contradictory nature of these reports and the difference of targeted OXTR genomic loci, these findings indicate that maternal prenatal stress, which is highly prevalent in ELS-survivors, via fetal OXTR methylation may prepare the developing fetus for a postnatal environment characterized by low maternal care, PPD, or both. It is now crucial to extend these finding by incorporating the influence of maternal ELS.
4.1.2 Maternal oxytocinergic adaptions during pregnancy
Major adaptations in the maternal OT/OTR system already occur during the time of pregnancy to optimally prepare for motherhood (Brunton and Russell, 2008; Hillerer et al., 2014). Through a complex inhibitory mechanism including actions of progesterone, its neuroactive metabolite allopregnanolone, and endogenous opioids, OT-release from magnocellular neurons is restrained throughout pregnancy. This inhibitory mechanism allows the storage of large amounts of OT in neurohypophyseal large dense-core vesicles necessary to induce labor and immediate postpartum onset of maternal behaviors while simultaneously preventing premature onset of uterus contractions. In addition to hypothalamic OT accumulation, rodent studies show that central OTRs are upregulated during the gestational period (Bealer et al., 2006; Meddle et al., 2007; Young et al., 1997) in brain areas known to be involved in maternal behavior such as the PVN, SON, BNST, and the MPOA. This gestational “priming” of the central maternal attachment system to prepare for onset of maternal behavior immediately after birth is sensitive to the effects of E and could be affected by ELS-exposure (Champagne, 2008). Considering the highly E-dependent upregulation of OTRs during the gestational period, we hypothesize that via ELS-associated epigenetic modifications (DNA methylation) of estrogen-responsive loci in the OTR-gene, the OT/OTR system lacks sufficient E-induced upregulation during late gestation in ELS-exposed women. Via this pathway, ELS may contribute to maternal risk for PPD and suboptimal parenting behavior. To our knowledge, to date there is no available data in humans that would directly support the claim of ELS-associated alterations of maternal OT adaptations during gestation. Phenotypically however and regardless of ELS, inter-individual differences in patterns of plasma OT during the gestational period are associated with differences in maternal-fetal attachment (Levine et al., 2007), mother-infant bonding (Feldman et al., 2007), and risk for PPD (Skrundz et al., 2011). These studies are consistent with the assumed antidepressant and attachment-facilitating effects of OT. Accordingly, women at risk for PPD exhibit lower OT plasma concentrations during pregnancy than women who are not at risk for PPD (Skrundz et al., 2011). Moreover, plasma OT concentrations during pregnancy are a positive predictor of certain maternal behaviors that support attachment formation such as child-directed gaze and affectionate touch (Feldman et al., 2007). Finally, Levine et al. (2007) reported that women with an increase of OT during gestation scored higher on a measure of maternal-fetal attachment compared to those women that either showed a decrease or a stable pattern of OT concentrations throughout pregnancy.
To conclude, there is convincing preliminary evidence that already during pregnancy, fetal and maternal OT/OTR adaptations occur to facilitate maternal-child bonding or to predict the risk to develop PPD and offspring socio-behavioral problems. It remains to be studied whether these adaptations are altered in the context of maternal ELS. In light of the findings of ELS-associated alterations in OT pathways in non-pregnant women (e.g., lower CSF-OT), it should be investigated in future studies whether these alterations extend to the gestational period and may therefore represent prenatal OT associated intergenerational transmission pathways of maternal ELS.
4.2 Transmission of Maternal ELS during the Postnatal Period and the Role of OT
At early stages in the postpartum period, the formation of selective and enduring interpersonal bonds between a mother and her newborn is the basis of healthy, normative child development (Bowlby, 1977; Bowlby, 1980). Critical for the successful establishment of this attachment is the mother’s ability to adequately use a behavioral repertoire, which includes child-directed gaze, affectionate touch, “motherese” vocalizations, establishment of physical proximity, and the expression of positive affect. The readiness of the mother to adapt her own behavior to the child’s state during episodes of dyadic interaction enables reciprocal bio-behavioral synchrony (Feldman, 2012; Feldman, 2015b) between the mother and child. Briefly, this synchrony refers to the temporally coordinated activity of social behaviors (e.g., social gaze) and biological processes during social contact. Experience of such synchrony is fundamentally important for social and emotional growth in children (Feldman, 2015b) and critically depends on OT (Apter-Levi et al., 2014; Atzil et al., 2011; Feldman et al., 2010; Kim et al., 2014). For instance, the increase of maternal plasma OT levels following interaction with her child predicts maternal infant-directed gaze (Kim et al., 2014), thereby facilitating the perception of infant social cues, a necessary prerequisite to react promptly and adequately to these cues. Moreover, only under conditions of high affective synchrony do parents and children show concordance of their saliva OT concentrations (Feldman et al., 2010). No such endocrine coupling occurs in low-synchrony parent-infant dyads. Compared to non-maltreated mothers, ELS-exposed mothers exhibit less sensitivity (Mielke et al., 2016), more hostility (Collishaw et al., 2007; Rijlaarsdam et al., 2014), more rejection (Miranda et al., 2013) and higher intrusiveness (Zvara et al., 2015) towards their children. In other words, ELS-exposed mothers may have greater difficulties to elicit bio-behavioral synchrony in interaction with their children. These findings imply that neural circuits in the maternal brain that involve OT signaling and support sensitive parenting, as well as emergence of bio-behavioral synchrony, might be critically altered in ELS survivors (also see chapter 2.2.1). In the case of maternal depression, which is highly prevalent in ELS survivors, bio-behavioral synchrony is lower than in non-depressed mothers (Feldman et al., 2009), possibly due a tendency of the depressed mother to withdraw from the interaction. Accordingly, depressed mothers present with lower baseline concentrations of OT and lower OT responses following interaction with their children (Pratt et al., 2015). Interestingly, children of depressed mothers also exhibit lower baseline concentrations of OT and lower OT responses following social interaction. This intergenerational transmission of OT deficiency in children of depressed mothers corresponds to impairments in important psychosocial domains such as reduced empathy or lower readiness to engage in social interactions (Apter-Levy et al., 2014), as well as poor emotion regulation and increased cortisol reactivity (Feldman et al., 2009). As shown here, ELS-associated asynchrony in mother-child interactions represents a promising approach to investigate OT postnatal pathways of intergenerational transmission of maternal ELS.
5. Methodological Challenges, Future Directions and Translational Implications
The reviewed evidence pertaining to OT/OTR pathways in the intergenerational transmission of maternal ELS can be considered preliminary. We are fully aware of common statistical (e.g., small effects, poor replication rates) methodological (e.g., intranasal OT), and biological limitations (peripheral vs. central DNA-methylation) associated with studying OT/OTR pathways in humans that have already been discussed above. Moreover, most human studies rely on plasma OT concentrations for the prediction of behavioral phenotypes, assuming that these peripherally derived measures reflect central OT concentrations. This basic premise has been challenged by studies showing that there is no correlation between plasma and CSF OT concentrations (Altemus et al., 2004; Kagerbauer et al., 2013), but confirmed by others, which found significant between-compartment correlations (Carson et al., 2015). Divergent findings like these, whose clarification is pivotal for advancement of behavioral and psychiatric neuroendocrinology, may partly result from methodological heterogeneity, which leads to an issue of particular concern. Reliability and validity of widely-used commercially available immunoassays to quantify peripheral OT concentrations have been put into question (McCullough et al., 2013). Across studies, the reported peripheral OT concentrations are highly variable depending on the applied methods, with values ranging from < 10 to over 1200 pg/ml (Brandtzaeg et al., 2016; McCullough et al., 2013). Thus, there is an urgent need to standardize OT assay protocols to guarantee high-quality research, which is indispensable for obtaining meaningful results as well as reliability and comparability between studies. In light of these concerns, we advocate a critical awareness when interpreting studies in the realm of human OT research.
Nevertheless, animal research strongly supports the notion of inter- and transgenerational effects of adverse early life experience that crucially involve OT pathways (Champagne, 2008). Moreover, a highly relevant study in rodents recently showed that a polymorphism in the Oxtr gene explains a large proportion of variance in accumbal OTR expression, which is known to be functionally relevant for attachment formation and parenting (King et al., 2015). A recent human study by Dannlowski et al. (2015) identified an ELS-associated reduction in bilateral gray matter volume of the ventral striatum. Intriguingly, this brain area is known to be involved in parenting behavior and depression risk, and the ELS-associated structural alterations were only present in individuals with a susceptible genotype (OXTR rs53576 GG), thus providing an example of a promising approach for the elucidation of intergenerational transmission of maternal ELS in humans. As a consequence of the complexity of developmental processes and genetic factors that mediate and/or moderate the intergenerational transmission of maternal ELS, future investigations would benefit from longitudinal research designs, beginning during the gestational period, with frequent postnatal follow-up assessments during critical developmental periods until adulthood, in clinical and non-clinical populations and adopt mechanism-based research strategies to answer the question of how and when specific transmission processes occur. Such longitudinal observational studies in the parental and offspring generation should be accompanied by examination of post mortem brain tissue to shed light on genotype-specific and methylation-associated brain OTR expression profiles helping to validate psychiatric and behavioral genetics and epigenetics to substantially increase translational significance and acceptance.
