Abstract
The hippocampus is most notably known for its role in cognition and spatial memory; however it also plays an essential role in emotional behaviors and neuroendocrine responses. The current study investigated the long-term effects of neonatal hippocampal lesions (Neo-Hibo) on emotional and hypothalamic-pituitary-adrenal (HPA) axis functioning. During infancy, unlike controls, Neo-Hibo monkeys exhibited enhanced expression of emotional behaviors (e.g. freezing, anxiety-like, and self-directed behaviors) when exposed to a human intruder (HI task). Upon reaching adulthood, they exhibited reduced freezing and hostility, but increased anxiety-like and self-directed behaviors during the HI task. Neo-Hibo monkeys behaved as if they systematically over-rated the risk inherent in the HI task, which supports Gray and McNaughton's septo-hippocampal theory of anxiety. Also, in adulthood, the increased levels of anxiety-like behaviors in Neo-Hibo monkeys were associated with a blunted cortisol response to the HI task. Examination of basal HPA axis function revealed that Neo-Hibo monkeys exhibited the typical diurnal cortisol decline throughout the day, but had lower cortisol concentrations in the morning as compared to controls. Taken together these data suggest that an intact hippocampus during development plays a larger role beyond that of inhibitory/negative feedback regulation of the HPA axis stress-activation, and may be critical for HPA axis basal functioning as well as for the stress response. The behavioral and neuroendocrine changes demonstrated in the current study are reminiscent of those seen in human or nonhuman primates with adult-onset hippocampal damage, demonstrating little functional compensation following early hippocampal damage.
Keywords: Emotion, Development, HPA-axis, Diurnal Rhythm, Macaque
1. Introduction
Our previous studies have demonstrated that neonatal lesion of the hippocampus critically alter the development of memory processes, including visual recognition memory (Zeamer et al., 2010; Zeamer & Bachevalier, 2013), spatial memory (Blue et al., 2013; Glavis-Bloom et al., 2013), and working memory processes (Heuer & Bachevalier, 2011, 2013). These studies provide strong evidence that damage to the hippocampus has a deleterious and long-lasting effect on the development of memory as it does in adult subjects (Nemanic, et al., 2004; Alvarado & Bachevalier, 2005; Bachevalier & Nemanic, 2008). Yet, the hippocampus is also known to play an essential role in emotional and defensive behaviors (Andersen, et al., 2007; de Kloet, 2012), as well as in negative control of neuroendocrine response to potential threats (Herman, et al., 2003). As reviewed below, such a role has largely been derived from studies of adult animals in which both the hippocampus and hypothalamic-pituitary-adrenal (HPA) axis are fully developed but little is known about how the hippocampus may also regulate the development of emotional behavior and neuroendocrine response to stress.
When faced with a potential threat, adult rodents and primates with hippocampal lesions exhibit less freezing/fear and more anxiety-like behaviors (Phillips & LeDoux, 1992; Chudasama, et al., 2008, 2009; Machado & Bachevalier, 2008; Buchanan, et al., 2009; Wang et al., 2013). Also electrical stimulation of the hippocampus reduces glucocorticoid secretion, whereas lesions of the hippocampus prolong the HPA-axis response to a stressor (see review Herman et al., 2003) and promote hypersecretion of basal glucocorticoids (Fendler et al., 1961; Sapolsky et al., 1991). Additionally, patients with hippocampal damage exhibit a blunted cortisol awakening response (Buchanan, et al., 2004; Wolf, et al., 2005) and demonstrate a lack of cortisol response to a psychological stressor (Tuvnes et al., 2003; Buchanan et al., 2009). Although these data provide converging evidence that the hippocampus contributes to the inhibitory/negative feedback regulation of the HPA axis (Herman et al., 2003) and regulates the amplitude of the HPA axis stress response, only a handful of studies have begun to assess whether the hippocampus is critical for the development of emotional and neuroendocrine responses.
Few studies have shown that neonatal hippocampal damage yields abnormal emotional reactivity to objects and social partners (Bauman et al., 2004; Bliss-Moreau et al., 2010, 2011a, 2013), but normal HPA-axis response to pharmacological challenges assessed at 2.5-4.5 months after the lesion (Goursaud et al., 2006). Yet, none of these studies have investigated the impact of early hippocampal damage on both behavioral and physiological (HPA axis) stress reactivity. The present study examined whether neonatal hippocampal damage in monkeys alter the development of emotional responses from infancy (2 and 4.5 months) to adulthood (6-8 years) and how these early lesions impacted the HPA axis stress response, as well as the basal HPA rhythm in adulthood. To this end, control animals and animals with neonatal hippocampal lesions that served in our cognitive testing battery, were tested on the Human Intruder (HI) paradigm to assess the effects of neonatal hippocampal lesions on the development of behavioral and neuroendocrine stress reactivity.
2. Methods
2.1 Subjects
Twelve rhesus monkeys (Macaca mulatta) surrogate-peer reared in a socially enriched environment that promoted species-specific socioemotional skills were used in this study (Goursaud & Bachevalier, 2007; Rommeck et al., 2011; Burbacher et al., 2013; Raper et al., 2013). Animals received neonatal neurotoxic lesions of the hippocampus (Neo-Hibo; males = 4, females = 2) or sham operations (Neo-C; males = 3, females = 3) at 7-25 days of age. During infancy, surgical procedures and behavioral testing were performed at the University of Texas Health Science Center (UTHSC, Houston, TX). In adulthood, behavioral testing and neuroendocrine measures were performed at the Yerkes National Primate Research Center (YNPRC, Atlanta, GA). At both institutions, animals were housed under a 12 hour light/dark cycle and all procedures were approved by the respective Institutional Animal Care and Use Committees of the UTHSC and of Emory University in accordance with the Guide for the Care and Use of Laboratory Animals, 8th edition (National Research Council) and the Animal Welfare Act. The animals used here have been described in previous publications (Goursaud & Bachevalier, 2007; Zeamer et al., 2010; Heuer & Bachevalier, 2011a,b; Blue et al., 2013; Glavis-Bloom et al., 2013; Zeamer & Bachevalier, 2013; Meng et al., 2014,2016), which provide details on procedures for rearing conditions, neuroimaging, neurosurgery, and lesion extent estimation (Goursaud & Bachevalier, 2007; Zeamer et al., 2010). These procedures are briefly summarized below.
2.2 Surrogate Peer-rearing Conditions
Upon arrival at the nursery, infants were housed individually in cages (40 cm × 30 cm × 40 cm) under open radiant incubators with contact comfort provided by a synthetic plush surrogate (30 cm in length) and visual, auditory, and olfactory contact with other infants in the nursery until 1 month of age. Daily care of animals was provided by a principal human caregiver 6 h daily, 5 days a week. On weekends, familiar human caregivers fed, handled and played with the infants 2–4 h daily. Beginning at 1 month of age subjects also received daily socialization with three other age- and sex-matched peers (3-4h, 5 days/week) in a large play cage, containing toys and towels, located in the primate nursery. At 3 months of age, animals were transferred to larger cages and housed individually, but visual and physical contact was possible between pairs of infants through the large central mesh separating two adjacent cages. Animals were housed in quads in large enclosures from 7 to 12 months of age and in pairs thereafter. Our surrogate-peer rearing is similar to the condition ‘continuous rotation peer rearing’ know to produce behavioral and temperament measures most comparable to those of mother-reared monkeys (Rommeck et al., 2011).
2.3 Neuroimaging Procedures
On the day of surgery, the infant was anesthetized (isoflurane, 1-2% to effect), its head was shaved and secured in a nonferromagnetic stereotaxic apparatus, then three MRI sequences were obtained with a GE Signa 1.5 Tesla Echo Speed scanner (GE Medical Systems, Milwaukee, WI) using a 3-inch surface coil. A 3D T1-weighted fast spoiled gradient (FSPGR)-echo sequence (TE = 2.6ms, TR = 10.2ms, 25° flip angle, contiguous 1 mm thick images, 256 × 256 matrix) was used to determine coordinates for each neurotoxin injection site in the hippocampus. A Fluid Attenuated Inversion Recovery (FLAIR) protocol (TE = 140 ms, TR = 10000 ms, inversion time [TI] = 2200, contiguous 3 mm thick images, 12 cm FOV, 256 × 160 matrix) was acquired for comparison with the post-surgical FLAIR images obtained 5-8 days post-surgery, and was used to accurately determine localized areas of edema and estimate the extent of lesion. At 1 year of age, animals underwent an additional T1 high-resolution MRI scan, following the same procedures described above, to calculate the percentage reduction of the hippocampal formation and provide an additional estimate of the extent of hippocampal lesion. As adults (8-10 years of age) animals underwent another structural MRI in a Siemens 3T Trio scanner (inversion time = 950 ms and TE / TR = 3.5 ms / 3 s, FOV = 96 mm × 96 mm, data matrix = 192 × 192) were acquired using the 3D MPRage sequence with GRAPPA (R=2) to verify that no other hippocampal shrinkage occurred with further maturation.
