Abstract
Recent advances in cryo-electron tomography (cryo-ET) have allowed direct visualization of the initial interactions between bacteriophages and their hosts. Previous studies focused on phage infection in Gram-negative bacteria but it is of particular interest how phages penetrate the thick, highly cross-linked Gram-positive cell wall. Here we detail structural intermediates of phage Φ29 during infection of Bacillus subtilis. Use of a minicell-producing strain facilitated in situ tomographic reconstructions of infecting phage particles. Φ29 initially contacts the cell wall at an angle through a subset of the twelve appendages, which are attached to the collar at the head proximal portion of the tail knob. The appendages are flexible and switch between extended and downward conformations during this stage of reversible adsorption; appendages enzymatically hydrolyze wall teichoic acids to bring the phage closer to the cell. A cell wall-degrading enzyme at the distal tip of the tail knob locally digests peptidoglycan, facilitating penetration of the tail further into the cell wall, and the phage particle reorients so that the tail becomes perpendicular to the cell surface. All twelve appendages attain the same “down” conformation during this stage of adsorption. Once the tail has become totally embedded in the cell wall, the tip can fuse with the cytoplasmic membrane. The membrane bulges out, presumably to facilitate genome ejection into the cytoplasm, and the deformation remains after complete ejection. This study provides the first visualization of the structural changes occurring in a phage particle during adsorption and genome transfer into a Gram-positive bacterium.
INTRODUCTION
Gram-positive bacteria, such as Bacillus subtilis, have a cell envelope very different from those of Gram-negative bacteria, like Escherichia coli. While Gram-negative bacteria contain an outer membrane and a thin peptidoglycan layer outside their cytoplasmic membrane, B. subtilis and other Gram-positive bacteria harbor only a thick cell wall (30–100 nm) (Silhavy et al., 2010). The cell wall is composed of highly cross-linked layers of peptidoglycan interwoven with teichoic and lipoteichoic acid, anchored to the peptidoglycan and cytoplasmic membrane respectively. Teichoic acids are thought to further crosslink the cell wall and create a charge barrier that protects the cytoplasmic membrane.
The thick and highly cross-linked Gram-positive cell wall presents an obstacle for bacteriophage infection, as they must completely penetrate the cell wall to deliver their genome into the cell cytoplasm. B. subtilis phage Φ29 has been extensively characterized genetically and was developed as a model organism for DNA replication, transcription, morphogenesis, DNA packaging studies and nanotechnology applications (Geng et al., 2013; Grimes et al., 2002; Guo et al., 2016; Meijer et al., 2001; Morais, 2012). Φ29 has also been the subject of much structure-based research; crystallographic structures of several virion proteins have been solved at atomic resolution (Guasch et al., 2002; Morais et al., 2008; Morais et al., 2003; Simpson et al., 2001; Xiang and Rossmann, 2011; Xiang et al., 2008; Xiang et al., 2009). In addition, atomic force and especially cryo-electron microscopy (cryo-EM) have provided a wealth of information on virion structure (Choi et al., 2006; Comolli et al., 2008; Hernando-Perez et al., 2012; Mao et al., 2016; Morais et al., 2005; Morais et al., 2008; Morais et al., 2001; Tang et al., 2008; Tao et al., 1998; Xiang et al., 2008).
The wild-type Φ29 capsid adopts a T=3, Q=5 prolate structure 54 nm long × 45 nm wide with prominent ~25 nm head fibers protruding from the quasi-3-fold axes (Tao et al., 1998). The homotrimeric head fibers are not essential for infection in the laboratory (Reilly et al., 1977; Salas et al., 1972) although they may enhance virus attachment to host cells. Thin section electron microscopy (EM) of resin-substituted infected cells revealed that fibered phages form more ordered arrays on the cell surface than do fiberless particles (Xiang and Rossmann, 2011). One vertex of the capsid is occupied by a neck, which consists of a dodecameric head-tail connector, the portal, and a lower collar attached to a non-contractile tail. Twelve homotrimeric appendages, attached to the head proximal end of the lower collar, are trimers of gp12*; the C-terminal chaperone domain of the primary gene 12 translation product is autocatalytically cleaved after trimerization (Moreno and Bluzat-Moreno, 1978; Villanueva and Salas, 1981; Xiang et al., 2009; Young, 1967). Appendages are required for virus adsorption to intact cells as they harbor an enzymatic activity that hydrolyzes wall teichoic acid and bring the tail knob closer to the cell surface (Moreno and Bluzat-Moreno, 1978; Villanueva and Salas, 1981; Xiang et al., 2009; Young, 1967). The tail knob is a complex of two proteins, one of which: gp13, has been shown to have a peptidoglycan hydrolyzing activity (Cohen et al., 2008; Cohen et al., 2009; Xiang et al., 2008). The 19.3 kb Φ29 dsDNA genome is attached to terminal protein gp3, with one molecule covalently bound to each 5′ end. Most of the DNA is tightly packed inside the capsid at near crystalline density, but the last end to be packaged (the first to be ejected into an infected cell), together with its gp3 molecule, extends into the lumen of the lower collar (Tang et al., 2008).
The overall process of Φ29 infection initiation is not well understood. Questions remain as to how Φ29 is targeted to the cell wall and how the phage structurally overcomes the physical barrier of the thick peptidoglycan layer. In B. subtilis, this layer is ~55 nm thick (Beeby et al., 2013; Matias and Beveridge, 2005; Zuber et al., 2006), considerably more than the 38 nm length of the phage tail. Direct visualization of Φ29 adsorption and infection would aid our understanding of how the tail penetrates the peptidoglycan, but the large size of B. subtilis cells and the thickness of its cell wall present challenges for high-resolution cryo-ET studies (Lucic et al., 2013; Milne and Subramaniam, 2009). Encouraged by recent applications using naturally thin cells or engineered skinny minicells of Gram-negative bacteria as hosts for phage adsorption and infection (Farley et al., 2016; Guerrero-Ferreira et al., 2011; Hu et al., 2013; Hu et al., 2015; Sun et al., 2014), we develop the first cryo-ET application of B. subtilis minicells as hosts to investigate structural intermediates during Φ29 infection.