Given the described insufficient OT signaling in depressed pregnant and postpartum women, intranasal OT administration has been discussed as a possible psychopharmacological treatment to ameliorate symptoms and maternal parenting behaviors. Clinical trials, however, have produced inconsistent results (Mah, 2016), and sometimes acute OT administration aggravated depressive symptoms (Mah et al., 2013) in postpartum women. Moreover, compared to placebo control, OT-treated women with PPD describe their children as being more difficult (Mah et al., 2013) and do not show beneficial effects on sensitive parenting behavior towards their children, but in contrary would use rather harsh parenting after hearing infant cry sounds (Mah et al., 2016). Also, synthetic intravenous OT, which is widely used in obstetrics to induce uterus contractions during parturition, was positively correlated with depression and anxiety symptoms at two months postpartum (Gu et al., 2015). Even more discouraging are animal studies that find no beneficial and at times even detrimental effects of chronic central or intranasal administration of OT. Chronic i.c.v. injection of high OT doses in mice produced higher anxiety-like behavior in a social stress paradigm, which was paralleled by reductions in OTR binding in various brain areas (e.g., amygdala, septum) relevant for stress-regulation (Peters et al., 2014). Similarly, chronic intranasal OT treatment resulted in a reduction of social behaviors and concurrent reductions of OTR binding in the nAcc, amygdala, and anterior olfactory nucleus amongst other brain regions (Huang et al., 2014). Moreover, in a mouse model of ASD, chronic intranasal OT administration did not lead to improvements in social behaviors (Bales et al., 2014). Lastly, exposure to ELS seems to alleviate or even reverse the presumed beneficial effects of intranasal OT treatment in humans (Bakermans-Kranenburg and Van Ijzendoorn, 2013; Grimm et al., 2014). Thus, premature use of intranasal OT as a long-term stand-alone treatment or as adjunctive pharmacotherapy in combination with psychotherapy in women with PPD and parenting difficulties that both may be a result of maternal ELS-exposure is potentially harmful to both mother and offspring.
It would also be highly informative to test whether treatment effects differ depending on OXTR genotype, thus empirically probing assumptions of differential susceptibility (see section 3.1). Given the empirical evidence in support of greater susceptibility to environmental exposures in individuals with certain OXTR gene polymorphisms (e.g., rs53576G), these individuals may also profit more from therapeutic interventions. For example, it was shown that rs53576 G allele carriers are more susceptible to develop depression after ELS exposure (McQuaid et al., 2013), but are also the ones who profit more from social support under acute psychosocial stress (Chen et al., 2011). As an example, Wilker et al. (2014) have shown in a sample of war survivors that a SNP in a stress-related gene (FK506-binding protein 51) moderates long-term effectiveness of an exposure-based therapy for posttraumatic stress disorder. Identifying genetic factors that moderate treatment responsiveness would help better identify women who have problems bonding with their children and with PPD and who would be more responsive to the effects of psychotherapy or other intervention strategies. From a public health perspective, there is now a growing awareness that the time around birth is a highly sensitive period for the mother and offspring with a comparatively high risk for maternal depression. Thus, the US Preventive Services Task Force published a recommendation statement to routinely screen pregnant and postpartum women for depression in primary care settings (Siu et al., 2016). This development is encouraging and necessary, bearing in mind the potential consequences and costs of maternal depression for the affected women, their children, and their families. Reliable and well-validated screening instruments for the identification of women at risk like the Edinburgh Postnatal Depression Scale (Cox et al., 1987) are available, and so are different targeted intervention strategies such as psychotherapy and parental education programs for antenatal and postpartum depression (Dennis and Hodnett, 2007; Dennis et al., 2007; Shaw et al., 2006). A better understanding of the biological underpinnings of gestational and postpartum depression would provide further targets for intervention.
6. Summary
The goal of the present work is to develop a framework of biological pathways in the intergenerational transmission of maternal ELS in humans that emphasizes the role of the OT/OTR system (see Figure 1). A summary of evidence is provided that shows that the OT/OTR system interacts with stress-related neuroendocrine and inflammatory pathways and is sensitive to environmental exposures (e.g., ELS). In addition, OT is a major modulator in neural circuits underlying parenting behaviors, attachment, stress reactivity, and risk for psychiatric disorders such as PPD. Emerging evidence of genetic variations in OT pathway genes that may moderate individual susceptibility after ELS exposure seems promising. First studies propose environmentally induced OXTR DNA methylation as a possible candidate mechanism of how ELS may become embedded in affected individuals, thereby contributing to alterations in OT signaling. Given the high biological plausibility of OT in the intergenerational transmission of maternal ELS stress, OT signaling pathways will likely attract considerable attention in future research with possible translational implications (see section 5.1). Given the ever growing complexity of research on human development, i.e., from molecular mechanisms to observations of complex behavioral phenotypes, the present review was aimed to provide an overview of critical recent advances of the field and to systematically review evidence for environmental, genetic, and epigenetic factors pertaining to OT signaling pathways.
Supplementary Material
Highlights.
Oxytocin plays a role in the intergenerational transmission of early adversity.
Putative pre- and postnatal OT pathways of transmission are proposed.
Oxytocin receptor gene (OXTR) variants affect susceptibility to the environment.
The role of OXTR DNA methylation is discussed as a mechanism of transmission.
Critical assessment of methodological challenges in human OT research is provided.
Acknowledgments
Authors report no competing interests. The conception and writing of this article was supported by the European Research Area Network NEURON (ERA-NET NEURON, MecTranGen 01EW1407A) and the National Institutes of Health (NIH R01 HD-060628, R01 MH105538, R01 MH091351).