2.4 Surgical Procedures
All surgical procedures were performed under aseptic conditions, an intravenous drip (0.5% dextrose and 0.45% sodium chloride) was placed to maintain normal hydration, and vital signs (heart rate, respiration, blood pressure, expired CO2) were monitored throughout the surgical procedures. The scalp was disinfected with Nolvasan solution and a local anesthetic (Marcain 25%, 1.5 ml) was injected subcutaneously along the incision line to reduce pain during skin incision. After the skin and underlying connective tissue were gently displaced to expose the skull, two craniotomies were made bilaterally above the injection sites, and the dura was cut and retracted to expose the brain.
For Neo-Hibo animals, injections of ibotenic acid (Biosearch Technologies, Novato, CA; 10 mg/ml in phosphate buffer saline, pH 7.4) were made at 7-8 sites along the rostro-caudal axis of the hippocampus using 10 μl Hamilton syringes. A total of 5.0μl of ibotenic acid was injected bilaterally at a rate of 0.4μl/min for each injection site, followed by a 3 min delay before retracting the needle to permit diffusion of the neurotoxin at the tip of the needle and minimize its spread along the needle track during retraction. At completion of the neurotoxic injection, the tissues were sutured in anatomical layers, the animal was removed from anesthesia, and placed in a temperature controlled incubator ventilated with oxygen until full recovery from anesthesia. All animals received acetaminophen (10 mg/kg), cephazolin (25 mg/kg), and dexamethasone sodium phosphate (0.4 mg/kg) to reduce pain, prevent infection and edema, respectively. Neo-C animals received sham-surgeries were treated in the same way as the experimental animals except that no needles were lowered and no injections were administered.
2.5 MRI-based lesion evaluation
Lesion extent was estimated at three time points: at 1-week using the post-surgery FLAIR images and at 1 year and in adulthood using the T1 high-resolution images obtained at those ages (Nemanic et al., 2002). Briefly, the hypersignals on the FLAIR images acquired at 1 mm intervals were visually identified and plotted onto corresponding coronal sections of a 1-week-old normal rhesus monkey brain (J. Bachevalier, unpublished data). These images were imported into ImageJ (version 1.44, http://rsbweb.nih.gov/ij/) to measure the surface area (in square pixels) of hypersignals within the left and right hippocampal formation (including all CA fields, dentate gyrus, and subicular complex) as well as adjacent neural structures (i.e., perirhinal cortex, entorhinal cortex, areas TH and TF on the parahippocampal gyrus, and amygdala), if any. Estimated percentage of hippocampal volume damaged was then calculated by dividing the total volume of hypersignals for the right and left hippocampus by the normal hippocampal volume obtained from the normal 1-week-old monkey brain. One year after surgery and in adulthood, the reduction in hippocampal volume was estimated, using the T1 images collected at 1 mm intervals throughout the entire hippocampus. Hippocampal surface area was measured (in square pixels) on each image using ImageJ. Percentage of reduction was then calculated using the following formula: 100 – [(total Hippocampal volume remaining / average Hippocampal volume in control monkeys) × 100].
2.6 Experiment 1: Behavioral reactivity in infancy
Behavioral reactivity was measured with the Human Intruder (HI) task. This paradigm has been used to effectively measure specific defensive and emotional responses in monkeys based on the salience of the threat presented by the intruder (Kalin et al., 1991). Using the HI paradigm, we previously reported that modulation of fearful defensive (freezing) and hostile defensive (hostility) behaviors emerged by 4.5 months of age in Neo-C surrogate-peer reared infants (Raper et al., 2013). Thus, we predicted that neonatal hippocampal lesions would alter the emergence and/or patterns of emotional behavior during infancy.
2.6.1 Human Intruder Infancy
At 2 and 4.5 months of age, animals were transferred to a testing room and placed in a stainless steel cage (53 cm × 53 cm × 55 cm) with one side made of clear lexan plastic for video recording. The HI paradigm lasted 43 minutes and consisted of four conditions (Alone 1, Profile [No Eye Contact in other publications], Stare, Alone 2). Animals were tested on two consecutive days during which the order of the Profile and Stare conditions were counterbalanced. For each day, the animal first remained alone in the cage for 10 minutes (Alone 1) to acclimate to the environment and obtain a baseline level of behavior. Then, the intruder (experimenter wearing a human rubber mask) entered the room, stood two meters from the test cage while presenting his/her profile to the animal for 10 minutes (Profile condition- No Eye Contact in other publications). The intruder then left the room while the animal remained alone in the cage for a 3-min period, after which the intruder re-entered the room and stared directly at the animal for 10 minutes (Stare condition). Finally, the intruder left the room leaving the animal alone for another 10 minutes (Alone 2). Researchers wore a different rubber mask at each age of testing. The animals’ behavioral reactivity during all conditions was videotaped and later coded using a detailed ethogram (Table 1) and the Observer 5 software (Noldus Inc.). Two trained experimenters unaware of the animals’ lesion group coded all the videotapes (inter-rater reliability: Cohen's Kappa = .81; average intra-rater reliability of Cohen's Kappa = 0.97).
Table 1.
Behavioral Ethogram
| Category and specific behavior | Measurement | Brief Definition |
|---|---|---|
| Fearful Defensive Behaviors | Cumulative Duration | |
| Freeze | duration | Rigid, tense, motionless posture except slight head movement |
| Hostile Defensive Behaviors | Cumulative Frequency | |
| Threat Bark Vocalization | frequency | Low pitch, high intensity, rasping, guttural |
| Threat (facial expression) | frequency | Any of the following: open mouth (no teeth exposed), head-bobbing, or ear flapping |
| Cage Aggression | frequency | Vigorously slaps, shakes or slams body against cage |
| Lunge | frequency | A quick, jerky movement toward the intruder |
| Anxiety-like Behaviors | Cumulative Frequency | |
| Scratch | frequency | Rapid scratching of body with hands or feet |
| Body Shake | frequency | Whole body or just head and shoulder region shakes |
| Tooth Grind | frequencya | Repetitive, audible rubbing of upper & lower teeth |
| Yawn | frequency | Open mouth widely, exposing teeth |
| Self-directed Behaviors | Cumulative Duration | |
| Self-grooming | duration | Use of hands or mouth to smooth or pick through fur |
| Self-clasping | duration | Non-manipulatory enclosing or holding of a limb or body part with arms |
| Other Self-directed | duration | Sucking thumb, eye poke |
| Stereotypies | Cumulative Duration | |
| Pacing | duration | Repetitive motor pattern around the test cage |
| Motor stereotypy | duration | Repetitive, abnormal voluntary or involuntary motor patterns (swinging, twirling, floating limb) |
| Affiliative Behaviors | Cumulative Frequency | |
| Coo Vocalization | frequency | Clear soft, moderate in pitch and intensity, usually “oooooh” sounding |
| Grunt Vocalization | frequency | Deep, muffled, low intensity, almost gurgling sound |
| Lipsmack | frequency | Rapid movement of pursed lips, accompanied by a smacking sound |
| Present | frequency | Rigid posture (knees locked) with tail elevated and rump oriented toward intruder |
List of all behaviors scored, how they are measured and a brief definitions.
Behavior for which total duration was also measured.
2.6.2 Data Analysis
Pearson's correlation coefficients were used to examine the relationship between the estimated lesion extent from the post-surgical FLAIR MRI with the percent reduction in hippocampal volume at 1 year old and in adulthood.
For the HI task, preliminary analyses were first performed to compare emotional reactivity during the two Alone conditions (Alone 1 & Alone 2). Repeated measures ANOVA (Group × Testing Day) revealed no significant main effects or interactions, therefore these two conditions were averaged over the two testing days to create a single Alone condition at each age. Similarly, data on the Profile and Stare conditions were compared between the first and second testing days (repeated measures ANOVA: Group × Testing Day). Again, no significant differences were detected between the two days of testing and a single measure averaging scores from the two Profile and the two Stare conditions were calculated for each animal. Thus, for the final analyses (see below), the combined scores of each animal were used for each of the 3 conditions (Alone, Profile, Stare). Behavioral data was transformed using a natural log plus one constant (LnX + 1) to achieve normality.
Twelve animals (Neo-C, n=6; Neo-Hibo, n=6) were tested at 2 months of age, whereas only eight (Neo-C, n=4; Neo-Hibo, n=4) were tested at 4.5 months. Assessments of the normal development of defensive behaviors between 2 and 4.5 months of age for group Neo-C were conducted and reported in a previous publication (Raper et al., 2013). Results are briefly described below for comparative purposes. To examine differences between Neo-C and Neo-Hibo animals, MANOVAs with Group (Neo-C, Neo-Hibo) and Condition (Alone, Profile, Stare) as main factors, and Age (2 months, 4.5 months-old) as a within subjects factor with repeated measures were used. Planned comparisons were performed with Mann-Whitney U tests to compare group differences in freezing at each age separately. Lastly, Friedman Test was used to examine the interaction between Age and Condition for hostile behaviors regardless of group assignment.