MATERIALS AND METHODS
Phage and bacteria
The B. subtilis minicell producing strain DS3774 (a minD mutant) (Patrick and Kearns, 2008) was grown in Luria–Bertani (LB) (10 g of tryptone, 5 g of yeast extract, 5 g of NaCl per liter) broth at 37°C to mid-log phase. The culture was centrifuged at 17,400 × g for 8 min to remove large cells, and the supernatant was again centrifuged at 53,200 × g for 25 min to pellet minicells. Minicells, resuspended in LB, remained motile as evaluated by light microscopy. Wild-type Φ29 was grown using B. subtilis A12; clarified lysates were concentrated by centrifugation and phage particles purified by equilibrium buoyant density centrifugation in buffer (tris-HCl, pH7.6, 10 mM MgCl2, 5 mM CaCl2) containing CsCl. The opalescent phage band was collected with a syringe through the side of the tube.
Phage infection, cryo-ET data collection and 3-D reconstruction
Minicells were infected with Φ29 and incubated at 37 °C from 3 to 30 min. Portions of the culture were mixed with 15 nm fiducial gold markers and deposited onto freshly glow-discharged, holey carbon grids. Grids were briefly blotted with filter paper and plunge frozen in liquid ethane.
Grids were imaged at −170 °C on a 300kv Technai F30 Polara (FEI) equipped with a K2 Summit direct electron detector (Gatan). Tilt series were collected in dose fractionation mode at a magnification of 9,400x, resulting in a final pixel size of 4.5 Å. Using SerialEM (Mastronarde, 2005), approximately 350 low-dose, single-axis tilt series were collected at −6 to −8 μm defocus with a cumulative dose of ~60 e−/Å2 distributed over 41 images and covering an angular range of −60° to +60°, with angular increments of 3°. Tilt series were drift corrected (Li et al., 2013), aligned using IMOD (Kremer et al., 1996) through Tomoauto (Morado et al., 2016), and reconstructed using Tomo3D (Agulleiro and Fernandez, 2015). In total, 308 tomographic reconstructions were generated by using Weighted Back Projection (WBP) and used for further processing (Table S1). To enhance contrast and speed up image analysis, we initially generated reconstructions from the binned by 4 aligned tilt series by using Simultaneous Iterative Reconstruction Technique (SIRT).
Subtomogram averaging
We manually selected several hundred particles from the SIRT reconstructions. For each phage particle, we picked two points (the center of the capsid and the tail) to estimate the particle position and initial orientation (two Euler angles) within the tomogram. A global average of all the extracted 4 × 4 × 4 binned sub-tomograms was generated after application of the two Euler angles. After multiple cycles of translational and rotational alignments, the global average was used as the reference for “template matching” as implemented in PyTom (Hrabe et al., 2012). In total, 21,048 particles were selected from 308 SIRT reconstructions (Table S1).
As the pixel size of the tomogram binned by 4 is 1.8 nm and the phage is about 120 nm in length, we extracted sub-tomogram of 128×128×128 voxels for each phage particle. Subtomograms were initially aligned and classified into 80 class averages (Fig. S1) by subtomogram alignment and classification (Winkler, 2007; Winkler et al., 2009). After removing bad particles (those including gold particles or ice contamination), 12,558 phage particles were selected. We extracted 360 × 360 × 360 voxels for each selected particle from the original WBP reconstructions for further processing at higher resolution as described previously (Hu et al., 2013; Hu et al., 2015). To accelerate image analysis, we also generated 4×4×4 binned subtomogram (90 × 90 × 90 voxels) and 2×2×2 binned subtomogram (180 × 180 × 180 voxels) for each particles.
Multivariate statistical analysis and hierarchical ascendant classification were applied in separating into four groups: DNA-filled free phage, cell-wall attached phage, cell-wall inserted phage, and empty phage particles. Fourier shell correlation coefficients were estimated by comparing the correlation between two randomly divided halves of the aligned images used to generate the final maps. The resolution of those asymmetric reconstructions is estimated as 3.4 nm (free virion), 3.8 nm (phage attached) and 4.0 nm (phage penetrating cell wall) (Fig. S2). We also did focused classification for each appendage by masking out its density from adjacent structures. 3-D visualization was performed in Chimera (Pettersen et al., 2004).
Molecular Modeling and 3-D visualization
UCSF Chimera was utilized for 3-D surface rendering of sub-tomogram averages and molecular modeling (Pettersen et al., 2004). We manually docked three structures gp8.5 (PDB ID: 3QC7) for the head fiber (Xiang and Rossmann, 2011), gp12* (PDB ID: 3GQ7) for the appendages (Xiang et al., 2009), and gp13 (PDB ID: 3CSQ) for the tail knob (Xiang et al., 2008). We then used the function “fit in map” in UCSF Chimera to refine the models.
RESULTS AND DISCUSSION
B. subtilis minicells as hosts to study Φ29 infection
Adsorption of Φ29 has long been known to be a slow process (Jacobson and Landman, 1977). To initiate Φ29 infection, we incubated purified Φ29 particles with freshly prepared minicells of a B. subtilis minD mutant strain for up to 30 min, and then prepared frozen hydrated samples for cryo-ET studies (Table S1). Our minicell preparations contained a small proportion of full-sized cells and both were imaged as comparison. B. subtilis cells are long and rod-shaped (Fig. 1A), while minicells are approximately spherical due to aberrant cell division (Fig. 1B). However, both cell types share a similar cell wall morphology with a thickness of ~55 nm. The majority of cells visualized exhibited a zone of lower density immediately outside the cytoplasmic membrane whereas other cells had the cell wall directly abutting the membrane. The lower density zone may correspond to the Gram-positive periplasm (Beeby et al., 2013; Matias and Beveridge, 2005; Zuber et al., 2006).
Figure 1. Tomographic reconstructions of a B. subtilis cell and a minicell infected by Φ29.

Free and bound phages are visible. CW = cell wall; CM = cytoplasmic membrane.