List of abbreviations
- 5HTTLPR
Serotonin Transporter Length Polymorphic Region
- BPD
Borderline Personality Disorder
- CNS
Central Nervous System
- CpG
Cytosine-Guanine DNA Sequence
- CRH
Corticotropin Releasing Hormone
- CSF
Cerebrospinal Fluid
- DA
Dopamine
- DAT1
Dopamine Transporter Gene 1
- DNA
Deoxyribonucleic Acid
- DRD2, DRD4
Dopamine Receptor 2 and Dopamine Receptor 4 Genes
- E
Estrogens
- ELS
Early Life Stress
- GABA
Gamma Amino Butric Acid
- HPA
Hypothalamic Pituitary Adrenal Axis
- i.c.v
Intracerebroventricular
- IL-1β
Interleukin 1 beta
- IL-6
Interleukin 6
- LG
Licking and Grooming
- MDD
Major Depressive Disorder
- MPF
Maternal-Placental-Fetal
- MPOA
Medial Preoptic Area
- mRNA
Messenger Ribonucleic Acid
- nAcc
Nucleus Accumbens
- OT
Oxytocin
- OTR
Oxytocin Receptor
- OXTR
Oxytocin Receptor Gene (human)
- Oxtr
Oxytocin Receptor Gene (rodents)
- PPD
Postpartum Depression
- PVN
Paraventricular Nucleus
- Rs
Reference Number for Single Nucleotide Polymorphism (e.g., rs53576)
- SNP
Single Nucleotide Polymorphism
- TNF-α
Tumor Necrosis Factor alpha
- ToM
Theory of Mind
- VMN
Ventromedial Nucleus
- VS
Ventral Striatum
- VTA
Ventral Tegmental Area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altemus M, Fong J, Yang R, Damast S, Luine V, Ferguson D. Changes in cerebrospinal fluid neurochemistry during pregnancy. Biological psychiatry. 2004;56:386–392. doi: 10.1016/j.biopsych.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, Dube SR, Giles WH. The enduring effects of abuse and related adverse experiences in childhood. European archives of psychiatry and clinical neuroscience. 2006;256:174–186. doi: 10.1007/s00406-005-0624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apter-Levi Y, Zagoory-Sharon O, Feldman R. Oxytocin and vasopressin support distinct configurations of social synchrony. Brain research. 2014;1580:124–132. doi: 10.1016/j.brainres.2013.10.052. [DOI] [PubMed] [Google Scholar]
- Apter-Levy Y, Feldman M, Vakart A, Ebstein RP, Feldman R. Impact of maternal depression across the first 6 years of life on the child’s mental health, social engagement, and empathy: the moderating role of oxytocin. American Journal of Psychiatry. 2014 doi: 10.1176/appi.ajp.2013.12121597. [DOI] [PubMed] [Google Scholar]
- Atzil S, Hendler T, Feldman R. Specifying the neurobiological basis of human attachment: brain, hormones, and behavior in synchronous and intrusive mothers. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36:2603–2615. doi: 10.1038/npp.2011.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van Ijzendoorn MH. Oxytocin receptor (OXTR) and serotonin transporter (5-HTT) genes associated with observed parenting. Social cognitive and affective neuroscience. 2008;3:128–134. doi: 10.1093/scan/nsn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, Van Ijzendoorn MH. Sniffing around oxytocin: review and meta-analyses of trials in healthy and clinical groups with implications for pharmacotherapy. Translational psychiatry. 2013;3:e258. doi: 10.1038/tp.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales K, Solomon M, Jacob S, Crawley J, Silverman JL, Larke R, Sahagun E, Puhger K, Pride M, Mendoza S. Long-term exposure to intranasal oxytocin in a mouse autism model. Translational psychiatry. 2014;4:e480. doi: 10.1038/tp.2014.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bealer SL, Lipschitz DL, Ramoz G, Crowley WR. Oxytocin receptor binding in the hypothalamus during gestation in rats. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2006;291:R53–R58. doi: 10.1152/ajpregu.00766.2005. [DOI] [PubMed] [Google Scholar]
- Beaver KM, Belsky J. Gene-environment interaction and the intergenerational transmission of parenting: testing the differential-susceptibility hypothesis. Psychiatric Quarterly. 2012;83:29–40. doi: 10.1007/s11126-011-9180-4. [DOI] [PubMed] [Google Scholar]
- Beck CT. The effects of postpartum depression on maternal-infant interaction: a meta-analysis. Nursing research. 1995;44:298–305. [PubMed] [Google Scholar]
- Beery AK, McEwen LM, MacIsaac JL, Francis DD, Kobor MS. Natural variation in maternal care and cross-tissue patterns of oxytocin receptor gene methylation in rats. Hormones and behavior. 2015 doi: 10.1016/j.yhbeh.2015.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell AF, Carter C, Steer CD, Golding J, Davis JM, Steffen AD, Rubin LH, Lillard TS, Gregory SP, Harris JC. Interaction between oxytocin receptor DNA methylation and genotype is associated with risk of postpartum depression in women without depression in pregnancy. Frontiers in genetics. 2015:6. doi: 10.3389/fgene.2015.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychological bulletin. 2009;135:885. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Bergman K, Sarkar P, O’Connor TG, Modi N, Glover V. Maternal stress during pregnancy predicts cognitive ability and fearfulness in infancy. Journal of the American Academy of Child & Adolescent Psychiatry. 2007;46:1454–1463. doi: 10.1097/chi.0b013e31814a62f6. [DOI] [PubMed] [Google Scholar]
- Boccia M, Petrusz P, Suzuki K, Marson L, Pedersen C. Immunohistochemical localization of oxytocin receptors in human brain. Neuroscience. 2013;253:155–164. doi: 10.1016/j.neuroscience.2013.08.048. [DOI] [PubMed] [Google Scholar]
- Bowers ME, Yehuda R. Intergenerational Transmission of Stress in Humans. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2016;41:232–244. doi: 10.1038/npp.2015.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowlby J. The making and breaking of affectional bonds. I. Aetiology and psychopathology in the light of attachment theory. An expanded version of the Fiftieth Maudsley Lecture, delivered before the Royal College of Psychiatrists, 19 November 1976. Br J Psychiatry. 1977;130:201–210. doi: 10.1192/bjp.130.3.201. [DOI] [PubMed] [Google Scholar]
- Bowlby J. Attachment and loss. Basic books 1980 [Google Scholar]
- Bradley B, Westen D, Mercer KB, Binder EB, Jovanovic T, Crain D, Wingo A, Heim C. Association between childhood maltreatment and adult emotional dysregulation in a low-income, urban, African American sample: moderation by oxytocin receptor gene. Development and psychopathology. 2011;23:439–452. doi: 10.1017/S0954579411000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandtzaeg OK, Johnsen E, Roberg-Larsen H, Seip KF, MacLean EL, Gesquiere LR, Leknes S, Lundanes E, Wilson SR. Proteomics tools reveal startlingly high amounts of oxytocin in plasma and serum. Scientific Reports. 2016:6. doi: 10.1038/srep31693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummelte S, Galea LA. Postpartum depression: etiology, treatment and consequences for maternal care. Hormones and behavior. 2015 doi: 10.1016/j.yhbeh.2015.08.008. [DOI] [PubMed] [Google Scholar]
- Brüne M. Does the oxytocin receptor polymorphism (rs2254298) confer ’vulnerability’ for psychopathology or’ differential susceptibility’? insights from evolution. BMC medicine. 2012;10:38. doi: 10.1186/1741-7015-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton PJ, Russell JA. The expectant brain: adapting for motherhood. Nature Reviews Neuroscience. 2008;9:11–25. doi: 10.1038/nrn2280. [DOI] [PubMed] [Google Scholar]
- Bublitz MH, Stroud LR. Childhood sexual abuse is associated with cortisol awakening response over pregnancy: preliminary findings. Psychoneuroendocrinology. 2012;37:1425–1430. doi: 10.1016/j.psyneuen.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülbül M, Babygirija R, Cerjak D, Yoshimoto S, Ludwig K, Takahashi T. Hypothalamic oxytocin attenuates CRF expression via GABA A receptors in rats. Brain research. 2011;1387:39–45. doi: 10.1016/j.brainres.2011.02.091. [DOI] [PubMed] [Google Scholar]
- Burkhouse KL, Woody ML, Owens M, McGeary JE, Knopik VS, Gibb BE. Sensitivity in detecting facial displays of emotion: Impact of maternal depression and oxytocin receptor genotype. Cognition and Emotion. 2015:1–13. doi: 10.1080/02699931.2014.996531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C, Davis E, Hobel C, Sandman C. Maternal pregnancy-specific anxiety is associated with child executive function at 6–9 years age. Stress. 2011;14:665–676. doi: 10.3109/10253890.2011.623250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C, Davis EP, Shahbaba B, Pruessner JC, Head K, Sandman CA. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proceedings of the National Academy of Sciences. 2012;109:E1312–E1319. doi: 10.1073/pnas.1201295109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso C, Kingdon D, Ellenbogen MA. A meta-analytic review of the impact of intranasal oxytocin administration on cortisol concentrations during laboratory tasks: Moderation by method and mental health. Psychoneuroendocrinology. 2014;49:161–170. doi: 10.1016/j.psyneuen.2014.07.014. [DOI] [PubMed] [Google Scholar]
