Abstract
Rats reared in hyperoxia hypoventilate in normoxia and exhibit progressive blunting of the hypoxic ventilatory response, changes which are at least partially attributed to abnormal carotid body development. Since the carotid body also responds to changes in arterial CO2/pH, we tested the hypothesis that developmental hyperoxia would attenuate the hypercapnic ventilatory response (HCVR) of neonatal rats by blunting peripheral and/or central chemoreceptor responses to hypercapnic challenges. Rats were reared in 21% O2 (Control) or 60% O2 (Hyperoxia) until studied at 4, 6–7, or 13–14 days of age. Hyperoxia rats had significantly reduced single-unit carotid chemoafferent responses to 15% CO2 at all ages; CO2 sensitivity recovered within 7 days after return to room air. Hypercapnic responses of CO2-sensitive neurons of the caudal nucleus tractus solitarius (cNTS) were unaffected by chronic hyperoxia, but there was evidence for a small decrease in neuronal excitability. There was also evidence for augmented excitatory synaptic input to cNTS neurons within brainstem slices. Steady-state ventilatory responses to 4% and 8% CO2 were unaffected by developmental hyperoxia in all three age groups, but ventilation increased more slowly during the normocapnia-to-hypercapnia transition in 4-day-old Hyperoxia rats. We conclude that developmental hyperoxia impairs carotid body chemosensitivity to hypercapnia, and this may compromise protective ventilatory reflexes during dynamic respiratory challenges in newborn rats. Impaired carotid body function has less of an impact on the HCVR in older rats, potentially reflecting compensatory plasticity within the CNS.
Keywords: developmental plasticity, carotid body, central chemoreceptor, nucleus tractus solitarius, hypercapnic ventilatory response
Introduction
Development of the respiratory control system is influenced by the oxygen levels experienced during early life (Carroll, 2003; Bavis, 2005). Neonatal rats exposed to chronic hyperoxia hypoventilate when returned to room air (Bavis et al., 2010, 2014) and exhibit a progressive blunting of their hypoxic ventilatory response (HVR) over the course of the hyperoxic exposure (Bavis et al., 2010). The blunted HVR is an example of developmental plasticity, defined as a form of phenotypic plasticity that is unique to developing organisms (Bavis and Mitchell, 2008). Indeed, long-lasting blunting of the HVR only occurs when rats experience hyperoxia during the first two postnatal weeks (Ling et al., 1996; Bavis et al., 2002) and persists for months after return to room air (Ling et al., 1996; Fuller et al., 2002). Chronic hyperoxia is thought to blunt the HVR by causing abnormal development of the carotid body, the principal site for arterial O2 chemoreception (Bavis et al., 2013). Hyperoxia-treated individuals have fewer O2-sensitive glomus (type I) cells in their carotid bodies and fewer chemoafferent axons in the associated carotid sinus nerve (CSN) (Erickson et al., 1998; Dmitrieff et al, 2012; Chavez-Valdez et al., 2012); these morphological changes lead to lifelong reductions in whole-nerve CSN responses to hypoxia (Fuller et al., 2002; Bisgard et al., 2003). The surviving glomus cells are also less responsive to hypoxia when tested immediately after the hyperoxic exposure (Donnelly et al., 2005, 2009; Bavis et al., 2011; Kim et al., 2013), but their O2 sensitivity recovers shortly after return to normoxia (Bavis et al., 2011).
In contrast to lifelong impairment of the HVR, previous studies have not detected plasticity in the hypercapnic ventilatory response (HCVR) in mammals after developmental hyperoxia. Indeed, the HCVR was normal in adult rats (Ling et al., 1996) and adult mice (Dauger et al., 2003) that had been exposed to 60–65% O2 for the first month of life. However, the HCVR has never been assessed for hyperoxia-treated rats as neonates. This is potentially important since some respiratory effects of developmental hyperoxia may only be apparent during the neonatal period. For example, neonatal rats hypoventilate and exhibit reduced carotid body glomus cell O2 sensitivity immediately after chronic hyperoxia, but neither of these effects persist after a few days in room air (Bavis et al., 2011, 2014b). Moreover, chickens incubated in hyperoxia exhibit a modest reduction in the HCVR when tested shortly after hatching (Mortola, 2011).
Developmental hyperoxia has the potential to alter both peripheral and central components of the respiratory control system responsible for the HCVR. Carotid body glomus cells depolarize in response to increasing CO2 and decreasing pH in the arterial blood, ultimately resulting in neurotransmitter secretion and the activation of afferent neurons that conduct action potentials to the nucelus tractus solitarius (NTS) in the brainstem (Lahiri and Forster, 2003; Kumar and Prabhakar, 2012). Across a range of species and experimental approaches, carotid body responses have been estimated to explain up to one-third of the increase in ventilation during hypercapnic challenges, particularly during mild hypercapnia (Forster & Smith, 2010; Smith et al., 2010). Carotid bodies also drive the initial ventilatory increase observed in neonatal rats immediately after a change in inspired CO2 (Cummings and Frappell, 2009). Therefore, hyperoxia-induced carotid body hypoplasia and loss of carotid chemoafferent neurons could directly impact the HCVR, as could any changes to glomus cell CO2/pH sensitivity. Moreover, there is evidence that carotid chemoreceptor activity modulates the CO2 sensitivity of central chemoreceptors (i.e., central and peripheral chemoreceptors are “interdependent”; cf. Forster and Smith, 2010; Smith et al., 2010). This model is supported by neuroanatomical and neurophysiological studies showing that (1) neurons in the NTS project to the retrotrapezoid nucleus (RTN), one of the putative sites for central CO2 sensitivity (Takakura et al., 2006; Guyenet et al., 2009) and (2) many NTS neurons are themselves CO2-sensitive chemoreceptors (Conrad et al., 2009). However, the generality of this interaction across species and physiological states and its ultimate effect on respiratory control (i.e., additive, hypoadditive, or hyperadditive) remains a matter of debate (e.g., Duffin and Mateika, 2013; Teppema and Smith, 2013; Wilson and Day, 2013; Cummings, 2014).
Central CO2 chemoreceptors are located throughout the brainstem and include neurons in the NTS, dorsal motor neucleus of vagus (DMV), RTN, medullary raphé, rostroventrolateral medulla, pre-Bötzinger complex, and locus coeruleus (LC) (Dean and Putnam, 2010; Nattie and Li, 2012). The integrated activity from these neuronal populations is the primary determinant of the whole-animal HCVR. Importantly, hyperoxia may alter the behavior of at least some of these cell populations. Acute exposure to normobaric or hyperbaric hyperoxia (i.e., minutes to hours) stimulates CO2-sensitive and CO2-insensitive neurons of the NTS and DMV and repeated exposure to hyperoxia may diminish CO2 sensitivity (Dean and Putnam, 2010; Mattot et al., 2014). Therefore, the impact of developmental hyperoxia on CO2-sensitive neurons in the NTS warrants further investigation.
In the present study, we tested the hypothesis that developmental hyperoxia would attenuate the HCVR of neonatal rats by blunting peripheral and/or central chemoreceptor responses to hypercapnic challenges. Specifically, neonatal rats were exposed to 60% O2 from birth and the HCVR, peripheral (carotid body) CO2 chemosensitivity, and central (NTS) CO2 chemosensitivity were examined at several ages during the first two weeks of life (P4 – P14). This age range was selected to encompass much of the postnatal maturation of the HCVR (Stunden et al., 2001; Putnam et al., 2005) and the critical window for hyperoxia-induced carotid body plasticity (Bavis et al., 2002; Bavis et al., 2013) in rats.
Methods
2.1. Experimental animals
Experiments were conducted on Sprague-Dawley rat pups of both sexes. Timed-pregnant rats were obtained from a commercial supplier (Charles River Laboratories; Portage, MI, USA) and placed into environmental chambers on the day before they were expected to give birth. Chambers were flushed with room air and oxygen at sufficient flow rates to maintain 60% O2. The resulting pups (“Hyperoxia”) were raised in 60% O2 with their mothers until studied (i.e., 4 – 14 days of age (P4 – P14), where P0 is the day of birth). Additional litters were reared in identical chambers flushed with 21% O2 to serve as age-matched control groups (“Control”). One Hyperoxia litter was removed from the chamber when pups reached P7 and permitted to recover in room air prior to study at P13-15 (“Recovery”). All rats were maintained on a 12:12 light cycle throughout the study and provided food and water ad libitum.
All experimental procedures were approved by the Institutional Animal Care and Use Committees at Bates College (carotid chemoafferent nerve recordings and ventilation measurements) and Wright State University (NTS brainstem slice recordings).
2.2. Single-unit carotid chemoafferent nerve recordings
Carotid chemoafferent nerve recordings were made at P4, P7, and P14 (or P13-15 for the Recovery group) using the same methods used in our previous studies (e.g., Bavis et al., 2011). Recordings were made from pups derived from 5–8 different litters per treatment group at each age, except for the Recovery group which consisted of pups from one litter; the number of recordings made for each group is reported in Figure 2.
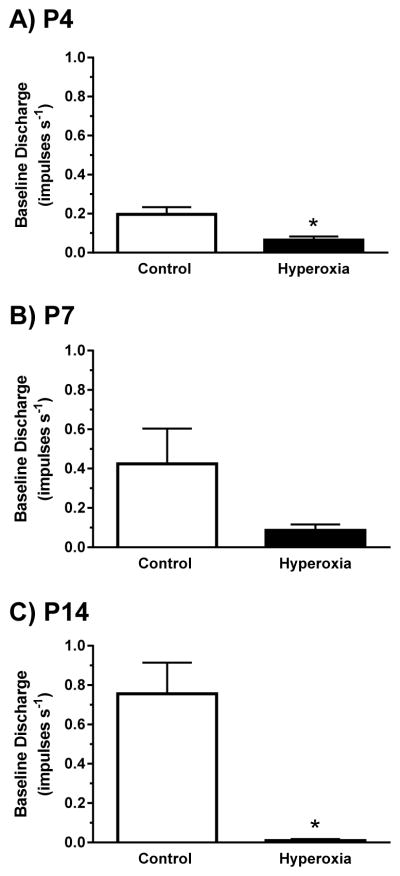
Fig. 2.
Single-unit carotid chemoafferent activity recorded under baseline conditions (21% O2/5% CO2) for (A) P4, (B) P7, and (C) P14 neonatal rats reared in 21% O2 (Control) or 60% O2 (Hyperoxia) from birth. Values are mean±SEM. Number of observations (Control, Hyperoxia): P4 (n=17, 20), P7 (n=16, 18), P14 (n=16, 11). * P<0.05 vs. Control within the same age group.
