Abstract
Objective
We examine race/ethnic, gender, and age differences in telomere length (TL) within a diverse, nationally representative sample of older adults.
Method
Data come from 5,228 White, Black, and Hispanic respondents aged 54+ in the 2008 Health and Retirement Study. TL was assayed from saliva using quantitative polymerase chain reaction (qPCR) by comparing telomere sequence copy number with a single gene copy number (T/S ratio). Linear regression was used to examine TL by race/ethnicity, gender, and age adjusting for social, economic, and health characteristics.
Results
Women had longer TL than men (p < .05). Blacks (p < .05) and Hispanics (p < .10) had longer TL than Whites. Black women and men had the longest TL relative to other groups (p < .05), while White men had the shortest TL (p < .05). Black women and Hispanic men showed greater differences in TL with age.
Discussion
Findings indicate social patterns in TL by race/ethnicity, gender, and age among older adults do not reflect differences observed in most population health outcomes.
Keywords: biomarkers of aging, health disparities, minority aging, accelerated aging, social epidemiology
Background
Biomarkers of aging, which are biological indicators of the progression of the aging process, are increasingly being used to identify individuals at greater risk of developing age-related diseases and functional impairment. In addition, biomarkers are useful tools to gain insights into the underlying social and biological processes driving differences in health risks across the adult life span. Biomarkers of aging are particularly useful for identifying populations at risk of premature aging, even prior to the onset of disease and disability (Worthman & Costello, 2009). Previous research suggests social differences in health at older ages may be the result of accelerated biological aging among certain subgroups of the U.S. population (Levine & Crimmins, 2014). Ideally, biomarkers of aging help to characterize accelerated biological aging, yet we are still hunting for biological markers that reliably index how fast the process is unfolding. One potential aging biomarker that may inform our understanding of population-level group differences in health and aging among older adults is telomere length (TL).
Telomere shortening is recognized as fundamental to the human aging process, and TL is hypothesized to be a biomarker of aging. Telomeres are repeating DNA sequences that cap the ends of chromosomes and gradually shorten with age. This shortening has been linked to biological and genetic factors of aging including oxidative stress, inflammation, chronic disease, cellular senescence, and mortality (Bakaysa et al., 2007; Blackburn, 2000, 2005; Brouilette, Singh, Thompson, Goodall, & Samani, 2003; Fitzpatrick et al., 2007; Needham et al., 2013; Sanders et al., 2012; von Zglinicki, 2002; Willeit et al., 2010). Some studies, however, find no association between TL and mortality (e.g., Harris et al., 2006). Prior researchers have suggested TL is a measure of biological weathering and accelerated aging, two processes argued to be key factors in producing population-level race/ethnic, gender, and socioeconomic health disparities (Geronimus et al., 2010; Geronimus et al., 2015; Sanders et al., 2012).
The value of TL as a biomarker of aging, and in particular as a biomarker that can be used to describe and explain social differences in aging trajectories among older adults, is unclear. Evidence for gender and race/ethnic differences in TL is based primarily on data from younger adult populations and is mixed. For instance, a meta-analysis of 32 studies concluded that women had longer TL than men (Gardner et al., 2014). Yet, some studies have reported longer TL in men (Adams et al., 2007; Martin-Ruiz et al., 2011), or no gender differences in TL (Hunt et al., 2008; Needham et al., 2013; Shiels et al., 2011). There is a similar lack of consensus on race and ethnic differences in TL. In studies that consider race differences in TL, several report longer TL in African Americans compared with Whites (Adler et al., 2013; Hunt et al., 2008; Needham et al., 2013), but at least two studies report shorter TL in African Americans (Diez Roux et al., 2009; Geronimus et al., 2010). Only three studies, to our knowledge, have examined TL in Hispanics; one study found Hispanics had shorter TL compared with Whites (Diez Roux et al., 2009), while two others reported no difference (Geronimus et al., 2015; Needham et al., 2013). There may be important race-by-gender differences in TL as well. One study, for instance, found that gender differences were only present in African Americans (Needham et al., 2013).
