Not all children with prenatal alcohol exposure exhibit cognitive and behavioral impairments. Given that early intervention can be more effective and cost-efficient than later treatment of developmental disabilities, it is critical to identify early markers indicating which exposed children are impaired. Information regarding the amount of alcohol the mother consumed during pregnancy is not sufficient to identify affected children because two individuals with the same exposure can differ markedly in degree of impairment depending on timing and pattern of drinking during pregnancy, maternal diet, and genetic vulnerability. Moreover, not all affected children exhibit the facial dysmorphology that characterizes fetal alcohol syndrome (FAS) and partial FAS, and this dysmorphology is particularly difficult to diagnose during infancy. The findings reported by Kable, Coles, and colleagues in this issue of Alcoholism: Clinical and Experimental Research regarding the sensitivity of the cardiac orienting (OR) response to prenatal alcohol exposure contribute new information addressing this important issue and provide support for a promising new approach to early identification of adverse effects in fetal alcohol spectrum disorders (FASD).
Because cognitive test performance is highly variable until 7–8 years of age, the utility of infant markers for identifying cognitive impairment can best be assessed in terms of their predictive validity for IQ in childhood. Prior to the 1970s, it was assumed that infant assessments could be used to predict later IQ. However, the conventional standardized tests of cognitive function in infancy, such as the Bayley Scales of Infant Development, have poor predictive validity until after 18 months of age, ranging from r = 0.04 to 0.29 (Kavsek, 2004). Conventional infant tests rely heavily on sensorimotor manipulation of objects to assess cognition and on the age at which the infant reaches standard developmental milestones. Beginning in the 1980s a new generation of assessments emerged, which focus on narrow-band measures of infant performance in cognitive domains that are already mature during infancy, including visual fixation, eye tracking, and the neurophysiological cardiac OR paradigm proposed by Kable et al. Visual fixation patterns have been shown to provide an assessment of early cognitive function with substantially better predictive validity than conventional infant tests (Kavsek, 2004). In a comprehensive meta-analysis of the relation of performance on these paradigms to childhood and adolescent IQ, predictive validity averaged r = 0.53.
The visual fixation measures, which also demonstrated impressive sensitivity to prenatal alcohol exposure, generated developmental findings similar to those assessed in the cardiac OR studies. One of the first such measures, the Fagan Test of Infant Intelligence (FTII), is a visual fixation paradigm that has been used in fetal alcohol and environmental contaminant research to examine memory and processing speed in exposed infants. Novelty preference (measured as proportion of looking time to a novel rather than a familiarized stimulus) provides an index of recognition memory. Duration of visual fixations to the stimuli provides a measure of information processing speed (Colombo and Mitchell, 1990); shorter fixations, which indicate faster information processing, predict better performance on childhood IQ. The FTII was used in studies of two prospective longitudinal cohorts with prenatal alcohol exposure, one a moderate-to-heavy exposed inner city Detroit cohort; the other, conducted in Cape Town, South Africa, where the incidence of heavy prenatal alcohol exposure and FAS are among the highest in the world. In Detroit, prenatal alcohol exposure was related to slower processing speed at 6.5 and 12 months but not to recognition memory (Jacobson et al., 1993) and to markedly slower reaction time on an eye tracking paradigm at 6.5 months (Jacobson et al., 1994). The slower processing speed findings were confirmed in the Cape Town cohort at both ages, but at 12 months FTII recognition memory was also impaired in this more heavily exposed cohort (Jacobson et al., 2002). The latter deficit had not been seen at the lower levels of exposure in Detroit, possibly due to the relatively few infants with full FAS. These measures also both demonstrated discriminant validity; they were sensitive to prenatal alcohol exposure but not affected by exposure to smoking, and exposure to cocaine during pregnancy was associated with faster processing speed but poorer recognition memory (Jacobson et al., 1993).
