Abstract
Objectives
Elevated serum phosphate levels have been associated with increased risks of cardiovascular events and death in several patient populations. The effects of serum phosphate on outcomes in patients with critical limb ischemia (CLI) have not been evaluated. In this study, we assessed the effect of abnormal phosphate levels on mortality and major limb events following surgical intervention for critical limb ischemia (CLI).
Methods
A retrospective review was undertaken to identify all patients at a single institution who underwent a first-time open or endovascular intervention for CLI between 2005 and 2014. Patients without recorded post-operative phosphate levels were excluded. Post-operative phosphate levels within 30-days of the initial operation were recorded and the mean was calculated. Patients were stratified according to mean phosphate levels (low <2.5, normal 2.5–4.5, high >4.5). Patient demographics, comorbidities, and operative details were compared in univariate analysis. Multivariable regression and cox-proportional hazard modeling were utilized to account for patient demographics and comorbid conditions.
Results
941 patients were identified including 42(5%) with low phosphate, 768(82%) with normal phosphate, and 131(14%) with high phosphate. Patients with elevated phosphate were younger and had higher rates of congestive heart failure, diabetes, and dialysis dependence. Bypass was more common among patients with normal phosphate as compared to high or low phosphate levels. There was no difference in WiFi or TASC classification between cohorts. There were significant differences in 1-year mortality (low: 19%, normal: 17%, high: 33%, p < .01) and 3-year mortality (low 38%, normal: 34%, high: 56%, p <.01) between phosphate cohorts. Major amputation (low: 12%, normal: 12%, high: 15%) and restenosis (low: 21%, normal: 24%, high: 28%) tended toward worse outcomes among patients with elevated phosphate levels, but did not reach statistical significance. After adjustment for baseline characteristics, mortality was higher (HR: 1.7, 95% CI: 1.3–2.2) and amputation free survival was lower (HR: 1.5, 95% CI: 1.2–1.9) among patients with elevated as compared with normal phosphate levels. A subgroup analysis was then performed to assess dialysis and non-dialysis patients separately. Patients with elevated serum phosphate levels maintained a significantly higher risk of mortality in each group (Dialysis HR: 1.8 95% CI 1.2–2.6, Non-dialysis: HR 1.5, 95% CI 1.04–2.10).
Conclusion
Elevated phosphate levels are associated with increased mortality and decreased amputation free survival following interventions for critical limb ischemia. Future studies evaluating the effects of phosphate reduction in patients with CLI are warranted.
Introduction
Patients undergoing intervention for critical limb ischemia (CLI) are at increased risk of cardiovascular events due in part to the high incidence of associated cardiovascular risk factors.1,2 Associations between ambulatory status, cardiac and renal disease, and amputation free survival have been identified previously in retrospective studies.3,4 Despite this, our ability to determine which patients are at increased risk for early and delayed limb and cardiovascular events as well as mortality remains challenging.
Elevated serum phosphate levels have been associated with increased morbidity and mortality in patients with coronary disease, chronic kidney disease, and within the general population.5–8 Among patients with a prior myocardial infarction, increased levels are associated with adverse events including new heart failure, myocardial infarction, and death.6 Similarly, in studies of the general population without cardiac or renal dysfunction, including the Framingham offspring study, elevated serum phosphate levels were associated with increased risk of cardiovascular disease including myocardial infarction, stroke, peripheral vascular disease and congestive heart failure.8
Despite growing evidence on the adverse effects associated with hyperphosphatemia, the effect of abnormal phosphate levels on patients undergoing vascular interventions have not been previously studied. We therefore sought to evaluate the effect of abnormal post-operative phosphate levels on perioperative outcomes and long-term survival in patients undergoing intervention for CLI.