- Carson D, Berquist S, Trujillo T, Garner J, Hannah S, Hyde S, Sumiyoshi R, Jackson L, Moss J, Strehlow M. Cerebrospinal fluid and plasma oxytocin concentrations are positively correlated and negatively predict anxiety in children. Molecular psychiatry. 2015;20:1085–1090. doi: 10.1038/mp.2014.132. [DOI] [PubMed] [Google Scholar]
- Carter AS, Garrity-Rokous FE, Chazan-Cohen R, Little C, Briggs-Gowan MJ. Maternal depression and comorbidity: predicting early parenting, attachment security, and toddler social-emotional problems and competencies. Journal of the American Academy of Child & Adolescent Psychiatry. 2001;40:18–26. doi: 10.1097/00004583-200101000-00012. [DOI] [PubMed] [Google Scholar]
- Cecil CA, Lysenko LJ, Jaffee SR, Pingault JB, Smith RG, Relton CL, Woodward G, McArdle W, Mill J, Barker ED. Environmental risk, Oxytocin Receptor Gene (OXTR) methylation and youth callous-unemotional traits: a 13-year longitudinal study. Molecular psychiatry. 2014;19:1071–1077. doi: 10.1038/mp.2014.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne F. Epigenetic mechanisms and the transgenerational effects of maternal care. Frontiers in neuroendocrinology. 2008;29:386–397. doi: 10.1016/j.yfrne.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne F, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:12736–12741. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen FS, Kumsta R, von Dawans B, Monakhov M, Ebstein RP, Heinrichs M. Common oxytocin receptor gene (OXTR) polymorphism and social support interact to reduce stress in humans. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:19937–19942. doi: 10.1073/pnas.1113079108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clodi M, Vila G, Geyeregger R, Riedl M, Stulnig TM, Struck J, Luger TA, Luger A. Oxytocin alleviates the neuroendocrine and cytokine response to bacterial endotoxin in healthy men. American Journal of Physiology-Endocrinology and Metabolism. 2008;295:E686–E691. doi: 10.1152/ajpendo.90263.2008. [DOI] [PubMed] [Google Scholar]
- Collishaw S, Dunn J, O’connor TG, Golding J. Maternal childhood abuse and offspring adjustment over time. Development and psychopathology. 2007;19:367–383. doi: 10.1017/S0954579407070186. [DOI] [PubMed] [Google Scholar]
- Corwin EJ, Johnston N, Pugh L. Symptoms of postpartum depression associated with elevated levels of interleukin-1 beta during the first month postpartum. Biological Research for Nursing. 2008;10:128–133. doi: 10.1177/1099800408323220. [DOI] [PubMed] [Google Scholar]
- Cox E, Stuebe A, Pearson B, Grewen K, Rubinow D, Meltzer-Brody S. Oxytocin and HPA stress axis reactivity in postpartum women. Psychoneuroendocrinology. 2015;55:164–172. doi: 10.1016/j.psyneuen.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. The British journal of psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- Danese A, Moffitt TE, Pariante CM, Ambler A, Poulton R, Caspi A. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Arch Gen Psychiatry. 2008;65:409–415. doi: 10.1001/archpsyc.65.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannlowski U, Kugel H, Grotegerd D, Redlich R, Opel N, Dohm K, Zaremba D, Grögler A, Schwieren J, Suslow T. Disadvantage of social sensitivity: interaction of oxytocin receptor genotype and child maltreatment on brain structure. Biological psychiatry. 2015 doi: 10.1016/j.biopsych.2015.12.010. [DOI] [PubMed] [Google Scholar]
- Davies MN, Volta M, Pidsley R, Lunnon K, Dixit A, Lovestone S, Coarfa C, Harris RA, Milosavljevic A, Troakes C. Functional annotation of the human brain methylome identifies tissue-specific epigenetic variation across brain and blood. Genome biology. 2012;13:1. doi: 10.1186/gb-2012-13-6-r43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis CL, Hodnett ED. Psychosocial and psychological interventions for treating postpartum depression. The Cochrane Library. 2007 doi: 10.1002/14651858.CD006116.pub2. [DOI] [PubMed] [Google Scholar]
- Dennis CL, Ross LE, Grigoriadis S. Psychosocial and psychological interventions for treating antenatal depression. The Cochrane Library. 2007 doi: 10.1002/14651858.CD006309.pub2. [DOI] [PubMed] [Google Scholar]
- Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- Dubowitz H, Black MM, Kerr MA, Hussey JM, Morrel TM, Everson MD, Starr RH. Type and timing of mothers’ victimization: effects on mothers and children. Pediatrics. 2001;107:728–735. doi: 10.1542/peds.107.4.728. [DOI] [PubMed] [Google Scholar]
- Entringer S, Buss C, Wadhwa PD. Prenatal stress, development, health and disease risk: A psychobiological perspective—2015 Curt Richter Award Paper. Psychoneuroendocrinology. 2015;62:366–375. doi: 10.1016/j.psyneuen.2015.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes ML, McAllister AK. Maternal immune activation: Implications for neuropsychiatric disorders. Science. 2016;353:772–777. doi: 10.1126/science.aag3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farré P, Jones MJ, Meaney MJ, Emberly E, Turecki G, Kobor MS. Concordant and discordant DNA methylation signatures of aging in human blood and brain. Epigenetics & chromatin. 2015;8:1. doi: 10.1186/s13072-015-0011-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R. Bio-behavioral synchrony: A model for integrating biological and microsocial behavioral processes in the study of parenting. Parenting. 2012;12:154–164. [Google Scholar]
- Feldman R. The adaptive human parental brain: implications for children’s social development. Trends in neurosciences. 2015a;38:387–399. doi: 10.1016/j.tins.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Feldman R. Sensitive periods in human social development: New insights from research on oxytocin, synchrony, and high-risk parenting. Development and psychopathology. 2015b;27:369–395. doi: 10.1017/S0954579415000048. [DOI] [PubMed] [Google Scholar]
- Feldman R, Gordon I, Influs M, Gutbir T, Ebstein RP. Parental oxytocin and early caregiving jointly shape children’s oxytocin response and social reciprocity. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38:1154–1162. doi: 10.1038/npp.2013.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R, Gordon I, Zagoory-Sharon O. The cross-generation transmission of oxytocin in humans. Hormones and behavior. 2010;58:669–676. doi: 10.1016/j.yhbeh.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Feldman R, Granat A, Pariente C, Kanety H, Kuint J, Gilboa-Schechtman E. Maternal depression and anxiety across the postpartum year and infant social engagement, fear regulation, and stress reactivity. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48:919–927. doi: 10.1097/CHI.0b013e3181b21651. [DOI] [PubMed] [Google Scholar]
- Feldman R, Weller A, Zagoory-Sharon O, Levine A. Evidence for a neuroendocrinological foundation of human affiliation plasma oxytocin levels across pregnancy and the postpartum period predict mother-infant bonding. Psychological Science. 2007;18:965–970. doi: 10.1111/j.1467-9280.2007.02010.x. [DOI] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The Adverse Childhood Experiences (ACE) Study. American journal of preventive medicine. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Field T. Postpartum depression effects on early interactions, parenting, and safety practices: a review. Infant Behavior and Development. 2010;33:1–6. doi: 10.1016/j.infbeh.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman DR, O’hara MW, Stuart S, Gorman LL, Larsen KE, Coy KC. Effective treatment for postpartum depression is not sufficient to improve the developing mother–child relationship. Development and psychopathology. 2007;19:585–602. doi: 10.1017/S0954579407070289. [DOI] [PubMed] [Google Scholar]
- Freeman SM, Smith AL, Goodman MM, Bales KL. Selective localization of oxytocin receptors and vasopressin 1a receptors in the human brainstem. Social neuroscience. 2016 doi: 10.1080/17470919.2016.1156570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfield L, Mathews HL, Janusek LW. Inflammatory and Epigenetic Pathways for Perinatal Depression. Biological research for nursing. 2015 doi: 10.1177/1099800415614892. 1099800415614892. [DOI] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiological reviews. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Glover V. Perinatal Programming of Neurodevelopment. Springer; 2015. Prenatal stress and its effects on the fetus and the child: possible underlying biological mechanisms; pp. 269–283. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004;305:1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- Glynn LM, Davis EP, Sandman CA. New insights into the role of perinatal HPA-axis dysregulation in postpartum depression. Neuropeptides. 2013;47:363–370. doi: 10.1016/j.npep.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Goodman SH, Rouse MH, Connell AM, Broth MR, Hall CM, Heyward D. Maternal depression and child psychopathology: A meta-analytic review. Clinical child and family psychology review. 2011;14:1–27. doi: 10.1007/s10567-010-0080-1. [DOI] [PubMed] [Google Scholar]