Asymmetric reconstruction of free Φ29
Evident in the tomographic reconstructions are free Φ29 particles (Fig. 1B) and absorbed phages. To localize over thousands of phages, we used “template matching” implemented in Pytom (Hrabe et al., 2012). In total, we identified 12,558 particles in 307 reconstructions. Over 5,000 particles were associated with the cell wall, and about 7,000 particles were free in solution. We used subtomogram averaging to generate an asymmetric reconstruction of the free virion at 3.4 nm resolution (Fig. 2A, B; Fig. S2); the structure is comparable to single particle cryo-EM structures of mature Φ29 (Morais et al., 2005; Tang et al., 2008; Tao et al., 1998).
Figure 2. Asymmetric reconstruction of free Φ29 virions.

A) A central section of an asymmetric reconstruction of Φ29 as determined by subtomogram averaging. No rotational symmetry was applied to the portal and tail assembly, which includes the lower collar, appendages, tail knob and tail tip protein. B) A side view of the surface rendering of a Φ29 virion. C) Surface rendering with crystal structures for the gp8.5 head fiber (PDB 3QC7), gp12* appendages (PDB 3GQ7), and gp13 tail tip protein (PDB 3CSQ) manually docked. In surface renderings and crystal models, head fibers are shown in light blue, the capsid in darker blue, appendages in oranges and yellow, and the tail is shown as a gradient from blue to green, terminating with the tail knob and tip protein. D) A cross-section of the asymmetric reconstruction clearly shows five head fibers (outside of the larger circle), twelve appendages (A1–A12, defined by the larger circle) and the tail knob (TK, defined by the smaller circle). E) A bottom view shows the five head fibers; the twelve appendages are displayed in either an extended (yellow) or down (orange) orientation. F) Two class averages show appendages with the gp12* crystal structures docked into both extended (right panel) and down conformations (left panel).
Fifty-five head fibers, each homotrimers of gp8.5, are attached to the prolate capsid at the quasi-three-fold axes (Fig. 2A–E) (Tao et al., 1998; Xiang and Rossmann, 2011). Each head fiber comprises a cylindrical fiber radiating from a disk-like platform attached to the capsid, with the fiber containing three domains: stem, neck and head. The neck density, connecting the fiber stem and head, is well resolved in the subtomogram average (Fig. 2A, B). The crystal structure of gp8.5 (Xiang and Rossmann, 2011) fits well into the density map (Fig. 2C).
The lower collar of Φ29 is composed of a non-contractile tail that terminates with a tail knob (Fig. 2A, B). Twelve tailspikes, called appendages in Φ29, are attached to the head-proximal region of the lower collar (Fig. 2A, B). Two distinct densities were found for the appendages on free phage as shown in a cross-section (Fig. 2D), supporting previous observations that they exist in two different conformations: up, or extended, and down (Tang et al., 2008; Xiang et al., 2006). As evident from a bottom view of the surface rendering, the majority of the 12 appendages prefer the down position. However, appendages 1 (A1) and 6 (A6) exhibited increased density in the extended position, suggesting that these two spend more time in the extended conformation than the others (Fig. 2D). Appendages A2, A5, A7, and A12 exhibited the lowest density in the extended conformation, suggesting they rarely occupy that state (Fig. 2D).
To provide a quantitative estimate of the conformational distribution for each appendage, we defined a mask for each appendage. The mask is large enough to contain one appendage in two possible conformations, but also smaller enough to exclude two adjacent appendages. Each of a total of twelve masks allows us to perform a focused classification for each individual appendage. Two distinct conformations for each appendage are observed and their distribution is calculated based on the focused classification. As an example, one class average of appendage A1 shows the down conformation (Fig. 2F, left), another class average displays the extended conformation (Fig. 2F, right). The observed occupancy in the extended conformation is 53%. In other words, about half of the A1 appendage adopts the extended conformation whereas the other half is “down”. Similarly, the A6 appendage has a slight preference for being extended: 59% of phages display A6 in the extended conformation. The extended conformation for other appendages is relatively rare (14–21%; Table S2). The overall distribution of all twelve appendages in the down or extended conformations is similar to that previously determined (Tang et al., 2008; Xiang et al., 2006). Although most appendages have a preference for the down conformation, the crystal structure of the gp12* appendage fits well into both maps (Fig. 2F), suggesting that the appendage itself is rigid but its attachment to the lower collar is flexible and that the extended and down conformations may be in a dynamic equilibrium whose value is different for each specific appendage (Table S2). The lower head fibers therefore cannot confer any significant steric hindrance to the appendages switching conformations as suggested in a previous cryo-EM study of a Φ29 mutant lacking head fibers (Tang, Olson et al. 2008).
Visualization of Φ29 infection - Adsorption
To begin to address how Φ29 attaches to and traverses the ~55 nm thick Gram-positive cell wall, which is significantly thicker than the 38 nm non-contractile tail, over 300 tomographic reconstructions were evaluated at different stages of infection (Fig. 3). On simple geometric grounds, the majority of random collisions between Φ29 and a bacterium should involve the head fibers or the tail knob, but we did not observe virions bound solely through those proteins, suggesting that they make only transient or weak interactions. Instead, we commonly found images similar to those shown in Figure 3B and 3E, where the infecting phage appears bound through head fibers, appendages and the tail knob. The phage is typically angled so that the tail is neither perpendicular nor parallel to the cell surface (Fig. 3B, E). Because the tail extends beyond the distal plane of the appendages, if the appendages had initially bound to wall teichoic acid, the virion would naturally angle itself with the head fibers then providing a minor supporting function. Even in those collisions where the phage tip of the tail knob makes the first contact with the cell, subsequent binding of one or more appendages to teichoic acid would again angle the virion to the cell surface (Fig. 3B, E). These images probably correspond to an early infection intermediate. Our observations are consistent with the idea that tight binding by head fibers to the cell surface may preclude a productive infection by preventing the tail knob from interacting with its receptor. In contrast, weak head fiber binding would keep a phage in the vicinity of the cell, providing additional opportunities for the appendages to recognize surface teichoic acid.
