Abstract
Background
Response inhibition is a distinct aspect of executive function that is frequently impaired in children with fetal alcohol spectrum disorders (FASD). We used a Go/NoGo (GNG) task in a functional MRI (fMRI) protocol to investigate differential activation of brain regions in the response inhibition network in children diagnosed with full or partial fetal alcohol syndrome (FAS/PFAS), compared with healthy controls.
Methods
A rapid, event-related task with 120 Go and 60 NoGo trials was used to study children aged 8–12 years—8 with FAS/PFAS, 17 controls. Letters were projected sequentially, with Go and NoGo trials randomly interspersed across the task. BOLD signal in the whole brain was contrasted for the correct NoGo minus correct Go trials between the FAS/PFAS and control groups.
Results
Compared to the FAS/PFAS group, controls showed greater activation of the inferior frontal and anterior cingulate network linked to response inhibition in typically developing children. By contrast, the FAS/PFAS group showed greater BOLD response in dorsolateral prefrontal cortex (dlPFC) and other middle prefrontal regions, suggesting compensation for inefficient function of pathways that normally mediate inhibitory processing. All group differences were significant after control for potential confounding variables. None of the effects of prenatal alcohol exposure on activation of the regions associated with response inhibition were attributable to the effects of this exposure on IQ.
Conclusions
This is the first FASD GNG study in which all participants in the exposed group met criteria for a diagnosis of full fetal alcohol syndrome (FAS) or partial FAS (PFAS). Although FASD is frequently co-morbid with attention deficit hyperactivity disorder (ADHD), the pattern of brain activation seen in these disorders differs, suggesting that different neural pathways mediate response inhibition in FASD and that different interventions for FASD are, therefore, warranted.
Keywords: fetal alcohol syndrome, prenatal alcohol exposure, response inhibition, Go/NoGo, functional magnetic resonance imaging
Introduction
The teratogenicity of prenatal alcohol exposure has been extensively documented during the past four decades, and alcohol consumption during pregnancy has been linked to a broad range of impairments known collectively as fetal alcohol spectrum disorders (FASD) (Hoyme et al. 2005; Cook et al. 2016). In addition to growth and cognitive problems (e.g., Carter et al. 2016), children with FASD have numerous behavioral problems including difficulty in response inhibition, as evidenced in problems with emotional regulation, impulsivity, attention, hyperactivity, and socially inappropriate behaviors (Mattson and Riley 1998; Carmichael-Olson et al. 1998; Jacobson et al. 2006; Dodge et al. 2014; Lindinger et al. 2016).
FASD is frequently co-morbid with attention deficit hyperactivity disorder (ADHD). About 60% of children prenatally exposed to alcohol in a clinic-referred sample (Rasmussen et al. 2010) and 32% in our prospectively recruited, community-based Detroit alcohol exposed cohort (Jacobson et al. 2011a) were diagnosed as having ADHD. In a case-control study, children who were diagnosed with ADHD were more than twice as likely to have been exposed to alcohol prenatally than children without a diagnosis of ADHD (Mick et al. 2002). Given that the cognitive and behavioral impairment in FASD is attributable to prenatal alcohol exposure, whereas the etiology of idiopathic ADHD is unknown, deficits in response inhibition in these disorders are likely mediated by different underlying neural mechanisms (Coles et al. 1997; Jacobson et al. 2011a).
Response inhibition, the ability to suppress prepotent or ongoing responses, is a distinct and important aspect of executive function (Barkley et al. 1997, 2002; Nigg et al. 2001, 2003) that has been frequently reported to be impaired in children with FASD (Mattson et al. 2011). In addition to clinical observations of contextual or socially inappropriate behaviors, studies have shown that children with prenatal alcohol exposure perform more poorly on tests of response inhibition. In one study of school-aged children (8–15 years of age) performance was poor compared with typically developing controls on the Stroop Color-Word Test in both the switching and interference conditions (Mattson et al. 1999). In another study, which used a Go/NoGo (GNG) task, children (7–15 years of age) with FASD made fewer correct responses during the inhibitory NoGo trials (Kodituwakku et al. 1995). The GNG task is well suited for assessing response inhibition because it requires the participant to inhibit very rapid prepotent responses.
Response inhibition has been extensively studied in typically developing children and adults using functional MRI (fMRI) imaging protocols. Greater activation of the prefrontal cortex, more specifically the more ventral and orbitofrontal regions, is associated with higher rates of behavioral inhibition in adults, suggesting that these regions play an important role in mediating the inhibition of a behavioral response (Bunge et al. 2002). An fMRI study of response inhibition in typically developing children distinguished between patterns of neural activation during the Go and NoGo trials (Liddle et al. 2001). Regional activations during the NoGo trials included the dorsolateral and ventrolateral prefrontal cortex, which were similar to those seen in the earlier studies. Activation of the bilateral dorsal anterior cingulate regions was seen during both the Go and NoGo trials. Dorsal anterior cingulate cortex is thought to play an important role in decision making in tasks entailing conflict (Botvinick et al. 1999), whereas areas of the prefrontal cortex, such as the ventral, orbitofrontal, and dorsolateral regions, have been found to play a specific role in mediating response inhibition (Durston et al. 2002; Tamm et al. 2002, Zhai et al. 2015).
Numerous studies have used fMRI to assess response inhibition in ADHD, which is frequently impaired in that disorder (Barkley et al. 1997; Nigg et al. 2001; Pliszka et al. 2006) (see Table 1 for a review). Studies using GNG tasks have found lower levels of activation in children and adolescents with ADHD in the regions of the inferior frontal cortex that are typically activated during the NoGo trials in normal controls (Rubia et al. 2008; Janssen et al. 2015; Hart et al. 2014; Morein-Zamir et al. 2014). In an adult study that used a GNG task, amount of activation in the inferior frontal cortex was negatively related to severity of the patient’s ADHD behavioral problems (Cubillo et al. 2010).
Table 1.