- Gopal Rao V, Löffler C, Battey J, Hansmann I. The human gene for oxytocin-neurophysin I (OXT) is physically mapped to chromosome 20p13 by in situ hybridization. Cytogenetic and Genome Research. 1992;61:271–273. doi: 10.1159/000133420. [DOI] [PubMed] [Google Scholar]
- Gordon I, Zagoory-Sharon O, Leckman JF, Feldman R. Oxytocin and the development of parenting in humans. Biological psychiatry. 2010;68:377–382. doi: 10.1016/j.biopsych.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory R, Cheng H, Rupp HA, Sengelaub DR, Heiman JR. Oxytocin increases VTA activation to infant and sexual stimuli in nulliparous and postpartum women. Hormones and behavior. 2015;69:82–88. doi: 10.1016/j.yhbeh.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory SG, Connelly JJ, Towers AJ, Johnson J, Biscocho D, Markunas CA, Lintas C, Abramson RK, Wright HH, Ellis P, Langford CF, Worley G, Delong GR, Murphy SK, Cuccaro ML, Persico A, Pericak-Vance MA. Genomic and epigenetic evidence for oxytocin receptor deficiency in autism. BMC medicine. 2009;7:62. doi: 10.1186/1741-7015-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm S, Pestke K, Feeser M, Aust S, Weigand A, Wang J, Wingenfeld K, Pruessner JC, La Marca R, Böker H. Early life stress modulates oxytocin effects on limbic system during acute psychosocial stress. Social cognitive and affective neuroscience. 2014:nsu020. doi: 10.1093/scan/nsu020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu V, Feeley N, Gold I, Hayton B, Robins S, Mackinnon A, Samuel S, Carter CS, Zelkowitz P. Intrapartum Synthetic Oxytocin and Its Effects on Maternal Well-Being at 2 Months Postpartum. Birth. 2015 doi: 10.1111/birt.12198. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27:199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- Halligan SL, Herbert J, Goodyer IM, Murray L. Exposure to postnatal depression predicts elevated cortisol in adolescent offspring. Biological psychiatry. 2004;55:376–381. doi: 10.1016/j.biopsych.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Hammen C, Bower JE, Cole SW. Oxytocin receptor gene variation and differential susceptibility to family environment in predicting youth borderline symptoms. Journal of personality disorders. 2015;29:177–192. doi: 10.1521/pedi_2014_28_152. [DOI] [PubMed] [Google Scholar]
- Hannon E, Lunnon K, Schalkwyk L, Mill J. Interindividual methylomic variation across blood, cortex, and cerebellum: implications for epigenetic studies of neurological and neuropsychiatric phenotypes. Epigenetics. 2015;10:1024–1032. doi: 10.1080/15592294.2015.1100786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Binder EB. Current research trends in early life stress and depression: review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Experimental neurology. 2012;233:102–111. doi: 10.1016/j.expneurol.2011.10.032. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Heim C, Young LJ, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. Lower CSF oxytocin concentrations in women with a history of childhood abuse. Molecular psychiatry. 2009;14:954–958. doi: 10.1038/mp.2008.112. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biological psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Meinlschmidt G, Neumann I, Wagner S, Kirschbaum C, Ehlert U, Hellhammer DH. Effects of suckling on hypothalamic-pituitary-adrenal axis responses to psychosocial stress in postpartum lactating women. The journal of clinical endocrinology & metabolism. 2001;86:4798–4804. doi: 10.1210/jcem.86.10.7919. [DOI] [PubMed] [Google Scholar]
- Herpertz SC, Bertsch K. A New Perspective on the Pathophysiology of Borderline Personality Disorder: A Model of the Role of Oxytocin. The American journal of psychiatry. 2015;172:840–851. doi: 10.1176/appi.ajp.2015.15020216. [DOI] [PubMed] [Google Scholar]
- Hill KT, Warren M, Roth TL. The influence of infant–caregiver experiences on amygdala Bdnf, OXTr, and NPY expression in developing and adult male and female rats. Behavioural brain research. 2014;272:175–180. doi: 10.1016/j.bbr.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillerer KM, Jacobs VR, Fischer T, Aigner L. The maternal brain: an organ with peripartal plasticity. Neural plasticity. 2014;2014:574159. doi: 10.1155/2014/574159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes GE, Kana V, Menard C, Merad M, Russo SJ. Neuroimmune mechanisms of depression. Nature Neuroscience. 2015;18:1386–1393. doi: 10.1038/nn.4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, Cicchetti D, Rogosch FA. Oxytocin receptor gene polymorphism, perceived social support, and psychological symptoms in maltreated adolescents. Development and psychopathology. 2014;26:465–477. doi: 10.1017/S0954579414000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Michetti C, Busnelli M, Managò F, Sannino S, Scheggia D, Giancardo L, Sona D, Murino V, Chini B. Chronic and acute intranasal oxytocin produce divergent social effects in mice. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39:1102–1114. doi: 10.1038/npp.2013.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308:245–248. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- Insel TR. Translating oxytocin neuroscience to the clinic: a National Institute of Mental Health perspective. Biological psychiatry. 2016;79:153–154. doi: 10.1016/j.biopsych.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Jolley SN, Elmore S, Barnard KE, Carr DB. Dysregulation of the hypothalamic-pituitary-adrenal axis in postpartum depression. Biological Research for Nursing. 2007;8:210–222. doi: 10.1177/1099800406294598. [DOI] [PubMed] [Google Scholar]
- Jonas W, Mileva-Seitz V, Girard AW, Bisceglia R, Kennedy J, Sokolowski M, Meaney M, Fleming A, Steiner M. Genetic variation in oxytocin rs2740210 and early adversity associated with postpartum depression and breastfeeding duration. Genes, Brain and Behavior. 2013;12:681–694. doi: 10.1111/gbb.12069. [DOI] [PubMed] [Google Scholar]
- Jurek B, Slattery DA, Hiraoka Y, Liu Y, Nishimori K, Aguilera G, Neumann ID, van den Burg EH. Oxytocin regulates stress-induced Crf gene transcription through CREB-regulated transcription coactivator 3. The Journal of Neuroscience. 2015;35:12248–12260. doi: 10.1523/JNEUROSCI.1345-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagerbauer S, Martin J, Schuster T, Blobner M, Kochs E, Landgraf R. Plasma oxytocin and vasopressin do not predict neuropeptide concentrations in human cerebrospinal fluid. Journal of neuroendocrinology. 2013;25:668–673. doi: 10.1111/jne.12038. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Gouin JP, Weng N-p, Malarkey WB, Beversdorf DQ, Glaser R. Childhood adversity heightens the impact of later-life caregiving stress on telomere length and inflammation. Psychosomatic medicine. 2011;73:16. doi: 10.1097/PSY.0b013e31820573b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Fonagy P, Koos O, Dorsett K, Strathearn L. Maternal oxytocin response predicts mother-to-infant gaze. Brain research. 2014;1580:133–142. doi: 10.1016/j.brainres.2013.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel M, Clive M, Gispen F, Guintivano J, Brown T, Cox O, Beckmann MW, Kornhuber J, Fasching PA, Osborne LM. Oxytocin Receptor DNA Methylation in Postpartum Depression. Psychoneuroendocrinology. 2016 doi: 10.1016/j.psyneuen.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T, Tanizawa O, Mori K, Brownstein MJ, Okayama H. Structure and expression of a human oxytocin receptor. 1992 doi: 10.1038/356526a0. [DOI] [PubMed] [Google Scholar]