Figure 3. Visualization of B. subtilis infected by Φ29.
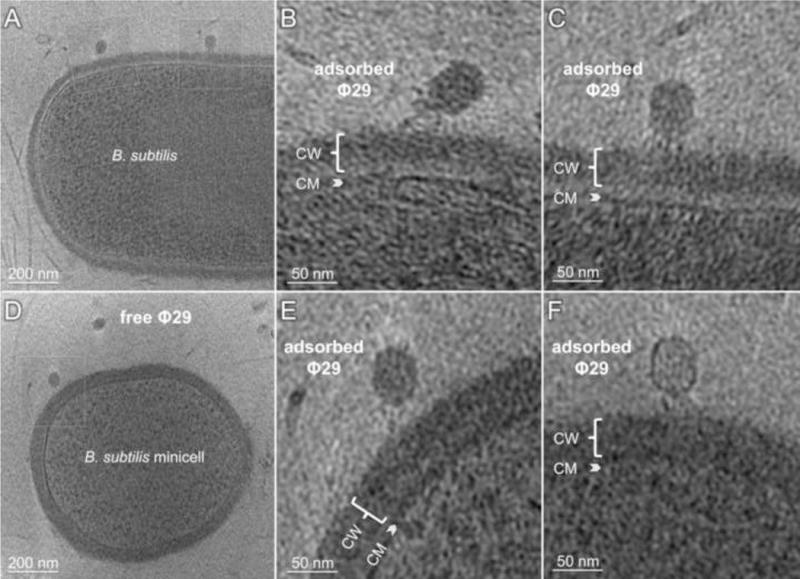
A) A central tomographic slice of an infected rod-shaped cell (partial) with diameter ~1μm. B) and C) Zoom-in views highlight two adsorbed phages with different conformations visible in A). CM: cytoplasmic membrane, CW: cell wall. D) A central slice of an infected B. subtilis minicell. E) A zoom-in view from D) highlights an adsorbed Φ29 interacting with the cell wall. F) A separate minicell with an adsorbed Φ29 whose genome has been partially ejected. CW = cell wall; CM = cytoplasmic membrane.
When the virion is bound at an angle, both the appendages and tail knob appear to contact the cell surface (Fig. 4A, B). To visualize the interaction in more detail, we generated a subtomogram average of about 2,000 adsorbed Φ29 particles (Fig. 4C). The overall structure is very similar to that of a free virion. However, while the appendages of free phage exist in both extended and down positions (Fig. 2), in adsorbed particles the appendages closest to the cell wall are mainly in the down position (Fig. 4C, D; Fig. S3; Table S2). The observed conformational flexibility of appendages should increase the probability that they interact productively with their substrate.
Figure 4. Visualization of Φ29 adsorption.

A, B) Two tomographic slices show phage particles interacting with the cell wall at an angle. C) Averaged structure of adsorbed Φ29 and its interaction with the cell wall at an angle similar to that shown in A and B. D) Surface rendering of the average map. Crystal structures were manually docked into the cryo-ET densities for the gp8.5 head fibers (Xiang and Rossmann, 2011), gp12* appendages (Xiang et al., 2009) and gp13 tail tip protein (Xiang et al., 2008). The appendage in yellow is in the extended conformation. E) Model of the interaction between a “down” appendage based on tomographic observations with a juxtaposed schematic diagram of the Gram-positive cell wall. F) Model of the tail knob position from C and D with manual docking of the crystal structure of the distal tip protein gp13; a cell wall schematic is juxtaposed. In surface renderings and crystal models, head fibers are shown in light blue, the capsid in darker blue, appendages in oranges and yellow, and the tail is shown as a gradient from blue to green, terminating with the tail knob.
Appendages have long been predicted to be related to phage adsorption (Tosi and Anderson, 1973); more recently they were shown to bind and digest wall teichoic acids (Xiang et al., 2009). Therefore, we suggest that the interaction between appendages and the cell wall we captured at this stage of Φ29 infection likely represents a definitive stage towards irreversible phage adsorption (Fig. 4E).
A careful comparison of the two adsorbed phage particles (Fig. 4A, B) reveals that the tail knob in one (Fig. 4B) is embedded in the cell wall, supporting the idea that the distal tip protein (gp13) of the tail knob functions as a peptidoglycan-degrading enzyme during infection and plays an important role in penetrating the cell wall. The crystal structure of gp13 was manually docked into the surface rendering and the predicted peptidoglycan-degrading residues (Cohen et al., 2008; Cohen et al., 2009; Xiang et al., 2008) were highlighted in orange (Fig. 4F) to better understand how the phage could degrade the peptide cross-links between the glycan chains of the cell wall.
Visualization of Φ29 infection - Cell wall penetration
Over 850 phages were observed with the tail perpendicular to the cell surface and embedded in the Gram-positive cell wall but before there is contact with the cytoplasmic membrane (Figs. 3C, 5A). These phage particles therefore appear to have been caught while traversing the thick cell wall. The average structure from the penetrated Φ29 particles reveals that the entire lower collar becomes embedded in the cell wall as the tail tip protein hydrolyzes the peptidoglycan cross-links (Fig. 5B, C, Fig. S4). Enzyme activity presumably renders the polysaccharide chains more flexible, allowing the phage to penetrate the cell wall. The capsid, head fibers, tail and tail knob do not appear to have undergone any significant conformational changes during penetration; their structures are similar to those found in free phage (Fig. 2). However, appendages are found predominantly in the down conformation (Fig. 5B, C, D, S4; Table S2). Penetrating the cell wall therefore shifts the equilibrium of the appendages considerably. Only a subset of appendages, which are symmetrically attached around the head-proximal end of the lower collar, are likely bound to teichoic acid when the virion is adsorbed at an angle to the cell surface. Sequential binding of the remaining appendages to teichoic acid can then provide energy to reorient the virion from its initial angled adsorbed state such that the tail becomes perpendicular to the cell wall, poised for later steps in the infection process. Furthermore, positioning all appendages in the down position also reduces the cross-sectional area of the phage tail, facilitating movement through the cell wall as both teichoic acid and peptidoglycan is hydrolyzed by the concerted actions of, respectively, the appendages and tail tip protein (Cohen et al., 2008; Cohen et al., 2009; Xiang et al., 2008).