Summary of Neuroimaging and ERP Studies of Response Inhibition in ADHD and FASD
| Type | Study | Age range |
Number of subjects |
Ethnicity | Medication history |
Control variables | Task | Behavioral data in scanner |
ROI/ERP data (FASD or ADHD vs Controls) |
|---|---|---|---|---|---|---|---|---|---|
| ADHD | |||||||||
| fMRI | Janssen et al. 2015 | 8–13 yr | 21 ADHD, 17 Control |
Amsterdam, Netherlands area |
Stimulant medication discontinued 48 hr prior to scan |
None | Event-related visual-spatial GNG with airplanes |
Slower RTs and more omission errors in ADHD |
↓ right inferior frontal gyrus, anterior cingulate, dorsal medial prefrontal cortex |
| Spinelli et al. 2011 | 8–13 yr | 13 ADHD, 17 Control |
No information |
Stimulant medication discontinued 48 hr prior to scan |
None | Event-related visual-spatial GNG with spaceships |
More omission errors in ADHD, no RT differences |
Comparing pre- error vs pre-correct trials: healthy controls showed ↑ in precuneus/posterior cingulate, parahippocampal and middle frontal; ADHD showed ↑ in cerebellum, DLPFC, basal ganglia |
|
| Hart et al. 2014a | 10–17 yr |
30 ADHD, 30 Control |
No information |
Stimulant medication discontinued 48 hr prior to scan |
IQ | Event-related visual-spatial SST with arrows |
No RT or accuracy differences, ADHD showed more premature responses |
↓ ventrolateral prefrontal, striatum, temporoparietal areas, ↑ ventromedial fronto-limbic |
|
| Morein-Zamir et al. 2014 | M=28.6 | 19 ADHD, 19 Control |
No information |
Stimulant medication discontinued at least 24 hr prior to scan |
Medication, sex | Block design visual-spatial GNG with houses and faces superimposed |
ADHD showed higher rates of commission and omission errors and slower RT |
↓ right inferior frontal cortex when controlling for attentional processing |
|
| Cubillo et al. 2010a | 26–30 yr |
11 ADHD, 14 Control |
No information |
No medication | IQ | Event-related visual-spatial SST with arrows |
No RT or accuracy differences |
↓ bilateral inferior frontal, caudate, and thalamus; severity of behavioral symptoms negatively correlated with activation in those areas; ↓ connectivity between fronto- frontal, -striatal, and -parietal networks |
|
| Rubia et al. 2008a | 9–16 yr | 20 ADHD, 13 CD, 20 Control |
No information |
No medication | Age | Event-related visual-spatial SST with arrows |
No accuracy differences; ADHD had increased RT variability |
Successful inhibition: ADHD ↓ activation in left dorsolateral prefrontal compared to both other groups; Inhibition failures: ADHD and CD ↓ activation in posterior Cingulate |
|
| FASD | |||||||||
| fMRI | Fryer et al. 2007 | 8–18 yr | 13 FASD, 18 Control |
Caucasian FASD (69%), Caucasian Control (67%) |
Atomoxetine, antidepressants, antipsychotics |
None | Event-related visual-spatial GNG with shapes |
No RT or accuracy differences |
↑ left medial, right middle frontal gyri, ↓ right caudate |
| O’Brien et. al 2013 | 8–18 yr | 20 FASD, 15 Control |
Caucasian FASD (65%), Caucasian Control (73%) |
Atomoxetine, antidepressants, antipsychotics |
Age | Event-related visual-spatial cued GNG with shapes |
No RT or accuracy differences on non- cued trials; poorer go- trial accuracy on cued trials in FASD |
↑ left precuneus, cingulate, anterior cingulate, right medial frontal gyrus; ↓ left precentral and postcentral gyrus in cued trials |
|
| Ware et al. 2015 | 13–16 yr |
21 FASD, 21 Control |
Caucasian FASD (57.1%), Caucasian Control (61.9%) |
Atomoxetine, antidepressants, antipsychotics |
Psychostimulant. Medication, SES, sex, handedness, IQ |
Event-related letter parametric SST |
No RT or accuracy differences |
↑ frontal, sensorimotor, striatal, cingulate compared to controls, especially as difficulty increased |
|
| DTI | Paolozza et al. 2014 | 9–15 yr | 43 FASD, 35 Control |
Caucasian (37%), First Nations (30%) FASD; Caucasian Control (94%) |
Not available | Age | Antisaccade test |
FASD showed more antisaccade direction errors and timing errors, slower RT |
↓ Mean Diffusivity of splenium of CC, task errors not correlated to MD of splenium in FASD, but are correlated in controls |
| ERP | Burden et al. 2009 | 10–13 yr |
7 FASD, 6 Control |
Cape Coloured (100%) |
Medication naïve |
Maternal education and cigarettes |
Event-related letter GNG |
No RT or accuracy differences |
↓ P2 amplitude meaning later discrimination of stimulus, ↑ N2 latency and↓ N2 amplitude meaning less efficiency in stimulus classification |
| Burden et al. 2011 | 10–13 yr |
38 FASD, 101 Control |
Cape Coloured (100%) |
Medication naïve |
prenatal PCBs, prenatal Hg, 11- year PCBs, maternal age |
Event-related letter GNG |
No RT or accuracy differences |
↓ P2 latency in alcohol exposed – meaning altered initial visual processing |
|
| Steinmann et al. 2011 | 11–15 yr |
12 FASD, 11 Control |
Cape Coloured (100%) |
Medication naïve |
None | Event-related auditory tone GNG |
No RT or accuracy differences |
↑ N2 latency meaning less efficiency in stimulus classification |
|
FASD study populations were middle class except for Burden et al. (2009) and Steinmann et al. (2011), which were comprised of lower socioeconomic status; no socioeconomic status information available for the ADHD studies
All male study sample
FASD, fetal alcohol spectrum disorders; ADHD, attention deficit hyperactivity disorder; ROI, region of interest; ERP, event-related potential, GNG, Go/NoGo; SST, stop-signal task; RT, reaction time; CD, conduct disorder; MD, mean diffusivity
By contrast, only a few studies have examined the neural bases of response inhibition in children with prenatal alcohol exposure (Table 1). Event-related potential (ERP) studies of response inhibition comparing children with FASD to typically developing children during a GNG task have found that exposed children exhibit increased P2 latency, which is believed to reflect slower identification and classification of stimuli; increased N2 peak latency, suggesting that the exposed children are slower to distinguish between the Go and NoGo stimuli (Steinmann et al. 2011); and decreased N2 amplitude, suggesting increased cognitive effort (Burden et al. 2009). In a more recent study, alcohol-exposed children also showed an increased latency and decreased amplitude of P3, suggesting poor allocation of attention (Gerhold et al. 2016).
Two fMRI studies administered a visual-spatial GNG task to children with FASD. These studies found patterns of greater neural activation in medial and middle frontal regions, which were consistent with the less efficient frontal functioning suggested by the data in the ERP studies and differed from the pattern of decreased prefrontal activation found in ADHD. In the first study, Fryer et al. (2007) found increased blood oxygen level dependent (BOLD) response across the prefrontal cortex (right medial and left middle frontal gyri) and decreased BOLD response in the caudate nucleus in children with FASD compared with controls. Using the same GNG task with children from the same population, the second study also found increased right medial frontal lobe activation during inhibition in alcohol-exposed children compared to normal controls (O’Brien et al. 2013). A recent study that assessed response inhibition using a verbal stop signal task with a parametric design similarly found increases in middle frontal activation, as well as widespread increases across superior frontal, cingulate, and sensorimotor and striatal regions in relation to increasing difficulty in children with prenatal alcohol exposure (Ware et al. 2015).
Thus, to date few fMRI studies have examined response inhibition in FASD. The current study, the first in which all participants in the exposed group met criteria for a diagnosis of full fetal alcohol syndrome (FAS) or partial FAS (PFAS), was conducted in Cape Town, South Africa, where the prevalence of FASD, at 13.6–20.9% (May et al. 2013), is among the highest in the world. This study is the first to administer a GNG task with a large number of distinct Go stimuli (20 different letters, compared with 1–3 in previous studies) to children with prenatal alcohol exposure. Unlike the prior studies, none of the children had a history of stimulant medication or had to be medicated during the scan. Based on findings from the previous studies using GNG paradigms, we hypothesized that children with FASD would perform a simple GNG task successfully but that fMRI would reveal weaker activation in the inferior frontal and dorsal anterior cingulate regions that have been linked to response inhibition in typically developing children. Instead, we predicted greater activation in other frontal regions associated with working memory and executive function, including lateral frontal regions such as the dorsolateral prefrontal cortex (dlPFC) in the middle frontal gyrus, suggesting compensation for weaker activation by children with FASD of the regions relied on for efficient inhibitory processing in typically developing children.
Methods
Data were collected from 25 right-handed, 8- to 12-year-old children (8 diagnosed with FAS or PFAS and 17 age- and sex-matched controls) from the Cape Coloured (mixed ancestry) community in Cape Town. Nine were the older siblings of participants in our Cape Town Longitudinal Cohort (Jacobson et al. 2008). The remaining children were identified by screening all of the 8- to 12-year-old children from an elementary school in a nearby rural section of Cape Town, where there is a very high incidence of alcohol abuse and heavy drinking during pregnancy among local farm workers (see Jacobson et al. 2011a for details). Use of psychostimulant medication is rare in this community. None of the children in the sample were exposed to psychostimulant medications prior to or during testing. The median time from screening to diagnosis was 4.7 months; from diagnosis to scan, 2.0 years.
Maternal alcohol consumption during pregnancy was assessed using a timeline follow-back interview (Jacobson et al. 2002) and summarized in terms of oz absolute alcohol (AA) per day, oz AA per occasion, and number of drinking days per week. The timeline follow-back approach is used to determine incidence and amount of drinking on a day-by-day basis during pregnancy. Any child whose mother reported consuming at least 14 standard drinks per week (equivalent to 2 drinks per day ≈ 1.0 oz AA per day) on average or engaged in binge drinking during pregnancy (4 or more drinks per occasion) was recruited into the alcohol exposed group. Controls were children whose mothers reported abstaining or drank only minimally and did not binge drink during pregnancy.