Kable and Coles (2004) have pioneered the use of cardiac OR during a visual habituation-dishabituation task as another early indicator of impairment in FASD. This paradigm is particularly promising because ORs have been shown to be sensitive to prenatal alcohol exposure in animal models and also linked to frontal cortical function in electrical stimulation studies in animals, suggesting that they may provide a precursor of executive function in childhood. Like the FTII, the OR paradigm measures both cognition (sustained attention, indicated by magnitude of response, i.e., maximum cardiac deceleration), and processing speed (how quickly the infant initiates a decelerative response, i.e., ≥2 beats/min deceleration from baseline). At 6 months, alcohol-exposed infants from Kable and Coles’ (2004) Atlanta cohort responded more slowly than controls but did not display differences in cardiac deceleration, suggesting a deficit in processing speed but not attention. By contrast, in the new Ukraine cohort of 12- to 18-month-olds, prenatal alcohol was related to poorer sustained attention in both the habituation and dishabituation phases of the test but not to processing speed (Kable et al., this issue). To date, few data are available on the predictive validity of the cardiac OR measures for childhood cognitive function, although one study has demonstrated prediction of Stanford Binet IQ scores at 5 years (see Kable et al., this issue).
In the new Kable et al. study, pregnant women were invited to participate if they “reported at least weekly binge-drinking episodes (5+ drinks), at least five episodes of 3–4 standard drinks, or at least 10 episodes of 1–2 standard drinks either in the month around conception or the most recent month of pregnancy.” Very little information is available in the literature regarding the levels of prenatal alcohol exposure associated with an increased risk of clinically significant adverse effects. The few studies conducted to date provide clear evidence of adverse effects at exposure levels averaging at least 1 standard drink/day (≈ 0.5 oz absolute alcohol) in women who concentrate their drinking during the weekends (Jacobson and Jacobson, 1994), but there is little evidence of effects at lower levels, that is, in infants whose mothers drink < 3–4 drinks/occasion frequently during pregnancy. It seems highly likely that the inclusion of lower levels of exposure in this sample led to a reduction in the power to detect fetal alcohol effects and/or to an underestimate of the magnitude of the observed effects. In fact, as the authors note, in this sample relatively few women reported continuing to drink after learning that they had become pregnant, suggesting that these effects reflect exposure during early pregnancy, and the effects are most clearly observed in relation to whether or not the mothers binge drank during pregnancy.
Within the auditory domain, only 1 of 32 comparisons (3.1%) were significant at p <0.05, and only 3 (9.4%) were significant at p < 0.10. As the authors acknowledge, it seems likely that these findings are due to chance. By contrast, in the visual domain, although none of the effects on processing speed approached statistical significance, 37.5% of the effects on magnitude of response were significant at p < 0.05 and 50.0%, at p < 0.10, indicating a highly robust effect on attentional function in the visual domain using the cardiac OR measure at the later age. These effects may have been seen only in the visual domain because the visual stimuli (chromatic faces) were more engaging than the pure tones used for the auditory processing assessment. Alternatively, Coles et al. (2002) have suggested that the visual domain may be more sensitive to fetal alcohol-related deficits in attention than the auditory domain.
The finding of slower processing speed at 6 months on the cardiac OR measure is consistent with the visual fixation paradigm studies suggesting a robust effect of prenatal alcohol exposure on processing speed during the first year of life. The absence of an alcohol effect on processing speed on the cardiac OR measure at 12–18 months may, as the authors suggest, be attributable to increased myelination of the prefrontal cortical areas by the second year. Conversely, the effect on the cardiac OR attention measure (magnitude of maximum deceleration response) was not seen until the later age and then only in the visual domain. These data illustrate a phenomenon very familiar to developmental researchers, which Kagan (1971) termed “heterotypic continuity” and Slater (1995) called a “temporal window of opportunity”; namely, that the measures of cognitive ability that are most sensitive at one point in development do not necessarily continue to be the most sensitive at later times during infancy. For example, brain development during the second year may enhance visual processing of faces such that the cardiac OR attention measure is the one that reveals the fetal alcohol-related impairment most efficiently.