Methods
Subject
All patients undergoing a first-time endovascular or open intervention for critical limb ischemia, defined as rest pain or tissue loss, at a single institution between 2005 and 2014 were identified using operative notes and billing data. Patients’ electronic medical records were reviewed to confirm the procedures performed. Patients who had undergone previous intervention for peripheral artery disease, those patients without a diagnosis of CLI, and those without documented post-operative phosphate levels were excluded from analysis. The Institutional Review Board of Beth Israel Deaconess Medical Center approved this study and waived the need for informed consent due to the retrospective study design.
Phosphate Measurement
Phosphate levels were obtained as part of routine care on admitted post-operative vascular patients because they are included in the basic metabolic panel. Pre-operative values were not used for analysis due to missing data (20%).Phosphate and calcium measurements obtained during the 30-day post-operative period were recorded, and a mean serum phosphate level was calculated from the first 3 phosphate levels collected for each patient. Phosphate levels were normally distributed and therefore mean values were used. Phosphate levels were then stratified as low (less than 2.5), normal (2.5–4.5), and high (greater than 4.5) as suggested by Food and Drug Administration guidelines and prior studies on serum phosphate.9,10, 11 Additionally, these values are similar to the National Kidney Foundation’s target serum phosphate levels for patients with chronic kidney disease of 2.7–4.6 mg/DL.12
Variable Definitions
Restenosis was defined as a peak systolic velocity ratio >3.5 in surveillance post-procedural ultrasound or documentation in physician progress notes. Physician progress notes were included in an effort to include patients who had their follow-up imaging at an outside institution. Reintervention was defined as either ipsilateral bypass or angioplasty after the initial procedure. Major amputation was defined as any ipsilateral amputation above the ankle. Mortality data were obtained from the Social Security Death Index. Data on wound infections were obtained from hospital discharge summaries and follow up clinic physician notes. A hypercoagulable state was defined as current cancer or documented coagulation disorder. The Society for Vascular Surgery’s Lower Extremity Threatened Limb Classification System (Wound, Ischemia, and foot Infection [WIfI]) was utilized to compare disease extent in each patient’s treated limb.13 Lesion anatomy was defined according to the modified Trans Atlantic Inter-society Consensus (TASC) classification described by Dormandy and Rutherford.14 Standard follow up included duplex imaging at 3–4 month intervals for the first year following intervention.
Statistics
Patient demographics, comorbid conditions, and operative details were compared across the 3 phosphate groups. Missing data were encountered in less than 3% of all patients for most variables studied. Only wound infection (13% missing) and TASC classification (11% missing) exceeded the 3% threshold. All statistical analyses were performed using Stata 12.0 (StataCorp, College Station, Texas). The Chi square statistic was used for comparisons of categorical variables. Normality of distribution was assessed and continuous variables were compared using Student t-test or Kruskal Wallis testing as appropriate. Kaplan Meier analysis was used to evaluate trends in mortality and amputation over time. Purposeful selection was utilized to identify covariates for inclusion in our multivariable model, which includes both variables identified on univariate analysis with P < 0.1 for each endpoint of interest, as well as clinically relevant factors shown to be predictive of adverse events in previous studies.15 Logistic regression and Cox modeling were utilized for multivariable analysis. For adjusted time-to-event analysis we used Cox proportional hazards models after visual inspection of the log-log plot to make sure the baseline hazards assumption held for each adverse event studied in this way. The Hosmer Lemshow Test was used to confirm goodness of fit for each multivariable model, and normal phosphate levels were utilized as the referent group. A p-value of less than 0.05 was considered significant.
Results
Baseline Characteristics
From a total of 1052 patients who underwent a first-time intervention for critical limb ischemia, 941(89%) had post-operative serum phosphate levels. These included 42 (5%) with low, 768 (82%) with normal, and 131 (14%) with high phosphate levels. There were significant differences in mean age (Low: 70, Normal: 72, High: 66, P < .01) diabetes (Low: 66%, Normal: 74%, High: 85%, P = 0.01), congestive heart failure (Low: 30%, Normal: 30%, High: 42%, P = 0.02), and dialysis (Low: 28% Normal: 15% High: 55%, P < .01). The proportion treated with bypass also varied (Low: 45%, Normal: 56%, High: 42%, P = 0.01). However, neither mean WIfI score nor TASC classification were different between the 3 phosphate level groups (Table I). There were no differences in median follow up time (26 months) identified between groups.