Figure 5. Visualization of Φ29 penetrating the cell wall.
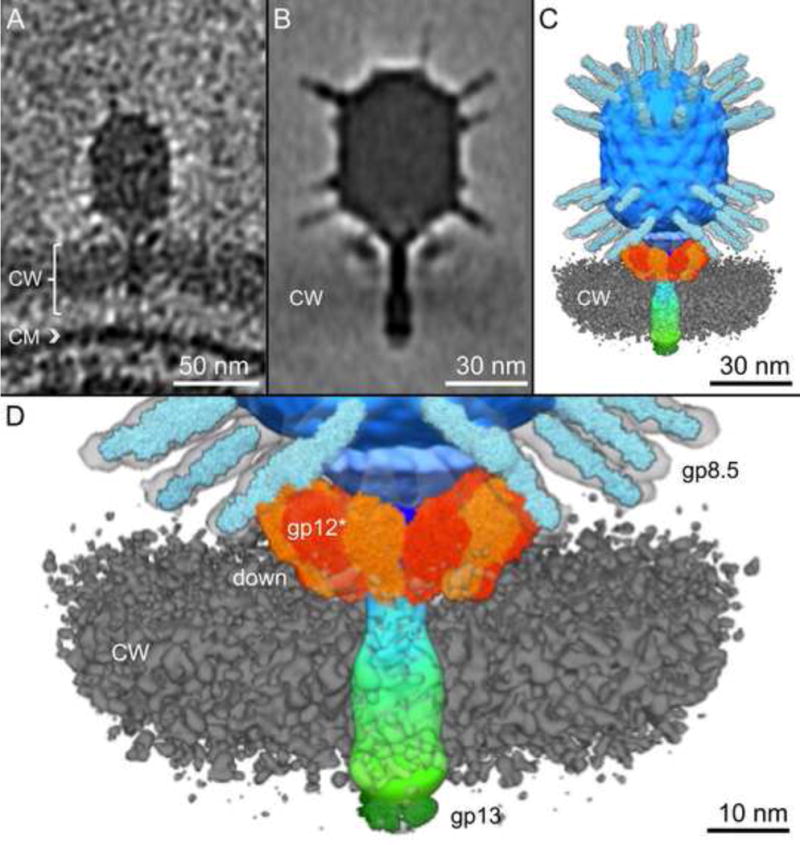
A) A slice through a tomographic reconstruction of a phage particle penetrating the cell wall (CW) perpendicular to the cytoplasmic membrane (CM). B) Density map of adsorbed Φ29 as determined by subtomogram averaging. C) Surface rendering of the adsorbed Φ29 embedded in the cell wall (colored in grey). Crystal structures for the gp8.5 head fibers (Xiang and Rossmann, 2011), gp12 appendages (Xiang et al., 2009) and gp13 tail tip protein (Xiang et al., 2008) have been manually docked into the map. D) Close up surface rendering of appendages in the down position. The cell wall (CW) has been made transparent to allow visualization of the tail knob. Head fibers are shown in light blue, the capsid in darker blue, appendages in light and dark orange, and the tail is shown as a gradient from blue to green, terminating with the tail knob.
Visualization of Φ29 infection - Interaction with the cytoplasmic membrane
Phages were also observed with their tail in direct contact with the cytoplasmic membrane (Fig. 6). This was the least frequent stage of infection we observed in this study (Table S1). The relative paucity of this intermediate structure is almost certainly due to the conditions we chose for Φ29 infection in this pilot study, but this stage of infection is also hard to visualize clearly because of the thickness of the cell wall. In particular, we cannot determine whether the tail tip protein gp13 is still associated with the virion. Nevertheless, our images provide direct evidence that the tail tip is able to penetrate the cell wall and interact with the cytoplasmic membrane, which is critical step to allow DNA ejected into the cytoplasmic.
Figure 6. Interaction with the cytoplasmic membrane.
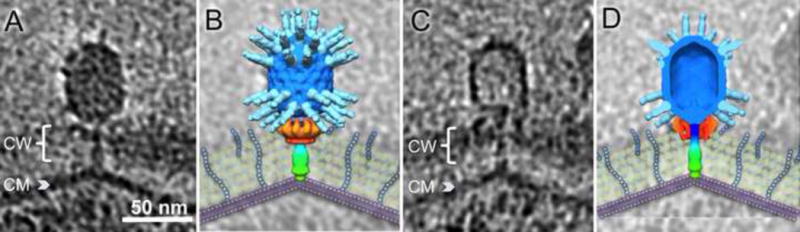
A) Slice through a tomographic reconstruction of Φ29 after penetration of the cell wall and with the tail fused to the cytoplasmic membrane, prior to DNA ejection. B) Surface rendering of Φ29 modeled on the tomogram from A) with a schematic diagram of the Gram-positive cell wall to better visualize this stage of infection. C) Slice through a tomographic reconstruction of an empty phage particle after DNA ejection. D) Sliced view of the Φ29 surface rendering shown on top of the tomogram from C) with cell wall diagram to better visualize this stage of infection C). Scales are the same for all panels. CW: cell wall; CM cytoplasmic membrane. In surface renderings, head fibers are shown in light blue, the capsid in darker blue, appendages in oranges, and the tail is shown as a gradient from blue to green, terminating with the tail knob.
Phages were seen before a significant amount of genome ejection had occurred (Fig. 6A), after completion of genome ejection (Fig. 6C), as well during the ejection process, where a substantial amount of DNA remains in the capsid (Fig. 3F). Notable is the localized outward deformation of the cytoplasmic membrane at its point of contact with the phage tail. Deformation apparently persists after the genome has been completely ejected (Fig. 6C), reminiscent of the structure seen during T4 infection of E. coli (Hu et al., 2015). Both Φ29 and T4 are therefore different from T7 (Hu et al., 2013), where the trans-membrane channel disassembles after genome ejection.