Rat pups were deeply anesthetized by exposure to 100% CO2 and then decapitated. The left and right carotid bifurcations (containing the carotid bodies) and nodose-petrosal ganglion complexes were isolated en bloc and placed into an ice-cold, oxygenated (95% O2, 5% CO2) saline solution containing (in mM): 125 NaCl, 5 KCl, 2 CaCl2, 1 Na2HPO4, 1 MgSO4, 26 NaHCO3, and 5 dextrose. The preparation was cleaned initially using a solution of 0.15–0.25% collagenase (collagenase P; Roche Diagnostics) and 0.01% protease (type XIV; Sigma-Aldrich) in saline (30 min at 37°C), and then cleaned manually (in ice-cold saline) until only the carotid body, carotid sinus nerve (CSN), glossopharyngeal nerve, and petrosal ganglion remained. The preparation was transferred to a perfusion chamber (RC-22C; 140 μL; Warner Instruments) on the stage of an inverted microscope (Eclipse TE-300; Nikon). The chamber was perfused with saline solution equilibrated with 21% O2 and 5% CO2 (balance N2) and delivered at a rate of ~3 ml min−1 through stainless steel tubing. The solution was passed through an in-line heater (SH-27B; Warner Instruments) so that bath temperature was 37°C.
Single-unit activity was recorded from the soma of carotid chemoafferent neurons using a suction electrode advanced into the petrosal ganglion. The pipette potential was amplified 2,000–5,000× with an extracellular amplifier (EX-1; Dagan Instruments), passband-filtered (0.1–2 kHz), and digitized (10 kHz sample rate; Powerlab 8/30 and Chart 5.2 software; ADInstruments). For identification of individual chemoafferent cells under baseline conditions (21% O2, 5% CO2, balance N2), the carotid body was stimulated (~200 μA × 0.05 ms pulse duration) at 0.5–1 Hz (Isostim A320; World Precision Instruments) using a glass electrode (filled with 1 M NaCl). If a stimulus elicited a single orthodromic action potential, the stimulator was turned off and baseline nerve activity was recorded for 2 min. The CO2 responsiveness was then assessed during two 2-min hypercapnic challenges by switching to saline solutions equilibrated with 10% and 15% CO2 (21% O2, balance N2); approximately half of the preparations received 10% CO2 first while the other half received 15% CO2 first. The hypercapnic challenges were separated by 5 min recovery in baseline conditions (21% O2, 5% CO2, balance N2). The peak responses to 10% CO2 and 15% CO2 were similar in magnitude (data not shown), suggesting that near maximal responses to CO2 were achieved; therefore, only responses to 15% CO2 are reported. After the second hypercapnic challenge, the preparation was exposed to hypoxia (5% O2, 5% CO2, balance N2) for 2 min. This sequence was repeated (at least 15 min later) if a second chemoafferent neuron was identified; no more than two recordings were made per preparation.
Action potentials were discriminated off-line to confirm single unit activity (Spike Histogram Module v. 1; ADInstruments). Baseline discharge rate was calculated from the number of action potentials over the final 60 s under baseline conditions (21% O2, 5% CO2, balance N2). For hypercapnic and hypoxic challenges, the number of individual action potentials per second was calculated and peak discharge frequency was determined after applying a 3-s moving average. Only neurons that exhibited spontaneous activity during the protocol (i.e., at least one spontaneous action potential) were accepted as viable units for analysis. Chronic hyperoxia may increase the proportion of petrosal ganglion neurons that exhibit no spontaneous activity under baseline or hypoxic conditions (Donnelly et al., 2005); accordingly, applying this exclusion criterion could underestimate the differences between Control and Hyperoxia treatment groups reported in Section 3.1.
2.3. Electrophysiological recordings from caudal NTS slice preparations
Medullary brainstem slices (~300 μm thick) were prepared from neonatal Sprague-Dawley rats (P4-P7) of both sexes as previously described (Conrad et al., 2009); recordings were made from slices derived from 2–3 different litters per treatment group. Briefly, The slices were cut transversely at the level of the NTS using a vibratome (Pelco Vibratome 1000) into aCSF at 4–6° C (composition (in mM): 124 NaCl, 3 KCl, 1.3 MgSO4, 26 NaHCO3, 1.24 NaH2PO4, 10 glucose and 2.4 CaCl2, equilibrated with 95% O2/5% CO2). Three successive slices were cut between 300 μm rostral to and 300 μm caudal to the area postrema. These slices from the caudal NTS (cNTS) were incubated in aCSF at room temperature until used.
Slices were placed in a perfusion chamber, held in place with a nylon grid and superfused with aCSF at ~35° C (pH ~7.45) as described in Conrad et al. (2009). Neurons were visualized with an upright microscope (Nikon Optiphot-2) with a 40× water-immersion objective (Hoffmann Modulated Contrast, N.A. 0.55, 3.0 mm working distance) using near-infrared illumination. In most experiments electrophysiological recordings were done using whole cell recording techniques (Conrad et al., 2009). In some experiments, after recording the spontaneous firing response to hypercapnic acidosis in current clamp mode, whole cell voltage clamp was used to simultaneously record fast spontaneous postsynaptic currents including AMPA-receptor induced excitatory (downward deflection) and GABA-A receptor-induced inhibitory (upward deflection) postsynaptic currents at a holding potential of −40 mV in aCSF. The frequency and amplitude of postsynaptic currents were analyzed offline using Clampfit software. Recordings were made with glass pipettes (borosilicate glass; TW150-3, World Precision Instruments, Inc.) pulled to a tip resistance of ~5 MΩ on a vertical electrode puller (Narishige PP-830). These pipettes were filled with a solution containing (in mM): 130 K-gluconate, 1 MgCl2, 10 Na-HEPES, 0.4 EGTA, 2 Na2ATP, 0.3 Na2GTP (pH ~7.4); the pipette filling solution was modified (no added CaCl2 and 0.4 mM EGA) to minimize washout of the electrical response to hypercapnia (Filosa and Putnam; 2003). Positive pressure was maintained in the pipette until the tip touched the neuronal soma and then negative pressure was used to form a tight seal. Additional negative pressure was then applied to rupture the patch and form a whole cell patch.
Membrane potential (Vm) was measured using current clamp with an Axopatch 200B amplifier, a Digidata 1440A-A/D converter and pCLAMP 10.2 software (all from Molecular Devices Co.). Data were filtered at 2 kHz and sampled at 10 kHz and saved on a computer for later analysis. The neurons were discarded if the series resistance changed by >20% during the experiment. Membrane potential was corrected for the measured liquid junction potential of 16 mV. The integrated firing rate (10 s bins) response to 5–10 minutes of exposure to hypercapnia (aCSF equilibrated with 85% O2/15% CO2, pH ~6.8–6.9) was determined in cNTS neurons from Control and Hyperoxia neonates. Some of the experiments were done in synaptic blockade solution (SNB; in mM: 121 NaCl, 3 KCl, 11.4 MgSO4, 26 NaHCO3, 1.25 NaH2PO4, 10 glucose and 0.2 CaCl2) containing carbenoxolone (CAR: 100 μm) to block gap junctions (Conrad et al., 2009) to assure that the responses we observed were intrinsic to the neuron or whether synaptic transmission would affect the chemosensitive firing rate response of cNTS neurons.
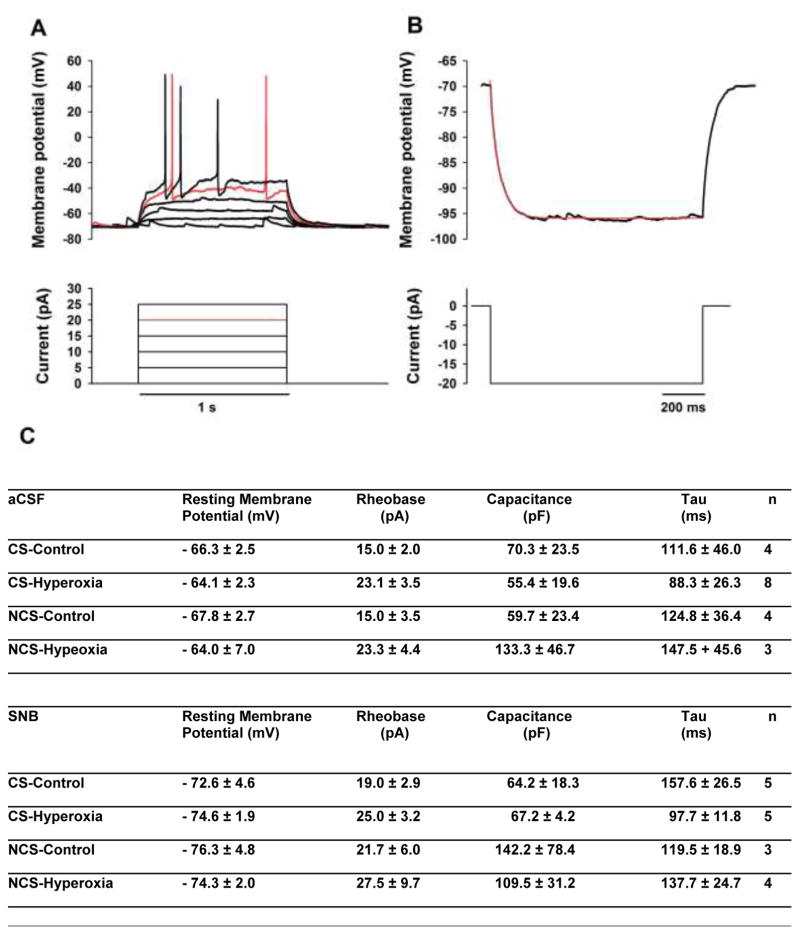
To measure the rheobase of cNTS neurons, a set of depolarizing current pulses with increments of 5 pA and duration of 1 s were injected into each neuron, which was held at −70 mV to eliminate spontaneous firing. The rheobase was the minimum amplitude of the injected current that could just induce an action potential. Capacitance values (Cm) for cNTS neurons were measured in current clamp mode according to the method of Golowasch et al. (2009). In brief, a hyperpolarizing DC current pulse (Iext, −20 pA, 1 s) was applied from a membrane potential (−70 mV) at which neurons had no spontaneous firing. The resulting hyperpolarization (20–30 mV) was recorded. Four consecutive traces in each neuron were averaged to remove noise. In order to get the membrane time constant (τm) and the change of membrane potential, the average trace was fitted from t=0 to a time at which the voltage had reached steady state with two or three exponential terms using the Lenenberg-Marquardt (LM) algorithm of the Clampfit analysis software in the pClamp package. The slowest component of the fit curve represents the charging of the membrane and was used to determine τm. Cm was obtained by dividing τm by the resistance coefficient Rm (= Vm/Iext) and thus Cm = τm/Rm = τm* Iext/Vm.
