Abstract
Abrogation of endoplasmic reticulum (ER) protein folding triggered by exogenous or endogenous factors, stimulates a cellular stress response, termed ER stress. ER stress reestablishes ER homeostasis through integrated signaling termed the ER-unfolded protein response (UPRER). In the presence of severe toxic or prolonged ER stress, the pro-survival function of UPRER is transformed into a lethal signal transmitted to and executed through mitochondria. Mitochondria are key for both apoptotic and autophagic cell death. Thus ER is vital in sensing and coordinating stress pathways to maintain overall physiological homeostasis. However, this function is deregulated in cancer, resulting in resistance to apoptosis induction in response to various stressors including therapeutic agents. Here we review the connections between ER stress and mitochondrial apoptosis, describing potential cancer therapeutic targets.
Keywords: mitochondria, apoptosis, autophagy, endoplasmic reticulum, unfolded protein response
1. Introduction
Coordination of external or internal cellular stress into overall stress signaling is an essential cellular process vital for cell growth and survival during organism development. The endoplasmic reticulum (ER) is a central eukaryotic cellular organelle that provides crucial biosynthetic, stress-sensing, and signaling functions [1, 2]. In addition to its role in folding and posttranslational modifications of proteins that are destined for the secretory pathway, the ER also maintains an efficient oxidizing and Ca2+-rich folding environment within cells [3–5]. ER-resident chaperones, such as calnexin, calreticulin, the glucose-regulated protein GRP78 (HSPA5, also called BiP), and protein disulfide isomerases, are involved in protein folding, signal transduction, and maintenance of Ca2+ buffering [6–10]. Pathophysiological conditions, including hypoxia, ER Ca2+ depletion, oxidative injury, hypoglycemia, and viral infections, affect ER homeostasis and hinder protein folding processes [11–15]. These activities ultimately result in an imbalance between protein folding load/capacity and the physiological condition is known as “ER stress.” ER stress initiates a stress response via a well-coordinated and integrated signal transduction pathway, called the unfolded protein response (UPR) [6, 11, 16–19], which primarily re-establishes ER homeostasis by harmonizing various processes of ER-UPR (UPRER) to promote cell survival [20]. In the presence of severe and irreparable ER stress, a switch in UPRER from a pro-survival mode towards a pro-death response occurs through mitochondrial engagement, leading to the activation of the intrinsic apoptosis pathway [17, 21–26]. Autophagy, another mode of inducing cell death, can also be activated in response to such unresolved stress, maintaining cellular integrity under ER stress [27–30].
Mitochondria play pivotal role in the cellular processes of bioenergetics and apoptosis [31, 32]. In the intrinsic apoptosis pathway, a signaling platform containing oligomers of the BCL2 family proteins BAX or BAK assembles on the mitochondrion to induce mitochondrial outer membrane permeabilization (MOMP), leading to release of apoptogenic factors, including cytochrome c and Smac/DIABLO, ultimately triggering apoptosis [33, 34]. The assembly of this platform is influenced by the dynamic behavior of the mitochondria and vice versa, ultimately regulating initiation of the cell death process. Such dynamic mitochondrial behavior is dependent on mitochondrial division (i.e., fission) and fusion processes that determine cellular characteristics including morphology and cellular distribution [35, 36]. In turn, these changes modulate overall cellular physiology by impacting cellular bioenergetics as well as the apoptosis potential in response to stress [37].
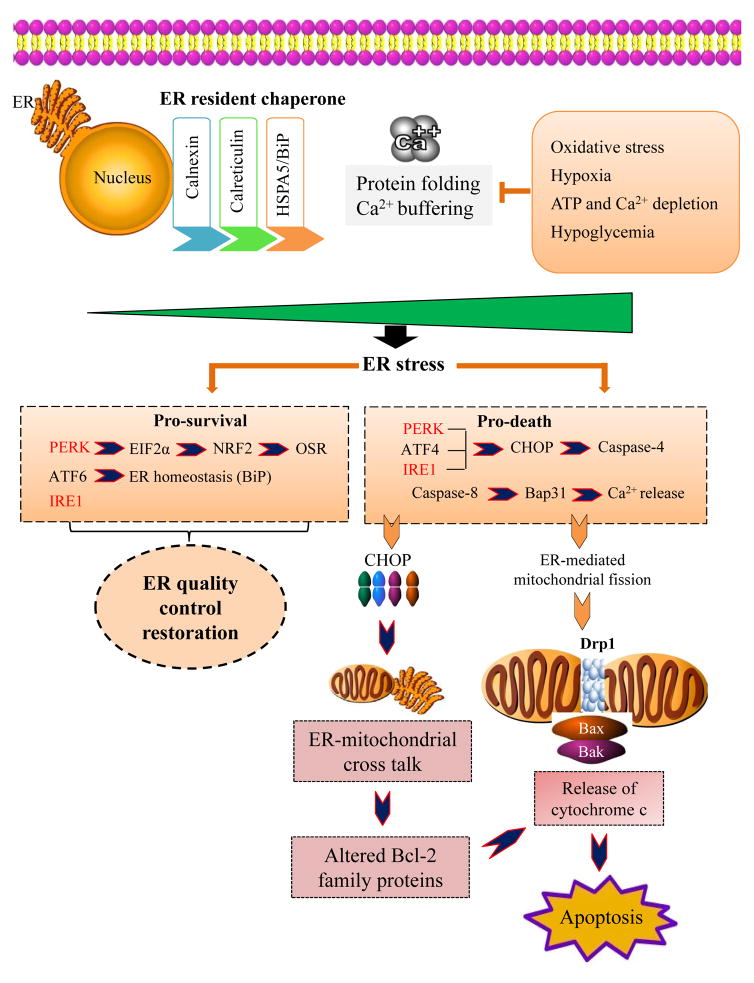
Mitochondrial division and fusion events have been linked with the ultimate cellular stress response, which is cell death [38]. Furthermore, the ER actively participates in mitochondrial division (fission), suggesting a new model that links ER stress with cell death induction involving mitochondrial dynamics and apoptosis [39]. Extrinsic or intrinsic stress signals are normally processed via the ER system and result in induction of cell survival. In the case of unresolved stress signals, cross talk with the mitochondrial system results in differential processing and induction of cell death [22, 40]. Such a relationship between the ER and mitochondria in response to various stressors could be exploited for potential development of anticancer therapies that would link the stress processed in the ER with mitochondrial apoptosis. Here, we describe the different mechanisms employed by the ER system to process and resolve various stress signals and to engage the mitochondrial system for cell death induction in the case of unresolved cellular stress (Figure 1).
Figure 1.
A schematic representation of ER-mitochondrial crosstalk. The UPRER is initiated by ER stress, which modulates ER function and stimulates mitochondrial-mediated intrinsic apoptosis via crosstalk with mitochondria and nucleus. See text for details. OSR, oxidative stress response
2. The role of ER stress and UPRER in prosurvival signaling
The ER is an essential cellular organelle involved in several processes, including protein homeostasis, stress response, survival signaling, and Ca2+ homeostasis [28]. The ER is responsible for protein folding and import as part of the cellular secretory machinery pathway, and this organelle functions to maintain tightly regulated oxidizing conditions and a Ca2+-rich environment [6, 22, 40, 41]. These functions of ER have been attributed to ER-resident chaperones, such as calnexin, calreticulin, BiP/GRP78, and protein disulfide isomerases, and Ca2+ buffering in the ER. Pathophysiological conditions, such as oxidative insult, hypoxia, Ca2+ depletion, hypoglycemia, ATP depletion, and viral infections affect ER homeostasis and interfere with protein folding. This triggers an imbalance between protein folding load and capacity, generating ER stress [6, 22, 40]. In response to such stress conditions, the ER induces the UPRER signaling pathway [16, 20]. The UPRER initially restores ER homeostasis by relieving stress conditions. However, when the stress conditions are too severe and cannot be reversed, the UPRER activates a cell death pathway, usually via intrinsic apoptosis, which involves the mitochondria [16, 22]. Thus, under toxic and unresolved stress conditions, UPRER alters the cell fate from a pro-survival pathway to a pro-death mechanism, eventually inducing cell death [17, 22, 40].
The UPRER is primarily mediated by three main signaling cascades, which are activated by three unique ER stress sensors: pancreatic ER kinase-like ER kinase (PERK), inositol requiring enzyme 1 (IRE1), and activating transcription factor 6 (ATF6) [42]. These ER transmembrane proteins are negatively regulated and maintained in an inactive state via binding of their luminal domains to the inhibitory chaperone BiP/GRP78 [2]. Under conditions of ER stress, BiP/GRP78 inhibition is titrated downward by the accumulation of unfolded or misfolded proteins, activating ER sensors. The Ser/Thr kinase PERK phosphorylates eukaryotic initiation factor 2 alpha (EIF2α) and nuclear factor E2-related factor 2 (NRF2) under stress conditions [42]. Phosphorylation of EIF2α inhibits global translation while preferentially promoting expression of the UPR transcription factor ATF4 [43, 44]. ATF4 regulates many genes involved in ER homeostasis, including BiP/GRP78 and GRP94, the oxidative stress response, apoptosis and amino acid biosynthesis and transport [45–49]. NRF2, a cytoprotective transcription factor, is pivotal to the antioxidant response and cell survival under oxidative stress conditions. PERK-mediated phosphorylation of NRF2 leads to its dissociation from its cytosolic repressor kelch-like Ech-associated protein 1 (KEAP1). This dissociation frees NRF2 to facilitate nuclear translocation, which ultimately induces the expression of genes involved in the antioxidant stress response [50–52].