- King LB, Walum H, Inoue K, Eyrich NW, Young LJ. Variation in the oxytocin receptor gene predicts brain region specific expression and social attachment. Biological psychiatry. 2015 doi: 10.1016/j.biopsych.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, Gruppe H, Mattay VS, Gallhofer B, Meyer-Lindenberg A. Oxytocin modulates neural circuitry for social cognition and fear in humans. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klengel T, Dias BG, Ressler KJ. Models of intergenerational and transgenerational transmission of risk for psychopathology in mice. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, Osten P, Schwarz MK, Seeburg PH, Stoop R, Grinevich V. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73:553–566. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Kumsta R, Heinrichs M. Oxytocin, stress and social behavior: neurogenetics of the human oxytocin system. Current opinion in neurobiology. 2013;23:11–16. doi: 10.1016/j.conb.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Kumsta R, Hummel E, Chen FS, Heinrichs M. Epigenetic regulation of the oxytocin receptor gene: implications for behavioral neuroscience. Frontiers in neuroscience. 2013;7:83. doi: 10.3389/fnins.2013.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusui C, Kimura T, Ogita K, Nakamura H, Matsumura Y, Koyama M, Azuma C, Murata Y. DNA methylation of the human oxytocin receptor gene promoter regulates tissue-specific gene suppression. Biochemical and biophysical research communications. 2001;289:681–686. doi: 10.1006/bbrc.2001.6024. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Frontiers in neuroendocrinology. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Macbeth AH, Pagani JH, Young WS. Oxytocin: the great facilitator of life. Progress in neurobiology. 2009;88:127–151. doi: 10.1016/j.pneurobio.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng G, Ludwig M. Intranasal oxytocin: myths and delusions. Biological psychiatry. 2015 doi: 10.1016/j.biopsych.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Levine A, Zagoory-Sharon O, Feldman R, Weller A. Oxytocin during pregnancy and early postpartum: individual patterns and maternal-fetal attachment. Peptides. 2007;28:1162–1169. doi: 10.1016/j.peptides.2007.04.016. [DOI] [PubMed] [Google Scholar]
- LoParo D, Waldman I. The oxytocin receptor gene (OXTR) is associated with autism spectrum disorder: a meta-analysis. Molecular psychiatry. 2015;20:640–646. doi: 10.1038/mp.2014.77. [DOI] [PubMed] [Google Scholar]
- Love TM. Oxytocin, motivation and the role of dopamine. Pharmacology Biochemistry and Behavior. 2014;119:49–60. doi: 10.1016/j.pbb.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nature reviews Neuroscience. 2006;7:126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Sabatier N, Bull PM, Landgraf R, Dayanithi G, Leng G. Intracellular calcium stores regulate activity-dependent neuropeptide release from dendrites. Nature. 2002;418:85–89. doi: 10.1038/nature00822. [DOI] [PubMed] [Google Scholar]
- Maes M, Leonard B, Myint A, Kubera M, Verkerk R. The new ‘5-HT’hypothesis of depression: cell-mediated immune activation induces indoleamine 2, 3-dioxygenase, which leads to lower plasma tryptophan and an increased synthesis of detrimental tryptophan catabolites (TRYCATs), both of which contribute to the onset of depression. Progress in neuro-psychopharmacology and biological psychiatry. 2011;35:702–721. doi: 10.1016/j.pnpbp.2010.12.017. [DOI] [PubMed] [Google Scholar]
- Mah BL. Oxytocin, Postnatal Depression, and Parenting: A Systematic Review. Harvard review of psychiatry. 2016;24:1–13. doi: 10.1097/HRP.0000000000000093. [DOI] [PubMed] [Google Scholar]
- Mah BL, Ijzendoorn MH, Out D, Smith R, Bakermans-Kranenburg MJ. The Effects of Intranasal Oxytocin Administration on Sensitive Caregiving in Mothers with Postnatal Depression. Child Psychiatry & Human Development. 2016:1–8. doi: 10.1007/s10578-016-0642-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah BL, Van IJzendoorn MH, Smith R, Bakermans-Kranenburg MJ. Oxytocin in postnatally depressed mothers: its influence on mood and expressed emotion. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2013;40:267–272. doi: 10.1016/j.pnpbp.2012.10.005. [DOI] [PubMed] [Google Scholar]
- Malek A, Blann E, Mattison DR. Human placental transport of oxytocin. Journal of Maternal-Fetal Medicine. 1996;5:245–255. doi: 10.1002/(SICI)1520-6661(199609/10)5:5<245::AID-MFM3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Marusak HA, Furman DJ, Kuruvadi N, Shattuck DW, Joshi SH, Joshi AA, Etkin A, Thomason ME. Amygdala responses to salient social cues vary with oxytocin receptor genotype in youth. Neuropsychologia. 2015 doi: 10.1016/j.neuropsychologia.2015.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough ME, Churchland PS, Mendez AJ. Problems with measuring peripheral oxytocin: can the data on oxytocin and human behavior be trusted? Neuroscience & Biobehavioral Reviews. 2013;37:1485–1492. doi: 10.1016/j.neubiorev.2013.04.018. [DOI] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonté B, Szyf M, Turecki G, Meaney MJ. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nature neuroscience. 2009;12:342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnis OA, McQuaid RJ, Matheson K, Anisman H. The moderating role of an oxytocin receptor gene polymorphism in the relation between unsupportive social interactions and coping profiles: implications for depression. Frontiers in psychology. 2015:6. doi: 10.3389/fpsyg.2015.01133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLearn KT, Minkovitz CS, Strobino DM, Marks E, Hou W. Maternal depressive symptoms at 2 to 4 months post partum and early parenting practices. Archives of Pediatrics & Adolescent Medicine. 2006;160:279–284. doi: 10.1001/archpedi.160.3.279. [DOI] [PubMed] [Google Scholar]
- McQuaid RJ, McInnis OA, Abizaid A, Anisman H. Making room for oxytocin in understanding depression. Neuroscience & Biobehavioral Reviews. 2014;45:305–322. doi: 10.1016/j.neubiorev.2014.07.005. [DOI] [PubMed] [Google Scholar]
- McQuaid RJ, McInnis OA, Matheson K, Anisman H. Distress of ostracism: oxytocin receptor gene polymorphism confers sensitivity to social exclusion. Social cognitive and affective neuroscience. 2015:nsu166. doi: 10.1093/scan/nsu166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuaid RJ, McInnis OA, Stead JD, Matheson K, Anisman H. A paradoxical association of an oxytocin receptor gene polymorphism: early-life adversity and vulnerability to depression. Frontiers in neuroscience. 2013:7. doi: 10.3389/fnins.2013.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annual review of neuroscience. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Epigenetics and the biological definition of gene× environment interactions. Child development. 2010;81:41–79. doi: 10.1111/j.1467-8624.2009.01381.x. [DOI] [PubMed] [Google Scholar]
- Meddle SL, Bishop VR, Gkoumassi E, van Leeuwen FW, Douglas AJ. Dynamic changes in oxytocin receptor expression and activation at parturition in the rat brain. Endocrinology. 2007;148:5095–5104. doi: 10.1210/en.2007-0615. [DOI] [PubMed] [Google Scholar]
- Meinlschmidt G, Heim C. Sensitivity to intranasal oxytocin in adult men with early parental separation. Biological psychiatry. 2007;61:1109–1111. doi: 10.1016/j.biopsych.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Meltzer-Brody S, Bledsoe-Mansori SE, Johnson N, Killian C, Hamer RM, Jackson C, Wessel J, Thorp J. A prospective study of perinatal depression and trauma history in pregnant minority adolescents. American journal of obstetrics and gynecology. 2013;208:211.e211–211.e217. doi: 10.1016/j.ajog.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nature reviews Neuroscience. 2011;12:524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- Mielke EL, Neukel C, Bertsch K, Reck C, Möhler E, Herpertz SC. Maternal sensitivity and the empathic brain: Influences of early life maltreatment. Journal of psychiatric research. 2016;77:59–66. doi: 10.1016/j.jpsychires.2016.02.013. [DOI] [PubMed] [Google Scholar]
- Mileva-Seitz V, Steiner M, Atkinson L, Meaney MJ, Levitan R, Kennedy JL, Sokolowski MB, Fleming AS. Interaction between oxytocin genotypes and early experience predicts quality of mothering and postpartum mood. PloS one. 2013;8:e61443. doi: 10.1371/journal.pone.0061443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biological psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E. Harsh family climate in early life presages the emergence of a proinflammatory phenotype in adolescence. Psychological science. 2010 doi: 10.1177/0956797610370161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychological bulletin. 2011;137:959. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda JK, de la Osa N, Granero R, Ezpeleta L. Multiple mediators of the relationships among maternal childhood abuse, intimate partner violence, and offspring psychopathology. Journal of interpersonal violence. 2013;28:2941–2965. doi: 10.1177/0886260513488686. [DOI] [PubMed] [Google Scholar]
- Montag C, Brockmann E-M, Bayerl M, Rujescu D, Müller DJ, Gallinat J. Oxytocin and oxytocin receptor gene polymorphisms and risk for schizophrenia: a case–control study. The world journal of biological psychiatry. 2013 doi: 10.3109/15622975.2012.677547. [DOI] [PubMed] [Google Scholar]
- Moog NK, Buss C, Entringer S, Shahbaba B, Gillen DL, Hobel CJ, Wadhwa PD. Maternal exposure to childhood trauma is associated during pregnancy with placental-fetal stress physiology. Biological psychiatry. 2015 doi: 10.1016/j.biopsych.2015.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers AJ, Williams L, Gatt JM, McAuley-Clark EZ, Dobson-Stone C, Schofield PR, Nemeroff CB. Variation in the oxytocin receptor gene is associated with increased risk for anxiety, stress and depression in individuals with a history of exposure to early life stress. Journal of psychiatric research. 2014;59:93–100. doi: 10.1016/j.jpsychires.2014.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA. The mesolimbic dopamine reward circuit in depression. Biological psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Norman GJ, Hawkley L, Luhmann M, Ball AB, Cole SW, Berntson GG, Cacioppo JT. Variation in the oxytocin receptor gene influences neurocardiac reactivity to social stress and HPA function: a population based study. Hormones and behavior. 2012;61:134–139. doi: 10.1016/j.yhbeh.2011.11.006. [DOI] [PubMed] [Google Scholar]