2.4 Ventilation measurements
Ventilation was measured in P4, P6-7, and P13-14 rats using head-body plethysmography (Bavis et al., 2010). Measurements were made for pups derived from 6–8 different litters per treatment group at each age; the number of individuals studied in each group is reported in Table 1.
Table 1.
Respiratory frequency and tidal volume during baseline conditions (0% O2) and during exposure to two levels of hypercapnia for rats reared in 21% O2 (Control) or 60% O2 (Hyperoxia).
| Age | Treatment | n | Mass (g) | Respiratory Frequency(breaths min−1) | Tidal Volume (ml 100g−1) | ||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Baseline | 4% CO2 | 8% CO2 | Baseline | 4% CO2 | 8% CO2 | ||||
| P4 | Control | 20 | 10.6±0.2 | 164±4 | 166±4 | 159±5 | 0.93±0.05 | 1.22±0.07 # | 1.47±0.07 # |
| Hyperoxia | 19 | 10.3±0.3 | 129±4 * | 132±5 * | 130±4 * | 0.74±0.04 * | 0.89±0.04 *, # | 1.11±0.05 *, # | |
|
| |||||||||
| P6-7 | Control | 17 | 15.1±0.3 | 161±8 | 156±6 | 149±6 # | 0.74±0.03 | 0.94±0.04 # | 1.17±0.04 # |
| Hyperoxia | 16 | 14.8±0.4 | 132±3 * | 133±3 * | 129±2 *, # | 0.66±0.06 | 0.83±0.09 # | 1.05±0.11 # | |
|
| |||||||||
| P13-14 | Control | 18 | 32.2±1.0 | 143±5 | 172±7 # | 180±6 # | 0.75±0.02 | 1.02±0.03 # | 1.31±0.04 # |
| Hyperoxia | 15 | 29.9±0.9 | 130±5 * | 158±5 *, # | 162±5 *, # | 0.69±0.03 * | 0.88±0.04 *, # | 1.19±0.05 *, # | |
Data are expressed as mean ± SEM.
P<0.05 vs. Control at the same level of inspired CO2 (i.e., post hoc test for main effect of developmental treatment) and
P<0.05 vs. Baseline within the same developmental treatment (i.e., post hoc test for main effect of inspired CO2). Body mass did not differ between treatment groups at any age.
The plethysmograph consisted of two cylindrical compartments, a head compartment (4.75 cm ID; 70 ml) and a body compartment (3.1 cm ID; length adjusted based on rat size). After weighing, the rat was sealed into the plethysmograph chamber with a flexible, latex rubber collar separating the head and body compartments; petroleum jelly was applied around the rat’s neck to obtain an airtight seal. Test gases were delivered to the head compartment at 1500 ml min−1 to ensure rapid changeover. Airflow in and out of the body compartment as the rat breathed was detected using a pneumotach coupled to a differential pressure transducer (MLT1L and ML141; ADInstruments); the system was calibrated before each trial by injecting 0.5 ml of air. Output from the pressure transducer was recorded to a computer at a sampling rate of 1000 Hz, integrated, and digitally filtered (high-pass, 0.1 Hz) to obtain respiratory volumes (PowerLab 8SP and LabChart 7 software with the Spirometry extension; ADInstruments). Air temperature in the body compartment was monitored continuously with a T-type thermocouple probe (Physitemp Instruments) and maintained at 32–34°C (i.e., within the thermoneutral zone; Malik and Fewell, 2003) by placing the plethysmograph chamber inside an incubator.
Following a 10 min adjustment period (21% O2, 0% CO2, balance N2), baseline ventilation was recorded for 5 minutes. Rats were then exposed to 5 min hypercapnic challenges with 4% CO2 and 8% CO2 (21% O2, balance N2) separated by a 5 min recovery period (21% O2, 0% CO2, balance N2). Ventilatory parameters were calculated for each breath and averaged over 45–60 sec at the end of the baseline and recovery periods and over 10–15 sec at 0.5 and 5 min into each hypercapnic challenge, excluding movement artifacts and sighs. Data from the baseline and recovery periods were averaged for each individual and used as a single “baseline” (i.e., 0% CO2) value for subsequent analysis.
2.5. Statistical analysis
Carotid chemoafferent baseline activity and peak responses to hypercapnia and hypoxia were compared between Control and Hyperoxia treatment groups using Student’s t-tests with Welch’s correction for unequal variances (Prism 6, GraphPad). For cNTS neuron properties, all neurons were initially classified as either chemosensitive (CS) or non-chemosensitive (NCS) based on their increased firing rate in response to hypercapnia. Specifically, the magnitude of the firing rate response to hypercapnia was quantified using a chemosensitivity index (CI), calculated according to the equation in Wang et al. (1998). The CI is the ratio of firing rates in hypercapnia vs. normocapnia normalized to a 0.2 pH unit change in external pH. A CI of 100% indicates an NCS neuron. A neuron was identified as a CS neuron if its CI was 120% or greater and was identified as an NCS neurons if its CI was between 120% and 80%; below 80% CI a neuron is considered inhibited by hypercapnia (usually less than 10% of cNTS neurons are inhibited by hypercapnia (Conrad et al., 2009) and are thus very rare; in this study we did not continue the rare recording in which a cNTS neuron was inhibited by hypercapnia). For comparisons of basic electrophysiological properties of cNTS neurons, data for CS and NCS neurons were pooled and subsequently subjected to two-way ANOVA (factor 1: developmental treatment group; factor 2: aCSF vs. SNB solution). Ventilation measurements were compared across treatment groups by two-way repeated measures ANOVA (factor 1: developmental treatment group; factor 2: level of inspired CO2) followed by Tukey’s multiple comparison test where appropriate (SigmaPlot 13.0, Systat Software). To determine whether the magnitude of the HCVR varied with age, ventilatory responses (expressed as a percentage increase from baseline) were compared across ages separately for each level of inspired CO2 using a two-way ANOVA (factor 1: developmental treatment group; factor 2: age) followed by Tukey’s multiple comparison test (SigmaPlot 13.0, Systat Software); values were log-transformed prior to running this test in order to meet the equal variance assumptions.
Preliminary analysis including sex as a factor yielded no significant sex or treatment × sex interactions for ventilation data, so data for males and females were pooled for all statistical analysis; approximately equal numbers of males and females were used for all studies. P≤0.05 was considered significant for all statistical tests. Values are reported as mean ± SEM.
3. Results
3.1. Carotid chemoafferent responses to hypercapnia and hypoxia
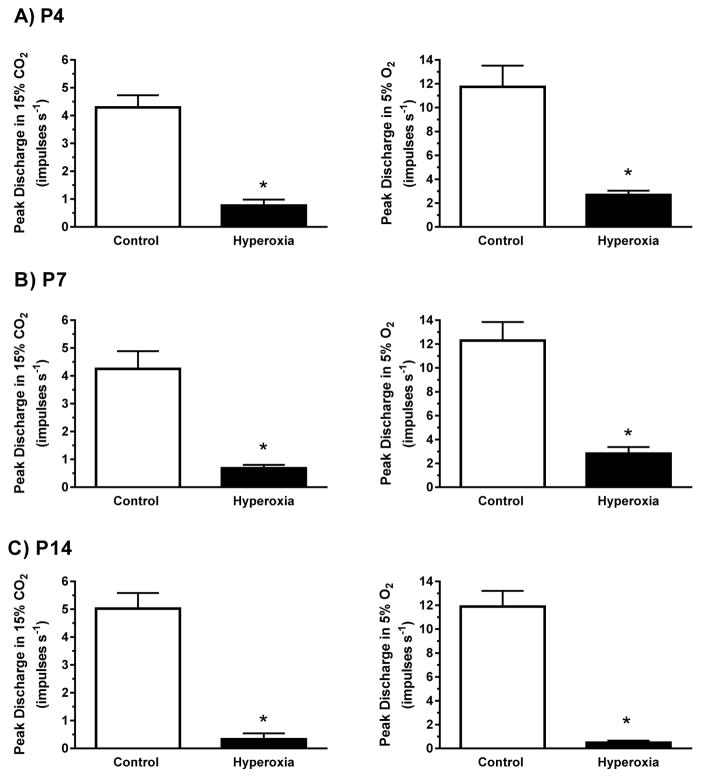
Baseline (21% O2/5% CO2) chemoafferent activity was low in both treatment groups at all ages, with mean discharge rates between 0.01 and 0.76 impulses s−1 (Figs. 1 and 2). Baseline activity was significantly lower in Hyperoxia rats than in age-matched Controls at P4 (P=0.004) and P14 (P<0.001) (Fig. 2); a similar trend was apparent at P7 although it did not quite reach statistical significance (P=0.08). Hypercapnia generally increased single-unit chemoafferent activity (Fig. 1), but the peak response to 15% CO2 was much lower in Hyperoxia rats at all ages (all P<0.001) and was nearly abolished after two weeks of developmental hyperoxia (Fig. 3, left panels). Similar results were obtained during 10% CO2 challenges (data not shown). Consistent with previous studies (e.g., Donnelly et al., 2005, 2009; Bavis et al., 2011), the chemoafferent response to 5% O2 was lower in Hyperoxia rats compared to age-matched Controls at all three ages (all P<0.001) (Fig. 3, right panels).
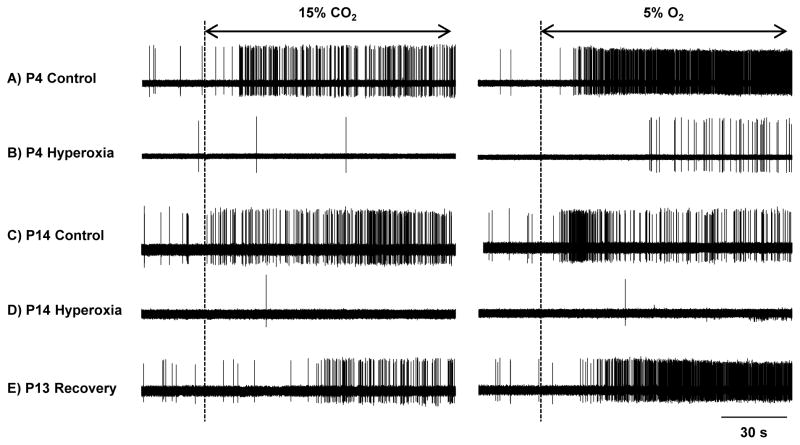
Fig. 1.