Overall model of Φ29 infection
Together with many key intermediate structures determined by cryo-ET, we propose a new model of Φ29 adsorption and infection as presented in Fig. 7. Moving by Brownian motion, Φ29 randomly collides with a bacterium. Perhaps facilitated by transient interactions of the head fibers, the flexibly oriented appendages reversibly bind the wall teichoic acids that protrude from the B. subtilis cell wall and initiate their hydrolysis. Degradation of the teichoic acids results in the appendages pulling the capsid and tail knob closer to the peptidoglycan layers of the cell wall. Once in contact with the peptidoglycan the tail tip protein digests the peptide crosslinks, rendering the glycan strands more flexible and allowing the tail knob to penetrate deeper into the cell wall. As digestion of both wall teichoic acid and peptidoglycan proceeds, the virion becomes embedded in the cell wall. Appendages that were not initially in contact with wall teichoic acid now bind, adopting the down position as the entire virion moves closer to the cytoplasmic membrane. Although wall teichoic acids are required for Φ29 adsorption to intact cells, the phage adsorbs to and grows on protoplasts of both wild-type and teichoic acid glycosylation-deficient strains (Jacobson and Landman, 1977; Moreno and Bluzat-Moreno, 1978; Young, 1967). There must therefore be a cytoplasmic membrane component that is recognized by the phage. This component has not yet been identified but it is likely to be bound by the tail knob protein gp9. The recent determination of the atomic structure of gp9 (Xu et al., 2016) reveals flexible hydrophobic loops that were suggested to form the trans-membrane pore for DNA transport into the cell cytoplasm. If this suggestion is confirmed, the tail tip protein must first change structure or dissociate from the knob to allow the loops to penetrate the membrane. Further studies will be necessary to determine whether the tip protein remains in contact with the knob, enters the cytoplasm, or remains bound to a portion of the cell wall, in addition to understanding how the Φ29 gp3-genome complex is then triggered to enter the cell cytoplasm.
Figure 7. Schematic model of Φ29 infection of wild-type B. subtilis.

Φ29 attaches to the bacteria cell wall through interactions between the appendages and wall teichoic acid. The varying conformations of the appendages presumably increase the odds of the phage making contact with wall teichoic acid, which the appendages are able to digest. Shortening of the wall teichoic acid chains pulls the phage and phage tail closer to the cell wall, while the head fibers help stabilize the capsid on the cell surface, facilitating interaction of the tail knob with the cell wall peptidoglycan. Enzymatic activity of the gp13 tail tip protein hydrolyzes the cross-linking amino acids in peptidoglycan and aids the phage in traversing the cell wall in order to make contact with the cytoplasmic membrane. Once in contact with the cytoplasmic membrane a pore is created to allow the phage to eject its DNA into the cell. CW: cell wall; WTA: wall teichoic acid; LTA: lipoteichoic acid. Head fibers are shown in light blue, the capsid in darker blue, appendages in oranges and yellows, and the tail is shown as a gradient from blue to green, terminating with the tail knob.
Recent advances in cryo-EM have revolutionized structural biology and have allowed high-resolution structure determination of large macromolecular assemblies, such as bacteriophages. However, it remains technically challenging to visualize phage-host interactions at high resolution, particularly because cryo-EM is fundamentally limited by specimen size. Our use of skinny E. coli minicells as hosts has revealed unprecedented details of structural remodeling of phage virions during infection (Hu et al., 2013; Hu et al., 2015; Liu et al., 2011), demonstrating the value of producing thin specimens for in situ structural analysis. As a first attempt to characterize the structural arrangement of a phage infecting Gram-positive bacteria, we used B. subtilis minicells as hosts for visualizing phage Φ29 adsorption and infection initiation. These minicells are substantially larger than we employed for our coliphage studies and, as a consequence, the resolution of the Φ29 reconstructions is not as high. Our initial attempts to make skinny B. subtilis minicells were unsuccessful but we expect that a reduction of cell size is achievable through additional genetic and biochemical approaches. The combination of reduced host size and the further development of high-throughput cryo-ET will reveal other key intermediates during Φ29 infection, providing additional mechanistic insights into the versatile mechanisms used by phages when interacting with their hosts.