- Norman GJ, Karelina K, Morris JS, Zhang N, Cochran M, DeVries AC. Social interaction prevents the development of depressive-like behavior post nerve injury in mice: a potential role for oxytocin. Psychosomatic medicine. 2010;72:519–526. doi: 10.1097/PSY.0b013e3181de8678. [DOI] [PubMed] [Google Scholar]
- Numan M, Stolzenberg DS. Medial preoptic area interactions with dopamine neural systems in the control of the onset and maintenance of maternal behavior in rats. Frontiers in neuroendocrinology. 2009;30:46–64. doi: 10.1016/j.yfrne.2008.10.002. [DOI] [PubMed] [Google Scholar]
- O’hara MW, McCabe JE. Postpartum depression: current status and future directions. Annual review of clinical psychology. 2013;9:379–407. doi: 10.1146/annurev-clinpsy-050212-185612. [DOI] [PubMed] [Google Scholar]
- Opacka-Juffry J, Mohiyeddini C. Experience of stress in childhood negatively correlates with plasma oxytocin concentration in adult men. Stress. 2012;15:1–10. doi: 10.3109/10253890.2011.560309. [DOI] [PubMed] [Google Scholar]
- Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, Heim CM. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. American Journal of Psychiatry. 2006 doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- Pace TW, Wingenfeld K, Schmidt I, Meinlschmidt G, Hellhammer DH, Heim CM. Increased peripheral NF-κB pathway activity in women with childhood abuse-related posttraumatic stress disorder. Brain, behavior, and immunity. 2012;26:13–17. doi: 10.1016/j.bbi.2011.07.232. [DOI] [PubMed] [Google Scholar]
- Patient C, Davison J, Charlton L, Baylis P, Thornton S. The effect of labour and maternal oxytocin infusion on fetal plasma oxytocin concentration. BJOG: An International Journal of Obstetrics & Gynaecology. 1999;106:1311–1313. doi: 10.1111/j.1471-0528.1999.tb08188.x. [DOI] [PubMed] [Google Scholar]
- Pedersen CA. Oxytocin control of maternal behavior regulation by sex steroids and offspring stimulia. Annals of the New York Academy of Sciences. 1997;807:126–145. doi: 10.1111/j.1749-6632.1997.tb51916.x. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Caldwell JD, Walker C, Ayers G, Mason GA. Oxytocin activates the postpartum onset of rat maternal behavior in the ventral tegmental and medial preoptic areas. Behavioral neuroscience. 1994;108:1163. doi: 10.1037//0735-7044.108.6.1163. [DOI] [PubMed] [Google Scholar]
- Pena CJ, Neugut YD, Calarco CA, Champagne FA. Effects of maternal care on the development of midbrain dopamine pathways and reward-directed behavior in female offspring. European Journal of Neuroscience. 2014;39:946–956. doi: 10.1111/ejn.12479. [DOI] [PubMed] [Google Scholar]
- Peters S, Slattery DA, Uschold-Schmidt N, Reber SO, Neumann ID. Dose-dependent effects of chronic central infusion of oxytocin on anxiety, oxytocin receptor binding and stress-related parameters in mice. Psychoneuroendocrinology. 2014;42:225–236. doi: 10.1016/j.psyneuen.2014.01.021. [DOI] [PubMed] [Google Scholar]
- Plant D, Barker ED, Waters C, Pawlby S, Pariante C. Intergenerational transmission of maltreatment and psychopathology: the role of antenatal depression. Psychological medicine. 2013;43:519–528. doi: 10.1017/S0033291712001298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt M, Apter-Levi Y, Vakart A, Feldman M, Fishman R, Feldman T, Zagoory-Sharon O, Feldman R. Maternal depression and child oxytocin response; moderation by maternal oxytocin and relational behavior. Depression and anxiety. 2015;32:635–646. doi: 10.1002/da.22392. [DOI] [PubMed] [Google Scholar]
- Puglia MH, Lillard TS, Morris JP, Connelly JJ. Epigenetic modification of the oxytocin receptor gene influences the perception of anger and fear in the human brain. Proceedings of the National Academy of Sciences. 2015;112:3308–3313. doi: 10.1073/pnas.1422096112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends in immunology. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijneveld SA, Van der Wal MF, Brugman E, Sing RAH, Verloove-Vanhorick SP. Infant crying and abuse. The Lancet. 2004;364:1340–1342. doi: 10.1016/S0140-6736(04)17191-2. [DOI] [PubMed] [Google Scholar]
- Reiner I, Van IMH, Bakermans-Kranenburg MJ, Bleich S, Beutel M, Frieling H. Methylation of the oxytocin receptor gene in clinically depressed patients compared to controls: The role of OXTR rs53576 genotype. Journal of psychiatric research. 2015;65:9–15. doi: 10.1016/j.jpsychires.2015.03.012. [DOI] [PubMed] [Google Scholar]
- Riem MM, Bakermans-Kranenburg MJ, Pieper S, Tops M, Boksem MA, Vermeiren RR, van IJzendoorn MH, Rombouts SA. Oxytocin modulates amygdala, insula, and inferior frontal gyrus responses to infant crying: a randomized controlled trial. Biological psychiatry. 2011;70:291–297. doi: 10.1016/j.biopsych.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Riem MM, Bakermans-Kranenburg MJ, van IJzendoorn MH, Out D, Rombouts SA. Attachment in the brain: adult attachment representations predict amygdala and behavioral responses to infant crying. Attachment & human development. 2012;14:533–551. doi: 10.1080/14616734.2012.727252. [DOI] [PubMed] [Google Scholar]
- Righetti-Veltema M, Conne-Perréard E, Bousquet A, Manzano J. Postpartum depression and mother–infant relationship at 3 months old. Journal of affective disorders. 2002;70:291–306. doi: 10.1016/s0165-0327(01)00367-6. [DOI] [PubMed] [Google Scholar]
- Rijlaarsdam J, Stevens GW, Jansen PW, Ringoot AP, Jaddoe VW, Hofman A, Ayer L, Verhulst FC, Hudziak JJ, Tiemeier H. Maternal childhood maltreatment and offspring emotional and behavioral problems maternal and paternal mechanisms of risk transmission. Child maltreatment. 2014 doi: 10.1177/1077559514527639. 1077559514527639. [DOI] [PubMed] [Google Scholar]
- Rilling JK. The neural and hormonal bases of human parentalcare. Neuropsychologia. 2013;51:731–747. doi: 10.1016/j.neuropsychologia.2012.12.017. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Young LJ. The biology of mammalian parenting and its effect on offspring social development. Science. 2014;345:771–776. doi: 10.1126/science.1252723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AL, Chen Y, Slopen N, McLaughlin KA, Koenen KC, Austin SB. MATERNAL EXPERIENCE OF ABUSE IN CHILDHOOD AND DEPRESSIVE SYMPTOMS IN ADOLESCENT AND ADULT OFFSPRING: A 21-YEAR LONGITUDINAL STUDY. Depression and anxiety. 2015;32:709–719. doi: 10.1002/da.22395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AL, Galea S, Austin SB, Corliss HL, Williams MA, Koenen KC. Women’s experience of abuse in childhood and their children’s smoking and overweight. American journal of preventive medicine. 2014;46:249–258. doi: 10.1016/j.amepre.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AL, Lyall K, Rich-Edwards JW, Ascherio A, Weisskopf MG. Association of maternal exposure to childhood abuse with elevated risk for autism in offspring. JAMA psychiatry. 2013;70:508–515. doi: 10.1001/jamapsychiatry.2013.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid B, Wong S, Mitchell B. Transcriptional regulation of oxytocin receptor by interleukin-1β and interleukin-6. Endocrinology. 2001;142:1380–1385. doi: 10.1210/endo.142.4.8107. [DOI] [PubMed] [Google Scholar]
- Schreier HM, Enlow MB, Ritz T, Gennings C, Wright RJ. Childhood abuse is associated with increased hair cortisol levels among urban pregnant women. Journal of epidemiology and community health. 2015;69:1169–1174. doi: 10.1136/jech-2015-205541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahrokh DK, Zhang TY, Diorio J, Gratton A, Meaney MJ. Oxytocin-dopamine interactions mediate variations in maternal behavior in the rat. Endocrinology. 2010;151:2276–2286. doi: 10.1210/en.2009-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory SG. The neural bases for empathy. The Neuroscientist. 2011;17:18–24. doi: 10.1177/1073858410379268. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Abu-Akel A. The social salience hypothesis of oxytocin. Biological psychiatry. 2015 doi: 10.1016/j.biopsych.2015.07.020. [DOI] [PubMed] [Google Scholar]
- Shaw E, Levitt C, Wong S, Kaczorowski J. Systematic review of the literature on postpartum care: effectiveness of postpartum support to improve maternal parenting, mental health, quality of life, and physical health. Birth. 2006;33:210–220. doi: 10.1111/j.1523-536X.2006.00106.x. [DOI] [PubMed] [Google Scholar]