Representative recordings of carotid chemoafferent activity in response to hypercapnia (15% CO2) and hypoxia (5% O2) for neonatal rats reared in 21% O2 (Control) or 60% O2 (Hyperoxia) from birth. Prior to initiating the hypercapnic or hypoxic challenge, preparations were bathed in saline equilibrated with 21% O2/5% CO2 (baseline). Data are shown for rats at P4 (panels A and B) and at P14 (panels C and D). Additionally, panel E provides an example of a rat that was exposed to 60% O2 through P7 and then returned to room air for 6 days (i.e., “Recovery”); note the brisk responses to hypercapnia and hypoxia in the Recovery rat compared to Hyperoxia rats studied immediately following chronic hyperoxia (i.e., panels B and D).
Fig. 3.
Single-unit carotid chemoafferent responses to 15% CO2 (left panels) and 5% O2 (right panels) for (A) P4, (B) P7, and (C) P14 neonatal rats reared in 21% O2 (Control) or 60% O2 (Hyperoxia) from birth. Note that the y-axis scales are different for the hypercapnic and hypoxic responses. Values are mean±SEM. Number of observations (Control, Hyperoxia): P4 (n=17, 20), P7 (n=16, 18), P14 (n=16, 11). * P<0.05 vs. Control within the same age group.
One litter of P7 Hyperoxia rats was returned to room air for 6–8 days prior to study at P13-15. Carotid chemoafferent activity for these rats (“Recovery” in Fig. 1E) tended to be greater than that observed in either P7 Hyperoxia or P14 Hyperoxia rats during baseline conditions (0.29±0.16 imp s−1, n=6) and during the 15% CO2 (3.11±0.70 imp s−1, n=6) and 5% O2 (14.17±4.11 imp s−1, n=6) challenges. Indeed, baseline chemoafferent activity and peak responses to 15% CO2 and 5% O2 were no longer different from those of P14 Control rats (all P>0.05).
3.2. cNTS neuronal excitability and chemosensitivity
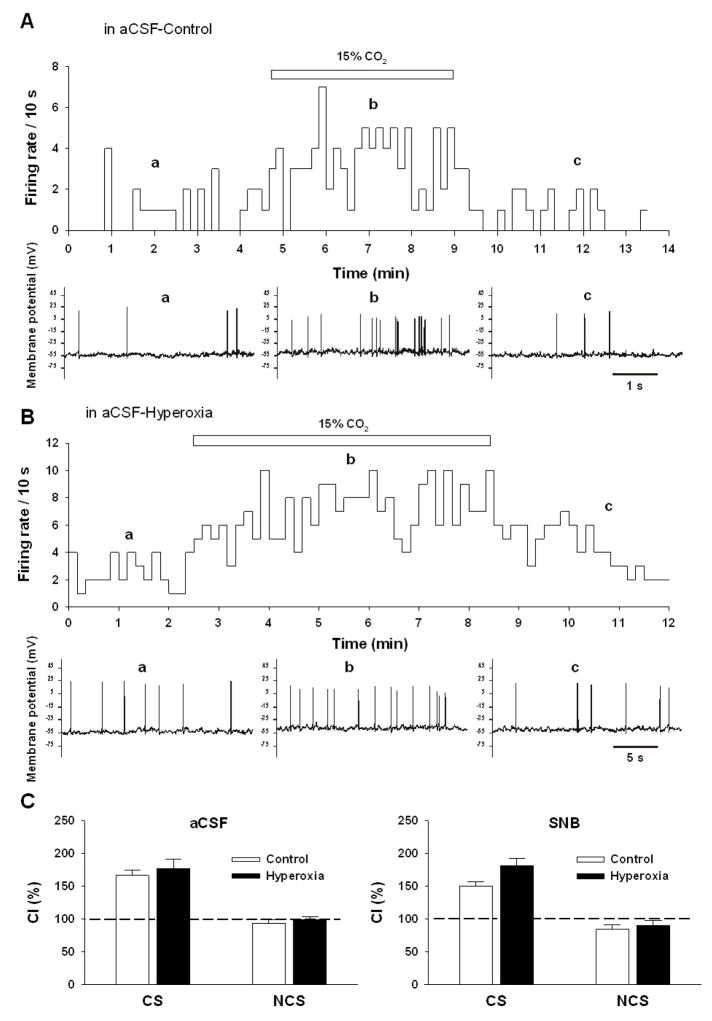
Recordings were made from a total of 42 cNTS neurons from Control rats and 44 cNTS neurons from Hyperoxia rats (age range P4-P7). 43% of Control neurons and 52% of Hyperoxia neurons exhibited chemosensitivity (a greater than 20% increase in CI with hypercapnia) when perfused with artificial aCSF equilibrated with 15% CO2 (Fig. 4A, B); these percentages did not change in the presence of SNB solution, suggesting that these are intrinsically chemosensitive neurons. The magnitude of the hypercapnic response (i.e., the CI) was not different in cNTS neurons from Control and Hyperoxia rats, either in the presence or absence of SNB (Fig. 4C).
Fig. 4.
The firing rate response to hypercapnia in representative cNTS neurons from neonatal rats reared in (A) 21% O2 (Control) or (B) 60% O2 (Hyperoxia) from birth. The top panel shows integrated firing rate (action potentials/10 s) (a) in normocapnic (5% CO2) artificial cerebrospinal fluid (aCSF), (b) in hypercapnic (15% CO2) aCSF, and (c) upon return to normocapnic aCSF. The bottom panels show representative membrane potential tracings with action potentials in (a) normocapnia, (b) hypercapnia, and (c) normocapnia. It can be seen that hypercapnia increases the firing rate of cNTS neurons from both Control and Hyperoxia rats. (C) The chemosensitivity index (CI) was calculated from the firing rate increase induced by hypercapnia for both chemosensitive (CS) and nonchemosensitive (NCS) cNTS neurons. A value of 100% indicates no firing rate increase in response to hypercapnia. Values are mean±SEM. Number of neurons: (Control, Hyperoxia): CS in aCSF (n=10, 11), NCS in aCSF (n=12, 11), CS in synaptic blockade (SNB) solution (n=8, 11), NCS in SNB (n=12, 9). No significant differences in CI were detected between Control and Hyperoxia rats (P>0.05).
Four basic electrophysiological parameters were characterized for cNTS neurons: resting membrane potential (Vm), capacitance (Cm), membrane time constant (Tau: τm), and rheobase (Fig. 5). The average values for each parameter for CS and NCS neurons under each condition and in each solution are shown in Figure 5C. No statistical differences (P-values ranging from 0.08 to 1.0) were found in CS vs. NCS neurons from rats reared in a given condition (Control or Hyperoxia) or exposed to a given solution (aCSF or SNB), so the values for each electrophysiological parameter were pooled from CS and NCS neurons for further statistical analysis. The pooled values for capacitance were 65.0±26.1 pF (Control, aCSF; n=8), 76.7±20.2 pF (Hyperoxia, aCSF; n=11), 93.4±31.4 pF (Control, SNB; n=8) and 86.0±14.9 pF (Hyperoxia, SNB; n=9). The pooled values for τm were 118.2±27.3 ms (Control, aCSF; n=8), 104.4±23.2 ms (Hyperoxia, aCSF; n=11), 143.3±18.4 ms (Control, SNB; n=8), and 115.2±13.8 ms (Hyperoxia, SNB; n=9). Based on a two-way ANOVA, there was no significant difference in the pooled values for capacitance or for τm comparing Control with Hyperoxia or comparing aCSF with SNB and there was no significant interaction between developmental condition and the solution used (all P>0.05). The pooled values for rheobase were 15.0±1.9 pA (Control, aCSF; n=8), 23.2±3.2 pA (Hyperoxia, aCSF; n=11), 20.0±2.7 pA (Control, SNB; n=8) and 26.1±4.3 pA (Hyperoxia, SNB; n=9). There were no significant differences in these pooled rheobase values based on the solution (aCSF vs. SNB), but rheobase was slightly higher in cNTS neurons from rats reared in Hyperoxia compared to Controls (P=0.027); there was no significant interaction between developmental condition and the solution used. Finally, the pooled values for Vm were −67.0±1.7 mV (Control, aCSF; n=8), −64.1±2.3 mV (Hyperoxia, aCSF; n=11), −74.0±3.2 mV (Control, SNB; n=8) and −74.4±1.3 (Hyperoxia, SNB; n=9). Vm was similar for Control vs. Hyperoxia rats, but Vm was more negative in SNB (P<0.001); there was no interaction between developmental condition and the solution used.
Fig. 5.
A representative trace of (A) the effect of depolarizing current pulse injections on the membrane potential and (B) the effect of a hyperpolarizing current pulse injection on the membrane potential of a cNTS neuron from a neonatal rat. The top trace shows the membrane potential recorded in response to each pulse injection and the bottom trace shows the pulse injection protocol. Depolarizing current injections were used to determine the rheobase while the hyperpolarizing current pulse was used to determine the capacitance and Tau. (C) The mean (±SEM) values for these basic neuronal electrophysiological properties are shown for CS and NCS cNTS neurons from Control and Hyperoxia rats both in aCSF and in SNB.
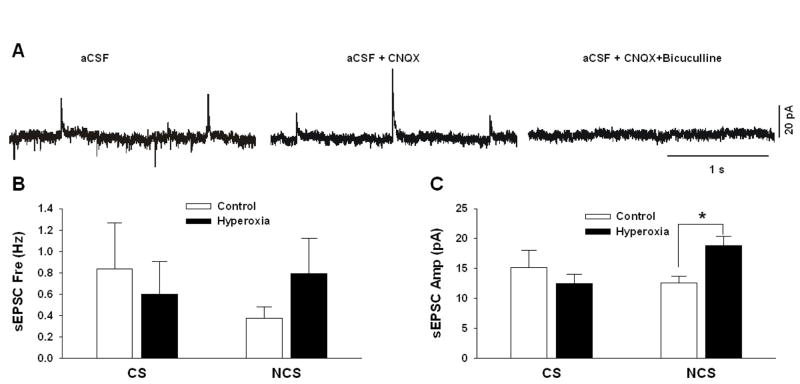
Overall, these data indicated that the basic electrophysiological parameters were similar in cNTS neurons from Control and Hyperoxia rats, both in the presence of aCSF and in SNB. There was a trend in the Vm data, however, that suggested that tonic excitatory input in cNTS neurons might be somewhat larger in Hyperoxia rats (i.e., Vm slightly more hyperpolarized by SNB solution). We therefore also measured excitatory and inhibitory currents in CS and NCS cNTS neurons. In aCSF, both excitatory (downward deflection) and inhibitory (upward deflection) post-synaptic currents were observed (Fig. 6A, left panel). In the presence of CNQX (an AMPA-receptor antagonist), only the inhibitory post-synaptic currents were seen (Fig. 6A, middle panel). In the presence of both CNQX and bicuculline (a GABA-receptor antagonist), both excitatory and inhibitory post-synaptic currents were eliminated (Fig. 6A, right panel). The spontaneous inhibitory post-synaptic currents (sIPSC) were too infrequent to obtain reliable frequency and amplitude values. We did measure the frequency (Fig. 6B) and the amplitude (Fig. 6C) of spontaneous excitatory post-synaptic currents (sEPSC). The amplitude of sEPSCs were greater in NCS neurons from Hyperoxia rats vs. Control rats (P=0.009; Fig. 6C). The frequency of sEPSCs did not differ between groups (P=0.66 and 0.22 for CS and NCS neurons, respectively).