Handedness was assessed on the Edinburgh Handedness Inventory (EHI; Oldfield, 1971), which examines hand preference across a number of domains, such as writing, eating and sports. Only right-handed children were recruited to participate in this neuroimaging study to minimize laterality differences. The children were administered 7 of the 10 subtests from the Wechsler Intelligence Scale for Children, Third edition (WISC-III)—Similarities, Arithmetic, Digit Span, Symbol Search, Coding, Block Design, and Picture Completion—and Matrix Reasoning from the WISC-IV. IQ was estimated from these eight subtests using Sattler’s (1992) formula; validity coefficients for Sattler Short Form IQ based on 5 or more subtests consistently exceed r = 0.90. We have also previously shown that these WISC IQ scores were strongly correlated with scores on the Junior South African Individual Scales (JSAIS; Madge et al. 1981), which has been normed for South African children, r = 0.77, p < 001 (Jacobson et al. 2011a).
In October 2005 and 2009 we organized clinics at which the children were each independently examined by two U.S. FAS expert dysmorphologists (HE Hoyme (HEH), MD, and LK Robinson, MD) for growth and dysmorphic features using the Revised Institute of Medicine criteria (Hoyme et al. 2005). A third clinic was held in 2013, where children were again examined by HEH, assisted by dysmorphologists G DeJong, MD, and P Shah, MD, and by RC Carter, MD. Case conferences were held following each clinic to reach consensus regarding FAS or PFAS diagnosis (see Jacobson et al. 2011a for procedure). Ten children who did not attend the 2005 clinic were examined by N Khaole, MD, a Cape Town-based expert FAS dysmorphologist, and these diagnoses were subsequently confirmed by HEH at the 2009 and 2013 clinics.
Each child was scanned on a 3T Allegra MR scanner (Siemens, Erlangen Germany). A magnetization-prepared rapid gradient echo (MPRAGE) structural image was acquired in a sagittal orientation with the following parameters: TR = 2300 ms, TE = 3.93 ms, TI = 1100 ms, 160 slices, flip angle 12 degrees, voxel size = 1.3 × 1.0 × 1.0 mm3, scan time = 6:03 min. During the fMRI protocol, 180 functional volumes sensitive to BOLD contrast were acquired with a T2*-weighted gradient echo, echo planar imaging sequence (TR = 2000 ms, TE = 30 ms, 34 interleaved slices, 3 mm thick, gap 0.9 mm, 200 × 200 mm2 field of view [in-plane resolution 3.125 × 3.125 mm2]). The first four volumes were discarded from all analyses to allow the signal to reach steady state. MR images were preprocessed and analyzed using SPM8 (Statistical Parametric Mapping, Wellcome Department of Imaging and Neuroscience, London, UK). For fMRI, all subjects’ images were co-registered to their own structural data and resliced (2×2×2 mm3). Preprocessing included motion correction, correction for different slice acquisition times, linear trend removal, and high frequency temporal filtering. Data were spatially smoothed using a 5mm full-width at half-maximum Gaussian filter.
An fMRI GNG task was administered to each child in the MR scanner. The task was a rapid, event-related task with 120 Go and 60 NoGo trials; thus, the probability of NoGo trials was 33.3%. Letters were projected in sequence (presentation time: 500ms; ISI: 1500ms). Go and NoGo trials were randomly interspersed throughout the whole 6-minute task. Children were instructed to focus on the screen on which the letters would appear. They were told to press a button with their right index finger in response to all letter stimuli presented on the screen except for the letter “X,” which was the NoGo stimulus. Twenty different letters were used in the Go condition, each letter appearing in six (5.0%) of the 120 Go trials. Prior to scanning, each child underwent a training session in which s/he practiced the task both outside and inside a mock scanner to insure that s/he understood the instructions and the importance of lying still within the scanner. The training and use of a mock scanner have been important in decreasing subject loss due to motion artifact, reducing anxiety, and facilitating completion of the fMRI scans. Two children from the control group with a correct inhibition rate below 60% in the scanner were dropped from the analysis due to poor performance on the task, leaving 15 children in the control group whose data are presented here. All the other children in the study had a performance score ≥ 65%.
The experimental task was programmed using E-Prime software (Psychology Software Tools, Inc., Pittsburgh, USA) and was presented using a data projector positioned in a room behind the scanner in line with the bore of the magnet. Images were projected through a waveguide onto a rear projection screen mounted behind the scanner, which subjects viewed using the standard mirror system that mounts to the single channel head coil. Responses were recorded using a Lumitouch response system (Photon Control Inc., Burnaby, Canada). The child was able to talk to the examiner using an intercom built into the scanner and could ask to stop the scan at any time by squeezing a ball held in his/her left hand.
Whole brain voxel-wise analyses were performed with between-group t-tests with four predictors for the correct and incorrect Go and NoGo trials convolved by the standard hemodynamic function. The six motion correction parameters were z-transformed and added as predictors of no interest. BOLD signal in the whole brain was contrasted for the correct NoGo minus correct Go conditions between the FAS/PFAS and control groups. The voxel-wise threshold was set at p < 0.01, and the clusterwise spatial extent threshold was set at p < 0.05, which for this study corresponded to ≥ 14 adjacent voxels. For each significant cluster, the eigenvalues (β) indicating average percent signal change between the correct NoGo and Go conditions for each subject were extracted for use in subsequent analyses.
Seven control variables were examined for consideration as potential confounders of the effects of prenatal alcohol exposure group on brain activation patterns during the GNG task: maternal years of education, marital status, age at delivery, parity, and cigarette smoking during pregnancy, and child sex and age at assessment. Each control variable was examined in relation to each behavioral outcome and percent signal change in each cluster. Any control variable related to an endpoint at p < 0.10 was considered a potential confounder of the effect of exposure group on that endpoint. Analysis of covariance was used to determine whether the effect of group on each outcome remained significant after adjustment for potential confounders. Given that prenatal alcohol exposure is known to be related to poorer overall intellectual function, hierarchical multiple regression was used to determine the degree to which the effects of alcohol on the regions activated during response inhibition were attributable to (i.e., mediated by) the effects of alcohol on IQ. Prenatal alcohol exposure (FAS/PFAS vs. control) was entered as a binary predictor in Step 1 of the analysis; IQ, in Step 2. Mediation was examined by testing whether the addition of IQ to the regression significantly reduced the effect of prenatal alcohol exposure (measured by its raw regression coefficient) on percent signal change, using the Clogg et al. (1992) test.
Results
Sample characteristics
Sample characteristics are summarized in Table 2. The women in the FAS/PFAS group reported very heavy drinking during pregnancy (range = 2.9 – 10.0 drinks/occasion on an average of 2 to 7 days/week). All but one met the NIAAA criterion for binge drinking for women (4 or more drinks/occasion). One drank daily, and the others all concentrated their drinking on 2–3 days of the week, generally beginning on Friday afternoon and continuing through the end of the weekend. By contrast, all but two women in the control group reported abstaining from drinking during pregnancy. These two controls drank only minimally during pregnancy: one reported 2 drinks on one occasion during pregnancy; the other, 2 drinks/occasion once/month. Alcohol users smoked more than women in the control group. None of the women reported using cocaine or methaqualone (“mandrax”), and two mothers of children with PFAS reported light marijuana use (1–3 days/month) during pregnancy.
Table 2.
Sample Characteristics
| FAS/PFAS (n = 8) |
Controls (n = 15) |
t or χ2 | |
|---|---|---|---|
| Maternal | |||
| Mother’s age at delivery | 28.8 (6.0) |
27.5 (4.7) |
0.60 |
| Married (%) | 37.5 | 60.0 | 1.06 |
| Years of education | 7.1 (3.4) |
8.3 (1.8) |
1.11 |
| Alcohol consumption (oz) across pregnancy |
|||
| AA/day | 2.8 (2.8) |
0.0 (0.0) |
8.68** |
| AA/occasion | 6.2 (0.4) |
0.1 (0.4) |
14.08** |
| Frequency (days/week) | 2.8 (1.4) |
0.0 (0.0) |
6.52** |
| Cigarettes/day | 7.3 (6.0) |
2.7 (4.2) |
2.57* |
| Child | |||
| Age at scan | 11.4 (1.4) |
11.7 (1.1) |
0.36 |
| Sex (% male) | 28.6 | 40.0 | 0.65 |
| WISC-IV IQ | 66.7 (10.7) |
76.1 (11.7) |
1.74† |
Values are Mean (SD). AA = absolute alcohol; WISC-IV = Wechsler Intelligence Scale for Children, Fourth Edition.
p < 0.10
p < 0.05
p < 0.001
There were no between-group differences for maternal marital status, age at delivery, years of education, or child sex or age at scan, all ps > 0.20. As expected, IQ scores of the exposed children were lower than for the controls but fell short of statistical significance, p = 0.073.