Nearly all of the prior studies examining sub-group differences in TL use select populations that may not reflect the gender and race/ethnic differences present in the broader U.S. older adult population. The lack of nationally representative data is particularly problematic for understanding racial and ethnic differences in TL, as these differences may vary across geographic contexts (Adler et al., 2013; Diez Roux et al., 2009; Geronimus et al., 2010; Geronimus et al., 2015; Hunt et al., 2008). The one study that examined TL differences in national data on U.S. adults did not focus on older adults specifically (Needham et al., 2013). The objective of this study is to determine whether there are gender and race/ethnic differences in TL in a national sample of older adults, and whether these differences vary with age. Examining TL in older adults is of particular importance because TL is hypothesized to be a biomarker of cellular aging that can be used to predict both health span and life span in older adults and explain environmentally induced differences in rates of aging. Based on previous research using national data on adults (Needham et al., 2013), we expect that (a) women and men will have similar TL, (b) Blacks will have longer TL than Whites, (c) Hispanics will have similar TL as Whites, and (d) TL will be shorter at older ages.
Data and Measures
We use data from the 2008 wave of the nationally representative U.S. Health and Retirement Study (HRS). In 2008, a random one half of sampled households were selected to receive an Enhanced Face-to-Face (EFTF) Interview, which included collection of saliva. Of those selected for the EFTF, 85% of respondents consented to providing saliva. The completion rate among those who consented was 99%, for an overall completion rate of 84% (HRS, 2013).
We restrict our analyses to 5,392 age-eligible respondents. We excluded 79 respondents who did not identify as White, Black, or Hispanic and 33 respondents with TL values greater than four standard deviations from the mean. We excluded an additional 52 respondents who had missing information on other analysis variables. The final analytic sample consisted of 5,228 community-dwelling adults aged 54 and older.
Telomere Length
TL is measured from buccal cells in saliva, which is highly correlated with blood leukocyte TL (r = .72), a measurement of TL that is frequently used in other studies (Mitchell et al., 2014). Saliva was collected using an Oragene Collection Kit, and all samples were stored in their original plates at −80°C. TL assays were performed by Telome Health (Aviv et al., 2011; Cawthon, 2002; Telomere Diagnostics, n.d.) using quantitative PCR (qPCR), a well-validated and now widely accepted technique to measure TL, by comparing telomere sequence copy number in each respondent’s sample (T) to a single-copy gene copy number (S), resulting in a T/S ratio (Aviv et al., 2011; Cawthon, 2009). DNA samples were assayed in 96 well plates. The HRS took effort to minimize experimental variability by testing coefficient of variation (CV) for each sample based on three runs (three pairs of T and S runs) for Plates 2 to 9, 11, and 13 and based on two runs for Plates 1, 10, and 13 to 64. Samples that had smaller than 12.5% CV were considered pass, and samples with greater than 12.5% CV were reassayed (overall pass rate > 98%). In addition, the HRS provides plate numbers to account for this variation in plate assay and dilution methods (HRS, 2013).
Measures
Our main variables of interest are age, gender, and race/ethnicity. Age was measured in years. Gender is a dichotomous variable with males treated as the reference. Race/ethnicity is a three-category variable representing non-Hispanic Whites, non-Hispanic Blacks, and Hispanics.
We include social and economic factors that might account for the race/ ethnic and gender differences in TL. Educational attainment was measured using number of years of completed education and categorized as less than 9 years, 9 to 11 years, 12 years, and 13 or more years. Total household income and wealth (assets minus debts) are categorized into quartiles because these variables were highly skewed. Marital status was categorized as married/partnered, divorced/separated, widowed, and never married. Employment status is a dichotomous variable indicating whether the respondent was employed.