Fig. 6.
Representative traces of post-synaptic currents for a cNTS neuron from a neonatal rat reared in 21% O2 in aCSF (A: left panel), in aCSF plus the AMPA-receptor antagonist CNQX (A: middle panel) and in aCSF plus CNQX and the GABA-A-receptor antagonist bicuculline (A: right panel). Excitatory post-synaptic currents (EPSC) are shown by downward deflections of the current trace and inhibitory post-synaptic currents (IPSC) are shown by upward deflections of the current trace. The frequency (B) and amplitude (C) of spontaneous EPSCs (sEPSC) are shown for both chemosensitive (CS) and non-chemosensitive (NCS) cNTS neurons from Control rats (reared in 21% O2) and from Hyperoxia rats (reared in 60% O2). Values are mean±SEM. Number of observations (Control, Hyperoxia): CS (n=4, 5), NCS (n=6, 5). * P<0.05 vs. Control.
3.3. Hypercapnic ventilatory response (HCVR)
Ventilation was measured in conscious rats at three steady-state levels of inspired CO2 (0, 4, and 8% CO2) (Fig. 7). As expected, minute ventilation increased during acute exposure to CO2 in Control and Hyperoxia rats at all ages (main effect for CO2 level, all P<0.001). Ventilation plateaued after the first minute of hypercapnia at all ages and remained unchanged over the remainder of the hypercapnic exposure (data not shown). The increase in ventilation was mediated exclusively by increases in tidal volume at P4 and P6-7 (CO2 level, both P<0.001) with no increase in respiratory frequency (Table 1); respiratory frequency actually decreased slightly during exposure to 8% CO2 in P6-7 rats (Tukey’s post hoc test, P=0.02 vs. 0% CO2). In contrast, both tidal volume and respiratory frequency increased with increasing CO2 levels at P13-14 (CO2 level, both P<0.001) (Table 1).
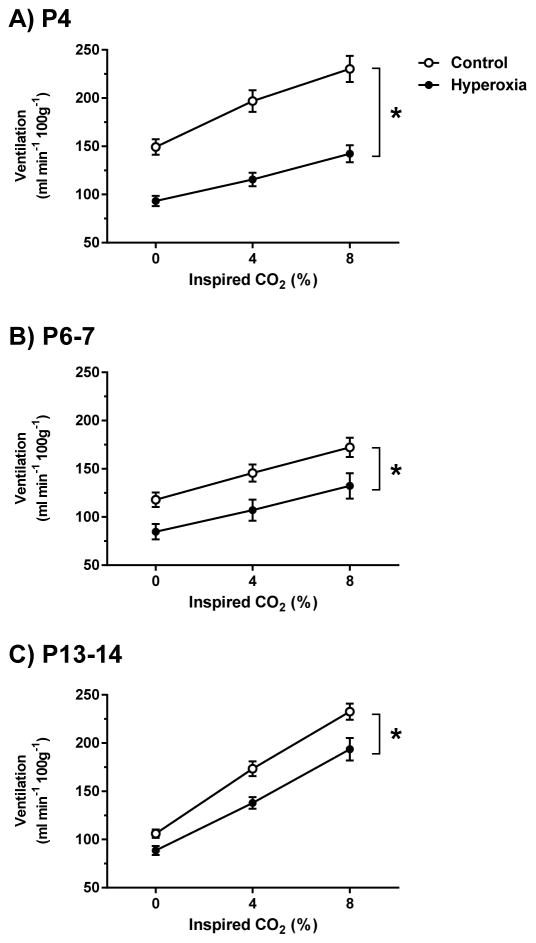
Fig. 7.
Minute ventilation during exposure to 0% (baseline), 4%, and 8% CO2 for (A) P4, (B) P6-7, and (C) P13-14 neonatal rats reared in 21% O2 (Control) or 60% O2 (Hyperoxia) from birth. Ventilation was measured at the end of the 5-min exposures to 4% and 8% CO2. Values are mean±SEM. Number of observations (Control, Hyperoxia): P4 (n=20, 19), P6-7 (n=17, 16), P13-14 (n=18, 15). * P<0.05 vs. Control at all time points (i.e., main effect for developmental treatment). Although not shown on the graph for clarity, ventilation increased significantly with increasing inspired CO2 level at all ages (i.e., 4% vs. 0% CO2 and 8% vs. 4% CO2, all P<0.001).
Minute ventilation was lower in Hyperoxia rats at all three ages studied (main effect for developmental treatment, P<0.001 at P4 and P13-14 and P=0.01 at P6-7). However, the increase in ventilation during 4% and 8% CO2 exposure was similar to that observed in Controls (i.e., similar slopes for the relationship between ventilation and inspired CO2 in Fig. 7) (treatment × CO2 level, P=0.25, 0.52, and 0.13 at P4, P6-7, and P13-14, respectively); the lower minute ventilation in Hyperoxia rats thus appears to reflect their decreased baseline (0% CO2) ventilation. Hyperoxia rats tended to have both reduced respiratory frequency and reduced tidal volume across all inspired CO2 levels compared to age-matched Controls (Table 1), although the decrease in tidal volume was not statistically significant at P6-7 (P=0.24).
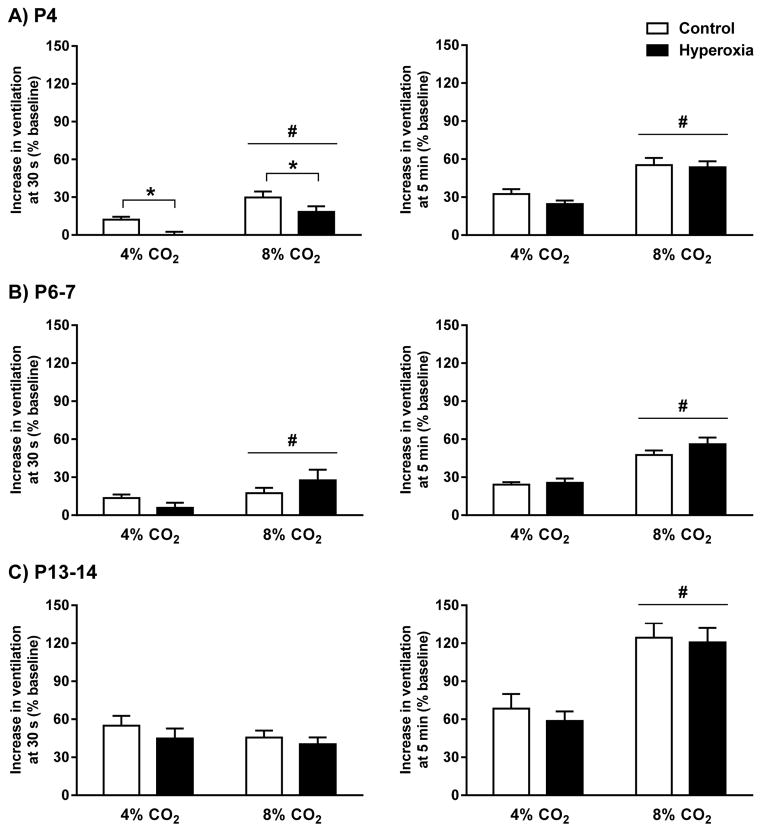
Ventilatory responses to 4% CO2 and 8% CO2 were also expressed as the percentage increase from baseline (Fig. 8). This analysis confirmed that the magnitudes of the HCVR at the end of the 5-minute hypercapnic challenges were similar between Control and Hyperoxia rats at all three ages (developmental treatment, P=0.40, 0.23, and 0.89 at P4, P6-7, and P13-14, respectively) (Fig. 8, right panels). Visual inspection of the data in Figure 8 (right panels) also suggested that the magnitude of the HCVR changed with age. This was confirmed by comparing the HCVR across ages separately at each level of inspired CO2 (main effect for age, P<0.001 for responses to both 4% and 8% CO2); although responses were similar between P4 and P6-7 rats, the HCVR more than doubled by P13-14 (Tukey’s post hoc test, P<0.001 for P13-14 vs. P4 and P6-7). This age-dependent increase in the HCVR was similar in Control and Hyperoxia rats (developmental treatment and developmental treatment × age, all P>0.05).
Fig. 8.
Hypercapnic ventilatory responses for (A) P4, (B) P6-7, and (C) P13-14 neonatal rats reared in 21% O2 (Control) or 60% O2 (Hyperoxia) from birth. The ventilatory response was measured approximately 30 seconds into the hypercapnic exposure (left panels) or at the end of the 5-min exposures to 4% and 8% CO2 (right panels). Values are mean±SEM. Number of observations (Control, Hyperoxia): P4 (n=20, 19), P6-7 (n=17, 16), P13-14 (n=18, 15). * P<0.05 vs. Control (i.e., main effect for developmental treatment), # P<0.05 vs. 4% CO2 (i.e., main effect for inspired CO2 level).
Although Control and Hyperoxia rats achieved similar increases in ventilation over the course of the hypercapnic challenges, we also examined ventilation at 30 seconds into the HCVR to determine whether decreased carotid body CO2 sensitivity (see section 3.1) slowed the onset of the HCVR. At P4, the early phase of the HCVR was significantly reduced in Hyperoxia rats at both 4% CO2 and 8% CO2 (main effect for developmental treatment, P=0.006) (Fig. 8A, left panel). This effect was no longer detected at P6-7 or P13-14 (both P>0.05) (Fig. 8B, C, left panels).
4. Discussion
Chronic perinatal hyperoxia substantially reduced the carotid body response to hypercapnia but had no effect on the CO2 sensitivity of cNTS neurons. The diminished carotid body chemosensitivity likely explains the slower increase in ventilation observed at the onset of hypercapnia in the youngest rats studied (P4) (e.g., Cummings and Frappell, 2009), but the magnitude of the HCVR under steady-state conditions was not affected by chronic hyperoxia at any age. This is consistent with previous studies that concluded that developmental hyperoxia has no lasting effect on the HCVR measured later in adult rats (Ling et al., 1996) or mice (Dauger et al., 2003). Although cNTS neuron chemosensitivity appeared normal after developmental hyperoxia, there was some evidence for a small decrease in neuronal excitability but enhanced excitatory synaptic input to cNTS neurons within brainstem slices. It is unclear how these small changes interact but the increased network excitability may (partially) compensate for impaired carotid body function and/or contribute to the early maturation of the hypoxic ventilatory response observed in hyperoxia-treated rats (Bavis et al., 2010, 2014a).