The relationship between the extent of hippocampal damage and behavior during the Human Intruder paradigm was also examined using Pearson correlations, however, no significant correlations were found (see Supplemental Materials). All analyses were conducted with SPSS 22 for Windows (IBM Corporation, USA), significance level was set at p < 0.05, and confidence intervals of 95% are available in the Supplemental Materials. Effect sizes for ANOVAs were calculated using partial eta squared (ηp2) and Cohen's d. Since the focus was the individual null hypotheses of each outcome, the Bonferroni correction for multiple comparisons was not applied, due to concerns over the risk of increased Type II error (Perneger, 1998).
3. Results: Experiment 1
3.1 Lesion Extent Verification
Estimation of hippocampal lesion extent from post-surgical FLAIR images for all 6 Neo-Hibo animals ranged from 3.9% to 87.4% (average, 48.1%), whereas the percentage of volume reduction ranged from 14.8% to 67% (average, 42.7%) at 1-year post-surgery and ranged from 6.4% to 65% (average, 38.5%) in adulthood (8-10 years). Lesion estimates from the post-surgical FLAIR strongly correlated with both 1 year and adult hippocampal volume reduction (r[6] = 0.81, p = 0.025 and r[6] = 0.80, p = 0.27, respectively). There was also a strong correlation between hippocampal volume reduction at 1 year and in adulthood (r[6] = 0.87, p = 0.012). A representative case (Neo-Hibo-3) is depicted in Figure 1. Additional cases have been presented in previous publications, Neo-Hibo-1 (Glavis-Bloom et al., 2013), Neo-Hibo-2 (Zeamer et al., 2010), Neo-Hibo-4 (Heuer & Bachevalier, 2011a), and will be briefly summarized here. Two cases (Neo-Hibo-2 and -3) received the most complete hippocampal lesions (67% and 87% damage to both hemispheres, respectively), whereas three cases (Neo-Hibo-1, -4, and -5) had significant damage in one hemisphere (64%, 67%, and 84%, respectively) but milder hippocampal damage in the other hemisphere (3%, 20.3%, and 20.7%, respectively). Lastly, Neo-Hibo-6 received the least hippocampal damage located primarily in the anterior portion of the hippocampus. As shown in Table 2, only Neo-Hibo-1 had over 10% damage to an unintended adjacent structure (left amygdala = 14%).
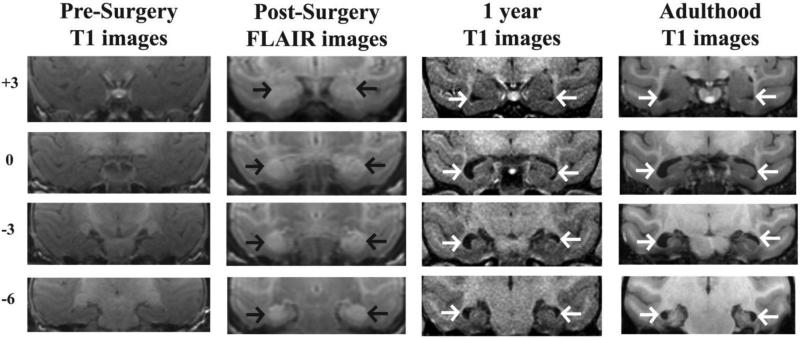
Figure 1.
Coronal sections through the anterior (top) to posterior (bottom) extent of the hippocampus. Far Left column: T1-weighted MR images through the hippocampus at pre-surgery in a representative case with neonatal hippocampal lesion (Neo-Hibo-3). Middle Left column: FLAIR images illustrating the location and extent of hypersignals (black arrows) within the hippocampus of the same animal post-surgery. Middle Right column: T1-weighted MR images through the hippocampus at 1 year of age in the same animal illustrating the enlargement of ventricles (white arrows) resulting from hippocampal volume reduction. Far Right column: T1-weighted MR images through the hippocampus in adulthood in the same animal white arrows indicate enlargement of ventricles from hippocampal volume reduction.
Table 2.
Intended and unintended damage after neurotoxic lesions of the hippocampus
| 1 week Post-surgical FLAIR MRI | 1 year T1 MRI | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adulthood T1 MRI Intended Damage | Unintended Damage | Hippocampal | ||||||||||
| Hippocampal Hippocampus | Amygdala | Entorhinal | Volume | |||||||||
| Reduction | Volume Reduction | |||||||||||
| Subjects | Lf% | Rt% | X% | Lf% | Rt% | X% | Lf% | Rt% | X% | Lf% | Rt% | X% |
| Lf% | Rt% | X% | ||||||||||
| Neo-Hibo-1 | 63.8 | 2.9 | 33.2 | 14.0 | 0.0 | 7.0 | 2.6 | 0.0 | 1.3 | 27.6 | 10.7 | |
| 19.1 | 50.4 | 14.3 | 32.4 | |||||||||
| Neo-Hibo-2 | 54.4 | 80.9 | 67.6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 61.2 | 72.9 | |
| 67.0 | 55.3 | 74.8 | 65.0 | |||||||||
| Neo-Hibo-3 | 78.5 | 96.3 | 87.4 | 1.7 | 0.0 | 0.8 | 0.0 | 0.0 | 0.0 | 54.7 | 47.8 | |
| 51.3 | 51.3 | 36.3 | 43.8 | |||||||||
| Neo-Hibo-4 | 20.3 | 67.3 | 43.8 | 0.0 | 4.7 | 2.4 | 0.0 | 0.0 | 0.0 | 33.6 | 61.7 | |
| 47.6 | 32.9 | 56.0 | 44.5 | |||||||||
| Neo-Hibo-5 | 20.7 | 84.4 | 52.6 | 0.0 | 4.9 | 2.4 | 0.0 | 1.5 | 0.7 | 49.2 | 64.0 | |
| 56.6 | 30.3 | 47.9 | 39.1 | |||||||||
| Neo-Hibo-6 | 7.9 | 0.0 | 3.9 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 21.3 | 8.25 | |
| 14.8 | 12.9 | 0.0 | 6.4 | |||||||||
| Mean | 40.9 | 55.3 | 48.1 | 2.6 | 1.6 | 2.1 | 0.4 | 0.2 | 0.3 | 41.3 | 44.2 | 42.7 |
| 38.8 | 38.2 | 38.5 | ||||||||||
Lf%: percent damage in the left hemisphere; Rt%: percent damage in the right hemisphere; X%: average damage to both hemispheres.
3.2 Effect of Neonatal Hippocampal Lesions on Development of Defensive Behaviors
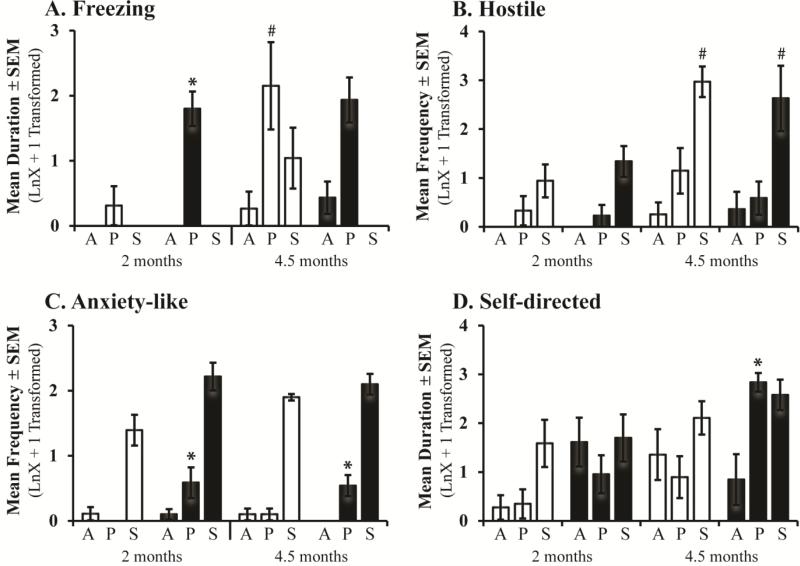
Unlike controls, which only exhibited increased fearful defensive freezing in the Profile condition at 4.5 months of age, Neo-Hibo animals exhibited similar amounts of freezing during the Profile condition at both 2 and 4.5 months (Age: F[1,6] = 2.53, p = 0.16, ηp2 = 0.29, d = 1.28; Figure 2A), and a trend with a large effect size for a Group × Condition interaction (F[2,18] = 2.89, p = 0.08, ηp2 = 0.24, d = 1.12). Planned comparisons for the Profile condition at the two ages separately demonstrated that Neo-Hibo animals spent significantly more time freezing than Neo-C animals at 2 months of age (U = 4.00, p = 0.026, ηp2 = 0.43, d = 1.75), but not at 4.5 months of age (U = 6.00, p = 0.69, ηp2 = 0.04, d = 0.42; Figure 2A). Groups did not differ in their expression of hostile defensive behaviors, but there was a significant Condition × Age interaction (F[2,18] = 6.79, p = 0.006, ηp2 = 0.43, d = 1.74; Figure 2B). Nonparametric Friedman Tests reveal that both groups exhibited an increase in hostile defensive behaviors with age during the Stare condition, (χ2[1, N=8] = 8.00, p = 0.005, ηp2 = 0.84, d = 4.57). Sham-operated infant monkeys expressed similar levels of anxiety-like behaviors at 2 and 4.5 months of age (Age: F[2,18] = 0.15, p = 0.70, ηp2 = 0.008, d = 0.18), but Neo-Hibo animals exhibited more anxiety-like behaviors in the Profile condition at both ages as compared to controls (Group × Condition: F[2,18] = 4.08, p = 0.035, ηp2 = 0.31, d= 1.34; Figure 2C). Interestingly, a significant Group × Condition × Age interaction for self-directed behaviors (F[2,18] = 3.53, p = 0.05, ηp2 = 0.28, d = 1.25) revealed that groups did not differ in the amount of self-directed behaviors at 2 months of age, whereas at 4.5 months Neo-Hibo animals exhibited significantly more self-directed behaviors during the Profile condition as compared to controls (Group: F[1,8] = 11.34, p = 0.015, ηp2 = 0.65, d = 2.73; Figure 2D). Conversely, although affiliative behaviors did not differ by age, Neo-Hibo animals exhibited fewer affiliative behaviors compared to controls (Group: F[1,18] = 4.11, p = 0.05, ηp2 = 0.19, d = 0.97; see Supplemental Materials). No significant main effects and no significant interactions were noted for stereotypies (Group: F[1,18] = 0.01, p = 0.9 ηp2 = 0.001, d = 0.06).