Because some studies have found that shorter TL is associated with obesity (Kim et al., 2009; Valdes et al., 2005), physical inactivity (Du et al., 2012; Ludlow & Roth, 2011), smoking (Gardner et al., 2014; Valdes et al., 2005), and chronic conditions, and because individual differences in health and health behaviors may account for race/ethnic and gender differences in TL, we include several key health measures in our analysis. Obesity is a dichotomous indicator comparing those with body mass index (BMI) of 30 and above with those with BMI less than 30. We used self-reports of frequency of moderate or vigorous physical activity in the past 12 months to create a dichotomous indicator of physical inactivity, representing those who engaged in activity less than twice a week. Smoking status was categorized as never, former, or current smoker. We also include a summary measure of chronic disease burden (range = 0-6), which sums the number of doctor-diagnosed self-reports of six major chronic conditions and diseases—hypertension, heart disease, diabetes, cancer, stroke, and lung disease (not including asthma)—that are among the leading causes of death and disability among older U.S. adults.
Analytic Strategy
Our measure of TL is approximately normally distributed. We used ordinary least squares (OLS) regression models to examine age, gender, and race/ethnic differences in TL. We first present an initial model with only these demographic characteristics. We then include in subsequent models social, economic, and health characteristics to determine whether race/ethnic and gender differences are attributed to other factors related to TL. To determine whether there are differences between all race/ethnic and gender groups, we also conducted pairwise comparisons using Stata’s pwcompare command. We then examined race/ethnic and age differences in TL in models stratified by gender. Using estimates from the gender-stratified models, we plot values of TL for each race/ethnic group by age for women and men. We also include unadjusted means by age (calculated using a 3-year moving average) for comparison with the predicted values from the fully adjusted model. All analyses were adjusted for the plate numbers used to assay TL based on the six different dilution factors (HRS, 2013) and were weighted to correct for differential probability of selection and non-response. Analyses were performed using Stata Version 13.
Results
Table 1 describes the distribution of TL and covariates in the full sample by race/ethnicity. On average, Whites were older, had higher education, and had higher levels of income and wealth relative to Blacks and Hispanics. The proportion of obese, inactive, and current smokers was higher among Blacks and Hispanics compared with Whites. Blacks had, on average, a higher chronic disease burden than both Whites and Hispanics. In addition, Blacks had longer mean TL length (M [SE] = 1.5 [0.02]) than Hispanics (M [SE] = 1.4 [0.02]) and Whites (M [SE] = 1.3 [0.01]).
Table 1.
Sample Characteristics by Race/Ethnicity and Gender, Health, and Retirement Study (N = 5,228).
| Whites |
Blacks |
Hispanics |
||
|---|---|---|---|---|
| Variables | Full sample | (n = 4,000) | (n = 703) | (n = 525) |
| Age, years | 66.9 (0.15) | 67.5 (0.18) | 64.7 (0.39) | 63.8 (0.39) |
| Female (%) | 53.2 | 52.8 | 56.2 | 54.1 |
| Education (%) | — | — | — | — |
| <High school | 16.9 | 12.1 | 29.1 | 53.9 |
| High school | 34.1 | 35.7 | 29.7 | 22.1 |
| Some college | 24.4 | 25.3 | 22.3 | 17.3 |
| ≥College degree | 24.6 | 27.0 | 18.9 | 6.7 |
| Marital status (%) | — | — | — | — |
| Married | 65.4 | 67.7 | 43.9 | 66.4 |
| Divorced/separated | 13.8 | 12.0 | 27.6 | 17.1 |
| Widowed | 17.1 | 17.1 | 19.7 | 13.6 |
| Never married | 3.7 | 3.2 | 8.8 | 2.9 |
| Employment status (%) | 42.2 | 42.7 | 42.4 | 36.8 |
| Income, dollars (%) | — | — | — | — |
| 1st quartile | 22.1 | 17.7 | 43.0 | 44.2 |
| 2nd quartile | 21.7 | 22.0 | 18.4 | 22.8 |
| 3rd quartile | 25.3 | 26.5 | 20.0 | 18.0 |
| 4th quartile | 31.0 | 33.8 | 18.6 | 15.1 |
| Wealth, dollars (%) | — | — | — | — |
| 1st quartile | 24.7 | 19.4 | 53.7 | 47.2 |
| 2nd quartile | 24.4 | 24.0 | 25.7 | 27.0 |
| 3rd quartile | 24.6 | 26.9 | 14.1 | 12.6 |
| 4th quartile | 26.4 | 29.8 | 6.5 | 13.2 |
| Obese (%) | 32.6 | 31.1 | 44.0 | 36.0 |
| Smoking status (%) | — | — | — | — |
| Never smoked | 42.1 | 42.2 | 41.5 | 41.9 |
| Former smoker | 43.3 | 44.1 | 38.6 | 40.7 |
| Current smoker | 14.3 | 13.5 | 19.6 | 16.7 |
| Physically inactive (%) | 40.8 | 39.2 | 51.6 | 45.1 |
| Chronic conditions | 1.3 (0.02) | 1.3 (0.02) | 1.6 (0.06) | 1.2 (0.06) |
| Telomere length | 1.3 (0.01) | 1.3 (0.01) | 1.5 (0.02) | 1.4 (0.02) |
Note. Numbers represent weighted means, with standard errors in parentheses, and weighted percentages.