4.1. Hyperoxia-induced changes in carotid body CO2 sensitivity
Chronic hyperoxia reduced, and in some cases abolished, carotid body responses to hypercapnia in neonatal rats. This finding contrasts markedly with the results reported by Lahiri et al. (1987) for adult cats subjected to 60–67 h of 100% O2. Specifically, Lahiri et al. observed that chronic hyperoxia enhanced the carotid chemoafferent responses to CO2 measured in anesthetized cats. Differences in species (rat vs. cat), age during hyperoxic exposure (neonate vs. adult), duration and severity of hyperoxia (>96 h of 60% O2 vs. <67 h of 100% O2), and the measurement conditions (in vitro vs. in vivo) are all potential explanations for the contrasting findings. Although we cannot rule out any of these possibilities without additional experiments, it is worth noting that chronic hyperoxia has similar effects on carotid body O2 sensitivity in neonatal rats (e.g., Donnelly et al., 2005, 2009; present study) and kittens (Hanson et al., 1989). Moreover, while some effects of chronic hyperoxia (e.g., carotid body hypoplasia) are limited to a critical window of plasticity during the early postnatal period (Bavis et al., 2002, 2013), attenuation of single-unit carotid chemoafferent responses to hypoxia can be elicited at later ages as well (Lahiri et al., 1987; Donnelly et al., 2005). Assuming that hyperoxia impairs carotid body O2 and CO2 sensing through a common mechanism (see below), it seems that neither species nor age of exposure are likely explanations for differences between studies. In contrast, short periods of hyperoxia augment carotid body O2 sensitivity while longer durations of hyperoxia decrease it, particularly when hyperoxia is initiated at later ages (i.e., P7 vs. P0; Donnelly et al., 2009; Roeser et al., 2011). Therefore, we speculate that the duration and/or severity of hyperoxia contribute to the apparently opposite results obtained in the present study and by Lahiri et al. (1987).
The transduction pathway for arterial CO2 in the carotid body glomus cell has not been fully explained, but it is widely accepted that high extracellular PCO2 decreases intracellular pH and that this leads to an inhibition of background K+ channels, membrane depolarization, voltage-gated Ca2+ entry into the cell, and secretion of neurotransmitters (Lahiri and Forster, 2003; Buckler, 2015); extracellular acidosis may also inhibit K+ channels directly. Carotid body glomus cells express two varieties of background K+ channels that are inhibited by both hypoxia and acidosis: Twik-related acid-sensitive K+ (TASK) channels (primarily heteromeric TASK-1/3 channels, but also homomeric TASK-1 and TASK-3 channels) and a calcium-activated K+ channel (BKCa) (Buckler et al., 2000; Kim et al. 2009; Peers, 1990; Peers and Green, 1991; Buckler, 2015). TASK channels in particular are proposed to be important mediators of carotid body responses to hypercapnia (Buckler, 2015). Although studies of mice lacking the gene for one or more TASK isoform have produced mixed results (Trapp et al., 2008; Ortega-Sáenz et al., 2010), one study found that TASK-1 and TASK-1/3 deficient mice had greatly reduced carotid chemoafferent responses to both hypoxia and hypercapnia (Trapp et al., 2008).
Carotid body hypoxic and hypercapnic responses decrease over a similar time course in hyperoxia and recover over a similar time course after return to normoxia (Bavis et al., 2011; present study), so it is possible that chronic postnatal hyperoxia impairs carotid body O2 and CO2 responses through a common mechanism. Given that O2 and CO2 chemotransduction pathways converge at the level of K+ channels within the carotid body (Kim et al., 2009; Buckler, 2015), hyperoxia-induced changes in K+ channel expression is one potential mechanism that warrants further investigation. Chronic postnatal hyperoxia (60% O2) has previously been shown to decrease carotid body mRNA expression for TASK channels and to increase mRNA expression for BKCa channels (Kim et al., 2006; Bavis et al., 2011). These data must be interpreted cautiously since changes in mRNA expression may not lead to changes in protein expression, but these findings are consistent with the hypothesis that hyperoxia depresses carotid body CO2 sensitivity (and O2 sensitivity) by downregulating TASK channels within the glomus cells. More specifically, of TASK-1, TASK-3, and BKCa, only TASK-1 expression changed after 7 days of hyperoxia (Bavis et al., 2011); TASK-3 and BKCa were affected after 14 days, but not after 7 days. Since carotid chemoafferent responses to CO2 were reduced by only 4–7 days of hyperoxia, this could indicate a relatively greater role for TASK-1 (and/or heteromeric TASK-1/3 channels) in hyperoxia-induced plasticity. Moreover, TASK-1 mRNA expression recovered within 7 days upon return to room air which correlates nicely with recovery of the carotid chemoafferent responses to hypercapnia and hypoxia observed in the present study. Decreases in TASK-3 and/or increases in BKCa mRNA expression presumably would only come into play during longer hyperoxic exposures.
4.2. cNTS neuron properties after developmental hyperoxia
While chronic postnatal hyperoxia markedly reduced the CO2 sensitivity of CB cells, it did not markedly change the HCVR. This implies some form of central compensation of the hypercapnic ventilatory control. Central ventilatory control is a complex process that involves numerous chemosensitive regions and complex interactions among these regions (Nattie and Li, 2012). Further, chemosensitive neurons from various central regions show different developmental patterns (Gargaglioni et al., 2010; Putnam et al., 2005). Our findings show that chronic postnatal hyperoxia did not alter the CO2 sensitivity of neurons within the cNTS: both the percentage of chemosensitive neurons and the magnitude of the chemosensitivity index were similar between developmental treatment groups and similar to those of untreated neonatal rats in a previous study (Conrad et al., 2009). It thus seems most likely that enhanced central CO2 chemosensitivity involves changes in neurons from another region of the brainstem other than the NTS, but such a conclusion must await future studies. The suggestion that chronic postnatal hyperoxia increases CO2-sensitivity of some central neurons raises an interesting possibility. If this is shown, the increased neuronal central chemosensitivity would be compensating the hyperoxia-induced reduction in CB CO2 sensitivity. This would raise mechanistic questions of how hyerpoxia decreases CO2 sensitivity in CB cells while increasing it in central chemosensitive neurons.
Basic electrophysiological properties of cNTS neurons were generally unaltered by chronic hyperoxia. There was a significant increase in the rheobase of cNTS neurons with chronic hyperoxia, suggesting a decrease in neuronal excitability, but this increase was very small compared to the two-fold increase in rheobase between cNTS neurons from adult rats versus neonatal rats (Vincent and Tell, 1997). However, two observations suggest a shift toward a more excitable network: (1) cNTS neurons for hyperoxia-treated rats tended to hyperpolarize to a somewhat greater extent when synaptic input was blocked (suggesting greater tonic excitatory input) and (2) the amplitude of spontaneous EPSCs on non-chemosensitive cNTS neurons was greater in slices from hyperoxia-treated rats. The latter result could indicate a greater density of glutamate receptors (likely AMPA receptors since the EPSCs were largely abolished by application of CNQX) on these neurons. Since the NTS receives and integrates afferent input from peripheral chemoreceptors, increased excitability of these neurons could increase CNS gain and contribute to compensation for diminished carotid body responses to hypoxia and hypercapnia.
This is not the first study to suggest that chronic hyperoxia increases network excitability in the NTS or in other CNS regions (reviewed in Bavis et al., 2013). Newborn mammals typically respond to acute hypoxia with only a transient increase in ventilation; within a few minutes, ventilation begins to decline toward, or below, baseline levels (Bissonnette, 2000). Current models suggest that postnatal maturation of the HVR (from the immature, biphasic response to the mature, sustained response) involves a shift in the balance of inhibitory and excitatory signaling pathways in the NTS (Gozal et al., 2000). Interestingly, rats reared in hyperoxia exhibit a sustained increase in ventilation during hypoxia (similar to adults) at an earlier age than untreated, control rats (Bavis et al., 2010, 2014a; Hill et al., 2013). Systemic blockade of NMDA glutamate receptors with MK-801 restores the biphasic HVR in hyperoxia-treated rats, suggesting that developmental hyperoxia strengthens glutamatergic signaling through NMDA receptors and/or its downstream signaling pathways (Bavis et al., 2014a); the role of AMPA glutamate receptors was not investigated. At the same time, blockade of inhibitory PDGF-β receptors had no effect on the HVR of hyperoxia-treated rats despite enhancing the HVR of age-matched control rats (Bavis et al., 2014a). It is interesting that this earlier study implicated glutamate signaling through NMDA receptors in hyperoxia-induced plasticity while the present study suggests augmented signaling through AMPA receptors. This could reflect different populations of cells or fundamental differences in the preparation (i.e., the role of NMDA receptors may not be evident in slice preparations that lack presynaptic activity from the peripheral chemoreceptors).
4.3. Implications for the interdependent model of central/peripheral chemoreception
It has been proposed that carotid chemoreceptor activity modulates the CO2 sensitivity of central chemoreceptors (i.e., central and peripheral chemoreceptors are “interdependent”; cf. Forster and Smith, 2010; Smith et al., 2010). According to this model, central CO2 sensitivity, and thus the HCVR, depends on the level of activation of carotid bodies. In awake dogs, for example, carotid body stimulation increases the slope of the ventilatory response to CNS hypercapnia while carotid body inhibition depresses it (Blain et al., 2010; Smith et al., 2015). However, the generality of this interaction across species and physiological states remains a matter of debate (e.g., Duffin and Mateika, 2013; Teppema and Smith, 2013; Wilson and Day, 2013; Cummings, 2014).
As shown in the present study, chronic hyperoxia dramatically reduces the activity of individual carotid chemoafferent neurons during acute hypercapnia. Moreover, previous studies have shown that developmental hyperoxia causes carotid body hypoplasia and the degeneration of chemoafferent neurons (Erickson et al., 1998; Chavez-Valdez et al., 2012; Dmitrieff et al., 2012; Bavis et al., 2013). Therefore, carotid body input to the CNS should have been quite low under baseline conditions (normocapnic normoxia) and during acute hypercapnia in the hyperoxia-treated rats. The reduced basal carotid body activity likely contributes to the overall hypopnea observed in this treatment group, along with diminished central respiratory drive (Bavis et al., 2014b). However, despite greatly reduced carotid body activity, the magnitude of the HCVR itself was unaffected by developmental hyperoxia whether it was expressed as the slope of the ventilation - inspired CO2 relationship (Fig. 7) or as the percentage increase in ventilation from baseline (Fig. 8). Thus, there was no evidence for a hyperadditive interaction between peripheral and central chemoreception in the present study. Indeed, there was no evidence that carotid bodies contribute significantly to the steady-state HCVR in neonatal rats (i.e., not even a simple additive effect). Carotid bodies may provide an initial defense to a hypercapnic challenge (Cummings and Frappell, 2009), but this contribution appears to be overshadowed by activation of the central CO2 chemoreceptors. Considered this way, the interaction between the peripheral and central chemoreceptors might be hypoadditive in neonatal rats. A similar conclusion (i.e., hypoadditive interaction) was reached for neonatal mice (Cummings, 2014).