As discussed earlier, activation of UPRER is primarily an anti-apoptotic adaptive response against toxic insults like ROS, whereas sustained activation of UPRER signaling causes cell death. Therefore, induction of UPRER is an attractive strategy to target cancer cells. Many drugs have shown promising results in inducing UPRER and some of these drugs are under clinical trials [11, 53–55]. Although, these UPRER inducing drugs are not as successful as expected (Reviewed in [56] and [11]), the activation of NRF2 by PERK has been considered as one of the main reasons for the pro-survival effect of UPRER. The activation of NRF2 induces antioxidant defense system as well as upregulates the expression of BCL-2 and Bcl-xL by binding to the ARE (antioxidant response element) leading to the prevention of apoptosis [57–60]. BCL-2 and Bcl-xL are anti-apoptotic proteins and prevent the oligomerization of pro-apoptotic proteins BAX and BAK, thus blocking the release of cytochrome c from mitochondria [61–63]. Bcl-xL also interacts with apoptotic protease activating factor 1 (Apaf-1) in the cytosol and inhibits the Apaf-1 dependent activation of caspase-9 [64]. Furthermore, prolonged UPRER induces apoptosis in p53-dependent manner via p53-up-regulated modulator of apoptosis (PUMA) and NOXA, pro-apoptotic members of Bcl-2 family proteins [65]. Thus, induction of UPRER results in the activation of the PERK-EIF2α-ATF4 and PERK-NRF2 signaling cascades, which promotes survival of ER-stressed cells by restoring ER quality control and enhancing oxidative stress adaptation along with overexpression of BCL-2 and Bcl-xL (Figure 1). These studies clearly suggest that inhibition of NRF2 along with UPRER induction is required for anti-cancer strategies.
Stimulation of IRE1, another ER-stress sensor, provides both protein kinase and endoribonuclease activity, although IRE1 itself is the only known direct substrate of this protein kinase activity. Auto phosphorylation of IRE1 is required for its endoribonuclease activity and splicing of XBP1u (u for unspliced) mRNA to yield mature XBP1s (s for spliced) mRNA, which encodes XBP1s, a potent transcription factor that induces expression of genes involved in ER quality control, ER/Golgi biogenesis, and ER-associated protein degradation (ERAD) components in addition to expression of genes involved in redox homeostasis and oxidative stress response, ultimately impacting cell fate decisions [66–68]. Finally, BiP/GRP78 dissociation activates ATF6 and induces its translocation to the Golgi apparatus. In the Golgi apparatus, proteases cleave and process ATF6 to produce an active transcription factor that regulates genes encoding ER chaperones and ERAD components and also those that play essential roles in lipid biogenesis and ER expansion [69, 70].
3. ER stress-mediated UPRER in pro-death signaling
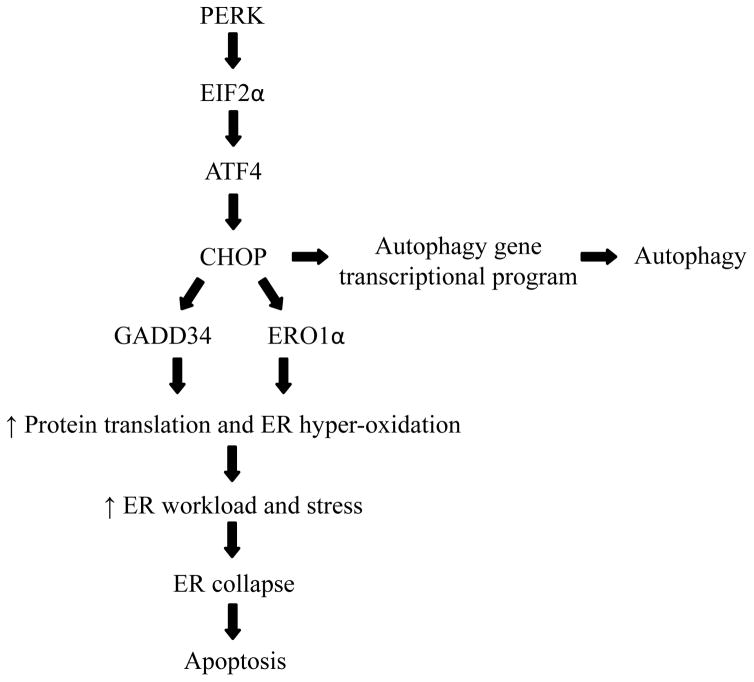
Under tolerable stress conditions UPRER signaling induces pro-survival pathways that lead to resistance and cell survival. While, in the presence of stress conditions that are severe, highly toxic, and irreversible, the same UPRER pathway induces pro-death mechanisms [22]. Although the molecular mechanisms behind such switching are poorly understood, it has been reported that the UPRER employs some of the sensors and executioners of pro-survival mechanisms to mediate pro-death pathways in response to toxic stress levels [71, 72]. Mild ER stress results in expression of ATF4-dependent pro-survival genes as well as a transient and limited activation of PERK. During severe ER stress, sustained PERK signaling induces phosphorylation of EIF2α, which in turn increases the translation of ATF4 [71]. ATF4 is a transcription factor known to bind the promoter region and enhance C/EBP homologous protein (CHOP) mRNA expression and thus protein levels [74]. CHOP plays an important role in ER stress-induced cellular death [2], as this factor is a target gene common to all three apical ER sensors/executioners. Under severe ER stress conditions, ATF4 and CHOP interact together and induces cell death via induction of genes involved in protein synthesis such as GADD34 and ERO 1α. The increased protein synthesis further worsens the ER stress load and leads to ATP depletion and oxidative stress resulting in a hyper-oxidizing ER environment [71]. Another, pro-survival IRE1-XBP1 signaling that exists under mild stress conditions is counterbalanced by IRE1 scaffold-signaling properties that are independent of its XBP1 splicing activity. IRE1 serves as a molecular platform to recruit the E3 ubiquitin ligase adaptor protein TNF receptor-associated factor 2 (TRAF2), and this factor then tethers IRE1 to the stress-activated ASK1-JNK/p38 MAPK cascade, which functions in cell fate decisions [20]. Both of these mechanisms lead to severe unresolved ER stress, ultimately resulting in apoptosis due to ER collapse [50, 76]. Thus, it is very clear that the ER stress response duality may be mediated by the differential stability of mRNA/protein factors under different levels of stress conditions [73] and the transition of UPRER from pro-survival to pro-apoptotic effects depends on sustained PERK and IRE1 signaling [75]. Furthermore, CHOP regulates BCL2 family protein expression, which regulates the intrinsic apoptosis pathway. Thus, ER stress-induced UPRER links mitochondrial apoptosis with ER stress by modulation of the intrinsic apoptosis cascade, and this cross talk alleviates any unresolved ER stress via induction of cell death [78–80].
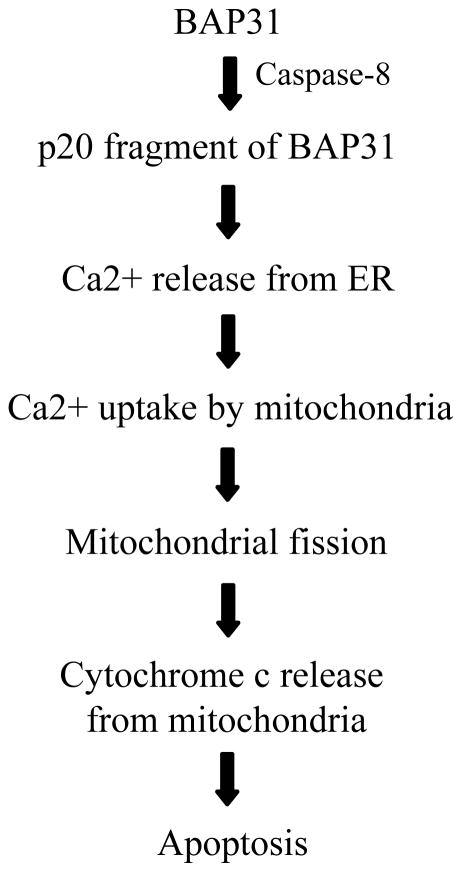
In addition to such cross talk between the UPRER and the intrinsic apoptotic pathway, cleavage and subsequent activation of ER-associated caspases, including caspase 12, during ER stress induces apoptosis in response to ER stress [81, 82]. Caspase-12 in rodents and caspase-4 in humans induce apoptosis in response to ER stress, typically in a CHOP-dependent manner [83, 84]. Regulation of Ca2+ homeostasis is another mechanism that modulates the mitochondrial apoptosis pathway via ER dynamics. Cleavage of the ER transmembrane protein BAP31 by ER-associated casapase-8 generates the p20 fragment, and abrogates the prosurvival functions of BAP31 [85]. In addition, p20, a caspase-8 cleavage fragment of BAP31 stimulates Ca2+ release from ER into the cytosol, resulting in mitochondrial uptake of Ca2+, fission, and cytochrome c release, all of which induce apoptosis (Figure 2). Anticancer agents, such as alkyl-lysophospholipid and edelfosine, have commandeered this mechanism to induce apoptosis following ER stress [86]. In addition, early cleavage of BAP31 by caspase-8 in anthracyclines-induced ER stress results in calreticulin mobilization from ER lumen to the plasma membrane, and this confers immunogenic properties to the apoptosis process [87].