Behavioral Performance
The task performance of the children in the scanner is summarized in Table 3. As planned, there were no significant between group differences in behavioral performance on this relatively simple response inhibition task, measured by percent correct inhibitions, number of omission errors, or reaction time across all trials, all ps > 0.20. Age and sex were also unrelated to behavioral performance, both ps > 0.10
Table 3.
Behavioral Performance on Go-No/Go Task during Scan
| FAS/PFAS (n = 8) |
Controls (n = 15) |
|
|---|---|---|
| % Correct Inhibitions | 83.8 (7.2) |
79.9 (10.1) |
| # of Omission Errors | 7.3 (11.0) |
5.3 (6.9) |
| Reaction Time (msec) | 499.5 (98.9) |
465.5 (64.2) |
Values are Mean (SD). None of the group comparisons are significant (all p’s > 0.20).
Neuroimaging Findings
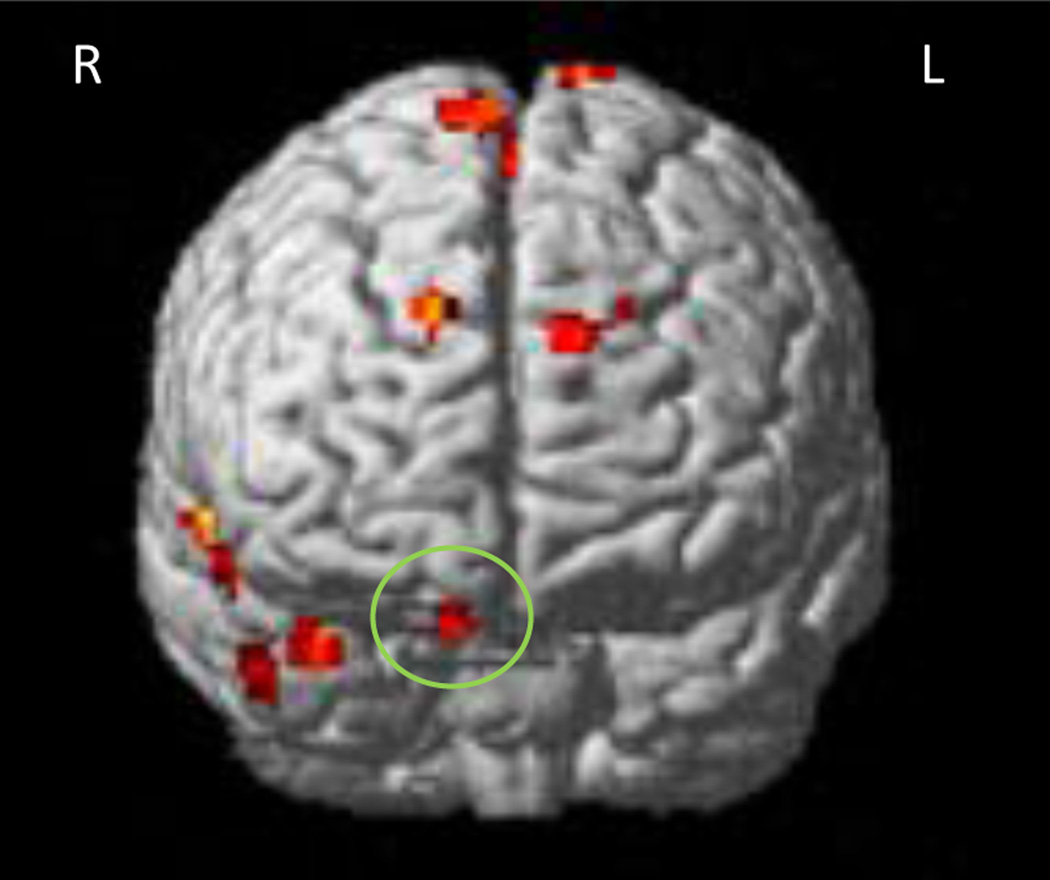
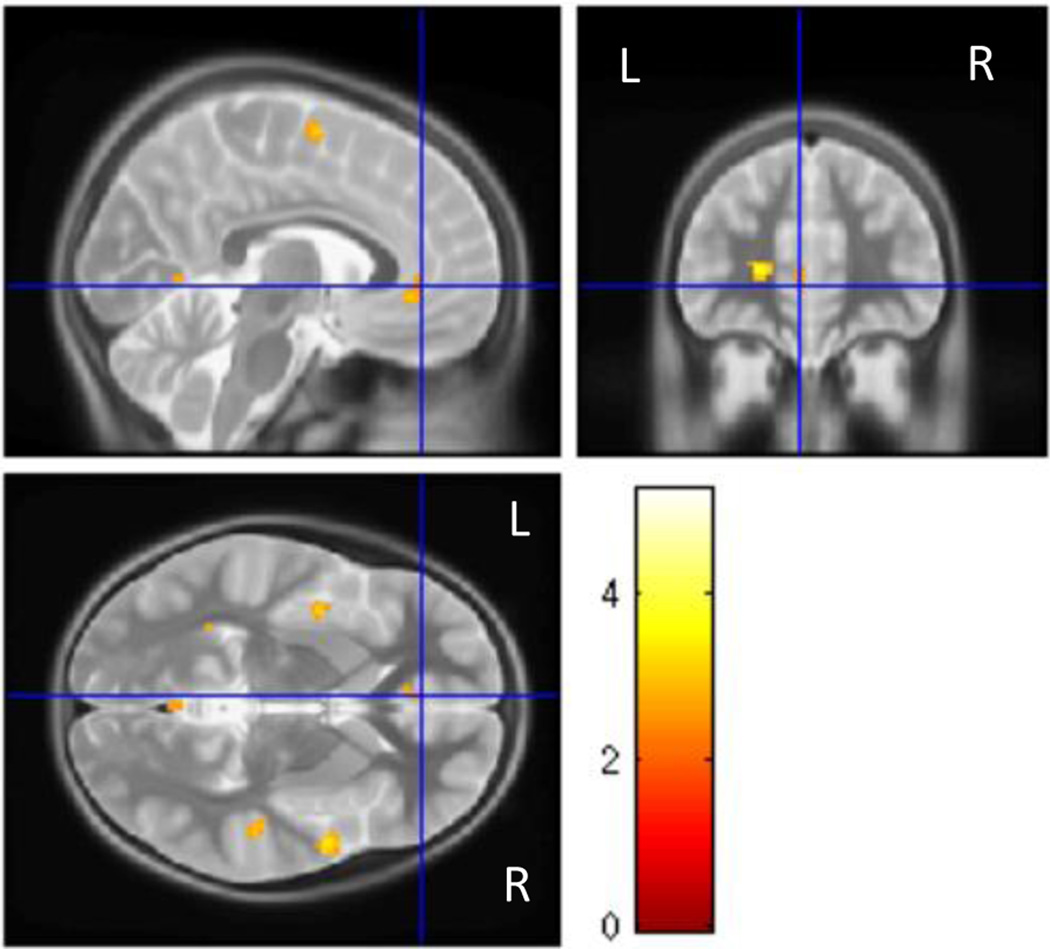
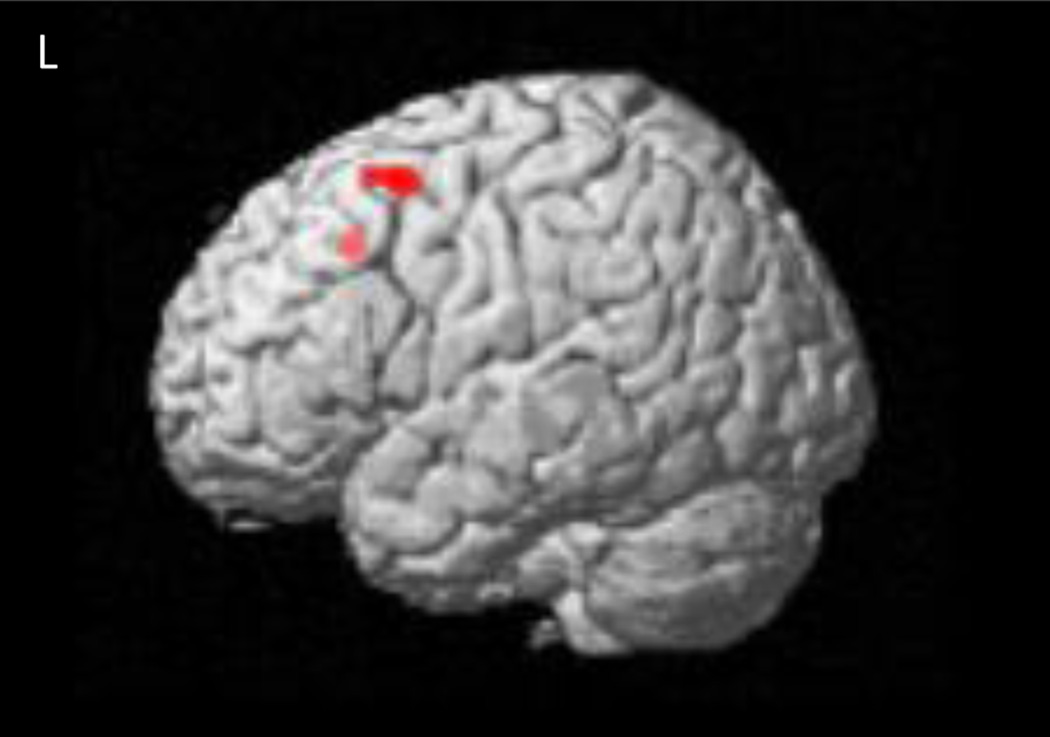
The neuroimaging group contrast findings are summarized in Table 4. Controls showed greater activation of the right inferior orbitofrontal cortex (Brodmann (BA) area 11; Fig. 1), anterior cingulate (BA 24, 32, 10; Fig. 2), and bilateral superior frontal cortex (BA 6, 9, 10), compared to the FAS/PFAS group. This pattern of activation was similar to that seen in previous studies of adults (Bunge et al. 2002) and typically developing children (Durston et al. 2002; Tamm et al. 2002). The control group also showed significantly increased activation in bilateral temporal lobe regions (BA 21, 22, 38) and insula (BA 13) when compared to the FAS/PFAS group. By contrast, the FAS/PFAS group showed greater BOLD response in the lateral middle frontal cortex, including the right dlPFC (BA 6, 8, 9; Fig. 3), and the left superior frontal cortex (BA 8), when compared to the control group.
Table 4.
Between group comparisons of BOLD response for correct NoGo vs Go trials
| Region | Brodmann areas | Peak Coordinates (x,y,z) |
Volume mm3 |
Peak T |
|---|---|---|---|---|
| Control > FAS/PFAS | ||||
| L Anterior Cingulate | 24 | −22, 42, 6 | 480 | 4.02 |
| 32, 10 | −6, 38, −8 | 488 | 3.03 | |
| L Superior Frontal | 9, 10 | −10, 64, 30 | 448 | 3.33 |
| R Superior Frontal | 6 | 10, 2, 72 | 1960 | 3.33 |
| 9 | 14, 58, 36 | 400 | 3.28 | |
| R Orbitofrontal Gyrus | 11 | 12, 50, −26 | 304 | 2.94 |
| R Precentral Gyrus | 6 | 46, −8, 22 | 248 | 3.15 |
| L Postcentral Gyrus | 1, 2, 3, 4, 5 | −12, −32, 80 | 624 | 3.75 |
| R Postcentral Gyrus | 3, 4, 40 | 32, −22, 42 | 720 | 3.94 |
| L Superior Temporal | 41 | −34, −42, 8 | 1296 | 5.26 |
| R Superior Temporal | 21, 38 | 38, 2, −30 | 1016 | 4.24 |
| 21, 22, 38 | 58, 2, −6 | 1088 | 4.23 | |
| L Middle Temporal | 21 | −48, −26, −16 | 120 | 2.99 |
| R Middle Temporal | 21, 22 | 50, −28, −6 | 544 | 4.07 |
| 21, 38 | 50, 14, −32 | 192 | 3.12 | |