The present experiments were not specifically designed to test the interdependent model of central/peripheral chemoreception, so it is appropriate to consider alternate explanations for the apparent lack of a hyperadditive interaction. First, previous studies investigating interdependence of central and peripheral chemoreceptors have used adult animals while the present study employed neonatal rats. It is possible that the relative influence of carotid bodies on central CO2 chemoreceptors increases with postnatal age. Second, studies demonstrating hyperadditive interactions generally have acutely isolated/controlled activation of peripheral and central chemoreceptors. In contrast, neonatal rats subjected to developmental hyperoxia undergo a prolonged, progressive loss of carotid chemoafferent input to the CNS. Accordingly, we cannot rule out compensatory plasticity in the cNTS or further downstream such that peripheral input to central chemoreceptors remains relatively unchanged. The latter hypothesis seems reasonable, particularly in light of observed changes in cNTS network excitability (see section 4.2) and since the early (presumably carotid body-mediated) phase of the HCVR “recovered” by P6-7 in hyperoxia-treated rats (Fig. 8B, C). If this were the case, however, it is somewhat surprising that no differences were detected in the steady-state HCVR at P4 when the early phase of the HCVR was blunted (Fig. 8A). It is possible that developmental hyperoxia may increase the chemosensitive response to hypercapnia of other brainstem neurons, such as those from the RTN, but additional experiments are needed to test this possibility.
4.4. Significance
Whereas the detrimental effects of developmental hyperoxia on hypoxic responses are well established (reviewed in Bavis, 2005 and Bavis et al., 2013), this is the first report that chronic hyperoxia also diminishes peripheral chemosensitivity to hypercapnic stimuli. As previously shown by others (e.g., Cummings and Frappell, 2009), and confirmed in the present study for P4 rats, the carotid bodies may be important to mounting a rapid response to acute hypercapnic challenges. Thus, even if the steady-state HCVR is normal, protective ventilatory reflexes to transient and/or dynamic respiratory challenges may be compromised after chronic hyperoxia. For example, blunted peripheral CO2 sensitivity might prolong apneas or delay arousal responses during rebreathing or asphyxic events. These effects are likely exacerbated by concurrent depression of peripheral O2 sensitivity.
Preterm infants experience an earlier increase in arterial PO2 than expected for their postconceptual age and, in addition to this relative hyperoxia, may also experience hyperoxia when treated with supplemental O2 (e.g., Hagadorn et al., 2006). The influence of developmental hyperoxia on the respiratory control of human infants has rarely been studied, but preterm infants that receive supplemental O2 tend to have blunted ventilatory responses to brief changes in inspired O2 (Calder et al., 1994; Katz-Salamon and Lagercrantz, 1994; Katz-Salamon et al., 1996). Whether these infants also exhibit abnormal CO2 sensitivity remains to be studied.
Highlights.
We studied whether postnatal hyperoxia alters respiratory responses to CO2 in rats.
Carotid body responses to CO2 were reduced at P4, P6-7, and P13-14.
cNTS neurons had normal CO2 responses.
Steady-state hypercapnic ventilatory responses were normal.
Acknowledgments
This work was supported by National Institutes of Health grants R15 HL-083972 and P20 GM103423. Joe Santin assisted with the statistical analysis of electrophysiological parameters for cNTS neurons and Dr. Debra Mayes assisted with the figures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bavis RW. Developmental plasticity of the hypoxic ventilatory response after perinatal hyperoxia and hypoxia. Respir Physiol Neurobiol. 2005;149:287–299. doi: 10.1016/j.resp.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Bavis RW, Mitchell GS. Long-term effects of the perinatal environment on respiratory control. J Appl Physiol. 2008;104:1220–1229. doi: 10.1152/japplphysiol.01086.2007. [DOI] [PubMed] [Google Scholar]
- Bavis RW, Olson EB, Jr, Mitchell GS. Critical developmental period for hyperoxia-induced blunting of hypoxic phrenic responses in rats. J Appl Physiol. 2002;92:1013–1018. doi: 10.1152/japplphysiol.00859.2001. [DOI] [PubMed] [Google Scholar]
- Bavis RW, Young KM, Barry KJ, Boller MR, Kim E, Klein PM, Ovrutsky AR, Rampersad DA. Chronic hyperoxia alters the early and late phases of the hypoxic ventilatory response in neonatal rats. J Appl Physiol. 2010;109:796–803. doi: 10.1152/japplphysiol.00510.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavis RW, Kim I, Pradhan N, Nawreen N, Dmitrieff EF, Carroll JL, Donnelly DF. Recovery of carotid body O2 sensitivity following chronic postnatal hyperoxia in rats. Respir Physiol Neurobiol. 2011;177:47–55. doi: 10.1016/j.resp.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavis RW, Fallon SC, Dmitrieff EF. Chronic hyperoxia and the development of the carotid body. Respir Physiol Neurobiol. 2013;185(1):94–104. doi: 10.1016/j.resp.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavis RW, DeAngelis KJ, Horowitz TC, Reedich LM, March RJ. Hyperoxia-induced developmental plasticity of the hypoxic ventilatory response in neonatal rats: contributions of glutamate-dependent and PDGF-dependent mechanisms. Respir Physiol Neurobiol. 2014a;191:84–94. doi: 10.1016/j.resp.2013.11.006. [DOI] [PubMed] [Google Scholar]
- Bavis RW, van Heerden ES, Brackett DG, Harmeling LH, Johnson SM, Blegen HJ, Logan S, Nguyen GN, Fallon SC. Postnatal development of eupneic ventilation and metabolism in rats chronically exposed to moderate hyperoxia. Respir Physiol Neurobiol. 2014b;198:1–12. doi: 10.1016/j.resp.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisgard GE, Olson EB, Jr, Wang ZY, Bavis RW, Fuller DD, Mitchell GS. Adult carotid chemoafferent responses to hypoxia after 1, 2, and 4 wk of postnatal hyperoxia. J Appl Physiol. 2003;95:946–952. doi: 10.1152/japplphysiol.00985.2002. [DOI] [PubMed] [Google Scholar]
- Bissonnette JM. Mechanisms regulating hypoxic respiratory depression during fetal and postnatal life. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1391–R1400. doi: 10.1152/ajpregu.2000.278.6.R1391. [DOI] [PubMed] [Google Scholar]
- Blain GM, Smith CA, Henderson KS, Dempsey JA. Peripheral chemoreceptors determine the respiratory sensitivity of central chemoreceptors to CO2. J Physiol. 2010;588:2455–2471. doi: 10.1113/jphysiol.2010.187211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler KJ. TASK channels in arterial chemoreceptors and their role in oxygen and acid sensing. Pflugers Arch. 2015;467:1013–1025. doi: 10.1007/s00424-015-1689-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler KJ, Williams BA, Honore E. An oxygen-, acid- and anaesthetic-sensitive TASK-like background potassium channel in rat arterial chemoreceptor cells. J Physiol. 2000;525:135–142. doi: 10.1111/j.1469-7793.2000.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder NA, Williams BA, Smyth J, Boon AW, Kumar P, Hanson MA. Absence of ventilatory response to alternating breaths of mild hypoxia and air in infants who have had bronchopulmonary dysplasia: implications for the risk of sudden infant death. Pediatr Res. 1994;35:677–681. doi: 10.1203/00006450-199406000-00011. [DOI] [PubMed] [Google Scholar]
- Carroll JL. Developmental plasticity in respiratory control. J Appl Physiol. 2003;94:375–389. doi: 10.1152/japplphysiol.00809.2002. [DOI] [PubMed] [Google Scholar]
- Chavez-Valdez R, Mason A, Nunes AR, Northington FJ, Tankersley C, Ahlawat R, Johnson SM, Gauda EB. Effect of hyperoxic exposure during early development on neurotrophin expression in the carotid body and nucleus tractus solitarii. J Appl Physiol. 2012;112:1762–1772. doi: 10.1152/japplphysiol.01609.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad SC, Nichols NL, Ritucci NA, Dean JB, Putnam RW. Development of chemosensitivity in neurons from the nucleus tractus solitarii (NTS) of neonatal rats. Respir Physiol Neurobiol. 2009;166:4–12. doi: 10.1016/j.resp.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings KJ. Interaction of central and peripheral chemoreflexes in neonatal mice: evidence for hypo-addition. Respir Physiol Neurobiol. 2014;203:75–81. doi: 10.1016/j.resp.2014.08.016. [DOI] [PubMed] [Google Scholar]
- Cummings KJ, Frappell PB. Breath-to-breath hypercapnic response in neonatal rats: temperature dependency of the chemoreflexes and potential implications for breathing stability. Am J Physiol Regul Integr Comp Physiol. 2009;297:R124–R134. doi: 10.1152/ajpregu.91011.2008. [DOI] [PubMed] [Google Scholar]
- Dauger S, Ferkdadji L, Saumon G, Vardon G, Peuchmaur M, Gaultier C, Gallego J. Neonatal exposure to 65% oxygen durably impairs lung architecture and breathing pattern in adult mice. Chest. 2003;123:530–538. doi: 10.1378/chest.123.2.530. [DOI] [PubMed] [Google Scholar]
- Davis SE, Solhied G, Castillo M, Dwinell M, Brozoski D, Forster HV. Postnatal developmental changes in CO2 sensitivity in rats. J Appl Physiol. 2006;101:1097–1103. doi: 10.1152/japplphysiol.00378.2006. [DOI] [PubMed] [Google Scholar]
- Dean JB, Putnam RW. The caudal solitary complex is a site of central CO2 chemoreception and integration of multiple systems that regulate expired CO2. Respir Physiol Neurobiol. 2010;173:274–287. doi: 10.1016/j.resp.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Dmitrieff EF, Piro SE, Broge TA, Jr, Dunmire KB, Bavis RW. Carotid body growth during chronic postnatal hyperoxia. Respir Physiol Neurobiol. 2012;180:193–203. doi: 10.1016/j.resp.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly DF, Kim I, Carle C, Carroll JL. Perinatal hyperoxia for 14 days increases nerve conduction time and the acute unitary response to hypoxia of rat carotid body chemoreceptors. J Appl Physiol. 2005;99:114–119. doi: 10.1152/japplphysiol.01009.2004. [DOI] [PubMed] [Google Scholar]