Table I.
Patient Demographics, Co-morbidities, and Operative Details (Univariate)
| Number (%) | Low Phosphate < 2.5 N = 42 |
Normal Phosphate 2.5–4.5 N = 768 |
High Phosphate > 4.5 N = 131 |
P-Value |
|---|---|---|---|---|
| White race | 32 (76) | 610 (80) | 93 (71) | 0.07 |
| Age –mean (SD) | 70 (14) | 72 (12) | 68 (13) | <.01 |
| Male Gender | 28 (67) | 458 (60) | 73 (56) | 0.43 |
| Coronary Artery Disease | 18 (45) | 387 (51) | 74 (58) | 0.29 |
| Congestive Heart Failure | 12 (30) | 224 (30) | 54 (42) | 0.02 |
| Recent history of MI | 14 (34) | 202 (27) | 38 (30) | 0.50 |
| COPD | 5 (12) | 93 (12) | 16 (12) | 1.00 |
| Dialysis | 11 (28) | 113 (15) | 72 (55) | <.01 |
| Hypertension | 37 (90) | 637 (84) | 117 (89) | 0.16 |
| Diabetes | 27 (66) | 559 (74) | 111 (85) | 0.01 |
| Hyperlipidemia | 22 (54) | 451 (59) | 81 (62) | 0.61 |
| Hypercoagulable State | 7 (18) | 145 (19) | 27 (21) | 0.87 |
| History of Tobacco Use | 23 (58) | 469 (62) | 74 (58) | 0.62 |
| Bypass | 21 (45) | 470 (56) | 64 (42) | <.01 |
| WiFi Score – mean (SD) | 3.8 (1.7) | 3.9 (1.7) | 4 (1.7) | 0.47 |
| TASC | 0.27 | |||
| A | 10 (30.4) | 122 (21.2) | 24 (27.3) | |
| B | 11 (35.5) | 203 (35.2) | 28 (31.8) | |
| C | 2 (6.5) | 81 (14.1) | 17 (19.3) | |
| D | 8 (25.8) | 170 (29.5) | 19 (21.6) | |
| Calcium Median (IQR) | 8.3 (8–9) | 8.5 (8–9) | 8.6 (8–9) | 0.20 |
SD: Standard Deviation, MI: Myocardial Infarction, COPD: Chronic Obstructive Pulmonary Disease, TASC: Trans-Atlantic Inter-Society Consensus
Outcomes
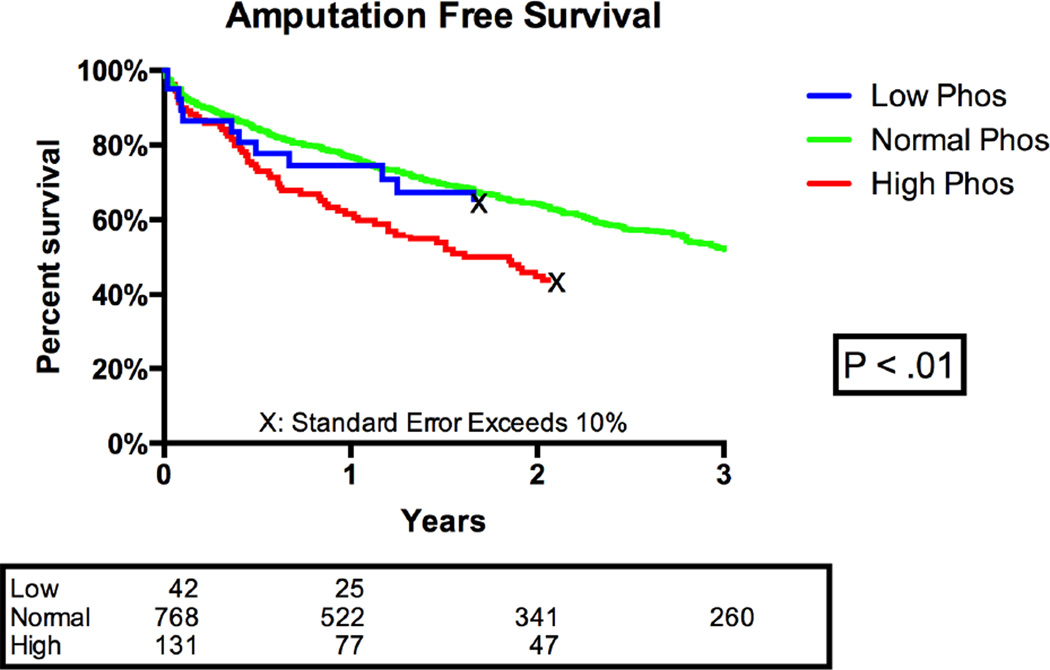
In Kaplan Meier analysis, there was a significant difference in overall mortality between the three groups with high phosphate patients showing significantly worse survival (1-year survival: low 19%, normal: 17% low, and high: 33%, P <.01). Patients with high post-operative phosphate levels also had significantly worse amputation free survival (P < .01) (Figure 1). Major amputation (low: 12%, normal: 12%, high: 15%) and restenosis (low: 21%, normal: 24%, high: 28%) tended toward worse outcomes among patients with elevated phosphate levels, but did not reach statistical significance. Additionally, there were no differences in 30-day mortality, major adverse limb events, or wound infection rates between the groups (Table II).