Figure 2.
Bars represent the mean ± SEM for 4 animals in each group tested at both 2 and 4.5 months of age on the Human Intruder paradigm. Freezing, hostile, anxiety-like, and self-directed behaviors during the Alone (A), Profile (P), and Stare (S) conditions for animals with sham operations (Neo-C, open bars) and animals with neonatal hippocampal lesions (Neo-Hibo, filled bars). # indicates significant age effect and * indicates significant group differences (p < 0.05).
4. Experiment 2: Behavioral reactivity in adulthood
Experiment 1 results reveal that Neo-Hibo animals exhibited more fearful defensive freezing at 2 months of age, less affiliative behaviors at both ages, and more anxiety-like and self-directed behaviors at 4.5 months of age as compared to controls. This suggests that early hippocampal damage did not abolish the animals’ ability to express species typical defensive and emotional behaviors, but altered the magnitude of their expression and their developmental trajectory. To investigate whether changes in emotional reactivity after Neo-Hibo lesions persisted in adulthood or whether the effects dissipated with further maturation, all animals were retested on the HI paradigm as adults. Furthermore, to assess whether changes in emotional reactivity after Neo-Hibo lesions were associated with alterations in HPA-axis stress reactivity, the HI paradigm acted as an acute stressor to measure cortisol stress response. Basal cortisol levels were also examined by sampling on a baseline (non-stressor) day and across the diurnal rhythm.
4.1 Human Intruder Adulthood
Animals were tested again on the HI paradigm in adulthood (6-8 years of age). The HI paradigm was slightly modified by reducing the length of the task to 30 minutes in order to capture the peak of the HPA axis stress response. The paradigm was given on only one day, all animals received the three conditions (Alone, Profile, Stare) presented in the same order for 9 minutes each and separated by a brief 3-min break between the Profile and Stare conditions. Emotional reactivity to the intruder was assessed via videotape recording for later coding using the Observer XT 10 software (Noldus, Inc.) and the same ethogram that was used from Experiment 1. One experimenter unaware of lesion status coded all of the videotapes in adulthood. For consistency across ages, this experimenter coded infant videos and demonstrated a high degree of inter-rater reliability (Cohen's Kappa = .84) with the previous experimenters that coded behavior in infancy (2 and 4.5 months). This experimenter also had an average intra-rater reliability of Cohen's Kappa = 0.98.
4.2 Blood Sampling
Animals were trained to voluntarily present a leg for awake blood collection and all samples were collected within 8 minutes or less of accessing the monkey. Plasma cortisol collected under these conditions reliably reflects basal levels (Blank et al., 1983; Raper et al., 2013). All animals were tested at the same time of day (Lights-On: 0700) and blood samples were collected immediately before (0700) and after the acute stressor (HI paradigm - 0730). To determine that changes in hormone levels were not due to handling, two blood samples were collected at the same time of day (0700 & 0730) in the absence of the acute stressor two days prior to the HI paradigm. Approximately one year later, the diurnal rhythm of cortisol secretion was characterized by collecting blood samples for each animal at Lights-On (0700), Mid-day (1300), and Lights-Off (1900). All blood samples were collected in pre-chilled 2-ml vacutainer tubes containing EDTA (3.6 mg) and immediately placed on ice. Samples were centrifuged at 3,000 rpm for 15 minutes in a refrigerated centrifuge (4°C) and plasma samples were stored at −80°C until assayed.
4.3 Plasma Hormone Assays
All assays were performed by the YNPRC Biomarker Core Laboratory. HI task and baseline day plasma samples were assayed for cortisol in duplicate by RIA using commercially available kits (DSL kit: Diagnostic Systems Laboratories, Webster, TX). The sensitivity of the DSL assay was 1.25μg/dl and intra- and inter-assay coefficients of variation in each assay were < 10%. Plasma samples for the diurnal cortisol rhythm were assayed using liquid chromatography – mass spectroscopy (LC-MS), because the Diagnostic Systems Laboratories discontinued their cortisol RIA by the time the diurnal blood samples had been obtained. LC-MS analyses were performed via reverse phase chromatography on an LTQ-Orbitrap mass spectrometer (Thermo Scientific, Waltham, MA). Quantitation was achieved using a deuterated cortisol internal standard (CDN Isotopes, Cortisol-9,11,-12,12-d4). The assay range was 2.5-60 μg/dl with intra- and inter-assay coefficients of variation < 15%.
4.4 Data Analysis
One Neo-C male was unavailable for testing as an adult, however, an unoperated female was added to the study at 1 year of age and served as a replacement. Preliminary analyses showed that this female did not differ from the other surrogate-peer reared Neo-C animals in either behavior responses or hormone levels. Thus, 12 animals (Neo-C, n = 6; Neo-Hibo, n = 6) were tested at this age. Prior to analysis, the behavioral data were examined for normality, when behaviors were not normally distributed they were transformed using a natural log plus one constant (LnX +1). Group differences in defensive and emotional behaviors during adulthood were examined with repeated measures ANOVA with Group (Neo-C, Neo-Hibo) as the between subject factor and Condition (Alone, Profile, Stare) as the within subjects repeated measure.
Cortisol levels were examined with repeated measures ANOVA with Group (Neo-C, Neo-Hibo) as the between subject factor and Time as the within subjects repeated measures (i.e. pre- and post-stressor; Lights-On and + 30 min for baseline day; Lights-On, Mid-day, Lights-Off for diurnal rhythm). Change in cortisol concentrations from pre- to post-stressor was calculated as a percent change ([post-stressor – pre-stressor]/pre-stressor × 100), whereas percent decline was calculated for changes in the diurnal rhythm ([Lights-On – Mid-day]/Lights-On × 100; [Mid-day – Lights-Off]/Mid-day × 100) for each animal and analyzed by GLM ANOVA with Group (Neo-C, Neo-Hibo) as the between subjects factor and changes in cortisol levels as the dependent variables.
The relationship between hippocampal volume loss and behavior or cortisol levels were also examined using Pearson correlations, however no significant correlations were found (see Supplemental Materials). All analyses were conducted with SPSS 22 for Windows (IBM Corporation, USA), significance level was set at p < 0.05, and confidence intervals of 95% are available in the Supplemental Materials. Effect sizes for ANOVAs were calculated using partial eta squared (ηp2) and Cohen's d. Since the focus was the individual null hypotheses of each outcome, the Bonferroni correction for multiple comparisons was not applied, due to concerns over the risk of increased Type II error (Perneger, 1998).