Table 2 shows multivariate models of race/ethnic, gender, and age differences in TL. Model 1 shows that TL was shorter at older ages (β = −0.004, p < .001), women had longer TL than men (β = 0.026, p < .05), and both Blacks (β = 0.118, p < .001) and Hispanics (β = 0.040, p < .10) had longer TL than Whites. Model 2 adjusts for marital status, education, employment status, income, and wealth. Age and gender differences in TL were largely unchanged. The differences between Whites and both Blacks (β = 0.124, p < .001) and Hispanics (β = 0.047, p < .05) remained, and were slightly larger after including social and economic factors. Model 3 further adjusts for smoking, obesity, physical inactivity, and chronic conditions. The age and race/ethnic differences were relatively unchanged with the inclusion of health and health behaviors, but the size of the gender difference was reduced and no longer statistically significant.
Table 2.
Linear Regression Models of TL on Age, Gender, and Race/Ethnicity (n = 5,228).
| Model 1 |
Model 2 (†demographics, SES) |
Model 3 (†health, behaviors) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| β | SE | β | SE | β | SE | ||||
| Age | −0.004 | (0.001) | *** | −0.004 | (0.001) | *** | −0.004 | (0.001) | *** |
| Female | 0.026 | (0.013) | * | 0.030 | (0.013) | * | 0.022 | (0.013) | — |
| Race/ethnicity (ref = White) |
— | — | — | — | — | — | — | — | — |
| Black | 0.118 | (0.024) | *** | 0.124 | (0.025) | *** | 0.122 | (0.025) | *** |
| Hispanic | 0.040 | (0.021) | † | 0.047 | (0.023) | * | 0.042 | (0.023) | † |
| Intercept | 1.608 | (0.046) | *** | 1.589 | (0.067) | *** | 1.617 | (0.068) | *** |
| R 2 | .027 | — | — | .034 | — | — | .036 | — | — |
Note. Model 2 adjusted for marital status, education, employment status, income, wealth. Model 3 adjusted for marital status, education, employment status, income, wealth, BMI, smoking, physical inactivity, chronic conditions. TL = telomere length; SES = socioeconomic status; SE = standard error; BMI = body mass index.
p < .10.
p < .05.
p < .01.
p < .001.
To show differences in TL among all race/ethnic and gender groups, we also conducted pairwise comparisons of all race/ethnic and gender combinations. Table 3 shows predicted TL by race/ethnicity and gender, accompanying confidence intervals, and tests of difference from other groups. Controlling for age, we found that Black women (M = 1.46, p < .05) and men (M = 1.43, p < .05) had longer TL than White and Hispanic men and women. Black women had longer telomeres than all other groups (p < .05), except Black men. White men had the shortest TL than any other group (M = 1.32, p < .05), except Hispanic men. Race/ethnic and gender group differences were largely the same after adjusting for marital status, employment status, SES, health, and health behavior characteristics in subsequent models. However, after accounting for differences in chronic conditions and health behaviors in Model 3, the differences between White men and women and Hispanic men and Black women were no longer statistically significant.