Supplementary Material
Acknowledgments
We thank Drs. Shelley Grimes, Peixuan Guo, and Margarita Salas for phage and bacterial strains, and for general advice on propagating Φ29. This work was supported by NIGMS grants R01GM110243 (JL and IJM), R01GM107629 (JL), R01GM093030 (DBK), and by AU-1714 from the Welch Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agulleiro JI, Fernandez JJ. Tomo3D 2.0–exploitation of advanced vector extensions (AVX) for 3D reconstruction. J Struct Biol. 2015;189:147–152. doi: 10.1016/j.jsb.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Beeby M, Gumbart JC, Roux B, Jensen GJ. Architecture and assembly of the Gram-positive cell wall. Mol Microbiol. 2013;88:664–672. doi: 10.1111/mmi.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KH, Morais MC, Anderson DL, Rossmann MG. Determinants of bacteriophage phi29 head morphology. Structure. 2006;14:1723–1727. doi: 10.1016/j.str.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Cohen DN, Erickson SE, Xiang Y, Rossmann MG, Anderson DL. Multifunctional roles of a bacteriophage phi 29 morphogenetic factor in assembly and infection. J Mol Biol. 2008;378:804–817. doi: 10.1016/j.jmb.2008.02.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen DN, Sham YY, Haugstad GD, Xiang Y, Rossmann MG, Anderson DL, Popham DL. Shared catalysis in virus entry and bacterial cell wall depolymerization. J Mol Biol. 2009;387:607–618. doi: 10.1016/j.jmb.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comolli LR, Spakowitz AJ, Siegerist CE, Jardine PJ, Grimes S, Anderson DL, Bustamante C, Downing KH. Three-dimensional architecture of the bacteriophage phi29 packaged genome and elucidation of its packaging process. Virology. 2008;371:267–277. doi: 10.1016/j.virol.2007.07.035. [DOI] [PubMed] [Google Scholar]
- Farley MM, Hu B, Margolin W, Liu J. Minicells, Back in Fashion. J Bacteriol. 2016;198:1186–1195. doi: 10.1128/JB.00901-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng J, Wang S, Fang H, Guo P. Channel size conversion of Phi29 DNA-packaging nanomotor for discrimination of single- and double-stranded nucleic acids. ACS nano. 2013;7:3315–3323. doi: 10.1021/nn400020z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes S, Jardine PJ, Anderson D. Bacteriophage phi 29 DNA packaging. Advances in virus research. 2002;58:255–294. doi: 10.1016/s0065-3527(02)58007-6. [DOI] [PubMed] [Google Scholar]
- Guasch A, Pous J, Ibarra B, Gomis-Ruth FX, Valpuesta JM, Sousa N, Carrascosa JL, Coll M. Detailed architecture of a DNA translocating machine: the high-resolution structure of the bacteriophage phi29 connector particle. J Mol Biol. 2002;315:663–676. doi: 10.1006/jmbi.2001.5278. [DOI] [PubMed] [Google Scholar]
- Guerrero-Ferreira RC, Viollier PH, Ely B, Poindexter JS, Georgieva M, Jensen GJ, Wright ER. Alternative mechanism for bacteriophage adsorption to the motile bacterium Caulobacter crescentus. Proc Natl Acad Sci U S A. 2011;108:9963–9968. doi: 10.1073/pnas.1012388108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo P, Noji H, Yengo CM, Zhao Z, Grainge I. Biological Nanomotors with a Revolution, Linear, or Rotation Motion Mechanism. Microbiology and molecular biology reviews: MMBR. 2016;80:161–186. doi: 10.1128/MMBR.00056-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernando-Perez M, Miranda R, Aznar M, Carrascosa JL, Schaap IA, Reguera D, de Pablo PJ. Direct measurement of phage phi29 stiffness provides evidence of internal pressure. Small. 2012;8:2366–2370. doi: 10.1002/smll.201200664. [DOI] [PubMed] [Google Scholar]
- Hrabe T, Chen Y, Pfeffer S, Cuellar LK, Mangold AV, Forster F. PyTom: a python-based toolbox for localization of macromolecules in cryo-electron tomograms and subtomogram analysis. J Struct Biol. 2012;178:177–188. doi: 10.1016/j.jsb.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Hu B, Margolin W, Molineux IJ, Liu J. The bacteriophage t7 virion undergoes extensive structural remodeling during infection. Science. 2013;339:576–579. doi: 10.1126/science.1231887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Margolin W, Molineux IJ, Liu J. Structural remodeling of bacteriophage T4 and host membranes during infection initiation. Proc Natl Acad Sci U S A. 2015;112:E4919–4928. doi: 10.1073/pnas.1501064112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson ED, Landman OE. Adsorption of bacteriophages phi 29 and 22a to protoplasts of Bacillus subtilis 168. J Virol. 1977;21:1223–1227. doi: 10.1128/jvi.21.3.1223-1227.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer JR, Mastronarde DN, McIntosh JR. Computer visualization of three-dimensional image data using IMOD. J Struct Biol. 1996;116:71–76. doi: 10.1006/jsbi.1996.0013. [DOI] [PubMed] [Google Scholar]
- Li X, Mooney P, Zheng S, Booth CR, Braunfeld MB, Gubbens S, Agard DA, Cheng Y. Electron counting and beam-induced motion correction enable near-atomic-resolution single-particle cryo-EM. Nature methods. 2013;10:584–590. doi: 10.1038/nmeth.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Chen CY, Shiomi D, Niki H, Margolin W. Visualization of bacteriophage P1 infection by cryo-electron tomography of tiny Escherichia coli. Virology. 2011;417:304–311. doi: 10.1016/j.virol.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucic V, Rigort A, Baumeister W. Cryo-electron tomography: the challenge of doing structural biology in situ. J Cell Biol. 2013;202:407–419. doi: 10.1083/jcb.201304193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao H, Saha M, Reyes-Aldrete E, Sherman MB, Woodson M, Atz R, Grimes S, Jardine PJ, Morais MC. Structural and Molecular Basis for Coordination in a Viral DNA Packaging Motor. Cell reports. 2016;14:2017–2029. doi: 10.1016/j.celrep.2016.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastronarde DN. Automated electron microscope tomography using robust prediction of specimen movements. J Struct Biol. 2005;152:36–51. doi: 10.1016/j.jsb.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Matias VR, Beveridge TJ. Cryo-electron microscopy reveals native polymeric cell wall structure in Bacillus subtilis 168 and the existence of a periplasmic space. Mol Microbiol. 2005;56:240–251. doi: 10.1111/j.1365-2958.2005.04535.x. [DOI] [PubMed] [Google Scholar]