5. Results: Experiment 2
5.1 Long-term effects of neonatal hippocampal lesions on emotional reactivity
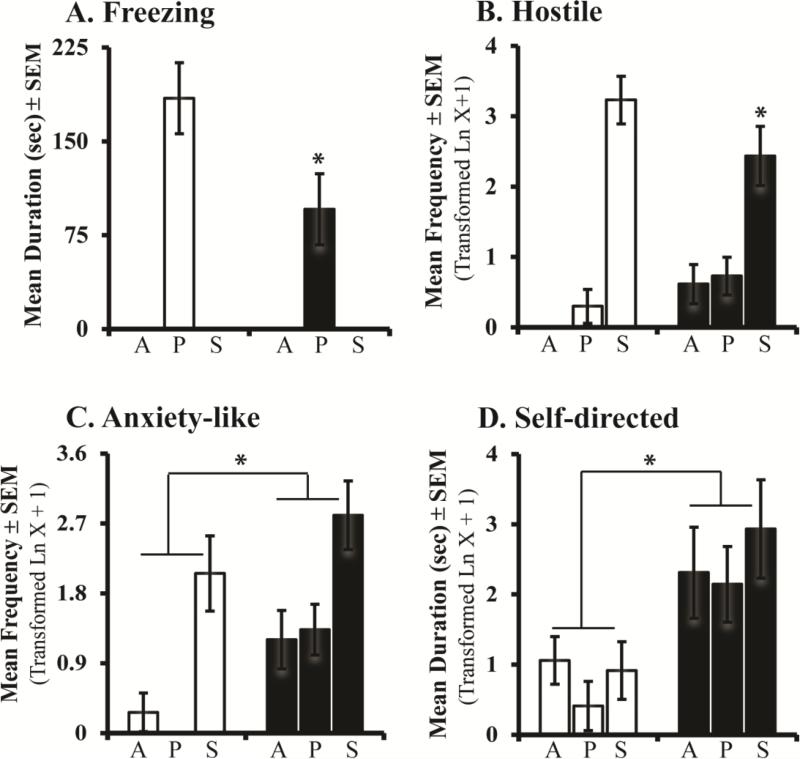
Group differences in behaviors observed during infancy persisted into adulthood (see Figure 3). Although control and Neo-Hibo animals both exhibit increased fearful defensive freezing during the Profile condition (Condition: F[2,20] = 48.765, p < 0.001, ηp2 = 0.83, d = 4.42), the freezing was dampened in Neo-Hibo as compared to controls (Group: F[1,10] = 4.89, p = 0.051, ηp2 = 0.33, d = 1.40; Figure 3A).
Figure 3.
Bars represent the mean ± SEM for freezing, hostile, anxiety-like and self-directed behaviors obtained in adulthood for each group during the three conditions of the Human Intruder paradigm. All other abbreviations as in Figure 2.
For hostile behaviors, there was a Group × Condition interaction (F[2,20] = 4.29, p = 0.033, ηp2 = 0.30, d = 1.31; Figure 3B), such that both groups showed increased hostile behaviors in the Stare condition, yet the magnitude of this increase was less in Neo-Hibo animals (repeated contrast: Alone vs Profile, F[1,10] = 0.29, p = 0.60, ηp2 = 0.03, d = 0.35; Profile vs Stare, F[1,10] = 3.94, p = 0.07, ηp2 = 0.28, d = 1.25). Both groups also expressed increased anxiety-like behaviors during the Stare condition (Condition: F[2,20] = 33.27, p < 0.001, ηp2 = 0.77, d = 3.66), although Neo-Hibo animals displayed more anxiety-like behaviors than controls overall (Group: F[1,10] = 4.70, p = 0.05, ηp2 = 0.32, d = 1.37; Figure 3C). Interestingly, Neo-Hibo animals also exhibited increased self-directed behaviors as compared to controls (Group: F[1,10] = 5.87, p = 0.036, ηp2 = 0.37, d = 1.53; Figure 3D). No significant interactions or group differences were revealed for any other behavior (Group effect: affiliative behavior F[1,10] = 1.21, p = 0.30, ηp2 = 0.10, d = 0.67; stereotypy F[1,10] = 0.04, p = 0.84, ηp2 = 0.004, d = 0.13; see Supplemental Materials).
5.2 Long-term effects of neonatal hippocampal lesions HPA-axis function
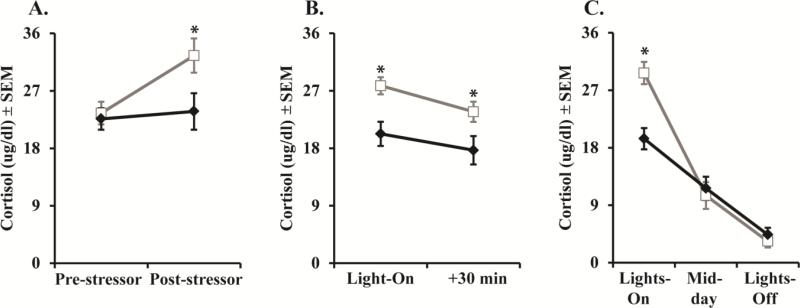
Early hippocampal damage impacted HPA functioning in adulthood. Cortisol response to the stressor revealed a Group × Time interaction (F[1,10] = 7.93, p = 0.018, ηp2 = 0.44, d = 1.77; Figure 4A), such that Neo-Hibo animals have lower post-stressor cortisol levels as compared to controls. Control animals had a greater percent change in cortisol secretion from pre- to post-stressor (M = 39.57 ± 8.80) than the Neo-Hibo animals (M = 3.74 ± 9.13; F[1,12] = 7.63, p = 0.02, ηp2 = 0.43, d = 1.74), further confirming that Neo-Hibo animals were hypo-responders to the stressor.
Figure 4.
Scores are mean ± SEM of (A) cortisol levels in adulthood during Human Intruder stressor, (B) cortisol levels on the Non-stress day, (C) cortisol levels across the diurnal rhythm. Neo-C is represented with open bars or open squares with grey lines, Neo-Hibo is represented with filled bars or filled diamonds with black lines. * indicates significance group differences (p < 0.05).
Cortisol levels were examined on a baseline day, two days prior to the HI stressor, to ensure that group differences in hormone secretion were due to the effects of the HI stressor and not merely the effect of handling. Both groups exhibited a small but significant decrease in cortisol levels from Lights-On to 30 minutes later (Time: F[1,10] = 13.61, p = 0.004, ηp2 = 0.58, d = 2.35; Figure 4B). Interestingly, Neo-Hibo animals had significantly lower cortisol levels at both time points as compared to controls (Group: F[1,10] = 5.91, p = 0.035, ηp2 = 0.37, d = 1.53; Figure 4B). The lower basal cortisol levels among Neo-Hibo animals was replicated a year later by examining the diurnal cortisol rhythm. Again, Neo-Hibo animals had lower cortisol levels at Lights-On as compared to controls (Group × Time: F[2,20] = 9.75, p = 0.001, ηp2 = 0.49, d = 1.96; Figure 4C), but did not differ from controls at Mid-day or Lights-Off (repeated contrast: Lights-On vs Mid-day F[1,10] = 24.1, p = 0.001, ηp2 = 0.71, d = 3.13; Mid-day vs Lights-Off F[1,10] = 0.33, p = 0.57, ηp2 = 0.03, d = 0.35). This Group effect was verified by a significant difference in the cortisol percent decline from Lights-On to Mid-day and no difference between groups from Mid-day to Lights-Off (F[1,12] = 12.71, p = 0.005, ηp2 = 0.56, d = 2.26; F[1,12] = 0.41, p = 0.54, ηp2 = 0.04, d = 0.41, respectively), such that controls had a great percent decline (M = 57.57 ± 3.39) compared to Neo-Hibo animals (M = 35.32 ± 5.24).
6. Discussion
The present study demonstrates that neonatal hippocampal damage causes lasting alterations in emotional behavior expression and neuroendocrine secretions. Although Neo-Hibo lesions did not impair or delay the emergence of defensive and emotional behaviors in early infancy, it did alter the overall magnitude of their expression, which became more pronounced in adulthood. Reduction in basal cortisol levels and a blunted cortisol stress response was also found after early hippocampal insult in adulthood. These behavioral and neuroendocrine changes after Neo-Hibo lesions are reminiscent to those seen after adult-onset lesions, suggesting little functional compensation after early hippocampal damage.
In the present study, early hippocampal insult altered the magnitude of defensive and emotional responses at the three ages tested (2 and 4.5 months, and 6-8 years) as compared to controls. First, the magnitude of fearful defensive freezing changed across age, such that as compared to controls, Neo-Hibo animals froze more at 2 months old, were no different at 4.5 months old, and then exhibited less freezing in adulthood. Although increased freezing at 2 months in Neo-Hibo animals is contradictory to some reports of reduced freezing to fearful cues subsequent to early hippocampal damage in rodents (Becker et al., 1999; Järlestedt et al., 2011), it is however similar to increased passivity during social interactions with peers at 2 months of age following neonatal hippocampal aspiration lesions in monkeys (Bachevalier, et al., 1999). In adulthood, reduced freezing on the intruder task suggests that the impact of Neo-Hibo lesions on emotional behavior becomes more severe with age. There was a similar maturational change of hostile behavior expression in Neo-Hibo animals, such that there were no group differences during infancy but decreased hostility in adulthood compared to controls. Reduced freezing and hostility during adulthood is consistent with reports of decreased defensive behaviors in monkeys with adult-onset hippocampal lesions (Machado & Bachevalier, 2008; Chudasama, et al., 2008, 2009) and decreased reactivity toward objects in other Neo-Hibo monkeys (Bliss-Moreau, et al., 2010, 2011a). Taken together, these results suggest that early hippocampal damage alters the typical developmental maturation of defensive behaviors, such that animals exhibit decreased defensive behaviors in adulthood.