Figure 1.
Kaplan Meier Analysis: Amputation Free Survival
Table II.
Patient outcomes (Univariate)
| Number (%) | Low Phosphate < 2.5 N = 42 |
Normal Phosphate 2.5–4.5 N = 768 |
High Phosphate > 4.5 N = 131 |
P-Value |
|---|---|---|---|---|
| Mortality | ||||
| 30-day Mortality | 3 (7) | 24 (3) | 6 (5) | 0.30 |
| 1 year Mortality | 8 (19) | 129 (17) | 43 (33) | <.01 |
| 3 year Mortality | 16 (38) | 264 (34) | 73 (56) | <.01 |
| Major Adverse Limb Event | 11 (26) | 286 (38) | 51 (39) | 0.31 |
| Reintervention | 7 (17) | 234 (31) | 41 (31) | 0.15 |
| Restenosis | 9 (21) | 186 (24) | 36 (28) | 0.64 |
| Major Amputation | 5 (12) | 88 (12) | 19 (15) | 0.61 |
| Wound Infection | 1 (2) | 37 (5) | 7 (5) | 0.73 |
Multivariable analysis was then utilized to account for demographics, comorbid conditions, and the index operation. Following multivariable analysis, amputation free survival (HR 1.5 95% CI 1.2–1.9) and overall mortality (HR 1.7, 95% CI 1.3–2.2) were significantly worse among patients with elevated compared with normal phosphate levels (Table III). A subgroup analysis was then performed to assess dialysis and non-dialysis patients separately. Patients with elevated serum phosphate levels maintained a significantly higher risk of mortality in each group (Dialysis HR: 1.8 95% CI 1.2–2.6, Non-dialysis: HR 1.5, 95% CI 1.04–2.10). Finally, to confirm that these findings were not the result of differences in renal function, each patient’s glomerular filtration rate (GFR) was included in the multivariable model. High post-op phosphate levels, however, maintained their association with increased mortality (HR 1.4, 95% 1.01–2.03). There were no differences in outcomes between patients with normal and low phosphate levels (Table IV).
Table III.