Table 3.
Predicted Mean Telomere Length by Race/Ethnicity and Gender Groups (n = 5,228).
| Model 1 |
Model 2 (†demographics, SES) |
Model 3 (†health, behaviors) |
||||
|---|---|---|---|---|---|---|
| M | CI | M | CI | M | CI | |
| White women | 1.34 | [1.33, 1.36] | 1.34 | [1.33, 1.36] | 1.34 | [1.32, 1.36] |
| White men | 1.32a | [1.29, 1.34] | 1.31a | [1.29, 1.33] | 1.32 | [1.29, 1.34] |
| Black women | 1.46a,b | [1.42, 1.51] | 1.47a,b | [1.42, 1.52] | 1.46a,b | [1.41, 1.51] |
| Black men | 1.43a,b | [1.35, 1.51] | 1.43a,b | [1.35, 1.51] | 1.44a,b | [1.36, 1.52] |
| Hispanic women | 1.37b,c | [1.32, 1.41] | 1.37c | [1.32, 1.42] | 1.36c | [1.31, 1.41] |
| Hispanic men | 1.37c | [1.31, 1.43] | 1.38c | [1.31, 1.45] | 1.38 | [1.31, 1.45] |
Note. Model 1 adjusted for age. Model 2 adds marital status, education, occupational status, income, and wealth. Model 3 further adds BMI, smoking, physical activity, chronic conditions. SES = socioeconomic status; CI = confidence interval; BMI = body mass index.
Different from White women (p < .05).
Different from White men (p < .05).
Different from Black women (p < .05).
p value < .05 based on pairwise comparison.
We next assessed age differences in TL by both gender and race/ethnicity. Age differences by race/ethnicity are presented separately for women and men in Table 4. Model 1 includes age, race/ethnicity, and Model 2 adds interactions between age and race/ethnicity. All models are adjusted for individual social, economic, health, and health behavior characteristics. Results from Model 1 for women show TL shortened with every 1-year increase in age (β = −0.004, p < .001). Interactions between age and race/ethnicity suggest an even larger decrease in TL with increasing age among Black women (β = −0.005, p ≤ .10). Age patterns in TL did not differ between White and Hispanic women; the interaction between age and Hispanic ethnicity was small and not statistically significant. The overall age pattern shown in Model 1 was similar for men (β = −0.005, p < .001), but interactions between age and race/ethnicity in Model 2 show Hispanic men have a sharper decline in TL across age relative to White men (β = −0.014, p ≤ .001) and relative to Black men (Adjusted Wald test, p < .05). There were no age differences between Black and White men. Results were the same in models with no adjustment for socioeconomic factors, health characteristics, or behaviors.
Table 4.
Gender Stratified Models Predicting TL (n = 5,228).
| Women |
Men |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 |
Model 2 |
Model 1 |
Model 2 |
|||||||||
| β | SE | β | SE | β | SE | β | SE | |||||
| Age | −0.004 | (0.001) | *** | −0.003 | (0.001) | ** | −0.005 | (0.001) | *** | −0.004 | (0.001) | ** |
| Race/ethnicity (ref = White) |
— | — | — | — | — | — | — | — | — | — | — | — |
| Black | 0.125 | (0.028) | *** | 0.458 | (0.200) | * | 0.121 | (0.043) | ** | 0.279 | (0.320) | — |
| Hispanic | 0.031 | 0.027 | — | 0.1 10 | (0.178) | — | 0.061 | 0.040 | — | 0.920 | (0.258) | *** |
| Race × Age | ||||||||||||
| Black × Age | — | — | — | −0.005 | (0.003) | † | — | — | — | −0.002 | (0.005) | — |
| Hispanic × Age |
— | — | — | −0.001 | (0.003) | — | — | — | — | −0.014 | (0.004) | *** |
| Intercept | 1.651 | (0.077) | *** | 1.615 | (0.081) | *** | 1.687 | (0.100) | *** | 1.61 1 | (0.102) | *** |
| R 2 | .044 | — | — | .045 | — | — | .047 | — | — | .052 | — | — |
| n | 3,010 | — | — | 3,010 | — | — | 2,218 | — | — | 2,218 | — | — |
Note. Models are adjusted for marital status, education, employment status, income, wealth, BMI, smoking, physical inactivity, chronic conditions. TL = telomere length; SE = standard error; BMI = body mass index.
p < . 10.
p < .05.
p < .01.
p < .001.