- Siu AL, Bibbins-Domingo K, Grossman DC, Baumann LC, Davidson KW, Ebell M, García FA, Gillman M, Herzstein J, Kemper AR. Screening for depression in adults: US Preventive Services Task Force recommendation statement. Jama. 2016;315:380–387. doi: 10.1001/jama.2015.18392. [DOI] [PubMed] [Google Scholar]
- Skrundz M, Bolten M, Nast I, Hellhammer DH, Meinlschmidt G. Plasma oxytocin concentration during pregnancy is associated with development of postpartum depression. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36:1886–1893. doi: 10.1038/npp.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuse DH, Gallagher L. Dopaminergic-neuropeptide interactions in the social brain. Trends in cognitive sciences. 2009;13:27–35. doi: 10.1016/j.tics.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Smearman EL, Winiarski DA, Brennan PA, Najman J, Johnson KC. Social stress and the oxytocin receptor gene interact to predict antisocial behavior in an at-risk cohort. Development and psychopathology. 2015;27:309–318. doi: 10.1017/S0954579414000649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AS, Tabbaa M, Lei K, Eastham P, Butler MJ, Linton L, Altshuler R, Liu Y, Wang Z. Local oxytocin tempers anxiety by activating GABA A receptors in the hypothalamic paraventricular nucleus. Psychoneuroendocrinology. 2016;63:50–58. doi: 10.1016/j.psyneuen.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AS, Wang Z. Hypothalamic oxytocin mediates social buffering of the stress response. Biological psychiatry. 2014;76:281–288. doi: 10.1016/j.biopsych.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoop R. Neuromodulation by oxytocin and vasopressin. Neuron. 2012;76:142–159. doi: 10.1016/j.neuron.2012.09.025. [DOI] [PubMed] [Google Scholar]
- Strathearn L. Maternal neglect: oxytocin, dopamine and the neurobiology of attachment. Journal of neuroendocrinology. 2011;23:1054–1065. doi: 10.1111/j.1365-2826.2011.02228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L, Fonagy P, Amico J, Montague PR. Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2009;34:2655–2666. doi: 10.1038/npp.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuebe AM, Grewen K, Meltzer-Brody S. Association between maternal mood and oxytocin response to breastfeeding. Journal of women’s health. 2013;22:352–361. doi: 10.1089/jwh.2012.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturge-Apple ML, Cicchetti D, Davies PT, Suor JH. Differential susceptibility in spillover between interparental conflict and maternal parenting practices: Evidence for OXTR and 5-HTT genes. Journal of Family Psychology. 2012;26:431. doi: 10.1037/a0028302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Kim P, Spicer J, Ho SS, Dayton CJ, Elmadih A, Abel KM. Approaching the biology of human parental attachment: brain imaging, oxytocin and coordinated assessments of mothers and fathers. Brain research. 2014;1580:78–101. doi: 10.1016/j.brainres.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RJ, Parker KJ, Hallmayer JF, Waugh CE, Gotlib IH. Oxytocin receptor gene polymorphism (rs2254298) interacts with familial risk for psychopathology to predict symptoms of depression and anxiety in adolescent girls. Psychoneuroendocrinology. 2011;36:144–147. doi: 10.1016/j.psyneuen.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SM, Hammen C, Starr LR, Najman JM. Oxytocin receptor gene polymorphism (rs53576) moderates the intergenerational transmission of depression. Psychoneuroendocrinology. 2014;43:11–19. doi: 10.1016/j.psyneuen.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tost H, Kolachana B, Hakimi S, Lemaitre H, Verchinski BA, Mattay VS, Weinberger DR, Meyer–Lindenberg A. A common allele in the oxytocin receptor gene (OXTR) impacts prosocial temperament and human hypothalamic-limbic structure and function. Proceedings of the National Academy of Sciences. 2010;107:13936–13941. doi: 10.1073/pnas.1003296107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unternaehrer E, Bolten M, Nast I, Staehli S, Meyer AH, Dempster E, Hellhammer DH, Lieb R, Meinlschmidt G. Maternal Adversities during Pregnancy and Cord Blood Oxytocin Receptor (OXTR) DNA Methylation. Social cognitive and affective neuroscience. 2016:nsw051. doi: 10.1093/scan/nsw051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unternaehrer E, Luers P, Mill J, Dempster E, Meyer AH, Staehli S, Lieb R, Hellhammer DH, Meinlschmidt G. Dynamic changes in DNA methylation of stress-associated genes (OXTR, BDNF ) after acute psychosocial stress. Translational psychiatry. 2012;2:e150. doi: 10.1038/tp.2012.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unternaehrer E, Meyer AH, Burkhardt SC, Dempster E, Staehli S, Theill N, Lieb R, Meinlschmidt G. Childhood maternal care is associated with DNA methylation of the genes for brain-derived neurotrophic factor (BDNF) and oxytocin receptor (OXTR) in peripheral blood cells in adult men and women. Stress. 2015:1–11. doi: 10.3109/10253890.2015.1038992. [DOI] [PubMed] [Google Scholar]
- Wade M, Hoffmann TJ, Jenkins JM. Gene-environment interaction between the oxytocin receptor (OXTR) gene and parenting behaviour on children’s theory of mind. Social cognitive and affective neuroscience. 2015:nsv064. doi: 10.1093/scan/nsv064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner NJ, Propper C, Gueron-Sela N, Mills-Koonce WR. Dimensions of maternal parenting and infants’ autonomic functioning interactively predict early internalizing behavior problems. Journal of abnormal child psychology. 2015:1–12. doi: 10.1007/s10802-015-0039-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Yang HP, Tian S, Wang L, Wang SC, Zhang F, Wang YF. Oxytocin-secreting system: A major part of the neuroendocrine center regulating immunologic activity. Journal of Neuroimmunology. 2015;289:152–161. doi: 10.1016/j.jneuroim.2015.11.001. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nature neuroscience. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Wilker S, Pfeiffer A, Kolassa S, Elbert T, Lingenfelder B, Ovuga E, Papassotiropoulos A, de Quervain D, Kolassa IT. The role of FKBP5 genotype in moderating long-term effectiveness of exposure-based psychotherapy for posttraumatic stress disorder. Translational psychiatry. 2014;4:e403. doi: 10.1038/tp.2014.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle M, Mrug S. Hypothesis-driven research for G× E interactions: the relationship between oxytocin, parental divorce during adolescence, and depression in young adulthood. Frontiers in psychology. 2015:6. doi: 10.3389/fpsyg.2015.01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow JT, Noble PL, Lyons CK, Sterk SM, Insel TR. Rearing effects on cerebrospinal fluid oxytocin concentration and social buffering in rhesus monkeys. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2003;28:910–918. doi: 10.1038/sj.npp.1300128. [DOI] [PubMed] [Google Scholar]
- Wismer Fries AB, Ziegler TE, Kurian JR, Jacoris S, Pollak SD. Early experience in humans is associated with changes in neuropeptides critical for regulating social behavior. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:17237–17240. doi: 10.1073/pnas.0504767102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim IS, Stapleton LRT, Guardino CM, Hahn-Holbrook J, Schetter CD. Biological and psychosocial predictors of postpartum depression: systematic review and call for integration. Clinical Psychology. 2015;11:99. doi: 10.1146/annurev-clinpsy-101414-020426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Muns S, Wang Z, Insel TR. Changes in oxytocin receptor mRNA in rat brain during pregnancy and the effects of estrogen and interleukin-6. Journal of neuroendocrinology. 1997;9:859–865. doi: 10.1046/j.1365-2826.1997.00654.x. [DOI] [PubMed] [Google Scholar]
- Yuan L, Liu S, Bai X, Gao Y, Liu G, Wang X, Liu D, Li T, Hao A, Wang Z. Oxytocin inhibits lipopolysaccharide-induced inflammation in microglial cells and attenuates microglial activation in lipopolysaccharide-treated mice. Journal of neuroinflammation. 2016;13:1. doi: 10.1186/s12974-016-0541-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler C, Dannlowski U, Brauer D, Stevens S, Laeger I, Wittmann H, Kugel H, Dobel C, Hurlemann R, Reif A, Lesch KP, Heindel W, Kirschbaum C, Arolt V, Gerlach AL, Hoyer J, Deckert J, Zwanzger P, Domschke K. Oxytocin receptor gene methylation: converging multilevel evidence for a role in social anxiety. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2015;40:1528–1538. doi: 10.1038/npp.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvara B, Mills-Koonce WR, Carmody KA, Cox M Investigators FLPK. Childhood sexual trauma and subsequent parenting beliefs and behaviors. Child abuse & neglect. 2015;44:87–97. doi: 10.1016/j.chiabu.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.