Figure 2.
The role of calcium in ER-mitochondrial crosstalk. Caspase-8 cleaved fragment of BAP31 stimulates Ca2+ release from the ER into the cytosol. This is followed by mitochondrial uptake of Ca2+, leading to mitochondrial fission, enhancement of cytochrome c release, and apoptosis.
4. UPRER-mediated autophagy
Macro-autophagy (or autophagy), a lysosomal degradation pathway for proteins and organelles, is essential for normal cellular homeostasis and serves as a major housekeeping mechanism in eukaryotic cells. In addition, autophagy is another cellular mechanism that can be employed to cope with ER stress [20, 30, 88]. The role of autophagy in ER stress is not completely understood; however, an overload of ER-resident unfolded proteins combined with an insufficient proteasome-mediated degradation system, resulting in persistence of the damage/stress, is thought to trigger autophagy to alleviate the ER stress, serving as an ER protein quality control system [27]. Autophagy induction exerts a pro-survival function by alleviating ER stress induced by multiple stressors [27, 88]. For example, activation of the ER-stress associated transcription factor CHOP induces the autophagy gene transcriptional program, which ultimately leads to autophagosome formation and autophagy (Figure 3). Essentially, autophagy ameliorates ER stress by removing aggregates or toxic proteins that were not removed by conventional proteasome-mediated degradation [89]. ER stress is often associated with Ca2+ release into the cytosol, and this increase in cytosolic Ca2+ levels activates regulatory pathways for both autophagy and apoptosis [90–92]. ER stress-mediated activation of autophagy is linked to increased adaptation and cell survival in response to physiological/pathological stress conditions [30, 88, 93, 94]; however, as in UPRER, autophagy also induce cell death via either enhancing apoptotic or non-apoptotic pathways during the ER stress response [93, 95, 96]. Importantly, since conventional apoptotic pathways are highly deregulated in cancer, cell death induction via autophagy is a seemingly attractive and promising option for killing of tumor cells.
Figure 3.
Role of PERK signaling in cell death. Sustained PERK signaling activates EIF2α, and in turn increases the translation of ATF4, which then targets transcription factor CHOP. CHOP can either activate autophagy program or induce GADD34 and ERO1α to promote hyperoxidizing ER environment, which further increases ER stress, leading to ER collapse and apoptosis.
5. Cross talk between UPRER and mitochondrial apoptosis
Stress-induced changes in ER physiology and the concomitant UPRER lead to modulation of mitochondrial function and ultimately to apoptosis in response to unresolved stress. This cross talk plays an important role in overall cellular physiology via involvement of mitochondrial apoptosis. Thus, cross-talk dynamics decide cell fate with respect to survival or death following ER stress. In addition to modulating ER stress-induced cell death, CHOP also regulates BCL2 protein family member expression [97, 98]. Although BCL2 family proteins directly regulate the intrinsic apoptotic pathway, these proteins also function as regulators of ER homeostasis through their participation in UPRER sensor mechanisms and cell fate decisions following ER stress [78].
The complex dynamic interplay between UPRER components and BCL2 family members determines cell fate following ER stress. In fact, the physical interaction between UPRER sensors and BCL2 family members appears to modulate ER stress-mediated activation of the intrinsic apoptosis pathway. Specifically, the pro-apoptotic proteins BAX and BAK not only regulate the Ca2+ levels in the ER but also modulate the activity of the UPRER sensor IRE1 via physical interaction, thus performing essential roles in the induction of mitochondrial apoptosis following ER stress-induced UPRER [78]. While BCL2 proteins can modulate IRE1 activity, IRE1 self-activation also activates the MAPK/JNK pathway, which in turn, activates BH3-only proteins, including BIM, and suppresses the anti-apoptotic activity of BCL2 [99, 100]. Augmenting this effect, CHOP induces BIM transcription while simultaneously suppressing BCL-2 induction, linking the ER stress response to mitochondrial apoptosis [97, 98, 101]. In addition, other BH3-only proteins, such as NOXA and PUMA, are also transcriptionally activated following ER stress, and such activation can occur in either a p53-dependent or -independent manner [65, 102–104].
Activation of the IRE1-TRAF2 signaling pathway appears to be important for linking ER stress to mitochondrial apoptosis. In BAX/BAK-deficient murine epidermal fibroblasts, reconstitution of BAK expression at the ER membrane reactivated IRE1-TRAF2 signaling as well as mitochondrial apoptosis mediated by reticular forms of BIM and PUMA, precluding the need for a mitochondrial localization event [105]. These events are vital for a rapid response following unresolved ER stress, and circumventing the need for mitochondrial localization for these proteins removes another possible regulatory step in apoptosis induction in response to ER stress. Furthermore, mobilization of Ca2+ promotes persistent JNK activation and mitochondrial apoptosis exclusively in an atypical IRE1-TRAF2 activation pathway that is dependent on ER BAK [105]. JNK is involved in regulation of both apoptotic and autophagic cell death pathways following ER stress; therefore, activation of the JNK pathway connects ER stress to mitochondrial apoptosis via UPRER [106]. These findings offer insight into the complex cross talk between ER stress and cell fate decisions via UPR. In many diseases, including cancer, this cross talk is deregulated and therefore offers an exciting target for intervention and treatment development [107]. Thus, manipulation of ER stress in such a way as to induce apoptosis through the mitochondrial pathway provides a desirable option for inducing cell death in otherwise resistant cancer cells. Agents that mediate such cross talk between ER stress and the intrinsic cell death pathway would likely rely on engaging the UPRER with the IRE1-TRAF2 circuit.
6. The role of ER in mitochondrial dynamics
Mitochondria were incorporated into the eukaryotic system early during evolution, and this event enabled these organisms to overcome the steep energetic barrier involved in continuous production of ATP [31]. This incorporated endosymbiont amplified the essential feature of eukaryotic programmed cell death, or apoptosis. During mitochondrial (intrinsic) apoptosis, a signaling platform is recruited for assembly on the mitochondria, leading to permeabilization of the outer mitochondrial membrane and release of apoptogenic proteins into the cytosol [33, 34]. Dramatic changes including fission and fusion processes in mitochondria influence cell death, determine the overall shape, connectedness, and distribution of mitochondria in cells. The ER influences mitochondrial dynamics and thus, links the cell’s physiological state with mitochondrial apoptosis [38]. Cross-talk between the ER and mitochondria ensures that cellular physiology is tightly regulated and that adequate responses to stress signals are initiated to avoid toxic physiological damage. In addition to connecting stress signals to the mitochondria via UPRER, the ER also participates in the division of mitochondria, leading to dynamic cellular changes affecting multiple cellular pathways, including those responsible for cell death [36, 38]. This new physiological concept links the ER with mitochondrial dynamics and cell death, ensuring coherence in cellular functioning in response to stress signals.
Large self-assembling dynamin-related guanosine triphosphatases (DRPs) regulate mitochondrial fission and fusion processes [35]. A single cytosolic DRP, termed DRP1, catalyzes mitochondria fission, while fusion requires the outer mitochondrial membrane proteins MFN1/MFN2 and the inner mitochondrial membrane protein OPA1. The self-assembling properties of DRP mediate the fission and fusion processes of mitochondria [108, 109]. In addition to these roles in fission and fusion, DRPs also function in pathways related to quality control and stress and affect BCL2-dependent MOMP, suggesting that DRPs directly link these processes with apoptosis in cells, although the mechanism of such modulation remains unclear. OPA1 negatively modulates MOMP through regulation of junctions near the mouths of cristae that regulate the release of apoptogenic factors from the intermembrane space [110, 111]. However, DRP1 recruitment to mitochondria modulates MOMP in a context-dependent manner. Massive recruitment and assembly of DRP1 into foci on the outer mitochondrial membrane occurs during apoptosis, resulting in fission and consequent dramatic fragmentation of the mitochondrial network. DRP1 behavior is thus similar to pro-apoptotic BAX, which is recruited to the outer mitochondrial membrane during apoptosis and oligomerizes into foci that are functionally linked to MOMP. Indeed, DRP1 has been found in foci with BAX on mitochondria during apoptosis [112–114], however, DRP1-mediated cell death may not necessarily require BAX and BAK in other cell types [115, 116]. MFN2 similarly behaves like DRP1 under apoptotic conditions and has been found in foci with BAX [112, 117]. Although these apoptotic foci mark the sites of mitochondrial fission, the role of DRP1 in modulating MOMP is independent of its role in the fission process.