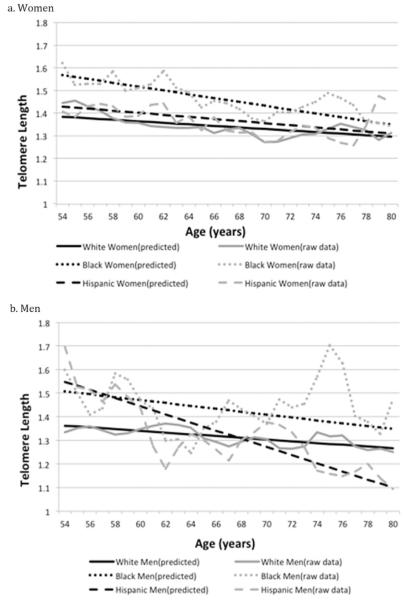
To visualize the age patterning in TL by gender and race/ethnicity, we plot predicted values from Model 2 for both men and women. Figure 1 shows both unadjusted means (using a 3-year moving average) and the fully adjusted predicted TL values by age for women (Panel a) and men (Panel b). TL is shorter at older ages for all race/ethnic and gender groups. The gap in TL length between Black and White women is slightly reduced at older ages. Although Hispanic men have longer TL than White men at younger ages, this gap narrows at older ages, and there is some indication that at the oldest-old ages, Hispanic men have shorter length than both White and Black men.
Figure 1. Race/ethnic differences in mean telomere length by age.
Note. Figure 1 shows both smoothed raw data (using a 3-year moving average) and predicted telomere length by race/ethnicity across age for women and men separately. Predicted values (from model estimates; Table 4, Model 2) of telomere length by age come from gender-stratified models with interactions between race/ethnicity and age adjusting for SES, marital status, employment status, health, and health behaviors. Means are weighted.
The purpose of this study was to examine differences in TL by race/ethnicity, gender, and age in a racially and ethnically diverse population of older U.S. adults. We found that women had longer TL than men, which is consistent with most of the literature examining gender differences in TL (Gardner et al., 2014). This gender difference was attenuated after accounting for differences in chronic conditions and health behaviors, suggesting women’s advantage in TL may be the result of gender differences in smoking, sedentary behavior, obesity, and chronic conditions. The only other study using nationally representative data similarly found gender differences were attenuated in fully adjusted models (Needham et al., 2013). We also found that Blacks had longer TL than Whites, confirming a pattern that has been reported in several other studies of race differences in TL (Adler et al., 2013; Hunt et al., 2008; Needham et al., 2013; Zhu et al., 2011). In addition, our results show Hispanics had longer TL than Whites, though this difference was only marginally statistically significant. Prior studies have reported shorter TL in Hispanics compared with Whites (Diez Roux et al., 2009), or have found no difference (Geronimus et al., 2015; Needham et al., 2013). The discrepancy between our study findings and previous research may reflect differences in study populations; in contrast to previous studies, we use national data on older adults and do not include younger Hispanics.
We also found race/ethnic differences in TL by both gender and age. Hispanic men had a sharper decline in TL across age relative to White men and other race/ethnic and gender groups. Diez Roux and colleagues (2009) also examined age differences by race/ethnicity in a sample of adults aged 45 and older but did not find differences in age patterns for Hispanic men as we did. This disadvantage in TL among older Hispanic men is counter to the relative health advantage known to be characteristic of Hispanic health in late life. Hispanics, despite having lower SES, have lower mortality rates for cancer, cardiovascular disease, and all-cause mortality compared with Whites, especially among middle to older age groups, contradicting the steeper decline in TL among older Hispanic men (Markides & Coreil, 1986).