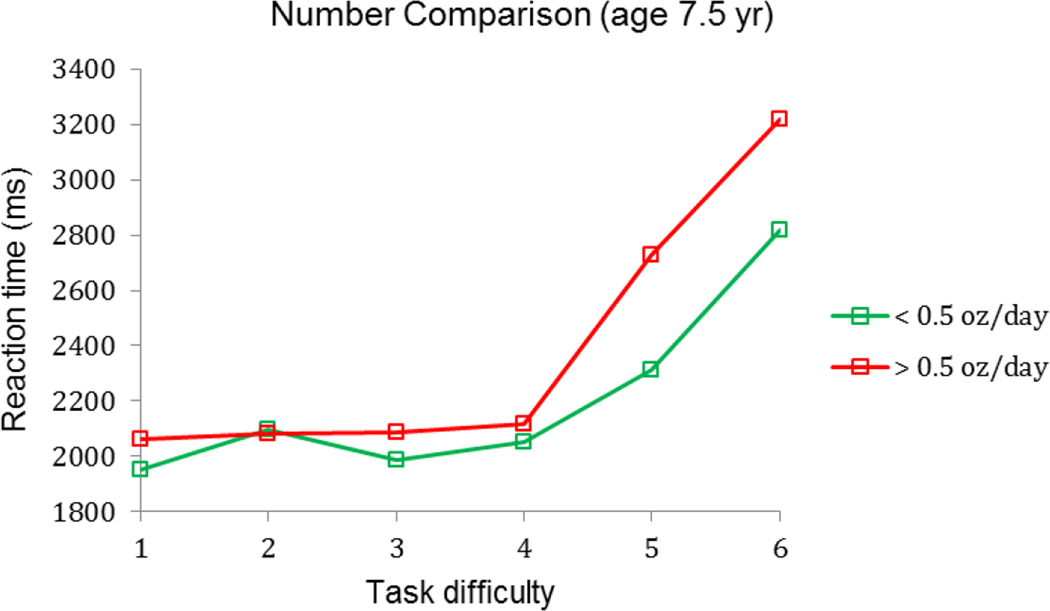
Although the cardiac OR paradigm no longer showed an alcohol effect on processing speed during the second year, studies of FASD in childhood and adolescence using more challenging assessment paradigms do provide ample evidence of long-term effects on processing speed. In the Seattle cohort, Streissguth et al. (1986) reported alcohol-related differences in processing speed on a continuous performance sustained attention task at 4 and 7 years. In Detroit at 7.5 years, reaction times on a Sternberg-type paradigm were unaffected by alcohol exposure for the easier problems in which numbers are farther apart in magnitude (e.g., 2 and 8) but were significantly slower once the task became more challenging (e.g., 4 and 5) in the moderate to heavy exposed group than controls, F(5,329) = 2.28, p < 0.05, presumably reflecting slower, less efficient information processing (Fig. 1; also see Burden et al., 2005).
Figure 1.
Relation of reaction time to magnitude comparison task difficulty, AA/day = oz absolute alcohol/day
Castellanos and Tannock (2002) have advocated going beyond descriptive symptom-based approaches to diagnosis to identify biomarkers with known underlying lesions or well-described neural circuitry. Consistent with their recommendation, a new generation of such indicators (see Kavsek, 2004) has emerged, including eyeblink conditioning, the cardiac OR paradigm used by Kable et al. and event-related potentials (ERP). The latter two are particularly well-suited for studying processing speed. By contrast to functional magnetic resonance imaging, which shows spatial patterns of regional brain activation during a cognitive task, ERP has excellent temporal resolution. In addition, ERP generates a series of wave forms, which can be linked to the distinct components of processing involved in a cognitive task. For example, one ERP study found evidence of slower processing speed in children with FASD in two components of a simple Go/NoGo response inhibition task—increased P2 latency, which has been shown to reflect slower identification and classification of stimuli, and increased N2 peak latency, which suggests that the exposed children are slower to distinguish between Go and NoGo stimuli (Burden et al. 2009). Like the cardiac OR measure, ERP data can be collected during infancy and are sensitive to prenatal alcohol exposure (Berger et al., 2015).