Direct involvement of ER with mitochondrial apoptosis can be attributed to the formation of ER-mitochondrial microdomains [38]. These microdomains are generated via ER-associated mitochondrial division (ERMD) and can be harnessed for diverse cellular functions, including apoptosis induction under unresolved stress conditions. During ERMD, the ER associates with mitochondria and marks the sites of division [36, 118]. These microdomains are enriched for mitochondrial division components, such as DRP1 and the DRP1 receptor and effector MFF. However, under conditions of unresolved stress, these microdomains can recruit pro-apoptotic BCL2 proteins, such as BAX, and regulate their activation to promote MOMP and subsequent release of apoptogenic factors, including cytochrome c, and then cell death via apoptosis [38].
ERMD sites that demarcate the ER-mitochondria microdomains are important for BAX insertion and oligomerization. In the absence of DRP1 due to recruitment to mitochondrial constriction sites, MFF accumulates at ER-mitochondrial contact sites [36]. A proposed role for mitochondria-associated membranes in BAX activation stems from the observation that sphingolipid metabolites from a non-mitochondrial membrane compartment directly promote the assembly and oligomerization of BAX in the mitochondrial outer membrane to induce MOMP [119]. In a similar manner, these ER-mitochondrial microdomains may facilitate the shuttling of key lipid effectors of BAX as a result of the ERMD process. Overall, ERMD appears to be a critical process in dynamic cellular physiology, linking the ER and mitochondria and mediating apoptosis via MOMP induction in response to unresolved ER stress. It is possible, however, that ERMD domains extend beyond the contact sites into the lumens of both the ER and mitochondria, thereby integrating the functional status of these organelles. Notably, ER-mitochondria contacts via formation of ERMD domains are not only responsible for normal cellular physiology, mediating mitochondrial fission/fusion or cell death, but may also be involved in pathologies, including cancer [120–122]. Therefore, ER-mitochondria interaction has important clinical relevance and offers an opportunity for therapeutic exploitation in oncology. Agents that induce unresolved stress conditions and also stimulate ERMD microdomain-induced MOMP and cell death would be highly desirable for cancer therapy, as suggested by the regulation of MOMP via ER stress-induced apoptosis. Moreover, mitochondrial dysfunction and ER stress have been implicated in diseases that have been associated with altered mitochondrial dynamics, such as neurodegeneration [120, 121].
7. Conclusions and future perspectives
The ER is one of the central organelles involved in the maintenance of cellular homeostasis. Disruption or malfunctioning of the ER due to ER stress has been associated with multiple pathological conditions, including cancer. This deregulation of ER function underlies the pro-apoptotic mechanism of various anticancer regimens; however, ER stress-induced signaling pathways and their molecular mechanisms are quite complex and have dual functions in cell survival and death (Figure 1). The ER and mitochondria communicate with each other to maintain normal physiological homeostasis at a steady-state level. Their individual and coordinated functions are essential for maintaining quality control and the overall physiological state of eukaryotic cellular systems (Figure 1). Disruption of this association commonly occurs in various malignancies and leads to pro-tumorigenic events, such as apoptosis resistance, which is an important hallmark of cancer. Elucidating the mechanisms of signaling by the ER stress pathways in order to promote cell death or cell survival induction comprises a major focus in the field and will provide an important aspect of rational drug design for therapeutic applications against diverse diseases, including cancer. Indeed, our recent findings demonstrate that activation of UPR including mitochondrial UPR plays critical role during anticancer induced apoptosis in cancer cells [123, 124], suggesting that targeting UPR may provide novel strategies for cancer therapeutics. In this regard, one possibility is the development of small molecule modulators of the kinase components of the UPRER, such as PERK and IRE1. A better mechanistic understanding of ER stress-induced intrinsic apoptosis via the UPRER demands immediate attention and could pave the way for rational design of UPRER-based anticancer drugs. One potential challenge will be the development of agents that specifically target the cyto-protective functions of the UPRER while either potentiating or maintaining the pro-apoptotic functions of this response. Promising agents [95, 125–136] are under investigation in various types of cancer, and possible combination therapies utilizing ER stress-inducing agents are encouraging approaches (Table 1). In conclusion, UPRER-mediated induction of the mitochondrial intrinsic apoptosis pathway in response to anticancer targeting is an attractive strategy for novel cancer therapy investigations and thus offers considerable potential for future drug design for the treatment of various malignancies.
Table 1.
ER stress-inducing agents in cancer.
| Agents | Cancer type | Mechanism of action | References |
|---|---|---|---|
| Resveratrol (RSV) | Prostate cancer | RSV triggers ER stress by depleting ER Ca2+ pool and induces autophagy-mediated apoptosis. | Selvaraj et al., 2016 |
| Withaferin A | Renal carcinoma | Withaferin A induces apoptosis in human renal carcinoma cells via ER stress. | Choi et al., 2011 |
| Withaferin A | Pancreatic cancer | Withaferin A causes impaired autophagy and ER stress-mediated apoptosis in pancreatic cancer cells. | Li et al., 2016 |
| β-phenethyl isothiocyanate (PEITC) | Ovarian cancer | PEITC promotes ROS accumulation and UPR- mediated apoptosis in ovarian cancer cells | Hong et al., 2015 |
| Silibinin | Prostate cancer | Silibinin induces ER stress in prostate cancer cells by inducing ROS generation. | Kim et al., 2016 |
| Subtilase cytotoxin AB (SubAB) | Colon cancer | ER stress induces cancer stem cell differentiation and sensitizes cells to traditional chemotherapy. | Wielenga et al., 2015 |
| Sulforaphane (SFN) | Prostate cancer | SFN induces ROS generation and initiates apoptosis. | Singh et al., 2005 |
| Delta(9)- tetrahydrocannabinol (THC) | Glioblastoma | THC induces ER stress via ceramide accumulation within the ER, which increases phosphorylation of eIf2alpha, ATF4 and CHOP upregulation, and promotes autophagy and apoptosis in cancer cells. | Salazar et al, 2009 |
| Cantharidin (CTD) | Lung Cancer | CTD induces ROS and Ca2+ production. ER-stress induced proteins associates with a decrease in mitochondrial membrane potential. Cells treated with CTD ultimately undergo apoptosis. | Hsia et al, 2014. |
| Bortezomib (BTZ) | Cholangiocarcinoma | BTZ induces ER-UPR and subsequently activates intrinsic apoptosis. | Vaeteewoottacharn et al., 2013 |
| GSK 2656157 | Pancreatic cancer | Induction of ER stress via inhibition of PERK decreases tumor size and vascularization in vivo. | Atkins et al., 2013 |
| Versipelostatin (VSL) | Stomach cancer, colon cancer, and fibrosarcoma | VSL inhibits transcription of ER-UPR gene GRP78, which in turn allows for selective killing of glucose- deprived cancer cells. | Park et al, 2004 |
| Tunicamycin (Tm) | Breast cancer | Tm activates ER-UPR in breast cancer stem cells (CSCs), thereby decreasing the subpopulation of CSCs as well as CSC invasiveness. | Nami et al., 2016 |
Highlights.
UPRER promotes switch from pro-survival to pro-death signaling under severe stress.
Mitochondria facilitate pro-death signaling initiated by UPRER.
ER-mediated mitochondrial dynamics affect mitochondrial function.
Deregulated UPRER-mitochondrial crosstalk confers resistance to apoptosis.
Promoting UPRER pro-death signaling is an intriguing strategy for cancer therapy.
Acknowledgments
This work was supported in part by the National Cancer Institute of the National Institutes of Health under Award Number R01CA160685, and the American Cancer Society Research Scholar Grant RSG-12-214-01 – CCG; and the National Cancer Institute Center Support Grant P30 CA016056 to the Roswell Park Cancer Institute. We apologize to those colleagues whose publications inadvertently were not cited.