The hippocampus plays an important role in anxiety (Gray & McNaughton, 2000; Phelps, et al., 2004). Thus, hippocampal metabolism is greater in animals with anxious temperament (Oler, et al., 2010) and hippocampal lesions in adult animals and humans leads to increased anxiety-like behaviors (Machado & Bachevalier, 2008; Buchanan, et al., 2009). The current developmental data indicates that Neo-Hibo lesions resulted in increased anxiety-like behaviors in the Profile condition during infancy, which was further amplified during adulthood in all conditions of the task. Even though these changes were not observed in previous studies of neonatal-onset hippocampal lesions (Bachevalier, et al., 1999; Bauman, et al., 2004, Bliss-Moreau, et al., 2010, 2011a, 2011b, 2013), the present results, together with similar results after adult-onset hippocampal damage (Machado & Bachevalier, 2008; Buchanan, et al., 2009), further support the role of the hippocampus in anxiety. One potential factor that could explain the different outcomes between previous studies and the current one is the sensitivity of the tasks to detect differences in anxiety-like behaviors. The HI paradigm has been proven to be a robust task for identifying anxious temperament (Kalin, et al., 2001; Fox, et al., 2008; Oler, et al., 2010; Shackman, et al., 2013), and may be more sensitive for detecting anxiety-like behaviors than social interaction observations or reactions to novel objects used in earlier studies. Contrary to previous studies indicating Neo-Hibo lesions increased motor stereotypies, with no difference in self-directed behaviors (Bauman, et al., 2008), the current study showed the reverse, i.e. no differences in stereotypies, and increased self-directed behaviors (self-grooming and self-clasping) during both infancy and adulthood. Interestingly, the increased self-grooming seen in the Neo-Hibo lesion animals is similar to a recent report indicating that monkeys with early hippocampal lesions exhibit increased partner grooming in adulthood (Moadab, et al., 2015). Self-directed behaviors are often regarded by primatologists as displacement behaviors because their occurrence appears out of context and consists of different body care activities (MacFarland, 1966). Additionally, displacement or self-directed behaviors have been used as an indicator of stress and anxiety more generally, such that anxiogenic drugs, catecholamines, or glucocorticoids cause an increase in self-grooming (Ninan, et al., 1982). Thus, it is interesting to note that damage to the hippocampus yielded increased anxiety-like and self-directed behaviors, yet blocked the animals’ ability to mount a cortisol stress response. This pattern of anxiety-like behaviors suggests increased corticotrophin-releasing-factor (Heinrichs & Koob, 2004), although this hypothesis will need to be further tested.
Exactly how hippocampal dysfunction could lead to decreased fearful defensive freezing and at the same time increased anxiety-like behaviors is still unknown. Although these two behavioral responses have usually been interpreted as manifestations of emotional reactivity, the present data suggest that they may be either differentially regulated by the same neurobiological system or regulated by two different systems. Further studies will need to parse apart the neural circuits underlying these emotional responses. Interestingly, our data showed that Neo-Hibo animals behaved as if they systematically over-rated the risk inherent in the HI task, such that they expressed more anxiety-like behaviors across all conditions of the task. This effect contrasts with that of neonatal amygdala lesions, which were raised and tested in exactly the same way (Raper, et al., 2013). Animals with neonatal amygdala lesions had normal levels of anxiety-like behaviors on the HI task, suggesting that the hippocampus plays a critical and unique role in the regulation of anxiety-like behaviors. This proposal is consistent with Gray and McNaughton's (2000) septo-hippocampal system theory of anxiety suggesting that the hippocampus is important for conflict resolution between approach and avoidance, especially during ambiguous situations. Thus, disruption of cognitive and memory processes after hippocampal damage could result in aberrant modulation of affective responses, such as anxiety. In this context, it is interesting to note that Neo-Hibo animals in this study are impaired in a variety of recognition and working memory tasks (Zeamer, et al., 2010; Heuer & Bachevalier, 2011; Glavis-Bloom, et al., 2013).
Although the hippocampus has been implicated in the negative feedback control of the HPA axis (Herman, et al., 2003), studies have shown that adult-onset hippocampal damage results in only a transient rise in glucocorticoids, which returns to normal within months in nonhuman primates (Sapolsky, et al., 1991). Unfortunately, the current study only examined HPA axis activity in adulthood, thus its potential transient rise in glucocorticoids immediately after the Neo-Hibo lesions cannot be addressed. However, the results replicate those seen in human patients (Buchanan, et al., 2004; 2009; Wolf, et al., 2005) and rodents (Tuvnes, et al., 2003) with hippocampal damage, such that Neo-Hibo animals exhibited a blunted cortisol response to the stressor and lower basal cortisol secretions in the morning as compared to controls. Taken together the data suggest that the hippocampus plays a larger role beyond that of inhibitory/negative feedback regulation of the HPA axis, and that an intact hippocampus during development may be critical for HPA axis response to a stressor and maintaining the basal cortisol rhythm.
An important caveat inherent in developmental studies is the interaction between genetic and environmental factors influencing brain and behavior, such that rearing conditions can alter brain maturation and behavior (Sanchez, et al., 1998; Rommeck, et al., 2011). Therefore, it is possible that rearing could have impacted emotional behavior expression in the hippocampal-operated animals. However, this is unlikely because we have previously shown that our surrogate-peer reared controls (similar to continuous rotational peer rearing; Rommeck, et al., 2011) exhibit the same developmental pattern in emotional behavior on the HI task, cognitive skills, as well as hippocampal maturation similar to mother-reared monkeys (Kalin, et al., 1991; Zeamer, et al., 2010; Raper, et al., 2013; Hunsaker, et al., 2014). A rearing by lesion interaction is also unlikely since behavioral and hormonal effects are structure specific. For example, although both neonatal amygdala lesioned and Neo-Hibo animals express less fearful defensive freezing compared to controls (M = 184.5 ± 28 sec), the magnitude of reduction varied across groups such that amygdala lesioned animals (M = 14.9 ± 7 sec; Raper, et al., 2013) also freeze significantly less than Neo-H (M = 95.7 ± 21 sec). Additionally, the groups showed distinct differences in anxiety-like behaviors, with amygdala lesions exhibiting similar anxiety-like behavior as controls, and Neo-Hibo animals exhibiting more anxiety-like behaviors. Likewise, Neo-Hibo animals were hypo-responders to stress, whereas neonatal amygdala lesioned animals only exhibit a slightly lower cortisol response compared to controls. Given the unique compilations of observed differences across the two lesion groups, it is unlikely that rearing conditions impacted the results of this study.
Finally, the lack of significant correlations between the extent of hippocampal lesions and the severity of emotional and neuroendocrine changes is surprising, especially given that bilateral hippocampal volume reduction in one case (Neo-Hibo-6) was less than 20%. However, in this case, the damage was mostly restricted to the anterior portion of the hippocampus, an area of the hippocampus strongly interconnected with the amygdala (Amaral, et al., 1992; Fudge et al, 2012) and implicated in anxiety and anxiety-like behaviors (Fanselow & Dong, 2010; Cha, et al, 2016; Poppenk, et al, 2013; Kober, et al, 2008). Furthermore, the lack of significant reduction in hippocampal volume as estimated with neuroimaging technique does not necessarily indicate that the remaining hippocampus is fully functional. Injections of ibotenic acid may not have resulted in cell death but could have disrupted the cellular connectional system of the hippocampus. Further post-mortem histological and pathological investigations of remaining hippocampal tissue in all cases will provide more in depth assessment of extent of lesion and hippocampal functionality.
The present results have important clinical significance considering that prominence of altered hippocampal structure and function, memory impairment, emotional dysregulation, and altered HPA axis functioning in many developmental neuropsychiatric disorders (e.g. anxiety disorders, autism spectrum disorders, and schizophrenia). Reduced hippocampal volume and lower cortisol secretions are common findings in patients with post-traumatic stress disorder (PTSD; Daskalakis, et al, 2013; O'Doherty, et al, 2015). In fact, recent studies have demonstrated that lower left hippocampal volume (van Rooij, et al, 2015) and lower cortisol response to a stressor (Steudte-Schmiedgen, et al, 2015) are risk factors for PTSD. Similarly, deficits in episodic memory and decreased hippocampal volume key findings in first episode and during the course of schizophrenia (Falkai, et al, 2012). Interestingly, more recent studies have also reported blunted cortisol awakening response in first-episode psychosis, which do not appear related to increased exposure to psychosocial stressors (Mondelli, et al., 2010; Aas, et al., 2011). Thus, lasting changes in emotional behavior, HPA axis functioning, and memory deficits reported here following early hippocampal insult, are strikingly similar to those reported in clinical populations. Accordingly, developmental animal models examining the neural circuitry subserving the expression and regulation of emotions can provide a foundation for understanding the neuroanatomical and neuropathological basis of human mood and anxiety disorders.
6.1 Conclusions
The current study demonstrates that an intact hippocampus is essential during development for acquiring normal emotional behavior expression and HPA axis functioning. Early hippocampal damage results in prolonged alterations of emotions (i.e. increased anxiety-like behaviors) and dampens both the cortisol basal rhythm and stress response. These data suggest that the hippocampus plays a larger role beyond that of inhibitory/negative feedback regulation of the HPA axis. The behavioral and neuroendocrine changes after neonatal hippocampal damage are reminiscent to those seen after adult-onset lesions suggesting little functional compensation after damage early in life.