- Meijer WJ, Horcajadas JA, Salas M. Phi29 family of phages. Microbiology and molecular biology reviews: MMBR. 2001;65:261–287. doi: 10.1128/MMBR.65.2.261-287.2001. second page, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne JL, Subramaniam S. Cryo-electron tomography of bacteria: progress, challenges and future prospects. Nat Rev Microbiol. 2009;7:666–675. doi: 10.1038/nrmicro2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morado DR, Hu B, Liu J. Using Tomoauto: A Protocol for High-throughput Automated Cryo-electron Tomography. J Vis Exp. 2016;(107) doi: 10.3791/53608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais MC. The dsDNA packaging motor in bacteriophage o29. Advances in experimental medicine and biology. 2012;726:511–547. doi: 10.1007/978-1-4614-0980-9_23. [DOI] [PubMed] [Google Scholar]
- Morais MC, Choi KH, Koti JS, Chipman PR, Anderson DL, Rossmann MG. Conservation of the capsid structure in tailed dsDNA bacteriophages: the pseudoatomic structure of phi29. Mol Cell. 2005;18:149–159. doi: 10.1016/j.molcel.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Morais MC, Koti JS, Bowman VD, Reyes-Aldrete E, Anderson DL, Rossmann MG. Defining molecular and domain boundaries in the bacteriophage phi29 DNA packaging motor. Structure. 2008;16:1267–1274. doi: 10.1016/j.str.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais MC, Tao Y, Olson NH, Grimes S, Jardine PJ, Anderson DL, Baker TS, Rossmann MG. Cryoelectron-microscopy image reconstruction of symmetry mismatches in bacteriophage phi29. J Struct Biol. 2001;135:38–46. doi: 10.1006/jsbi.2001.4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais MC, Kanamaru S, Badasso MO, Koti JS, Owen BA, McMurray CT, Anderson DL, Rossmann MG. Bacteriophage phi29 scaffolding protein gp7 before and after prohead assembly. Nat Struct Biol. 2003;10:572–576. doi: 10.1038/nsb939. [DOI] [PubMed] [Google Scholar]
- Moreno F, Bluzat-Moreno FG. Evidence that the neck appendages are adsorption organelles in Bacillus subtilis bacteriophage phi29. J Virol. 1978;27:831–834. doi: 10.1128/jvi.27.3.831-834.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick JE, Kearns DB. MinJ (YvjD) is a topological determinant of cell division in Bacillus subtilis. Mol Microbiol. 2008;70:1166–1179. doi: 10.1111/j.1365-2958.2008.06469.x. [DOI] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera–a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Reilly BE, Nelson RA, Anderson DL. Morphogenesis of bacteriophage phi 29 of Bacillus subtilis: mapping and functional analysis of the head fiber gene. J Virol. 1977;24:363–377. doi: 10.1128/jvi.24.1.363-377.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas M, Vasquez C, Mendez E, Vinuela E. Head fibers of bacteriophage phi 29. Virology. 1972;50:180–188. doi: 10.1016/0042-6822(72)90358-3. [DOI] [PubMed] [Google Scholar]
- Silhavy TJ, Kahne D, Walker S. The bacterial cell envelope. Cold Spring Harbor perspectives in biology. 2010;2:a000414. doi: 10.1101/cshperspect.a000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson AA, Leiman PG, Tao Y, He Y, Badasso MO, Jardine PJ, Anderson DL, Rossmann MG. Structure determination of the head-tail connector of bacteriophage phi29. Acta Crystallogr D Biol Crystallogr. 2001;57:1260–1269. doi: 10.1107/s0907444901010435. [DOI] [PubMed] [Google Scholar]
- Sun L, Young LN, Zhang X, Boudko SP, Fokine A, Zbornik E, Roznowski AP, Molineux IJ, Rossmann MG, Fane BA. Icosahedral bacteriophage PhiX174 forms a tail for DNA transport during infection. Nature. 2014;505:432–435. doi: 10.1038/nature12816. [DOI] [PubMed] [Google Scholar]
- Tang J, Olson N, Jardine PJ, Grimes S, Anderson DL, Baker TS. DNA poised for release in bacteriophage phi29. Structure. 2008;16:935–943. doi: 10.1016/j.str.2008.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Olson NH, Xu W, Anderson DL, Rossmann MG, Baker TS. Assembly of a tailed bacterial virus and its genome release studied in three dimensions. Cell. 1998;95:431–437. doi: 10.1016/s0092-8674(00)81773-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosi M, Anderson DL. Antigenic properties of bacteriophage phi 29 structural proteins. J Virol. 1973;12:1548–1559. doi: 10.1128/jvi.12.6.1548-1559.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva N, Salas M. Adsorption of bacteriophage phi 29 to Bacillus subtilis through the neck appendages of the viral particle. J Virol. 1981;38:15–19. doi: 10.1128/jvi.38.1.15-19.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. 3D reconstruction and processing of volumetric data in cryo-electron tomography. J Struct Biol. 2007;157:126–137. doi: 10.1016/j.jsb.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Winkler H, Zhu P, Liu J, Ye F, Roux KH, Taylor KA. Tomographic subvolume alignment and subvolume classification applied to myosin V and SIV envelope spikes. J Struct Biol. 2009;165:64–77. doi: 10.1016/j.jsb.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y, Rossmann MG. Structure of bacteriophage phi29 head fibers has a supercoiled triple repeating helix-turn-helix motif. Proc Natl Acad Sci U S A. 2011;108:4806–4810. doi: 10.1073/pnas.1018097108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y, Morais MC, Cohen DN, Bowman VD, Anderson DL, Rossmann MG. Crystal and cryoEM structural studies of a cell wall degrading enzyme in the bacteriophage phi29 tail. Proc Natl Acad Sci U S A. 2008;105:9552–9557. doi: 10.1073/pnas.0803787105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y, Leiman PG, Li L, Grimes S, Anderson DL, Rossmann MG. Crystallographic insights into the autocatalytic assembly mechanism of a bacteriophage tail spike. Mol Cell. 2009;34:375–386. doi: 10.1016/j.molcel.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y, Morais MC, Battisti AJ, Grimes S, Jardine PJ, Anderson DL, Rossmann MG. Structural changes of bacteriophage phi29 upon DNA packaging and release. EMBO J. 2006;25:5229–5239. doi: 10.1038/sj.emboj.7601386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Gui M, Wang D, Xiang Y. The bacteriophage varphi29 tail possesses a pore-forming loop for cell membrane penetration. Nature. 2016;534:544–547. doi: 10.1038/nature18017. [DOI] [PubMed] [Google Scholar]
- Young FE. Requirement of glucosylated teichoic acid for adsorption of phage in Bacillus subtilis 168. Proc Natl Acad Sci U S A. 1967;58:2377–2384. doi: 10.1073/pnas.58.6.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber B, Haenni M, Ribeiro T, Minnig K, Lopes F, Moreillon P, Dubochet J. Granular layer in the periplasmic space of gram-positive bacteria and fine structures of Enterococcus gallinarum and Streptococcus gordonii septa revealed by cryo-electron microscopy of vitreous sections. J Bacteriol. 2006;188:6652–6660. doi: 10.1128/JB.00391-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