Footnotes
Conflict of interest: All authors declare no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sasaki K, Yoshida H. Organelle autoregulation--stress responses in the ER, Golgi, mitochondria and lysosome. J Biochem. 2015;157:185–195. doi: 10.1093/jb/mvv010. [DOI] [PubMed] [Google Scholar]
- 2.Wang S, Kaufman RJ. The impact of the unfolded protein response on human disease. J Cell Biol. 2012;197:857–867. doi: 10.1083/jcb.201110131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banhegyi G, Margittai E, Szarka A, Mandl J, Csala M. Crosstalk and barriers between the electron carriers of the endoplasmic reticulum. Antioxid Redox Signal. 2012;16:772–780. doi: 10.1089/ars.2011.4437. [DOI] [PubMed] [Google Scholar]
- 4.Csala M, Kereszturi E, Mandl J, Banhegyi G. The endoplasmic reticulum as the extracellular space inside the cell: role in protein folding and glycosylation. Antioxid Redox Signal. 2012;16:1100–1108. doi: 10.1089/ars.2011.4227. [DOI] [PubMed] [Google Scholar]
- 5.Travers KJ, Patil CK, Wodicka L, Lockhart DJ, Weissman JS, Walter P. Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell. 2000;101:249–258. doi: 10.1016/s0092-8674(00)80835-1. [DOI] [PubMed] [Google Scholar]
- 6.Luo B, Lee AS. The critical roles of endoplasmic reticulum chaperones and unfolded protein response in tumorigenesis and anticancer therapies. Oncogene. 2013;32:805–818. doi: 10.1038/onc.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wada I, Rindress D, Cameron PH, Ou WJ, Doherty JJ, 2nd, Louvard D, Bell AW, Dignard D, Thomas DY, Bergeron JJ. SSR alpha and associated calnexin are major calcium binding proteins of the endoplasmic reticulum membrane. J Biol Chem. 1991;266:19599–19610. [PubMed] [Google Scholar]
- 8.David V, Hochstenbach F, Rajagopalan S, Brenner MB. Interaction with newly synthesized and retained proteins in the endoplasmic reticulum suggests a chaperone function for human integral membrane protein IP90 (calnexin) J Biol Chem. 1993;268:9585–9592. [PubMed] [Google Scholar]
- 9.Ng DT, Watowich SS, Lamb RA. Analysis in vivo of GRP78-BiP/substrate interactions and their role in induction of the GRP78-BiP gene. Mol Biol Cell. 1992;3:143–155. doi: 10.1091/mbc.3.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu Z, Luo H, Fu W, Mattson MP. The endoplasmic reticulum stress-responsive protein GRP78 protects neurons against excitotoxicity and apoptosis: suppression of oxidative stress and stabilization of calcium homeostasis. Exp Neurol. 1999;155:302–314. doi: 10.1006/exnr.1998.7002. [DOI] [PubMed] [Google Scholar]
- 11.Wang M, Kaufman RJ. The impact of the endoplasmic reticulum protein-folding environment on cancer development. Nat Rev Cancer. 2014;14:581–597. doi: 10.1038/nrc3800. [DOI] [PubMed] [Google Scholar]
- 12.Ozawa K, Tsukamoto Y, Hori O, Kitao Y, Yanagi H, Stern DM, Ogawa S. Regulation of tumor angiogenesis by oxygen-regulated protein 150, an inducible endoplasmic reticulum chaperone. Cancer Res. 2001;61:4206–4213. [PubMed] [Google Scholar]
- 13.Lodish HF, Kong N, Wikstrom L. Calcium is required for folding of newly made subunits of the asialoglycoprotein receptor within the endoplasmic reticulum. J Biol Chem. 1992;267:12753–12760. [PubMed] [Google Scholar]
- 14.Akihara R, Homma T, Lee J, Yamada K, Miyata S, Fujii J. Ablation of aldehyde reductase aggravates carbon tetrachloride-induced acute hepatic injury involving oxidative stress and endoplasmic reticulum stress. Biochem Biophys Res Commun. 2016;478:765–771. doi: 10.1016/j.bbrc.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 15.Zhang P, Su C, Jiang Z, Zheng C. Herpes Simplex Virus 1 UL41 Protein Suppresses the IRE1/XBP1 Signal Pathway of the Unfolded Protein Response via its RNase activity. J Virol. 2016 doi: 10.1128/JVI.02056-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 17.Schroder M. Endoplasmic reticulum stress responses. Cell Mol Life Sci. 2008;65:862–894. doi: 10.1007/s00018-007-7383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clarke HJ, Chambers JE, Liniker E, Marciniak SJ. Endoplasmic reticulum stress in malignancy. Cancer Cell. 2014;25:563–573. doi: 10.1016/j.ccr.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 19.Tameire F, Verginadis, Koumenis C. Cell intrinsic and extrinsic activators of the unfolded protein response in cancer: Mechanisms and targets for therapy. Semin Cancer Biol. 2015;33:3–15. doi: 10.1016/j.semcancer.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verfaillie T, Garg AD, Agostinis P. Targeting ER stress induced apoptosis and inflammation in cancer. Cancer Lett. 2013;332:249–264. doi: 10.1016/j.canlet.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 21.Jovaisaite V, Mouchiroud L, Auwerx J. The mitochondrial unfolded protein response, a conserved stress response pathway with implications in health and disease. J Exp Biol. 2014;217:137–143. doi: 10.1242/jeb.090738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maurel M, McGrath EP, Mnich K, Healy S, Chevet E, Samali A. Controlling the unfolded protein response-mediated life and death decisions in cancer. Semin Cancer Biol. 2015;33:57–66. doi: 10.1016/j.semcancer.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Shore GC, Papa FR, Oakes SA. Signaling cell death from the endoplasmic reticulum stress response. Curr Opin Cell Biol. 2011;23:143–149. doi: 10.1016/j.ceb.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Armstrong JL, Flockhart R, Veal GJ, Lovat PE, Redfern CP. Regulation of endoplasmic reticulum stress-induced cell death by ATF4 in neuroectodermal tumor cells. J Biol Chem. 2010;285:6091–6100. doi: 10.1074/jbc.M109.014092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park GB, Kim YS, Lee HK, Song H, Cho DH, Lee WJ, Hur DY. Endoplasmic reticulum stress-mediated apoptosis of EBV-transformed B cells by cross-linking of CD70 is dependent upon generation of reactive oxygen species and activation of p38 MAPK and JNK pathway. J Immunol. 2010;185:7274–7284. doi: 10.4049/jimmunol.1001547. [DOI] [PubMed] [Google Scholar]
- 26.Lei X, Zhang S, Bohrer A, Ramanadham S. Calcium-independent phospholipase A2 (iPLA2 beta)-mediated ceramide generation plays a key role in the cross-talk between the endoplasmic reticulum (ER) and mitochondria during ER stress-induced insulin-secreting cell apoptosis. J Biol Chem. 2008;283:34819–34832. doi: 10.1074/jbc.M807409200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoyer-Hansen M, Jaattela M. Connecting endoplasmic reticulum stress to autophagy by unfolded protein response and calcium. Cell Death Differ. 2007;14:1576–1582. doi: 10.1038/sj.cdd.4402200. [DOI] [PubMed] [Google Scholar]
- 28.Chevet E, Hetz C, Samali A. Endoplasmic reticulum stress-activated cell reprogramming in oncogenesis. Cancer Discov. 2015;5:586–597. doi: 10.1158/2159-8290.CD-14-1490. [DOI] [PubMed] [Google Scholar]
- 29.Vidal RL, Hetz C. Crosstalk between the UPR and autophagy pathway contributes to handling cellular stress in neurodegenerative disease. Autophagy. 2012;8:970–972. doi: 10.4161/auto.20139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yorimitsu T, Nair U, Yang Z, Klionsky DJ. Endoplasmic reticulum stress triggers autophagy. J Biol Chem. 2006;281:30299–30304. doi: 10.1074/jbc.M607007200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lane N, Martin W. The energetics of genome complexity. Nature. 2010;467:929–934. doi: 10.1038/nature09486. [DOI] [PubMed] [Google Scholar]
- 32.Wang C, Youle RJ. The role of mitochondria in apoptosis*. Annual review of genetics. 2009;43:95–118. doi: 10.1146/annurev-genet-102108-134850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tait SW, Green DR. Mitochondrial regulation of cell death. Cold Spring Harb Perspect Biol. 2013;5 doi: 10.1101/cshperspect.a008706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yadav N, Chandra D. Mitochondrial and postmitochondrial survival signaling in cancer. Mitochondrion. 2014;16:18–25. doi: 10.1016/j.mito.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoppins S, Lackner L, Nunnari J. The machines that divide and fuse mitochondria. Annu Rev Biochem. 2007;76:751–780. doi: 10.1146/annurev.biochem.76.071905.090048. [DOI] [PubMed] [Google Scholar]