These behavioral and neurophysiological findings of slower cognitive processing speed are supported by a growing body of evidence of white matter deficits in fetal alcohol-affected individuals based on diffusion tensor imaging (DTI), which can also already be detected in infancy (Taylor et al., 2015). An impressively large number of DTI studies have documented fetal-alcohol impairment in white matter microstructural integrity (see review Wozniak and Muetzel, 2011), which might be expected to lead to slower cognitive processing speed. We used DTI to study the neural bases of the effect of prenatal alcohol exposure on eyeblink conditioning, a culturally neutral, classical conditioning paradigm that involves contingent temporal pairing of a conditioned stimulus (e.g., a tone) with an unconditioned stimulus (e.g., air puff to the eye). Eyeblink conditioning is particularly sensitive to prenatal alcohol exposure. At 5 years none of the children with FAS in our Cape Town cohort met criterion for conditioning, as contrasted with 75.0% of the controls; two-thirds of the other heavily exposed children also failed to meet criterion for conditioning (Jacobson et al. 2011). Whereas, after training, onset of the conditioned eyeblink for the controls occurred at or in anticipation of the air puff, eyeblink responses occurred late (after the onset of the air puff) for the FAS group and at or after the puff in the heavily exposed nonsyndromal children. DTI data from two different Cape Town samples has provided evidence that poorer white matter integrity in an important region of the cerebellum partially mediates the effect of prenatal alcohol exposure on eyeblink conditioning (Spottiswoode et al., 2011; Fan et al., 2015). The pattern of white matter deficits in both DTI studies is consistent with poorer myelination, suggesting an impairment in speed of neural transmission that is likely related to the slower cognitive processing seen in visual fixation and the cardiac OR task used by Kable et al. during the first year of life and in other cognitively challenging tasks, such as magnitude comparison, at school age.
The two studies that Kable, Coles, and colleagues have conducted to examine the effects of prenatal alcohol exposure on cardiac OR demonstrate the challenges involved in the identification of which infants with prenatal alcohol exposure are adversely affected. The slower processing speed findings at 6 months and attention deficits at 12–18 months illustrate the degree to which rapid brain maturation and developmental change during the early postpartum years make it difficult to predict the long-term consequences of a prenatal insult. Ultimately, the utility of the cardiac OR paradigm for early identification of affected children will depend on the degree to which one or both of the deficits it measures prove to be predictive of cognitive impairment in childhood. It is impressive that findings from this new generation of infant assessments can be used to assess degree to which deficits found in the U.S. also identify affected individuals in cross-cultural settings, such as in the Ukraine and South Africa. The cardiac OR data from the first year add to the growing body of evidence linking prenatal alcohol exposure to impairment in cognitive processing speed, including evidence of longer latencies in ERP studies and poorer microstructural integrity in numerous DTI studies. Given these findings, it is critically important to begin to target impairment in processing speed when designing interventions for FASD, especially for the most affected children—those with full or partial FAS.
Acknowledgments
Supported by grants R01-AA06966, R01-AA09524, U01-AA014790, R01-AA016781, R21-AA020515, R21-AA020037 from the National Institute on Alcohol Abuse and Alcoholism, and a grant from the Lycaki-Young, Fund from the State of Michigan.
Footnotes
The authors declare no conflict of interest.
References
- Berger A, Jacobson SW, Jacobson JL. Atypical numerosity processing: ERP evidence in infants with prenatal alcohol exposure. International workshop on Cognitive and Neural Basis for the Development of Numerical Understanding, Center for the Neurocognitive Basis of Numerical Cognition; April, 2015; Be’er Sheva, Israel. [Google Scholar]
- Burden MJ, Jacobson SW, Jacobson JL. Relation of prenatal alcohol exposure to cognitive processing speed and efficiency in childhood. Alcohol Clin Exp Res. 2005;29:1473–1483. doi: 10.1097/01.alc.0000175036.34076.a0. [DOI] [PubMed] [Google Scholar]