Adjusted Time-to-Event Analysis
| Normal Phosphate 2.5–4.5 |
High Phosphate >4.5 HR (95% CI) |
P- Value |
Low Phosphate <2.5 HR (95% CI) |
P-Value | |
|---|---|---|---|---|---|
| Mortalitya | Ref | 1.7 (1.3–2.2) | <.01 | 1.1 (0.7–1.8) | 0.69 |
| Amputation Free Survivala | Ref | 1.5 (1.2–1.9) | <.01 | 1.1 (0.7–1.7) | 0.59 |
| Major Adverse Limb+ Eventb | Ref | 0.9 (0.7–1.4) | 0.84 | 0.6 (0.3–1.2) | 0.13 |
| Reinterventionb | Ref | 1.0 (0.7–1.5) | 0.83 | 1.2 (0.5–2.6) | 0.71 |
| Restenosisb | Ref | 0.8 (0.6–1.4) | 0.79 | 1.4 (0.7–2.7) | 0.37 |
HR: Hazard Ratio
. cox Analysis - Adjusted for age, dialysis, congestive heart failure, COPD, diabetes, index procedure
. Cox Analysis - Adjusted for age, dialysis, congestive heart failure, COPD, diabetes, index procedure, smoking
Table IV.
Adjusted Adverse Outcomes
| Normal Phosphate 2.5–4.5 |
High Phosphate >4.5 OR (95% CI) |
P-Value | Low Phosphate <2.5 OR (95% CI) |
P-Value | |
|---|---|---|---|---|---|
| 30-day Mortalitya | Ref | 1.8 (0.6–5.0) | 0.26 | 2.8 (0.8–10.3) | 0.12 |
| Wound Infectionb | Ref | 2.1 (0.8–5.3) | 0.13 | 0.6 (0.1–5.1) | 0.67 |
OR: Odds Ratio
. Multivariable Regression - Adjusted for age, dialysis, congestive heart failure, COPD, diabetes, index procedure
. Multivariable Regression - Adjusted for age, dialysis, congestive heart failure, COPD, diabetes, index procedure, smoking
Discussion
In this study, we found that patients with elevated post-operative serum phosphate levels undergoing first-time intervention for CLI had increased mortality and decreased amputation free survival after adjusting for relevant co-morbidities. Outcomes in patients with low compared with normal phosphate levels did not differ. Patients with high phosphate levels were more likely to have end-stage renal disease and diabetes but tended to be younger than those with normal or low levels. In univariate analysis we did not identify differences in major adverse limb events including reintervention, restenosis, major amputation, and wound infection between the three groups. Our analysis used the average post-procedural phosphate level that was available for 89% of our total CLI population. We also used pre-operative and maximum phosphate levels in analysis, but found similar effects on all listed outcomes including survival, amputation free survival, and major adverse limb events. Our time-to-event analysis, adjusted for all relevant risk factors including dialysis, congestive heart failure, diabetes, and tobacco use, demonstrated a persistent and durable effect of post-operative phosphate levels on survival.