Supplementary Material
Highlights.
Early hippocampal lesions cause increased anxiety-like and self-directed behaviors
Despite increased anxiety-like behaviors, monkey had a blunted cortisol stress response
Data is similar to adult-onset hippocampal damage in humans and monkeys
Implication for mood and psychiatric disorders with altered hippocampal development
Acknowledgements
Authors are grateful to Jairus O'Malley, Tammy Humbird, Keith Kline, PhD, Christopher Machado, PhD, and Alyson Zeamer, PhD for their assistance with data collection and coding. Additional thanks goes to Sarah Pruett, PhD in the Yerkes BioMarker Core Laboratory for the use of equipment and assistance with the hormone assays. This research was supported by the National Institute for Mental Health (MH58846), National Institute for Child Health and Development (HD35471), Integrated Training in Psychobiology and Psychopathology Fellowship (NIMH T32 MH732525), National Science Foundation (NSF IBN9876754), and the Yerkes National Primate Research Center is supported by the National Institutes of Health, Office of Research Infrastructure Programs (ORIP/OD P51-OD011132).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aas M, Dazzan P, Mondelli V, Toulopoulou T, Reichenberg A, Di Forti M, Fisher HI, et al. Abnormal cortisol awakening response predicts worse cognitive function in patients with first-episode psychosis. Psychol Med. 2011;41:463–476. doi: 10.1017/S0033291710001170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado M, Bachevalier J. Selective neurotoxic damage to the hippocampal formation impairs acquisition and performance of the transverse patterning task and location memory in rhesus macaques. Hippocampus. 2005;15:118–131. doi: 10.1002/hipo.20037. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL, Pitkanen A, Carmichael ST. Anatomical organization of the primate amygdaloid complex. In: Aggleton JP, editor. The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. John Wiley & Sons; 1992. pp. 1–66. [Google Scholar]
- Andersen P, Morris R, Amaral D, Bliss T, O'Keefe J. The hippocampus book. Oxford University Press; New York, NY: 2007. [Google Scholar]
- Bachevalier J, Alvarado MC, Malkova L. Memory and socioemotional behavior in monkeys after hippocampal damage incurred in infancy or in adulthood. Biol Psychi. 1999;46:329–339. doi: 10.1016/s0006-3223(99)00123-7. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Nemanic S. Spatial memory in monkeys as measured with the visual paired-comparison task: effects of selective hippocampal, perirhinal and areas TH/TF lesions. Hippocampus. 2008;18:64–80. doi: 10.1002/hipo.20369. [DOI] [PubMed] [Google Scholar]
- Bauman MD, Lavenex P, Mason WA, Capitanio JP, Amaral DG. The development of social behavior following neonatal amygdala lesions in rhesus monkeys. J Cogn Neurosci. 2004;168:1388–1411. doi: 10.1162/0898929042304741. [DOI] [PubMed] [Google Scholar]
- Bauman MD, Toscano JE, Babineau BA, Mason WA, Amaral DG. Emergence of stereotypies in juvenile monkeys (Macaca mulatta) with neonatal amygdala or hippocampus lesions. Behav Neurosci. 2008;122:1005–1015. doi: 10.1037/a0012600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker A, Grecksch G, Bernstein HG, Holt V, Bogerts B. Social behavior in rats lesioned with ibotenic acid in the hippocampus: Quantitative and qualitative analysis. Psychopharmacol. 1999;144:333–338. doi: 10.1007/s002130051015. [DOI] [PubMed] [Google Scholar]
- Blank MS, Gordon TP, Wilson ME. Effects of venipuncture on serum levels of prolactin, growth hormones, and cortisol in outdoor compound-housed female rhesus monkeys. Acta Endocrinol. 1983;102:190–195. doi: 10.1530/acta.0.1020190. [DOI] [PubMed] [Google Scholar]
- Bliss-Moreau E, Toscano JE, Bauman MD, Mason WA, Amaral DG. Neonatal amygdala or hippocampus lesions influence responsiveness to objects. Dev Psychobiol. 2010;52:487–503. doi: 10.1002/dev.20451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss-Moreau E, Toscano JE, Bauman MD, Mason WA, Amaral DG. Neonatal amygdala lesions alter responsiveness to objects in juvenile macaques. Neurosci. 2011a;178:123–132. doi: 10.1016/j.neuroscience.2010.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss-Moreau E, Bauman MD, Amaral DG. Neonatal amygdala lesions result in globally blunted affect in adult rhesus macaques. Behav Neurosci. 2011b;125:848–858. doi: 10.1037/a0025757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss-Moreau E, Moadab G, Bauman MD, Amaral DG. The impact of early amygdala damage on juvenile rhesus macaque social behavior. J Cogn Neurosci. 2013;25:2124–2140. doi: 10.1162/jocn_a_00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blue SN, Kazama AM, Bachevalier J. Development of memory for spatial locations and object/place associations in infant rhesus macaques with and without neonatal hippocampal lesions. J Int Neuropsychol Sci. 2013;19:1053–64. doi: 10.1017/S1355617713000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan TW, Kern S, Allen JS, Tranel D, Kirschbaum C. Circadian regulation of cortisol after hippocampal damage in humans. Bio Psychi. 2004;56:651–656. doi: 10.1016/j.biopsych.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Buchanan TW, Tranel D, Kirschbaum C. Hippocampal damage abolishes the cortisol response to psychosocial stress in humans. Horm Behav. 2009;56:44–50. doi: 10.1016/j.yhbeh.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbacher TM, Grant KS, Worlein J, Ha J, Curnow E, Juul S, Sackett GP. Four decades of leading-edge research in the reproductive and developmental sciences: the Infant Primate Research Laboratory at the University of Washington National Primate Research Center. Am J Primatol. 2013;75:1063–1083. doi: 10.1002/ajp.22175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha J, Greenberg T, Song I, Simpson HB, Posner J, Mujica-Parodi LR. Abnormal hippocampal structure and function in clinical anxiety and comorbid depression. Hippocampus. 2016;26:545–553. doi: 10.1002/hipo.22566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Izquierdo A, Murray EA. Distinct contributions of the amygdala and hippocampus to fear expression. Euro J Neurosci. 2009;30:2327–2337. doi: 10.1111/j.1460-9568.2009.07012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Wright KS, Murray EA. Hippocampal lesions in rhesus monkeys disrupt emotional responses but not reinforce devaluation effects. Biol Psychi. 2008;63:1084–1091. doi: 10.1016/j.biopsych.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Daskalakis NP, Lehrner A, Yehuda R. Endocrine aspects of post-traumatic stress disorder and implications for diagnosis and treatment. Endocrinol Metab Clin N Am. 2013;42:503–513. doi: 10.1016/j.ecl.2013.05.004. [DOI] [PubMed] [Google Scholar]
- de Kloet ER. Stress and the hippocampus. In: Thorsten Bartsch, editor. The Clinical Neurobiology of the Hippocampus: An Integrative View. Oxford University Press; Oxford, UK.: 2012. pp. 77–104. [Google Scholar]
- Falkai P, Gruber O, Schmitt A. Schizophrenia. In: Bartsch T, editor. The clinical neurobiology of the hippocampus. Oxford University Press; Oxford, UK: 2012. pp. 288–296. [Google Scholar]
- Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischette CT, Komisaruk BR, Edinger HM, Feder HH, Siegel A. Differential fornix ablations and the circadian rhythmicity of adrenal corticosteroid secretion. Brain Res. 1980;195:373–387. doi: 10.1016/0006-8993(80)90073-6. [DOI] [PubMed] [Google Scholar]
- Fox AS, Shelton SE, Oakes TR, Davidson RJ, Kalin NH. Trait-like brain activity during adolescence predicts anxious temperament in primates. PLoS One. 2008;3:e2570. doi: 10.1371/journal.pone.0002570. doi:10.1371/journal.pone.0002570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudge JL, Decamp DM, Becoats KT. Revisiting the hippocampal-amygdala pathway in primates: association with immature-appearing neurons. Neuroscience. 2012;212:104–119. doi: 10.1016/j.neuroscience.2012.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glavis-Bloom C, Alvarado MC, Bachevalier J. Neonatal hippocampal damage impairs specific food/place associations in adult macaques. Behav Neurosci. 2013;127:9–22. doi: 10.1037/a0031498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goursaud A-PS, Bachevalier J. Social attachment in juvenile monkeys with neonatal lesion of the hippocampus, amygdala, and orbital frontal cortex. Behav Brain Res. 2007;176:75–93. doi: 10.1016/j.bbr.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Goursaud A-PS, Mendoza SP, Capitanio JP. Do neonatal bilateral ibotenic acid lesions of the hippocampal formation or of the amygdala impair HPA axis responsiveness and regulation in infant rhesus macaques (Macaca mulatta)? Brain Res. 2006;1071:97–104. doi: 10.1016/j.brainres.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Gray JA, McNaughton N. The Neuropsychology of Anxiety. 2nd Ed Oxford University Press; New York, NY: 2000. [Google Scholar]