| L Insula | 13, 43, 6 | −26, −22, 30 | 2632 | 4.30 |
| 13, 21 | −40, −2, −6 | 464 | 4.03 | |
| R Insula | 13 | 36, −30, 4 | 400 | 3.70 |
| L Fusiform Gyrus | 36 | −32, −14, −26 | 120 | 3.15 |
| R Calcarine Sulcus | 17 | 32, −56, 6 | 760 | 4.86 |
| L Putamen | − | −24, −2, 20 | 584 | 4.08 |
| Cerebellar Vermis | − | −2, −62, 0 | 184 | 3.30 |
| L Cerebellar Culmen | − | −24, −32, −30 | 208 | 2.93 |
| R Cerebellar Culmen | − | 26, −48, −38 | 216 | 4.06 |
| − | 14, −36, −38 | 136 | 2.78 | |
| FAS/PFAS > Control | ||||
| L Superior Frontal | 32 | −14, 20, 40 | 160 | 3.73 |
| L Middle Frontal | 6 | −30, 8, 54 | 296 | 3.07 |
| 8 | −44, 18, 58 | 112 | 2.78 | |
| R Middle Frontal | 9 | 32, 14, 34 | 120 | 3.03 |
| R Cerebellar Tonsil | − | 20, −46, −46 | 280 | 4.94 |
All clusters survived correction for multiple comparisons using spatial extent cluster size correction at p < 0.05. Percent signal change for the following clusters was examined using analysis of covariance adjusting for maternal cigarettes/day during pregnancy: left middle frontal, postcentral, bilateral superior temporal gyri, and left insula; the following were adjusted for years of maternal education: left superior frontal, fusiform, and right postcental gyrus, left insula, and left anterior cingulate.
Fig. 1.
Region in the right orbitofrontal cortex showing greater activation increases during NoGo trials compared to Go trials in control children than children with FAS/PFAS.
Fig. 2.
Region in the left anterior cingulate showing greater activation increases during NoGo trials compared to Go trials in control children than children with FAS/PFAS.
Fig. 3.
Region in the left dlPFC showing greater activation increases during NoGo trials compared to Go trials in children with FAS/PFAS than control children.
All control variables were examined in relation to the percent signal change values for each cluster to identify potential confounders. Except for smoking and maternal education, none of the other control variables were related to the outcomes (all ps > 0.10). Although number of cigarettes smoked per day during pregnancy was related to percent signal change for five clusters and maternal years of education to percent signal change for an additional five clusters (see footnote to Table 4), the alcohol exposure group differences for all the clusters remained significant after statistical adjustment for their respective potential confounders (all ps < 0.05). The alcohol effect on IQ did not mediate the effect of alcohol on the anterior cingulate, orbital frontal gyrus, or any of the other frontal regions that were activated by the children in the FAS/PFAS and control groups (Table 5). The only effects significantly mediated by IQ were on regional brain activation in one right middle temporal cluster (50, 14, −32), the bilateral insula, and the right cerebellar tonsil.
Table 5.