The results of this study support findings from prior investigations on non-surgical patients with chronic kidney disease, cardiac disease, and within the general population.5–7 The relationship between serum phosphate levels and mortality is not well understood. Increasing evidence, however, supports a role for phosphate in the pathogenesis of medial and intimal calcification.16,17 For example, Ix et al evaluated the effect of serum phosphate on arterial calcification in patients with normal to moderate kidney function and found elevated serum levels were strongly associated with non-compressible vessels and increased ankle-brachial index after adjusting for renal function and cardiovascular risk factors. The authors suggest that increased cardiovascular events might be explained by increased arterial stiffness related to medial artery calcification.18 In the CARDIA study, Foley et al, also demonstrated that elevated serum phosphate levels were associated with coronary artery calcification in patients without a history of renal dysfunction.7 And in patients with peripheral arterial disease, calcification has been associated with increased rates of amputation and mortality, further supporting the hypothesis that decreased amputation free survival among patients with elevated phosphate may be related to increased arterial calcification.19, 20
The increased mortality in patients with hyperphosphatemia is demonstrated in multiple studies of patients with end-stage renal disease.21,22 Despite these findings, it remains possible that increased serum phosphate levels may reflect advanced coronary or renal disease. To further evaluate the impact of these factors on mortality, we performed a stratified analysis of dialysis and non-dialysis patients, adjusting for cardiac risk factors. This revealed a similar effect on mortality for both populations, suggesting that elevated serum phosphate levels have a detrimental effect on survival, independent of both renal and cardiac disease. Similarly, when glomerular filtration rate was included in the model, the effect on mortality remained. These findings support previous studies that demonstrated similar effects among patients without renal dysfunction. Dhingra et al described the effect of hyperphosphatemia on cardiovascular events in a large prospective study of Framingham offspring and found a significant association between elevated serum phosphate levels and increased risk of cardiovascular disease among individuals free of chronic kidney or cardiovascular disease in the community.8
The role of serum phosphate levels in heterotopic calcification has only recently been explored. Arterial calcification is a complex process promoted through an imbalance between endogenous stimulators and inhibitors.17 Vascular smooth muscle cells respond to increased phosphate levels by undergoing osteochondrogenic differentiation with subsequent mineralization of the extracellular matrix. This process can be amplified by cytokines that increase the expression of sodium dependent phosphate co-transporters in the smooth muscle cells thus increasing their susceptibility to calcification.17 The presence of coronary and tibial artery calcification is associated with increased cardiovascular events, lower extremity amputation, and death.20, 23–25 Currently, dietary phosphate restriction, oral phosphate binders, and Vitamin D (1,25) supplementation are the primary treatment approaches to hyperphosphatemia.26 Historically, treatment was limited to patients on hemodialysis, however recent guidelines now also recommend treatment for patients with chronic kidney disease due to the associated adverse outcomes of untreated hyperphosphatemia.12 Clinical trials have shown phosphate binders to be successful in reducing serum phosphate levels, as well as secondary hyperparathyroid hormone and FGF-23, which occur in conjunction with hyperphosphatemia.26 The efficacy of phosphate reduction on improving overall mortality, however, is unknown and large clinical trials have yet to evaluate the effect of serum phosphate reduction on mortality.12, 27, 28
Decreased amputation free survival associated with hyperphosphatemia may have important clinical implications for the treatment of patients with critical limb ischemia. Our results suggest that peri-operative serum phosphate levels provide valuable prognostic information regarding patient mortality and amputation free survival. This study also highlights the need to identify phosphate management strategies in patients with critical limb ischemia even when concurrent renal dysfunction is not present. While hyperphosphatemia is commonly treated among patients on hemodialysis, current management guidelines now emphasize the importance of maintaining normal phosphate levels in those with earlier states of renal disease.12 Measurement of serum phosphate in the perioperative period may thus provide an opportunity to identify those patients at elevated risk. Finally, our data provides a clinical rational for further investigations on the role of phosphate in long-term outcomes of CLI patients.
There are several limitations to this study. First, it is a retrospective review of patients at a single institution and re-interventions or amputation may be underestimated due to un-captured events. This study also had a small sample size of patients with low phosphate, and it is possible that differences in outcomes between low and normal phosphate levels may exist but were not identified due to inadequate power. Finally, lab values in the 30-day post-operative period may not represent a patient’s normal baseline, and due to missing data we were unable to assess preoperative phosphate levels for our analysis. However, when we ran our analysis using patients’ pre-operative phosphate levels (n=756), the statistical association of elevated phosphate levels with worse amputation free survival remained. Nonetheless, our data suggest that abnormal phosphate levels during the perioperative period identify a patient population at increased risk of mortality.
Conclusion
Elevated serum phosphate levels are associated with increased mortality and decreased amputation free survival in patients with critical limb ischemia. Future research focusing on the effects of serum phosphate in patients with critical limb ischemia are warranted.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Accepted for Presentation at the Vascular Annual Meeting, National Harbor, MD, June 8–11th 2016.