- Burden MJ, Andrew C, Saint- Amour D, Meintjes EM, Molteno CD, Hoyme HE, Robinson LK, Khaole N, Nelson CA, Jacobson JL, Jacobson SW. The effects of fetal alcohol syndrome on response execution and inhibition: an event- related potential study. Alcohol Clin Exp Res. 2009;33:1994–2004. doi: 10.1111/j.1530-0277.2009.01038.x. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nature Rev Neurosci. 2002;3:617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- Coles CD, Platzman KA, Lynch ME, Freides D. Auditory and visual sustained attention in adolescents prenatally exposed to alcohol. Alcohol Clin Exp Res. 2002;26:263–271. [PubMed] [Google Scholar]
- Colombo J, Mitchell DW. Individual differences in early visual attention: fixation time and information processing. In: Colombo J, Fagan J, editors. Individual Differences in Infancy: Reliability, Stability, Prediction. Hillsdale, NJ: Lawrence Erlbaum; 1990. pp. 193–227. [Google Scholar]
- Fan J, Meintjes EM, Molteno CD, Spottiswoode BS, Dodge NC, Alhamud AA, Stanton ME, Peterson BS, Jacobson JL, Jacobson SW. White matter integrity of the cerebellar peduncles as a mediator of effects of prenatal alcohol exposure on eyeblink conditioning. Hum Brain Mapp. 2015;36:2470–2482. doi: 10.1002/hbm.22785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW. Prenatal alcohol exposure and neurobehavioral development: where is the threshold? Alcohol Res Health. 1994;18:30–36. [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Sokol RJ, Martier SS, Ager JW. Prenatal alcohol exposure and infant information processing ability. Child Development. 1993;64:1706–1721. [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Sokol RJ. Effects of fetal alcohol exposure on infant reaction time. Alcohol Clin Exp Res. 1994;18:1125–1132. doi: 10.1111/j.1530-0277.1994.tb00092.x. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Hay A, Molteno C, Marais AS, Carter RC, September M, Chiodo LM, Wynn K, Jones KL, Khaole N, Viljoen D, Jacobson JL. FAS and neurobehavioral deficits in alcohol-exposed South African infants. Alcohol Clin Exp Res. 2002;26:175A. [Google Scholar]
- Jacobson SW, Stanton ME, Dodge NC, Pienaar M, Fuller DS, Molteno CD, Meintjes EM, Hoyme HE, Robinson LK, Khaole N, Jacobson JL. Impaired delay and trace eyeblink conditioning in school- age children with fetal alcohol syndrome. Alcohol Clin Exp Res. 2011;35:250–264. doi: 10.1111/j.1530-0277.2010.01341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JA, Coles CD. The impact of prenatal alcohol exposure on neurophysiological encoding of environmental events at six months. Alcohol Clin Exp Res. 2004;28:489–496. doi: 10.1097/01.alc.0000117837.66107.64. [DOI] [PubMed] [Google Scholar]
- Kagan J. Change and Continuity in Infancy. New York: Wiley; 1971. [Google Scholar]
- Kavšek M. Predicting later IQ from infant visual habituation and dishabituation: A meta-analysis. J Applied Dev Psychol. 2004;25:369–393. [Google Scholar]
- Slater A. Individual differences in infancy and later IQ. J Child Psychol Psychiatr Allied Disciplines. 1995;36:69–112. doi: 10.1111/j.1469-7610.1995.tb01656.x. [DOI] [PubMed] [Google Scholar]
- Spottiswoode BS, Meintjes EM, Anderson AW, Molteno CD, Stanton ME, Dodge NC, Gore JC, Peterson BS, Jacobson JL, Jacobson SW. Diffusion tensor imaging of the cerebellum and eyeblink conditioning in fetal alcohol spectrum disorder. Alcohol Clin Exp Res. 2011;35:2174–2183. doi: 10.1111/j.1530-0277.2011.01566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streissguth AP, Barr HM, Sampson PD, Parrish-Johnson JC, Kirchner GL, Martin DC. Attention, distraction and reaction time at age 7 years and prenatal alcohol exposure. Neurotoxicol Teratol. 1986;8:717–725. [PubMed] [Google Scholar]
- Taylor PA, Jacobson SW, van der Kouwe A, Molteno CD, Chen G, Wintermark P, Alhamud A, Jacobson JL, Meintjes EM. A DTI- based tractography study of effects on brain structure associated with prenatal alcohol exposure in newborns. Hum Brain Mapp. 2015;36:170–186. doi: 10.1002/hbm.22620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak JR, Muetzel RL. What does diffusion tensor imaging reveal about the brain and cognition in fetal alcohol spectrum disorders? Neuropsychol Rev. 2011;21:133–147. doi: 10.1007/s11065-011-9162-1. [DOI] [PubMed] [Google Scholar]