- 36.Friedman JR, Lackner LL, West M, DiBenedetto JR, Nunnari J, Voeltz GK. ER tubules mark sites of mitochondrial division. Science. 2011;334:358–362. doi: 10.1126/science.1207385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suen DF, Norris KL, Youle RJ. Mitochondrial dynamics and apoptosis. Genes Dev. 2008;22:1577–1590. doi: 10.1101/gad.1658508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoppins S, Nunnari J. Cell Biology. Mitochondrial dynamics and apoptosis--the ER connection. Science. 2012;337:1052–1054. doi: 10.1126/science.1224709. [DOI] [PubMed] [Google Scholar]
- 39.Hom JR, Gewandter JS, Michael L, Sheu SS, Yoon Y. Thapsigargin induces biphasic fragmentation of mitochondria through calcium-mediated mitochondrial fission and apoptosis. J Cell Physiol. 2007;212:498–508. doi: 10.1002/jcp.21051. [DOI] [PubMed] [Google Scholar]
- 40.Rainbolt TK, Saunders JM, Wiseman RL. Stress-responsive regulation of mitochondria through the ER unfolded protein response. Trends Endocrinol Metab. 2014;25:528–537. doi: 10.1016/j.tem.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 41.Gorlach A, Klappa P, Kietzmann T. The endoplasmic reticulum: folding, calcium homeostasis, signaling, and redox control. Antioxid Redox Signal. 2006;8:1391–1418. doi: 10.1089/ars.2006.8.1391. [DOI] [PubMed] [Google Scholar]
- 42.Maas NL, Diehl JA. Molecular Pathways: The PERKs and Pitfalls of Targeting the Unfolded Protein Response in Cancer. Clin Cancer Res. 2015;21:675–679. doi: 10.1158/1078-0432.CCR-13-3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dey S, Baird TD, Zhou D, Palam LR, Spandau DF, Wek RC. Both transcriptional regulation and translational control of ATF4 are central to the integrated stress response. J Biol Chem. 2010;285:33165–33174. doi: 10.1074/jbc.M110.167213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baird TD, Wek RC. Eukaryotic initiation factor 2 phosphorylation and translational control in metabolism. Adv Nutr. 2012;3:307–321. doi: 10.3945/an.112.002113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kilberg MS, Balasubramanian M, Fu L, Shan J. The transcription factor network associated with the amino acid response in mammalian cells. Adv Nutr. 2012;3:295–306. doi: 10.3945/an.112.001891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luo S, Baumeister P, Yang S, Abcouwer SF, Lee AS. Induction of Grp78/BiP by translational block: activation of the Grp78 promoter by ATF4 through and upstream ATF/CRE site independent of the endoplasmic reticulum stress elements. J Biol Chem. 2003;278:37375–37385. doi: 10.1074/jbc.M303619200. [DOI] [PubMed] [Google Scholar]
- 47.Liu F, Inageda K, Nishitai G, Matsuoka M. Cadmium induces the expression of Grp78, an endoplasmic reticulum molecular chaperone, in LLC-PK1 renal epithelial cells. Environ Health Perspect. 2006;114:859–864. doi: 10.1289/ehp.8920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lange PS, Chavez JC, Pinto JT, Coppola G, Sun CW, Townes TM, Geschwind DH, Ratan RR. ATF4 is an oxidative stress-inducible, prodeath transcription factor in neurons in vitro and in vivo. J Exp Med. 2008;205:1227–1242. doi: 10.1084/jem.20071460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao E, Ding J, Xia Y, Liu M, Ye B, Choi JH, Yan C, Dong Z, Huang S, Zha Y, Yang L, Cui H, Ding HF. KDM4C and ATF4 Cooperate in Transcriptional Control of Amino Acid Metabolism. Cell Rep. 2016;14:506–519. doi: 10.1016/j.celrep.2015.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sano R, Reed JC. ER stress-induced cell death mechanisms. Biochim Biophys Acta. 2013;1833:3460–3470. doi: 10.1016/j.bbamcr.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kobayashi A, Kang MI, Okawa H, Ohtsuji M, Zenke Y, Chiba T, Igarashi K, Yamamoto M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol. 2004;24:7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cullinan SB, Zhang D, Hannink M, Arvisais E, Kaufman RJ, Diehl JA. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol Cell Biol. 2003;23:7198–7209. doi: 10.1128/MCB.23.20.7198-7209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fribley AM, Evenchik B, Zeng Q, Park BK, Guan JY, Zhang H, Hale TJ, Soengas MS, Kaufman RJ, Wang CY. Proteasome inhibitor PS-341 induces apoptosis in cisplatin-resistant squamous cell carcinoma cells by induction of Noxa. J Biol Chem. 2006;281:31440–31447. doi: 10.1074/jbc.M604356200. [DOI] [PubMed] [Google Scholar]
- 54.Mimnaugh EG, Xu W, Vos M, Yuan X, Isaacs JS, Bisht KS, Gius D, Neckers L. Simultaneous inhibition of hsp 90 and the proteasome promotes protein ubiquitination, causes endoplasmic reticulum-derived cytosolic vacuolization, and enhances antitumor activity. Mol Cancer Ther. 2004;3:551–566. [PubMed] [Google Scholar]
- 55.Nawrocki ST, Carew JS, Pino MS, Highshaw RA, Dunner K, Jr, Huang P, Abbruzzese JL, McConkey DJ. Bortezomib sensitizes pancreatic cancer cells to endoplasmic reticulum stress-mediated apoptosis. Cancer Res. 2005;65:11658–11666. doi: 10.1158/0008-5472.CAN-05-2370. [DOI] [PubMed] [Google Scholar]
- 56.Nagelkerke A, Bussink J, Sweep FC, Span PN. The unfolded protein response as a target for cancer therapy. Biochim Biophys Acta. 2014;1846:277–284. doi: 10.1016/j.bbcan.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 57.Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 58.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 59.Niture SK, Jaiswal AK. Nrf2 protein up-regulates antiapoptotic protein Bcl-2 and prevents cellular apoptosis. J Biol Chem. 2012;287:9873–9886. doi: 10.1074/jbc.M111.312694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Niture SK, Jaiswal AK. Nrf2-induced antiapoptotic Bcl-xL protein enhances cell survival and drug resistance. Free Radic Biol Med. 2013;57:119–131. doi: 10.1016/j.freeradbiomed.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yin XM, Oltvai ZN, Korsmeyer SJ. BH1 and BH2 domains of Bcl-2 are required for inhibition of apoptosis and heterodimerization with Bax. Nature. 1994;369:321–323. doi: 10.1038/369321a0. [DOI] [PubMed] [Google Scholar]
- 62.Antonsson B, Conti F, Ciavatta A, Montessuit S, Lewis S, Martinou I, Bernasconi L, Bernard A, Mermod JJ, Mazzei G, Maundrell K, Gambale F, Sadoul R, Martinou JC. Inhibition of Bax channel-forming activity by Bcl-2. Science. 1997;277:370–372. doi: 10.1126/science.277.5324.370. [DOI] [PubMed] [Google Scholar]
- 63.Finucane DM, Bossy-Wetzel E, Waterhouse NJ, Cotter TG, Green DR. Bax-induced caspase activation and apoptosis via cytochrome c release from mitochondria is inhibitable by Bcl-xL. J Biol Chem. 1999;274:2225–2233. doi: 10.1074/jbc.274.4.2225. [DOI] [PubMed] [Google Scholar]
- 64.Hu Y, Benedict MA, Wu D, Inohara N, Nunez G. Bcl-XL interacts with Apaf-1 and inhibits Apaf-1-dependent caspase-9 activation. Proc Natl Acad Sci U S A. 1998;95:4386–4391. doi: 10.1073/pnas.95.8.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li J, Lee B, Lee AS. Endoplasmic reticulum stress-induced apoptosis: multiple pathways and activation of p53-up-regulated modulator of apoptosis (PUMA) and NOXA by p53. J Biol Chem. 2006;281:7260–7270. doi: 10.1074/jbc.M509868200. [DOI] [PubMed] [Google Scholar]
- 66.Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- 67.Liu Y, Adachi M, Zhao S, Hareyama M, Koong AC, Luo D, Rando TA, Imai K, Shinomura Y. Preventing oxidative stress: a new role for XBP1. Cell Death Differ. 2009;16:847–857. doi: 10.1038/cdd.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Prischi F, Nowak PR, Carrara M, Ali MM. Phosphoregulation of Ire1 RNase splicing activity. Nat Commun. 2014;5:3554. doi: 10.1038/ncomms4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yamamoto K, Sato T, Matsui T, Sato M, Okada T, Yoshida H, Harada A, Mori K. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6alpha and XBP1. Dev Cell. 2007;13:365–376. doi: 10.1016/j.devcel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 70.Bommiasamy H, Back SH, Fagone P, Lee K, Meshinchi S, Vink E, Sriburi R, Frank M, Jackowski S, Kaufman RJ, Brewer JW. ATF6alpha induces XBP1-independent expansion of the endoplasmic reticulum. J Cell Sci. 2009;122:1626–1636. doi: 10.1242/jcs.045625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Han J, Back SH, Hur J, Lin YH, Gildersleeve R, Shan J, Yuan CL, Krokowski D, Wang S, Hatzoglou M, Kilberg MS, Sartor MA, Kaufman RJ. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol. 2013;15:481–490. doi: 10.1038/ncb2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ma Y, Brewer JW, Diehl JA, Hendershot LM. Two distinct stress signaling pathways converge upon the CHOP promoter during the mammalian unfolded protein response. Journal of molecular biology. 2002;318:1351–1365. doi: 10.1016/s0022-2836(02)00234-6. [DOI] [PubMed] [Google Scholar]