Mediation by IQ of the effects of prenatal alcohol exposure on regional brain activations
| Region | B1 | B2 | ta |
|---|---|---|---|
| Control > FAS/PFAS | |||
| L Anterior Cingulate | −0.62 | −0.63 | 0.1 |
| −0.55 | −0.50 | −0.4 | |
| L Superior Frontal | −0.56 | −0.50 | −0.6 |
| R Superior Frontal | −0.59 | −0.46 | −1.4† |
| −0.54 | −0.43 | −0.8 | |
| R Orbitofrontal Gyrus | −0.53 | −0.60 | 0.4 |
| R Precentral Gyrus | −0.59 | −0.53 | −0.9 |
| L Postcentral Gyrus | −0.58 | −0.55 | −0.3 |
| R Postcentral Gyrus | −0.66 | −0.69 | 0.6 |
| L Superior Temporal | −0.74 | −0.76 | 0.4 |
| R Superior Temporal | −0.67 | −0.55 | −1.1 |
| −0.63 | −0.50 | −1.4† | |
| L Middle Temporal | −0.55 | −0.49 | −0.7 |
| R Middle Temporal | −0.62 | −0.58 | −0.5 |
| −0.52 | −0.33 | −1.8* | |
| L Insula | −0.74 | −0.65 | −1.9* |
| −0.61 | −0.63 | 0.2 | |
| R Insula | −0.69 | −0.59 | −2.2* |
| L Fusiform Gyrus | −0.56 | −0.50 | −0.8 |
| R Calcarine Sulcus | −0.62 | −0.58 | −0.6 |
| L Putamen | −0.58 | −0.62 | 0.6 |
| Cerebellar Vermis | −0.73 | −0.66 | −1.6† |
| L Cerebellar Culmen | −0.72 | −0.63 | −1.4† |
| R Cerebellar Culmen | −0.69 | −0.58 | −1.4† |
| FAS/PFAS > Control | |||
| L Superior Frontal | 0.62 | 0.63 | −0.2 |
| L Middle Frontal | 0.56 | 0.60 | −0.4 |
| 0.52 | 0.49 | 0.3 | |
| R Middle Frontal | 0.54 | 0.65 | −1.1 |
| R Cerebellar Tonsil | 0.67 | 0.78 | −2.3* |
B1 is the raw regression coefficient for the effect of prenatal alcohol exposure at the first step of the regression analysis; B2, the coefficient for the effect at the second step.
Difference in coefficients method (Clogg et al, 1992)
p < 0.10
p < 0.05
Discussion
This is the first study to examine neural bases of response inhibition in children with a diagnosis of FAS or PFAS using a GNG paradigm with a relatively large number of distinct Go stimuli. Although the alcohol exposed and control groups performed equally well on the GNG task administered in the scanner, these groups showed different neural activation patterns. Compared with the FAS/PFAS group, the controls showed greater activation in the inferior frontal region, which has been linked to response inhibition in typically developing children (e.g., Aron et al 2004). By contrast, as seen in previous studies of children with FASD (Fryer et al. 2007; O’Brien et al. 2013; Ware et al. 2015), the dlPFC in the middle frontal gyrus showed greater activation in the FAS/PFAS group compared to normal controls. The stronger dlPFC activations during this simple response inhibition task suggest compensation for immature and inefficient inferior frontal lobe function during inhibitory processing in children with FASD that was also seen in previous studies (Fryer et al. 2007; Ware et al. 2015). It is also of interest that, compared with the FAS/PFAS group, the controls showed greater activation of the dorsal anterior cingulate, a region linked to cognitive control that is activated in typically developing children performing this task. The greater activation seen in the control group in temporal and fusiform gyrus regions was also reported during a letter recognition task in typically developing young adults (Park et al. 2012), suggesting that this finding may be attributable to the relatively large number of letter Go stimuli in the current task.
The effects of prenatal alcohol exposure are extensive, including impairment in overall intellectual function, indicated by lower IQ scores. Mediation analysis was performed to determine if the alcohol effect on response inhibition might be attributable to the overall impairment in intellectual function associated with this exposure. This analysis showed that the frontal regions known to mediate response inhibition were directly affected by prenatal alcohol (over and above its effects on overall intellectual function), whereas in the temporal and insula regions, which are known to be involved in letter recognition, the effect of alcohol was mediated by its impact on IQ.
Our study adds to the body of literature comparing underlying neural activation patterns in normally developing children and those with pediatric disorders, such as FASD and ADHD, which are associated with problems in behavioral inhibition. As in previous studies of FASD, exposed children exhibited an increase in activation in the dlPFC, an area of frontal cortex associated with working memory and executive function that is not activated during response inhibition in typically developing children. These data thus suggest that, by contrast to the control children, who activate the inferior frontal region, which is believed to be specialized for response inhibition, alcohol-exposed children rely on middle frontal regions mediating higher order executive function to perform this relatively simple response inhibition task. Although behavioral between-group differences were not seen on this relatively simple GNG task, the reduced activation in the regions relied on by typically developing children and increased activation in regions known to mediate higher order cognitive function suggest that performance is likely to be poorer in alcohol-exposed children during more challenging response inhibition tasks and in real-life situations dependent on response inhibition skills.
Psychostimulant medications, such as methylphenidate and amphetamines, may affect BOLD response in pediatric samples, including children with ADHD, and are frequently used with alcohol-exposed children who exhibit ADHD-like symptoms (e.g., Ware et al. 2015; O’Malley et al. 2000). A strength of this study is that all of the children were unmedicated during and prior to the assessment since psychostimulant medications are virtually never prescribed in this disadvantaged population. The findings, thus, provide data on the impact of prenatal alcohol exposure on response inhibition in medication naïve children, thereby confirming and extending previous findings from studies that included children who were previously medicated and/or unable to abstain from their use during testing.
Although an ADHD comparison group was not recruited for this study, the literature on response inhibition shows that, like those with FASD, children with ADHD also exhibit reduced activation in the inferior frontal cortex during response inhibition compared with controls. However, the children with FAS and PFAS also show an increased dlPFC activation that is not seen in ADHD. Because poorer response inhibition is seen behaviorally in both disorders, the stimulant medications that have been developed for children diagnosed with ADHD are often also prescribed for children with behavioral problems whose root cause is prenatal alcohol exposure. However, methylphenidate and other psychostimulants used to treat ADHD have been found to be less consistently effective in children with FASD (Kodituwakku and Kodituwakku 2011; O’Malley et al. 2000; Frankel et al. 2006). The findings reported here and elsewhere indicating that different neural pathways may mediate response inhibition in FASD suggest that different behavioral interventions are likely to be necessary.
One limitation of this study is the relatively small sample size. However, the three previous fMRI studies that used GNG to study response inhibition in FASD were also conducted on similarly small samples; all three were performed on children from the same U.S. clinic. The evidence of similar activation patterns in children with FASD reported here in a very different population, therefore, provides important convergent validation of the findings reported in the previous U.S. studies. Because these children were not recruited until childhood, the maternal reports of alcohol consumption during pregnancy were by necessity retrospective. However, the validity of these reports was demonstrated in a previous study of children in this cohort, in which these maternal reports were found to be related to degree of activation in multiple brain regions associated with number processing (Woods et al. 2015). As in all correlational studies, the observed effects may be attributable to confounding from unmeasured control variables. However, all potential confounders for which data were available were controlled for and the alcohol effect persisted after adjustment for confounders. Although prenatal exposure to maternal smoking has been linked to changes in brain structure (Roza et al. 2007) as well as deficits in GNG task performance (Bennett et al. 2009) and was also associated with increased activation in postcentral gyrus, insula, bilateral superior temporal gyrus and dlPFC in the current study, statistical analysis showed that these increases in regional brain activation were attributable to prenatal alcohol exposure rather than maternal smoking.
In summary, control children showed greater activation increases in the inferior frontal region and anterior cingulate cortex during the NoGo trials compared to Go trials, a finding that is consistent with the literature on response inhibition in typically developing children. By contrast, children diagnosed with FAS or PFAS showed an increase in activation of the prefrontal regions, especially dlPFC, which is involved in executive function and working memory, suggesting that the neural pathways that mediate response inhibition in typically developing children do not function efficiently in children with FASD, requiring them to depend on alternative, compensatory cognitive processes. Although prenatal alcohol exposure did not affect behavioral performance on this simple GoNo task, the failure to activate the brain regions associated with response inhibition in the control group suggests that difficulties in response inhibition are likely when the child is confronted with more challenging cognitive tasks or more challenging social contexts. The pattern of brain activation seen in this study is consistent with previous reports in fMRI studies of response inhibition in FASD and is different from the pattern seen in fMRI studies of response inhibition in ADHD. Although behavioral deficits in response inhibition are commonly seen in children with both these disorders, the distinct pattern of neural activation seen in FASD suggests that different approaches to treatment are likely warranted.