- Donnelly DF, Bavis RW, Kim I, Dbouk HA, Carroll JL. Time-course of alterations in pre- and post-synaptic chemoreceptor function during developmental hyperoxia. Respir Physiol Neurobiol. 2009;168:189–197. doi: 10.1016/j.resp.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffin J, Mateika JH. Cross-Talk opposing view: peripheral and central chemoreflexes have additive effects on ventilation in humans. J Physiol. 2013;591:4351–4353. doi: 10.1113/jphysiol.2013.256800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JT, Mayer C, Jawa A, Ling L, Olson EB, Jr, Vidruk EH, Mitchell GS, Katz DM. Chemoafferent degeneration and carotid body hypoplasia following chronic hyperoxia in newborn rats. J Physiol. 1998;509:519–526. doi: 10.1111/j.1469-7793.1998.519bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filosa JA, Putnam RW. Multiple targets of chemosensitive signaling in locus coeruleus neurons: role of K+ and Ca2+ channels. Am J Physiol Cell Physiol. 2003;284:C145–C155. doi: 10.1152/ajpcell.00346.2002. [DOI] [PubMed] [Google Scholar]
- Forster HV, Smith CA. Contributions of central and peripheral chemoreceptors to the ventilatory response to CO2/H+ J Appl Physiol. 2010;108:989–994. doi: 10.1152/japplphysiol.01059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Bavis RW, Vidruk EH, Wang ZY, Olson EB, Jr, Bisgard GE, Mitchell GS. Life-long impairment of hypoxic phrenic responses in rats following 1 month of developmental hyperoxia. J Physiol. 2002;538:947–955. doi: 10.1113/jphysiol.2001.012908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargaglioni LH, Hartzler LK, Putnam RW. The locus coeruleus and central chemosensitivity. Resp Physiol Neurobiol. 2010;173:264–273. doi: 10.1016/j.resp.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golowasch J, Thomas G, Taylor AL, Patel A, Pineda A, Khalil C, Nadim F. Membrane capacitance measurements revisited: dependence of capacitance value on measurement method in nonisopotential neurons. J Neurophysiol. 2009;102:2161–2175. doi: 10.1152/jn.00160.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozal D, Gozal E, Simakajornboon N. Signaling pathways of the acute hypoxic ventilatory response in the nucleus tractus solitarius. Respir Physiol. 2000;121:209–221. doi: 10.1016/s0034-5687(00)00129-8. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Bayliss DA, Stornetta RL, Fortuna MG, Abbott SB, DePuy SD. Retrotrapezoid nucleus, respiratory chemosensitivity and breathing automaticity. Respir Physiol Neurobiol. 2009;168:59–68. doi: 10.1016/j.resp.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagadorn JI, Furey AM, Nghiem TH, Schmid CH, Phelps DL, Pillers DAM, Cole CH. Achieved versus intended pulse oximeter saturation in infants born less than 28 weeks’ gestation: the AVIOx study. Pediatrics. 2006;118(4):1574–1582. doi: 10.1542/peds.2005-0413. [DOI] [PubMed] [Google Scholar]
- Hanson MA, Eden GJ, Nijhuis JG, Moore PJ. Peripheral chemoreceptors and other oxygen sensors in the fetus and newborn. In: Lahiri S, Forster RE, Davies RO, Pack AI, editors. Chemoreceptors and Reflexes in Breathing: Cellular and Molecular Aspects. Oxford University Press; New York: 1989. pp. 113–120. [Google Scholar]
- Hill CB, Grandgeorge SH, Bavis RW. Developmental hyperoxia alters CNS mechanisms underlying hypoxic ventilatory depression in neonatal rats. Respir Physiol Neurobiol. 2013;189:498–505. doi: 10.1016/j.resp.2013.08.017. [DOI] [PubMed] [Google Scholar]
- Katz-Salamon M, Lagercrantz H. Hypoxic ventilatory defense in very preterm infants: attenuation after long term oxygen treatment. Arch Dis Child Fetal Neonatal Ed. 1994;70:F-90–95. doi: 10.1136/fn.70.2.f90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz-Salamon M, Eriksson M, Jonnson B. Development of chemoreceptor function in infants with chronic lung disease (CLD) with initially lacking hyperoxic response. Arch Dis Child. 1996;75:4–9. doi: 10.1136/fn.75.1.f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Cavanaugh EJ, Kim I, Carroll JL. Heteromeric TASK-1/TASK-3 is the major oxygen-sensitive background K+ channel in rat carotid body glomus cells. J Physiol. 2009;587:2963–2975. doi: 10.1113/jphysiol.2009.171181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, Donnelly DF, Carroll JL. Modulation of gene expression in subfamilies of TASK K+ channels by chronic hyperoxia exposure in rat carotid body. Adv Exp Med Biol. 2006;580:37–41. doi: 10.1007/0-387-31311-7_6. [DOI] [PubMed] [Google Scholar]
- Kim I, Yang D, Carroll JL, Donnelly DF. Perinatal hyperoxia exposure impairs hypoxia-induced depolarization in rat carotid body glomus cells. Respir Physiol Neurobiol. 2013;188:9–14. doi: 10.1016/j.resp.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Prabhakar NR. Peripheral chemoreceptors: function and plasticity of the carotid body. Compr Physiol. 2012;2:141–219. doi: 10.1002/cphy.c100069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri S, Forster RE., II CO2/H+ sensing: peripheral and central chemoreception. Int J Biochem Cell Biol. 2003;35:1413–1435. doi: 10.1016/s1357-2725(03)00050-5. [DOI] [PubMed] [Google Scholar]
- Lahiri S, Mulligan E, Andronikou S, Shirahata M, Mokashi A. Carotid body chemosensory function in prolonged normobaric hyperoxia in the cat. J Appl Physiol. 1987;62:1924–1931. doi: 10.1152/jappl.1987.62.5.1924. [DOI] [PubMed] [Google Scholar]
- Ling L, Olson EB, Jr, Vidruk EH, Mitchell GS. Attenuation of the hypoxic ventilatory response in adult rats following one month of perinatal hyperoxia. J Physiol. 1996;495:561–571. doi: 10.1113/jphysiol.1996.sp021616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik SS, Fewell JE. Thermoregulation in rats during early postnatal maturation: importance of nitric oxide. Am J Physiol. 2003;285(6):R1366–R1372. doi: 10.1152/ajpregu.00280.2003. [DOI] [PubMed] [Google Scholar]
- Mortola JP. Prenatal hyperoxia blunts the hypoxic ventilatory chemosensitivity of the 1-day old chicken hatchling. Respir Physiol Neurobiol. 2011;178:352–356. doi: 10.1016/j.resp.2011.06.015. [DOI] [PubMed] [Google Scholar]
- Nattie E, Li A. Central chemoreceptors: locations and functions. Compr Physiol. 2012;2:221–254. doi: 10.1002/cphy.c100083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Sáenz P, Levitsky KL, Marcos-Almaraz MT, Bonilla-Henao V, Pascual A, López-Barneo J. Carotid body chemosensory responses in mice deficient of TASK channels. J Gen Physiol. 2010;135:379–392. doi: 10.1085/jgp.200910302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peers C. Effect of lowered extracellular pH on Ca2+-dependent K+ currents in type I cells from the neonatal rat carotid body. J Physiol. 1990;422:381–395. doi: 10.1113/jphysiol.1990.sp017990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peers C, Green FK. Inhibition of Ca2+-activated K+ currents by intracellular acidosis in isolated type I cells of the neonatal rat carotid body. J Physiol. 1991;437:589–602. doi: 10.1113/jphysiol.1991.sp018613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam RW, Conrad SC, Gdovin MJ, Erlichman JS, Leiter JC. Neonatal maturation of the hypercapnic ventilatory response and central neural CO2 chemosensitivity. Respir Physiol Neurobiol. 2005;149:165–179. doi: 10.1016/j.resp.2005.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeser JC, Brackett DG, van Heerden ES, Young KM, Bavis RW. Potentiation of the hypoxic ventilatory response by 1 day of hyperoxia in neonatal rats. Respir Physiol Neurobiol. 2011;176:50–56. doi: 10.1016/j.resp.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CA, Forster HV, Blain GM, Dempsey JA. An interdependent model of central/peripheral chemoreception: Evidence and implications for ventilatory control. Respir Physiol Neurobiol. 2010;173:288–297. doi: 10.1016/j.resp.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CA, Blain GM, Henderson KS, Dempsey JA. Peripheral chemoreceptors determine the respiratory sensitivity of central chemoreceptors to CO2: role of carotid body CO2. J Physiol. 2015;593:4225–4243. doi: 10.1113/JP270114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stunden CE, Filosa JA, Garcia AJ, Dean JB, Putnam RW. Development of in vivo ventilatory and single chemosensitive neuron responses to hypercapnia in rats. Respir Physiol. 2001;127:135–155. doi: 10.1016/s0034-5687(01)00242-0. [DOI] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS, Colombari E, West GH, Stornetta RL, Guyenet PG. Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO2-sensitive neurons in rats. J Physiol. 2006;572:503–523. doi: 10.1113/jphysiol.2005.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teppema LJ, Smith CA. CrossTalk opposing view: peripheral and central chemoreceptors have hyperadditive effects on respiratory motor control. J Physiol. 2013;591:4359–4361. doi: 10.1113/jphysiol.2013.256818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp S, Aller MI, Wisden W, Gourine AV. A role for TASK-1 (KCNK3) channels in the chemosensory control of breathing. J Neurosci. 2008;28:8844–8850. doi: 10.1523/JNEUROSCI.1810-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent A, Tell F. Postnatal changes in electrophysiological properties of rat nucleus tractus solitarii neurons. Eur J Neurosci. 1997;9:1612–1624. doi: 10.1111/j.1460-9568.1997.tb01519.x. [DOI] [PubMed] [Google Scholar]
- Wang W, Pizzonia JH, Richerson GB. Chemosensitivity of rat medullary raphe neurones in primary tissue culture. J Physiol. 1998;511:433–450. doi: 10.1111/j.1469-7793.1998.433bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RJ, Day TA. CrossTalk opposing view: peripheral and central chemoreceptors have hypoadditive effects on respiratory motor output. J Physiol. 2013;591:4355–4357. doi: 10.1113/jphysiol.2013.256578. [DOI] [PMC free article] [PubMed] [Google Scholar]