- 73.Rutkowski DT, Arnold SM, Miller CN, Wu J, Li J, Gunnison KM, Mori K, Sadighi Akha AA, Raden D, Kaufman RJ. Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol. 2006;4:e374. doi: 10.1371/journal.pbio.0040374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Averous J, Bruhat A, Jousse C, Carraro V, Thiel G, Fafournoux P. Induction of CHOP expression by amino acid limitation requires both ATF4 expression and ATF2 phosphorylation. J Biol Chem. 2004;279:5288–5297. doi: 10.1074/jbc.M311862200. [DOI] [PubMed] [Google Scholar]
- 75.Lin JH, Li H, Zhang Y, Ron D, Walter P. Divergent effects of PERK and IRE1 signaling on cell viability. PLoS One. 2009;4:e4170. doi: 10.1371/journal.pone.0004170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Menegon S, Columbano A, Giordano S. The Dual Roles of NRF2 in Cancer. Trends Mol Med. 2016;22:578–593. doi: 10.1016/j.molmed.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 77.Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Invest. 2008;118:3378–3389. doi: 10.1172/JCI34587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Szegezdi E, Macdonald DC, Ni Chonghaile T, Gupta S, Samali A. Bcl-2 family on guard at the ER. Am J Physiol Cell Physiol. 2009;296:C941–953. doi: 10.1152/ajpcell.00612.2008. [DOI] [PubMed] [Google Scholar]
- 79.Ruiz-Vela A, Opferman JT, Cheng EH, Korsmeyer SJ. Proapoptotic BAX and BAK control multiple initiator caspases. EMBO Rep. 2005;6:379–385. doi: 10.1038/sj.embor.7400375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang D, Armstrong JS. Bax and the mitochondrial permeability transition cooperate in the release of cytochrome c during endoplasmic reticulum-stress-induced apoptosis. Cell Death Differ. 2007;14:703–715. doi: 10.1038/sj.cdd.4402072. [DOI] [PubMed] [Google Scholar]
- 81.Li Y, Guo Y, Tang J, Jiang J, Chen Z. New insights into the roles of CHOP-induced apoptosis in ER stress. Acta Biochim Biophys Sin. 2014;46:629–640. doi: 10.1093/abbs/gmu048. [DOI] [PubMed] [Google Scholar]
- 82.Chen BL, Sheu ML, Tsai KS, Lan KC, Guan SS, Wu CT, Chen LP, Hung KY, Huang JW, Chiang CK, Liu SH. CCAAT-Enhancer-Binding Protein Homologous Protein Deficiency Attenuates Oxidative Stress and Renal Ischemia-Reperfusion Injury. Antioxid Redox Signal. 2015;23:1233–1245. doi: 10.1089/ars.2013.5768. [DOI] [PubMed] [Google Scholar]
- 83.Lee DY, Lee KS, Lee HJ, Kim do H, Noh YH, Yu K, Jung HY, Lee SH, Lee JY, Youn YC, Jeong Y, Kim DK, Lee WB, Kim SS. Activation of PERK signaling attenuates Abeta-mediated ER stress. PLoS One. 2010;5:e10489. doi: 10.1371/journal.pone.0010489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Takemoto K, Miyata S, Takamura H, Katayama T, Tohyama M. Mitochondrial TRAP1 regulates the unfolded protein response in the endoplasmic reticulum. Neurochem Int. 2011;58:880–887. doi: 10.1016/j.neuint.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 85.Heath-Engel H, Wang B, Shore G. Bcl2 at the endoplasmic reticulum protects against a Bax/Bak-independent paraptosis-like cell death pathway initiated via p20Bap31. Biochim Biophys Acta. 2012;1823:335–347. doi: 10.1016/j.bbamcr.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 86.Nieto-Miguel T, Fonteriz RI, Vay L, Gajate C, Lopez-Hernandez S, Mollinedo F. Endoplasmic reticulum stress in the proapoptotic action of edelfosine in solid tumor cells. Cancer Res. 2007;67:10368–10378. doi: 10.1158/0008-5472.CAN-07-0278. [DOI] [PubMed] [Google Scholar]
- 87.Panaretakis T, Kepp O, Brockmeier U, Tesniere A, Bjorklund AC, Chapman DC, Durchschlag M, Joza N, Pierron G, van Endert P, Yuan J, Zitvogel L, Madeo F, Williams DB, Kroemer G. Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death. EMBO J. 2009;28:578–590. doi: 10.1038/emboj.2009.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kouroku Y, Fujita E, Tanida I, Ueno T, Isoai A, Kumagai H, Ogawa S, Kaufman RJ, Kominami E, Momoi T. ER stress (PERK/eIF2alpha phosphorylation) mediates the polyglutamine-induced LC3 conversion, an essential step for autophagy formation. Cell Death Differ. 2007;14:230–239. doi: 10.1038/sj.cdd.4401984. [DOI] [PubMed] [Google Scholar]
- 90.Hoyer-Hansen M, Bastholm L, Szyniarowski P, Campanella M, Szabadkai G, Farkas T, Bianchi K, Fehrenbacher N, Elling F, Rizzuto R, Mathiasen IS, Jaattela M. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol Cell. 2007;25:193–205. doi: 10.1016/j.molcel.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 91.Sakaki K, Kaufman RJ. Regulation of ER stress-induced macroautophagy by protein kinase C. Autophagy. 2008;4:841–843. doi: 10.4161/auto.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gozuacik D, Bialik S, Raveh T, Mitou G, Shohat G, Sabanay H, Mizushima N, Yoshimori T, Kimchi A. DAP-kinase is a mediator of endoplasmic reticulum stress-induced caspase activation and autophagic cell death. Cell Death Differ. 2008;15:1875–1886. doi: 10.1038/cdd.2008.121. [DOI] [PubMed] [Google Scholar]
- 93.Verfaillie T, Salazar M, Velasco G, Agostinis P. Linking ER Stress to Autophagy: Potential Implications for Cancer Therapy. Int J Cell Biol. 2010;2010:930509. doi: 10.1155/2010/930509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Criollo A, Maiuri MC, Tasdemir E, Vitale I, Fiebig AA, Andrews D, Molgo J, Diaz J, Lavandero S, Harper F, Pierron G, di Stefano D, Rizzuto R, Szabadkai G, Kroemer G. Regulation of autophagy by the inositol trisphosphate receptor. Cell Death Differ. 2007;14:1029–1039. doi: 10.1038/sj.cdd.4402099. [DOI] [PubMed] [Google Scholar]
- 95.Salazar M, Carracedo A, Salanueva IJ, Hernandez-Tiedra S, Lorente M, Egia A, Vazquez P, Blazquez C, Torres S, Garcia S, Nowak J, Fimia GM, Piacentini M, Cecconi F, Pandolfi PP, Gonzalez-Feria L, Iovanna JL, Guzman M, Boya P, Velasco G. Cannabinoid action induces autophagy-mediated cell death through stimulation of ER stress in human glioma cells. J Clin Invest. 2009;119:1359–1372. doi: 10.1172/JCI37948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ding WX, Ni HM, Gao W, Hou YF, Melan MA, Chen X, Stolz DB, Shao ZM, Yin XM. Differential effects of endoplasmic reticulum stress-induced autophagy on cell survival. J Biol Chem. 2007;282:4702–4710. doi: 10.1074/jbc.M609267200. [DOI] [PubMed] [Google Scholar]
- 97.Puthalakath H, O’Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, Hughes PD, Michalak EM, McKimm-Breschkin J, Motoyama N, Gotoh T, Akira S, Bouillet P, Strasser A. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129:1337–1349. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 98.McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7:880–885. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang T, Li X, Yang D, Zhang H, Zhao P, Fu J, Yao B, Zhou Z. ER stress and ER stress-mediated apoptosis are involved in manganese-induced neurotoxicity in the rat striatum in vivo. Neurotoxicology. 2015;48:109–119. doi: 10.1016/j.neuro.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 101.Gurlo T, Rivera JF, Butler AE, Cory M, Hoang J, Costes S, Butler PC. CHOP Contributes to, But Is Not the Only Mediator of, IAPP Induced beta-Cell Apoptosis. Mol Endocrinol. 2016;30:446–454. doi: 10.1210/me.2015-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang Q, Mora-Jensen H, Weniger MA, Perez-Galan P, Wolford C, Hai T, Ron D, Chen W, Trenkle W, Wiestner A, Ye Y. ERAD inhibitors integrate ER stress with an epigenetic mechanism to activate BH3-only protein NOXA in cancer cells. Proc Natl Acad Sci U S A. 2009;106:2200–2205. doi: 10.1073/pnas.0807611106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kapur A, Felder M, Fass L, Kaur J, Czarnecki A, Rathi K, Zeng S, Osowski KK, Howell C, Xiong MP, Whelan RJ, Patankar MS. Modulation of oxidative stress and subsequent induction of apoptosis and endoplasmic reticulum stress allows citral to decrease cancer cell proliferation. Sci Rep. 2016;6:27530. doi: 10.1038/srep27530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pagliarini V, Giglio P, Bernardoni P, De Zio D, Fimia GM, Piacentini M, Corazzari M. Downregulation of E2F1 during ER stress is required to induce apoptosis. J Cell Sci. 2015;128:1166–1179. doi: 10.1242/jcs.164103. [DOI] [PubMed] [Google Scholar]