Acknowledgments
We thank our University of Cape Town and Wayne State University research staff, including Maggie September, Mariska Pienaar, Mandy Cronje, and Renee Sun, for their work on subject recruitment and data collection. We also thank Vaibhav Diwadkar for his consultation and review of the manuscript; Dominic Cheng, for his consultation; H. Eugene Hoyme, MD, Luther Robinson, MD, and Nathaniel Khaole, MD, who conducted the FASD dysmorphology assessments in the 2005 and 2009 clinics; and H.E. Hoyme, MD, Greetje DeJong, MD, and Prachi Shah, MD, in 2013 clinic; and R. Colin Carter, who oversaw the growth assessments at our three clinics. We greatly appreciate the contributions of the mothers and children for their participation in the study.
This study was supported by a Fogarty International Research Collaboration Award from the National Institutes of Health [R03 TW007030 to S.W.J.]; a National Institute on Alcohol Abuse and Alcoholism (NIAAA) [R01 AA016781 to S.W.J.]; a Focus Area grant [FA2005040800024 to E.M.M.] from the National Research Foundation of South Africa; the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation of South Africa; Medical Research Council of South Africa; a Children’s Bridge grant from the Office of the President of Wayne State University; seed money grant from the University of Cape Town; and a grant from the Lycaki-Young Fund from the State of Michigan [to S.W.J. and J.L.J.] The FASD dysmorphology clinic assessments were supported in part by grants from the NIAAA Collaborative Initiative on Fetal Alcohol Spectrum Disorder [U01 AA014790 to S.W.J., U24 AA014815 to K. Jones, and U01 AA014809 to T. Foroud].
Footnotes
Conflict of Interest: None
References
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Response inhibition in attention-deficit hyperactivity disorder. Mental Retardation and Developmental Disability Research Reviews. 1999;5:177–184. [Google Scholar]
- Bennett DS, Mohamed FB, Carmody DP, Bendersky M, Patel S, Khorrami M, Faro SH, Lewis M. Response inhibition among early adolescents prenatally exposed to tobacco: An fMRI study. Neurotoxicology and Teratology. 2009;31:283–290. doi: 10.1016/j.ntt.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JD. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002;33:301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burden MJ, Andrew C, Saint-Amour D, Meintjes EM, Molteno CD, Hoyme EH, Robinson LK, Khaole N, Nelson CA, Jacobson JL, Jacobson SW. The effects of fetal alcohol syndrome on response execution and inhibition: an event-related potential study. Alcoholism: Clinical and Experimental Research. 2009;33:1994–2004. doi: 10.1111/j.1530-0277.2009.01038.x. [DOI] [PubMed] [Google Scholar]
- Carmichael-Olson H, Feldman JJ, Streissguth AP, Gonzalez RD. Neuropsychological deficits and life adjustment in adolescents and adults with fetal alcohol syndrome. Alcoholism: Clinical and Experimental Research. 1992;16:380. [PubMed] [Google Scholar]
- Carter RC, Jacobson JL, Molteno CD, Dodge NC, Meintjes EM, Jacobson SW. Fetal Alcohol Growth Restriction and Cognitive Impairment. Pediatrics. 2016;138(2) doi: 10.1542/peds.2016-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles CD, Platzman KA, Raskind-Hood CL, Brown RT, Falek A, Smith IE. A comparison of children affected by prenatal alcohol exposure and attention deficit, hyperactivity disorder. Alcoholism: Clinical and Experimental Research. 1997;21:150–161. [PubMed] [Google Scholar]
- Clogg CC, Petkova E, Shihadeh ES. Statistical methods for analyzing collapsibility in regression models. Journal of Education and Behavioral Statistics. 1992;17:51–74. [Google Scholar]
- Cook JL, Green CR, Lilley CM, Anderson SM, Baldwin ME, Chudley AE, Conry JL, LeBlanc N, Loock CA, Mallon BF, McFarlane AA, Temple VK, Rosales T. Fetal alcohol spectrum disorder: a guideline for diagnosis across the lifespan. Canadian Medical Association Journal. 2016;188:191–197. doi: 10.1503/cmaj.141593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubillo A, Halari R, Ecker C, Giampietro V, Taylor E, Rubia K. Reduced activation and inter-regional functional connectivity of fronto-striatal networks in adults with childhood Attention-Deficit Hyperactivity Disorder (ADHD) and persisting symptoms during tasks of motor inhibition and cognitive switching. Journal of Psychiatric Research. 2010;44:629–639. doi: 10.1016/j.jpsychires.2009.11.016. [DOI] [PubMed] [Google Scholar]
- Dodge NC, Jacobson JL, Jacobson SW. Protective effects of the alcohol dehydrogenase-ADH1B*3 allele on attention and behavior problems in adolescents exposed to alcohol during pregnancy. Neurotoxicology and Teratology. 2014;41:43–50. doi: 10.1016/j.ntt.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S, Thomas KM, Worden MS, Yang Y, Casey BJ. The effect of preceding context on inhibition: an event-related fMRI study. Neuroimage. 2002;16:449–453. doi: 10.1006/nimg.2002.1074. [DOI] [PubMed] [Google Scholar]
- Frankel F, Paley B, Marquardt R, O’Connor M. Stimulants, neuroleptics, and children’s friendship training for children with fetal alcohol spectrum disorders. Journal of Child Adolescent Psychopathology. 2006;16:777–789. doi: 10.1089/cap.2006.16.777. [DOI] [PubMed] [Google Scholar]
- Fryer SL, Tapert SF, Mattson SN, Paulus MP, Spadoni AD, Riley EP. Prenatal alcohol exposure affects frontal-striatal BOLD response during inhibitory control. Alcoholism: Clinical and Experimental Research. 2007;31:1415–1424. doi: 10.1111/j.1530-0277.2007.00443.x. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The Prefrontal Cortex: Anatomy, Physiology and Neuropsychology of the Frontal Lobe. New York, NY: Raven Press; 1989. [Google Scholar]
- Goldman-Rakic PS. Circuitry of primate prefrontal cortex and regulation of behavior by representational memory. Handbook of Physiology-The Nervous System. 1987;5:373–417. [Google Scholar]
- Gerhold MM, Jacobson SW, Jacobson JL, Molteno CD, Meintjes EM, Andrew CM. An ERP study of response inhibition in the auditory domain in children with fetal alcohol spectrum disorders. Alcoholism: Clinical and Experimental Research. doi: 10.1111/acer.13263. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart H, Chantiluke K, Cubillo AI, Smith AB, Simmons A, Brammer MJ, Marquand AF, Rubia K. Pattern classification of response inhibition in ADHD: Toward the development of neurobiological markers for ADHD. Human Brain Mapping. 2014;35:3083–3094. doi: 10.1002/hbm.22386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyme HE, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, Buckley DG, Miller JH, Aragon AS, Khaole N, Viljoen DL, Jones KL, Robinson LK. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: clarification of the 1996 institute of medicine criteria. Pediatrics. 2005;115:39–47. doi: 10.1542/peds.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson JL, Dodge ND, Burden MJ, Klorman R, Jacobson SW. Number processing in adolescents with prenatal alcohol exposure and ADHD: Differences in the neurobehavioral phenotype. Alcoholism: Clinical and Experimental Research. 2011;35:431–442. doi: 10.1111/j.1530-0277.2010.01360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW, Chiodo LM, Sokol RJ, Jacobson JL. Validity of maternal report of prenatal alcohol, cocaine, and smoking in relation to neurobehavioral outcome. Pediatrics. 2002;109:815–825. doi: 10.1542/peds.109.5.815. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Carr LG, Croxford J, Sokol RJ, Li T-K, Jacobson JL. Protective effects of the alcohol dehydrogenase-ADH1B allele in African American children exposed to alcohol during pregnancy. Journal of Pediatrics. 2006;148:30–37. doi: 10.1016/j.jpeds.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Stanton ME, Molteno CD, Burden MJ, Fuller DS, Hoyme EH, Robinson LK, Khaole N, Jacobson JL. Impaired eyeblink conditioning in children with fetal alcohol syndrome. Alcoholism: Clinical and Experimental Research. 2008;32:365–372. doi: 10.1111/j.1530-0277.2007.00585.x. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Stanton ME, Meintjes EM, Molteno CD. Biobehavioral markers of adverse effect in fetal alcohol spectrum disorders. Neuropsychology Review. 2011a;21:148–166. doi: 10.1007/s11065-011-9169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW, Stanton ME, Dodge NC, Pienaar M, Fuller DS, Molteno CD, Robinson LK, Khaole N, Jacobson JL. Impaired delay and trace eyeblink conditioning in school-age children with fetal alcohol syndrome. Alcoholism: Clinical and Experimental Research. 2011b;35:250–264. doi: 10.1111/j.1530-0277.2010.01341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen T, Heslenfeld DJ, van Mourik R, Logan GD, Oosterlaan J. Neural correlates of response inhibition in children with attention-deficit/hyperactivity disorder: A controlled version of the stop-signal task. Psychiatry Research: Neuroimaging. 2015;233:278–284. doi: 10.1016/j.pscychresns.2015.07.007. [DOI] [PubMed] [Google Scholar]