We also found Black men and women had the longest TL relative to all other groups. Differences between Blacks and other groups were largely unchanged after accounting for differences in sociodemographic characteristics, socioeconomic status, chronic conditions, and health behaviors. Moreover, Blacks had longer TL relative to Whites and Hispanics across age. Our findings also suggested, however, that TL among Black and White women was more similar at the oldest ages than at younger ages. One prior study found Black women had, on average, shorter TL than White women at older ages, but also found Black women had a steeper decline in TL across age (Diez Roux et al., 2009). Most studies to date, however, have reported longer TL in Black women compared with White women (Hunt et al., 2008; Needham et al., 2013; Zhu et al., 2011).
Prior theory and empirical evidence would suggest Blacks experience accelerated aging and higher mortality and morbidity rates (Geronimus et al., 2010; Levine & Crimmins, 2014) and thus would also have shorter TL in older age as a result of a lifetime of biological weathering. Yet, in the current study, Blacks have an advantage with respect to TL. One potential explanation is that the greater mortality selection among Blacks means Blacks who survive to older age are healthier and more robust, which may be reflected in longer TL. It is also possible that Blacks, and Black women in particular, are born with longer TL and as a result may have longer TL over the life course. Prior research suggests females are born with longer telomeres and this may explain women’s health advantage over men (Aubert, Hills, & Lansdorp, 2012). Some studies have also shown Blacks to have longer TL at birth (Drury et al., 2015; Rewak et al., 2014). Additional research has also shown that girls and Blacks maintain longer TL into adolescence relative to their male and White counterparts (Zhu et al., 2011). Thus, our findings extend prior evidence of the Black advantage in TL to the older adult life course.
This study raises important considerations in research on disparities in TL. First, focusing on differences between race/ethnic groups may obscure important heterogeneity within race/ethnic groups. It has been suggested that ignoring this heterogeneity risks miss-estimation of race/ethnic differences in TL (Geronimus et al., 2015). Second, our findings show Blacks have an advantage with respect to TL, but the rate of change (e.g., telomere shortening) may be a more relevant indicator of the wear and tear that results in accelerated biological aging (Aviv et al., 2009; Hunt et al., 2008). Longitudinal data are needed to determine whether there are gender and race/ethnic differences in this rate of change. Third, the Black advantage in TL and the Hispanic male disadvantage at older ages do not reflect the social patterning in health that is typically observed in the U.S. population relative to their White counterparts. Thus, our findings on differences in TL in an older adult population are inconsistent with the theories and evidence on health disparities among older adults. This suggests there may be questionable value in TL as a biomarker of aging, and particularly as a tool for characterizing and understanding race/ethnic disparities in health at older ages.
This is the first study to examine race/ethnic, gender, and age differences in TL in a racially, ethnically, and socioeconomically diverse sample of older U.S. adults; yet, this study has some limitations. First, we used cross-sectional data and thus cannot study changes in TL with age. Although our findings suggest race/ethnic and gender differences vary across age, we do not know whether there is variation in TL shortening with age. Second, method-specific biases have been shown to matter for estimating gender and race differences (Elbers et al., 2014; Gardner et al., 2014). For example, Southern blot has shown unequivocally that women have longer TL than men. This study uses qPCR, which has been shown to yield less consistent gender results across studies (Gardner et al., 2014). Last, race/ethnic and gender differences in TL may vary by cell type making comparing across studies problematic. For example, TL from lymphocytes has been shown to be shorter than TL from granulocytes (Aviv, Shay, Christensen, & Wright, 2005). Our TL data come from buccal cells in saliva, while other studies have used TL derived from blood leukocyte cells. However, TL from saliva has been shown to be highly correlated with blood leukocyte TL (Mitchell et al., 2014).