- 105.Klee M, Pallauf K, Alcala S, Fleischer A, Pimentel-Muinos FX. Mitochondrial apoptosis induced by BH3-only molecules in the exclusive presence of endoplasmic reticular Bak. EMBO J. 2009;28:1757–1768. doi: 10.1038/emboj.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shimizu S, Konishi A, Nishida Y, Mizuta T, Nishina H, Yamamoto A, Tsujimoto Y. Involvement of JNK in the regulation of autophagic cell death. Oncogene. 2010;29:2070–2082. doi: 10.1038/onc.2009.487. [DOI] [PubMed] [Google Scholar]
- 107.Hetz C, Chevet E, Harding HP. Targeting the unfolded protein response in disease. Nat Rev Drug Discov. 2013;12:703–719. doi: 10.1038/nrd3976. [DOI] [PubMed] [Google Scholar]
- 108.DeVay RM, Dominguez-Ramirez L, Lackner LL, Hoppins S, Stahlberg H, Nunnari J. Coassembly of Mgm1 isoforms requires cardiolipin and mediates mitochondrial inner membrane fusion. J Cell Biol. 2009;186:793–803. doi: 10.1083/jcb.200906098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science. 2012;337:1062–1065. doi: 10.1126/science.1219855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yamaguchi R, Lartigue L, Perkins G, Scott RT, Dixit A, Kushnareva Y, Kuwana T, Ellisman MH, Newmeyer DD. Opa1-mediated cristae opening is Bax/Bak and BH3 dependent, required for apoptosis, and independent of Bak oligomerization. Mol Cell. 2008;31:557–569. doi: 10.1016/j.molcel.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yamaguchi R, Perkins G. Dynamics of mitochondrial structure during apoptosis and the enigma of Opa1. Biochim Biophys Acta. 2009;1787:963–972. doi: 10.1016/j.bbabio.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Karbowski M, Lee YJ, Gaume B, Jeong SY, Frank S, Nechushtan A, Santel A, Fuller M, Smith CL, Youle RJ. Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J Cell Biol. 2002;159:931–938. doi: 10.1083/jcb.200209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang P, Wang P, Liu B, Zhao J, Pang Q, Agrawal SG, Jia L, Liu FT. Dynamin-related protein Drp1 is required for Bax translocation to mitochondria in response to irradiation-induced apoptosis. Oncotarget. 2015;6:22598–22612. doi: 10.18632/oncotarget.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Grosse L, Wurm CA, Bruser C, Neumann D, Jans DC, Jakobs S. Bax assembles into large ring-like structures remodeling the mitochondrial outer membrane in apoptosis. EMBO J. 2016;35:402–413. doi: 10.15252/embj.201592789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang Z, Liu L, Wu S, Xing D. Drp1, Mff, Fis1, and MiD51 are coordinated to mediate mitochondrial fission during UV irradiation-induced apoptosis. FASEB J. 2016;30:466–476. doi: 10.1096/fj.15-274258. [DOI] [PubMed] [Google Scholar]
- 116.Oettinghaus B, D’Alonzo D, Barbieri E, Restelli LM, Savoia C, Licci M, Tolnay M, Frank S, Scorrano L. DRP1-dependent apoptotic mitochondrial fission occurs independently of BAX, BAK and APAF1 to amplify cell death by BID and oxidative stress. Biochim Biophys Acta. 2016;1857:1267–1276. doi: 10.1016/j.bbabio.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 117.Cleland MM, Norris KL, Karbowski M, Wang C, Suen DF, Jiao S, George NM, Luo X, Li Z, Youle RJ. Bcl-2 family interaction with the mitochondrial morphogenesis machinery. Cell Death Differ. 2011;18:235–247. doi: 10.1038/cdd.2010.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hatch AL, Gurel PS, Higgs HN. Novel roles for actin in mitochondrial fission. J Cell Sci. 2014;127:4549–4560. doi: 10.1242/jcs.153791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chipuk JE, McStay GP, Bharti A, Kuwana T, Clarke CJ, Siskind LJ, Obeid LM, Green DR. Sphingolipid metabolism cooperates with BAK and BAX to promote the mitochondrial pathway of apoptosis. Cell. 2012;148:988–1000. doi: 10.1016/j.cell.2012.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schon EA, Przedborski S. Mitochondria: the next (neurode)generation. Neuron. 2011;70:1033–1053. doi: 10.1016/j.neuron.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 122.Iwasawa R, Mahul-Mellier AL, Datler C, Pazarentzos E, Grimm S. Fis1 and Bap31 bridge the mitochondria-ER interface to establish a platform for apoptosis induction. EMBO J. 2011;30:556–568. doi: 10.1038/emboj.2010.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kumar S, Chaudhary AK, Kumar R, O’Malley J, Dubrovska A, Wang X, Yadav N, Goodrich DW, Chandra D. Combination therapy induces unfolded protein response and cytoskeletal rearrangement leading to mitochondrial apoptosis in prostate cancer. Mol Oncol. 2016;10:949–965. doi: 10.1016/j.molonc.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chaudhary AK, Bhat TA, Kumar S, Kumar A, Kumar R, Underwood W, Koochekpour S, Shourideh M, Yadav N, Dhar S, Chandra D. Mitochondrial dysfunction-mediated apoptosis resistance associates with defective heat shock protein response in African-American men with prostate cancer. Br J Cancer. 2016;114:1090–1100. doi: 10.1038/bjc.2016.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Choi MJ, Park EJ, Min KJ, Park JW, Kwon TK. Endoplasmic reticulum stress mediates withaferin A-induced apoptosis in human renal carcinoma cells. Toxicol In Vitro. 2011;25:692–698. doi: 10.1016/j.tiv.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 126.Li X, Zhu F, Jiang J, Sun C, Zhong Q, Shen M, Wang X, Tian R, Shi C, Xu M, Peng F, Guo X, Hu J, Ye D, Wang M, Qin R. Simultaneous inhibition of the ubiquitin-proteasome system and autophagy enhances apoptosis induced by ER stress aggravators in human pancreatic cancer cells. Autophagy. 2016;12:1521–1537. doi: 10.1080/15548627.2016.1191722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hong YH, Uddin MH, Jo U, Kim B, Song J, Suh DH, Kim HS, Song YS. ROS Accumulation by PEITC Selectively Kills Ovarian Cancer Cells via UPR-Mediated Apoptosis. Front Oncol. 2015;5:167. doi: 10.3389/fonc.2015.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kim SH, Kim KY, Yu SN, Seo YK, Chun SS, Yu HS, Ahn SC. Silibinin induces mitochondrial NOX4-mediated endoplasmic reticulum stress response and its subsequent apoptosis. BMC Cancer. 2016;16:452. doi: 10.1186/s12885-016-2516-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Selvaraj S, Sun Y, Sukumaran P, Singh BB. Resveratrol activates autophagic cell death in prostate cancer cells via downregulation of STIM1 and the mTOR pathway. Mol Carcinog. 2016;55:818–831. doi: 10.1002/mc.22324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wielenga MC, Colak S, Heijmans J, van Lidth de Jeude JF, Rodermond HM, Paton JC, Paton AW, Vermeulen L, Medema JP, van den Brink GR. ER-Stress-Induced Differentiation Sensitizes Colon Cancer Stem Cells to Chemotherapy. Cell Rep. 2015;13:490–494. doi: 10.1016/j.celrep.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 131.Singh SV, Srivastava SK, Choi S, Lew KL, Antosiewicz J, Xiao D, Zeng Y, Watkins SC, Johnson CS, Trump DL, Lee YJ, Xiao H, Herman-Antosiewicz A. Sulforaphane-induced cell death in human prostate cancer cells is initiated by reactive oxygen species. J Biol Chem. 2005;280:19911–19924. doi: 10.1074/jbc.M412443200. [DOI] [PubMed] [Google Scholar]
- 132.Hsia TC, Yu CC, Hsu SC, Tang NY, Lu HF, Huang YP, Wu SH, Lin JG, Chung JG. Cantharidin induces apoptosis of H460 human lung cancer cells through mitochondria-dependent pathways. Int J Oncol. 2014;45:245–254. doi: 10.3892/ijo.2014.2428. [DOI] [PubMed] [Google Scholar]
- 133.Vaeteewoottacharn K, Kariya R, Matsuda K, Taura M, Wongkham C, Wongkham S, Okada S. Perturbation of proteasome function by bortezomib leading to ER stress-induced apoptotic cell death in cholangiocarcinoma. J Cancer Res Clin Oncol. 2013;139:1551–1562. doi: 10.1007/s00432-013-1473-6. [DOI] [PubMed] [Google Scholar]
- 134.Atkins C, Liu Q, Minthorn E, Zhang SY, Figueroa DJ, Moss K, Stanley TB, Sanders B, Goetz A, Gaul N, Choudhry AE, Alsaid H, Jucker BM, Axten JM, Kumar R. Characterization of a novel PERK kinase inhibitor with antitumor and antiangiogenic activity. Cancer Res. 2013;73:1993–2002. doi: 10.1158/0008-5472.CAN-12-3109. [DOI] [PubMed] [Google Scholar]
- 135.Park HR, Tomida A, Sato S, Tsukumo Y, Yun J, Yamori T, Hayakawa Y, Tsuruo T, Shin-ya K. Effect on tumor cells of blocking survival response to glucose deprivation. J Natl Cancer Inst. 2004;96:1300–1310. doi: 10.1093/jnci/djh243. [DOI] [PubMed] [Google Scholar]
- 136.Nami B, Donmez H, Kocak N. Tunicamycin-induced endoplasmic reticulum stress reduces in vitro subpopulation and invasion of CD44+/CD24− phenotype breast cancer stem cells. Exp Toxicol Pathol. 2016;68:419–426. doi: 10.1016/j.etp.2016.06.004. [DOI] [PubMed] [Google Scholar]