- Kodituwakku PW, Handmaker NS, Cutler EK, Weathersby EK, Handmaker SD. Specific impairments in self-regulation in children exposed to alcohol prenatally. Alcoholism: Clinical and Experimental Research. 1995;19:1558–1564. doi: 10.1111/j.1530-0277.1995.tb01024.x. [DOI] [PubMed] [Google Scholar]
- Kodituwakku PW, Kodituwakku EL. From research to practice: an integrative framework for the development of interventions for children with fetal alcohol spectrum disorders. Neuropsychology Review. 2011;21:204–223. doi: 10.1007/s11065-011-9170-1. [DOI] [PubMed] [Google Scholar]
- Liddle PF, Kiehl KA, Smith AM. Event-related fMRI study of response inhibition. Human Brain Mapping. 2001;12:100–109. doi: 10.1002/1097-0193(200102)12:2<100::AID-HBM1007>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindinger NM, Malcolm-Smith S, Dodge NC, Molteno CD, Thomas KGF, Meintjes EM, Jacobson SW. Theory of Mind in children with fetal alcohol spectrum disorders. Alcoholism: Clinical and Experimental Research. 2016;40:367–376. doi: 10.1111/acer.12961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madge EM, Van den Berg AR, Robinson M, Landman J. Junior South African Individual Scales. Human Sciences Research Council, Pretoria. 1981 [Google Scholar]
- Mattson SN, Crocker N, Nguyen TT. Fetal alcohol spectrum disorders: neuropsychological and behavioral features. Neuropsychology Review. 2011;21:81–101. doi: 10.1007/s11065-011-9167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Goodman AM, Caine C, Delis DC, Riley EP. Executive functioning in children with heavy prenatal alcohol exposure. Alcoholism: Clinical and Experimental Research. 1999;23:1808–1815. [PubMed] [Google Scholar]
- Mattson SN, Riley EP. A review of the neurobehavioral deficits in children with fetal alcohol syndrome or prenatal exposure to alcohol. Alcoholism: Clinical and Experimental Research. 1998;22:279–294. doi: 10.1111/j.1530-0277.1998.tb03651.x. [DOI] [PubMed] [Google Scholar]
- May PA, Blankenship J, Marais A-S, Gossage PJ, Kalberg WO, Joubert B, Cloete M, Barnard R, De Vries M, Hasken J, Robinson LK, Adnams CM, Buckley D, Manning M, Parry CD, Hoyme HE, Tabachnick B, Seedat S. Maternal alcohol consumption producing fetal alcohol spectrum disorders (FASD): Quantity, frequency, and timing of drinking. Drug and Alcohol Dependence. 2013;133:502–512. doi: 10.1016/j.drugalcdep.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mick E, Biederman J, Faraone SV, Sayer J, Kleinman S. Case-control study of attention-deficit hyperactivity disorder and maternal smoking, alcohol use, and drug use during pregnancy. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41:378–385. doi: 10.1097/00004583-200204000-00009. [DOI] [PubMed] [Google Scholar]
- Morein-Zamir S, Dodds C, van Hartevelt TJ, Schwarzkopf W, Sahakian B, Müller U, Robbins T. Hypoactivation in right inferior frontal cortex is specifically associated with motor response inhibition in adult ADHD. Human Brain Mapping. 2014;35:5141–5152. doi: 10.1002/hbm.22539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT. Is ADHD a disinhibitory disorder? Psychological Bulletin. 2001;127:571–598. doi: 10.1037/0033-2909.127.5.571. [DOI] [PubMed] [Google Scholar]
- Nigg JT. Response inhibition and disruptive behaviors: toward a multiprocess conception of etiological heterogeneity for ADHD combined type and conduct disorder early-onset type. Annals of the New York Academy of Sciences. 2003;1008:170–182. doi: 10.1196/annals.1301.018. [DOI] [PubMed] [Google Scholar]
- O’Brien JW, Norman AL, Fryer SL, Tapert SF, Paulus MP, Jones K, Riley EP, Mattson SN. Effect of predictive cuing on response inhibition in children with heavy prenatal alcohol exposure. Alcoholism: Clinical and Experimental Research. 2013;37:644–654. doi: 10.1111/acer.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- O’Malley KD, Koplin B, Dohner VA. Psychostimulant clinical response in fetal alcohol syndrome. Canadian Journal of Psychiatry. 2000;45:90–91. [PubMed] [Google Scholar]
- Park J, Hebrank A, Polk TA, Park DC. Neural dissociation of number from letter recognition and its relationship to parietal numerical processing. Journal of Cognitive Neuroscience. 2012;24:39–50. doi: 10.1162/jocn_a_00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliszka SR, Glahn DC, Semrud-Clikeman M, Franklin C, Perez R, Xiong J, Liotti M. Neuroimaging of inhibitory control areas in children with attention deficit hyperactivity disorder who were treatment naive or in long-term treatment. American Journal of Psychiatry. 2006;163:1052–1060. doi: 10.1176/ajp.2006.163.6.1052. [DOI] [PubMed] [Google Scholar]
- Rasmussen C, Benz J, Pei J, Andrew G, Schuller G, Abele-Webster L, Alton C, Lord L. The impact of an ADHD co-morbidity on the diagnosis of FASD. Canadian Journal of Clinical Pharmacology. 2010;17:165–176. [PubMed] [Google Scholar]
- Roza SJ, Verburg BO, Jaddoe VWV, Hofman A, Mackenbach JP, Steegers EA, Witteman JC, Verhulst FC, Tiemeier H. Effects of maternal smoking in pregnancy on prenatal brain development. The Generation R Study. European Journal of Neuroscience. 2007;25:611–617. doi: 10.1111/j.1460-9568.2007.05393.x. [DOI] [PubMed] [Google Scholar]
- Rubia K, Halari R, Smith AB, Mohammed M, Scott S, Giampietro V, Taylor E, Brammer MJ. Dissociated functional brain abnormalities of inhibition in boys with pure conduct disorder and in boys with pure attention deficit hyperactivity disorder. The American Journal of Psychiatry. 2008;165:889–897. doi: 10.1176/appi.ajp.2008.07071084. [DOI] [PubMed] [Google Scholar]
- Steinmann TP, Andrew CM, Thomsen CE, Kjær TW, Meintjes EM, Molteno CD, Jacobson JL, Jacobson SW, Sorensen HB. An auditory Go/No-Go study of event-related potentials in children with fetal alcohol spectrum disorders; Conference proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Annual Conference; 2011. pp. 789–792. [DOI] [PubMed] [Google Scholar]
- Tamm L, Menon V, Reiss AL. Maturation of brain function associated with response inhibition. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41:1231–1238. doi: 10.1097/00004583-200210000-00013. [DOI] [PubMed] [Google Scholar]
- Ware AL, Infante AM, O’Brien JW, Tapert SF, Jones K, Riley EP, Mattson SN. An fMRI study of behavioral response inhibition in adolescents with and without histories of heavy prenatal alcohol exposure. Behavioural Brain Research. 2015;278:137–146. doi: 10.1016/j.bbr.2014.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods KJ, Meintjes EM, Molteno CD, Jacobson SW, Jacobson JL. Parietal dysfunction during number processing in children with fetal alcohol spectrum disorders. NeuroImage: Clinical. 2015;8:594–605. doi: 10.1016/j.nicl.2015.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai ZW, Pajtek S, Luna B, Geier CF, Ridenour TA, Clark DB. Reward-modulated response inhibition, cognitive shifting, and the orbital frontal cortex in early adolescence. Journal of Research on Adolescence. 2015;25:753–764. doi: 10.1111/jora.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]