- Heinrichs SC, Koob GF. Corticotropin-releasing factor in brain: a role in activation, arousal, and affect regulation. J Pharmacol Exp Ther. 2004;311:427–440. doi: 10.1124/jpet.103.052092. [DOI] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front. Neuroendocrinol. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Heuer E, Bachevalier J. Effects of selective neonatal hippocampal lesions on tests of object and spatial recognition memory in monkeys. Behav Neurosci. 2011a;125:137–49. doi: 10.1037/a0022539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer E, Bachevalier J. Neonatal hippocampal lesions in rhesus macaques alter the monitoring, but not maintenance, of information in working memory. Behav Neurosci. 2011b;125:859–70. doi: 10.1037/a0025541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer E, Bachevalier J. Working memory for temporal order is impaired after selective neonatal hippocampal lesions in adult rhesus macaques. Behav Brain Res. 2013;239:55–62. doi: 10.1016/j.bbr.2012.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsaker MR, Scott JA, Bauman MD, Schumann CM, Amaral DG. Postnatal development of the hippocampus in rhesus macaque (Macaca mulatta): A longitudinal magnetic resonance imaging study. Hippocampus. 2014;24:794–807. doi: 10.1002/hipo.22271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn AL, Fox AS, Abercrombie HC, Shelton SE, Oakes TR, Davidson RJ, Kalin NH. Subgenual prefrontal cortex activity predicts individual differences in hypothalamic-pituitary-adrenal activity across different contexts. Biol Psychi. 2010;67:175–181. doi: 10.1016/j.biopsych.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järlested K, Atkins AL, Hagberg H, Pekna M, Mallard C. Trace fear conditioning detects hypoxicischemic brain injury in neonatal mice. Dev Neurosci. 2011;33:222–230. doi: 10.1159/000329710. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ, Kelley AE. The primate amygdala mediates acute fear but not the behavioral and physiological components of anxious temperament. J Neurosci. 2001;21:2067–2074. doi: 10.1523/JNEUROSCI.21-06-02067.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin N, Shelton SE, Takahashi LK. Defensive behaviors in infant rhesus monkeys: Ontogeny and context-dependent selective expression. Child Dev. 1991;62:1175–1183. [PubMed] [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: A meta-analysis of neuroimaging studies. NeuroImage. 2008;42:998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. Behavioral and hormonal reactivity to threat: Effects of selective amygdala, hippocampal or orbital frontal lesions in monkeys. Psychoneuroendocrinol. 2008;33:926–941. doi: 10.1016/j.psyneuen.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y, Hu X, Bachevalier J, Zhang X. Decreased functional connectivity in dorsolateral prefrontal cortical networks in adult macaques with neonatal hippocampal lesions: Relations to visual working memory deficits. Neurobiol Learn Mem. 2016 doi: 10.1016/j.nlm.2016.04.003. online ahead of press. doi: 10.1016/j.nlm.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y, Payne C, Li L, Hu X, Zhang X, Bachevalier J. Alterations of hippocampal projections in adult macaques with neonatal hippocampal lesions: a diffusion tensor imaging study. Neuroimage. 2014;102(Pt 2):828–37. doi: 10.1016/j.neuroimage.2014.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moadab G, Bliss-Moreau E, Amaral DG. Adult social behavior with familiar partners following neonatal amygdala and hippocampus damage. Behav Neurosci. 2015;129:339–350. doi: 10.1037/bne0000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondelli V, Dazzan P, Hepgul N, Di Forti M, Aas M, D'Albenzio A, Di Nicola M, et al. Abnormal cortisol levels during the day and cortisol awakening response in first-episode psychosis: The role of stress and of antipsychotic treatment. Schizophr Res. 2010;116:234–242. doi: 10.1016/j.schres.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemanic S, Alvarado MC, Price RE, Jackson EF, Bachevalier J. Assessment of locus and extent of neurotoxic lesions in monkeys using neuroimaging techniques: A replication. J Neurosci Meth. 2002;121:199–209. doi: 10.1016/s0165-0270(02)00264-9. [DOI] [PubMed] [Google Scholar]
- Nemanic S, Alvarado M, Bachevalier J. The hippocampal/parahippocampal regions and recognition memory: insights from visual paired-comparison versus object delayed nonmatching in monkeys. J Neurosci. 2004;24:2013–2026. doi: 10.1523/JNEUROSCI.3763-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninan PT, Insel TM, Cohen RM, Cook JM, Skolnick P, Paul SM. Benzodiazepene receptor-mediated experimental ‘anxiety’ in primates. Science. 1982;218:1332–1334. doi: 10.1126/science.6293059. [DOI] [PubMed] [Google Scholar]
- O'Doherty CM, Chitty KM, Saddiqui S, Bennett MR, Lagopoulos J. A systematic review and meta-analysis of magnetic resonance imaging measurement of structural volumes in posttraumatic stress disorder. Psychiatry Research: Neuroimaging. 2015;232:1–33. doi: 10.1016/j.pscychresns.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Oler JA, Fox AS, Shelton SE, Rogers J, Dyer TD, Davidson RJ, Shelledy W, Oakes TR, Blangero J, Kalin NH. Amygdalar and hippocampal substrates of anxious temperament differ in their heritability. Nature. 2010;466:864–868. doi: 10.1038/nature09282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA. Human emotion and memory: interactions of the amygdala and hippocampal complex. Curr Opin Neurobiol. 2004;14:198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Poppenk J, Evensmoen HR, Moscovitch M, Nadel L. Long-axis specialization of the human hippocampus. Trends Cogn Sci. 2013;17:230–240. doi: 10.1016/j.tics.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Raper J, Wilson ME, Sanchez M, Machado C, Bachevalier J. Pervasive alterations of emotional and neuroendocrine responses to an acute stressor after neonatal amygdala lesions in rhesus monkeys. Psychoneuroendocrinol. 2013;38:1021–1035. doi: 10.1016/j.psyneuen.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommeck I, Capitanio JP, Strand SC, McCowan B. Early social experience affects behavioral and physiological responsiveness to stressful conditions in infant rhesus macaques (Macaca mulatta). Am J Primatol. 2011;73:692–701. doi: 10.1002/ajp.20953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez MM, Hearn EF, Do D, Rilling JK, Herndon JG. Differential rearing affects corpus callosum size and cognitive function of rhesus monkeys. Brain Res. 1998;812:38–49. doi: 10.1016/s0006-8993(98)00857-9. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Zola-Morgan S, Squire LR. Inhibition of glucocorticoid secretion by the hippocampal formation in the primate. J Neurosci. 1991;11:3695–3704. doi: 10.1523/JNEUROSCI.11-12-03695.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Fox AS, Oler JA, Shelton SE, Davidson RJ, Kalin NH. Neural mechanisms underlying heterogeneity in the presentation of anxious temperament. Proc Natl Acad Sci USA. 2013;110:6145–6150. doi: 10.1073/pnas.1214364110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steudte-Schmiedgen S, Stalder T, Schonfeld S, Wittchen H-U, Trautmann S, Alexander N, Miller R, Kirschbaum C. Hair cortisol concentrations and cortisol stress reactivity predict PTSD symptom increase after trauma exposure during military deployment. Psychoneuroendocrinol. 2015;59:123–133. doi: 10.1016/j.psyneuen.2015.05.007. [DOI] [PubMed] [Google Scholar]
- Tuvnes FA, Steffenach H-A, Murison R, Moser M-B, Moser EI. Selective hippocampal lesions do not increase adrenocortical activity. J Neurosci. 2003;23:4345–4354. doi: 10.1523/JNEUROSCI.23-10-04345.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooij SJH, Kennis M, Sjouwerman R, van den Heuvel MP, Kahn RS, Geuze E. Smaller hippocampal volume as a vulnerability factor for persistence of post-traumatic stress disorder. Psychol Med. 2015;45:2737–2746. doi: 10.1017/S0033291715000707. [DOI] [PubMed] [Google Scholar]
- Wang ME, Fraize NP, Yin L, Yuan RK, Petsagourakis D, Wann EG, Muzzio IA. Differential roles of the dorsal and ventral hippocampus in predator odor contextual fear conditioning. Hippocampus. 2013;23:451–466. doi: 10.1002/hipo.22105. [DOI] [PubMed] [Google Scholar]
- Wolf OT, Fujiwara E, Luwinski G, Kirschbaum C, Markowitsch HJ. No morning cortisol response in patients with severe global amnesia. Psychoneuroendocrinol. 2005;30:101–105. doi: 10.1016/j.psyneuen.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Zeamer A, Heuer E, Bachevalier J. Developmental trajectory of object recognition memory in infant rhesus macaques with and without neonatal hippocampal lesions. J Neurosci. 2010;30:9157–9165. doi: 10.1523/JNEUROSCI.0022-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeamer A, Bachevalier J. Long-term effects of neonatal hippocampal lesions on novelty preference in monkeys. Hippocampus. 2013;23:745–50. doi: 10.1002/hipo.22139. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.