References
- 1.LaMuraglia GM, Conrad MF, Chung T, Hutter M, Watkins MT, Cambria RP. Significant perioperative morbidity accompanies contemporary infrainguinal bypass surgery: an NSQIP report. J Vasc Surg. 2009;50(2):299–304. e1–e4. doi: 10.1016/j.jvs.2009.01.043. [DOI] [PubMed] [Google Scholar]
- 2.Slovut DP, Lipsitz EC. Surgical technique and peripheral artery disease. Circulation. 2012;126(9):1127–1138. doi: 10.1161/CIRCULATIONAHA.111.059048. [DOI] [PubMed] [Google Scholar]
- 3.Engelhardt M, Boos J, Bruijnen H, Wohlgemuth W, Willy C, Tannheimer M, et al. Critical limb ischaemia: initial treatment and predictors of amputation-free survival. Eur J Vasc Endovasc Surg. 2012;43(1):55–61. doi: 10.1016/j.ejvs.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 4.Chung J, Modrall JG, Ahn C, Lavery LA, Valentine RJ. Multidisciplinary care improves amputation-free survival in patients with chronic critical limb ischemia. J Vasc Surg. 2015;61(1):162–169. doi: 10.1016/j.jvs.2014.05.101. [DOI] [PubMed] [Google Scholar]
- 5.Kestenbaum B, Sampson JN, Rudser KD, Patterson DJ, Seliger SL, Young B, et al. Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol. 2005;16(2):520–528. doi: 10.1681/ASN.2004070602. [DOI] [PubMed] [Google Scholar]
- 6.Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation. 2005;112(17):2627–2633. doi: 10.1161/CIRCULATIONAHA.105.553198. [DOI] [PubMed] [Google Scholar]
- 7.Foley RN. Phosphate levels and cardiovascular disease in the general population. Clin J Am Soc Nephrol. 2009;4(6):1136–1139. doi: 10.2215/CJN.01660309. [DOI] [PubMed] [Google Scholar]
- 8.Dhingra R, Sullivan LM, Fox CS, Wang TJ, D'Agostino RB, Sr, Gaziano JM, et al. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med. 2007;167(9):879–885. doi: 10.1001/archinte.167.9.879. [DOI] [PubMed] [Google Scholar]
- 9.The Investigation Operations Manual: Blood Serum Chemistry - Normal Values United States Food and Drug Administration. [22 February 2016];2015 :443–444. Available from: http://www.fda.gov/downloads/ICECI/Inspections/IOM/UCM135835.pdf.
- 10.Amanzadeh J, Reilly RF., Jr Hypophosphatemia: an evidence-based approach to its clinical consequences and management. Nat Clin Pract Nephrol. 2006;2(3):136–148. doi: 10.1038/ncpneph0124. [DOI] [PubMed] [Google Scholar]
- 11.Geerse DA, Bindels AJ, Kuiper MA, Roos AN, Spronk PE, Schultz MJ. Treatment of hypophosphatemia in the intensive care unit: a review. Crit Care. 2010;14(4):R147-R. doi: 10.1186/cc9215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42(4 Suppl 3):S1–S201. [PubMed] [Google Scholar]
- 13.Mills JL, Sr, Conte MS, Armstrong DG, Pomposelli FB, Schanzer A, Sidawy AN, et al. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: risk stratification based on wound, ischemia, and foot infection (WIfI) J Vasc Surg. 2014;59(1):220–234. e1–e2. doi: 10.1016/j.jvs.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Dormandy JA, Rutherford RB. Management of peripheral arterial disease (PAD). TASC Working Group. TransAtlantic Inter-Society Consensus (TASC) J Vasc Surg. 2000;31(1 Pt 2):S1–s296. [PubMed] [Google Scholar]
- 15.Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code for Biology and Medicine. 2008;3(1):1–8. doi: 10.1186/1751-0473-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shobeiri N, Adams MA, Holden RM. Phosphate: an old bone molecule but new cardiovascular risk factor. Br J Clin Pharmacol. 2014;77(1):39–54. doi: 10.1111/bcp.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giachelli CM. The Emerging Role of Phosphate in Vascular Calcification. Kidney Int. 2009;75(9):890–897. doi: 10.1038/ki.2008.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ix JH, De Boer IH, Peralta CA, Adeney KL, Duprez DA, Jenny NS, et al. Serum phosphorus concentrations and arterial stiffness among individuals with normal kidney function to moderate kidney disease in MESA. Clin J Am Soc Nephrol. 2009;4(3):609–615. doi: 10.2215/CJN.04100808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehto S, Niskanen L, Suhonen M, Rönnemaa T, Laakso M. Medial Artery Calcification: A Neglected Harbinger of Cardiovascular Complications in Non–Insulin-Dependent Diabetes Mellitus. Arterioscler Thromb Vasc Biol. 1996;16(8):978–983. doi: 10.1161/01.atv.16.8.978. [DOI] [PubMed] [Google Scholar]
- 20.Guzman RJ, Brinkley DM, Schumacher PM, Donahue RM, Beavers H, Qin X. Tibial artery calcification as a marker of amputation risk in patients with peripheral arterial disease. J Am Coll Cardiol. 2008;51(20):1967–1974. doi: 10.1016/j.jacc.2007.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Block GA, Hulbert-Shearon TE, Levin NW, Port FK. Association of serum phosphorus and calcium × phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998;31(4):607–617. doi: 10.1053/ajkd.1998.v31.pm9531176. [DOI] [PubMed] [Google Scholar]
- 22.Ganesh SK, Stack AG, Levin NW, Hulbert-Shearon T, Port FK. Association of elevated serum PO(4), Ca × PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol. 2001;12(10):2131–2138. doi: 10.1681/ASN.V12102131. [DOI] [PubMed] [Google Scholar]
- 23.Sangiorgi G, Rumberger JA, Severson A, Edwards WD, Gregoire J, Fitzpatrick LA, et al. Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: a histologic study of 723 coronary artery segments using nondecalcifying methodology. J Am Coll Cardiol. 1998;31(1):126–133. doi: 10.1016/s0735-1097(97)00443-9. [DOI] [PubMed] [Google Scholar]
- 24.Hollander M, Hak AE, Koudstaal PJ, Bots ML, Grobbee DE, Hofman A, et al. Comparison between measures of atherosclerosis and risk of stroke: the Rotterdam Study. Stroke. 2003;34(10):2367–2372. doi: 10.1161/01.STR.0000091393.32060.0E. [DOI] [PubMed] [Google Scholar]
- 25.London GM, Guerin AP, Marchais SJ, Metivier F, Pannier B, Adda H. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant. 2003;18(9):1731–1740. doi: 10.1093/ndt/gfg414. [DOI] [PubMed] [Google Scholar]
- 26.Martin KJ, Gonzalez EA. Prevention and control of phosphate retention/hyperphosphatemia in CKD-MBD: what is normal, when to start, and how to treat? Clin J Am Soc Nephrol. 2011;6(2):440–446. doi: 10.2215/CJN.05130610. [DOI] [PubMed] [Google Scholar]
- 27.Kendrick J, Kestenbaum B, Chonchol M. Phosphate and Cardiovascular Disease. Adv Chronic Kidney Dis. 2011;18(2):113–119. doi: 10.1053/j.ackd.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Navaneethan SD, Palmer SC, Vecchio M, Craig JC, Elder GJ, Strippoli GF. Phosphate binders for preventing and treating bone disease in chronic kidney disease patients. Cochrane Database Syst Rev. 2011;(2) doi: 10.1002/14651858.CD006023.pub2. Cd006023. [DOI] [PubMed] [Google Scholar]