The inclusion of biomarkers in sociologically driven population research has the potential to improve our understanding of the pervasive problem of health disparities. The recent emergence of TL in longitudinal, nationally representative, and diverse samples of older adults presents an opportunity to better characterize differences in health by social characteristics. Yet, the expanding use of TL in health disparities research has increased the need for a more precise understanding of what we are measuring and what TL tells us about the health and aging of diverse populations. Our findings indicate that social patterns in TL by race, gender, and age do not reflect differences observed in most other population health outcomes. This could be taken to suggest TL is not a biomarker of aging that identifies people at increased risk of disease, disability, and accelerated aging in late life across racially and ethnically diverse populations.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the Michigan Institute for Clinical and Health Research (MICHR) and the National Institute on Aging (NIA) of the National Institutes of Health, under Award P30AG043073 to the University of Southern California’s Minority Aging Health Economics Research Center (USC-RCMAR) and Awards T32AG0037 and R00AG039528. The HRS 2008 Telomere data collection was funded through NIA grant U01AG009740 to the University of Michigan.
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Adams J, Martin-Ruiz C, Pearce MS, White M, Parker L, von Zglinicki T. No association between socio-economic status and white blood cell telomere length. Aging Cell. 2007;6:125–128. doi: 10.1111/j.1474-9726.2006.00258.x. doi:10.1111/j.1474-9726.2006.00258.x. [DOI] [PubMed] [Google Scholar]
- Adler N, Pantell MS, O’Donovan A, Blackburn E, Cawthon R, Koster A, Epel E. Educational attainment and late life telomere length in the health, aging and body composition study. Brain, Behavior, and Immunity. 2013;27:15–21. doi: 10.1016/j.bbi.2012.08.014. doi:10.1016/j.bbi.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert G, Hills M, Lansdorp PM. Telomere length measurement-caveats and a critical assessment of the available technologies and tools. Mutation Research. 2012;730:59–67. doi: 10.1016/j.mrfmmm.2011.04.003. doi:10.1016/j.mrfmmm.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv A, Chen W, Gardner JP, Kimura M, Brimacombe M, Cao X, Berenson GS. Leukocyte telomere dynamics: Longitudinal findings among young adults in the bogalusa heart study. American Journal of Epidemiology. 2009;169:323–329. doi: 10.1093/aje/kwn338. doi:10.1093/aje/kwn338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv A, Hunt SC, Lin J, Cao X, Kimura M, Blackburn E. Impartial comparative analysis of measurement of leukocyte telomere length/DNA content by southern blots and qPCR. Nucleic Acids Research. 2011;39(20):e134. doi: 10.1093/nar/gkr634. doi:10.1093/nar/gkr634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv A, Shay J, Christensen K, Wright W. The longevity gender gap: Are telomeres the explanation? Science of Aging Knowledge Environment. 2005;2005(23):pe16. doi: 10.1126/sageke.2005.23.pe16. doi:10.1126/sageke.2005.23.pe16. [DOI] [PubMed] [Google Scholar]
- Bakaysa SL, Mucci LA, Slagboom PE, Boomsma DI, McClearn GE, Johansson B, Pedersen NL. Telomere length predicts survival independent of genetic influences. Aging Cell. 2007;6:769–774. doi: 10.1111/j.1474-9726.2007.00340.x. doi:10.1111/j.1474-9726.2007.00340.x. [DOI] [PubMed] [Google Scholar]
- Blackburn EH. Telomere states and cell fates. Nature. 2000;408:53–56. doi: 10.1038/35040500. doi:10.1038/35040500. [DOI] [PubMed] [Google Scholar]
- Blackburn EH. Telomeres and telomerase: Their mechanisms of action and the effects of altering their functions. FEBS Letters. 2005;579:859–862. doi: 10.1016/j.febslet.2004.11.036. doi:10.1016/j. febslet.2004.11.036. [DOI] [PubMed] [Google Scholar]
- Brouilette S, Singh RK, Thompson JR, Goodall AH, Samani NJ. White cell telomere length and risk of premature myocardial infarction. Arteriosclerosis, Thrombosis, and Vascular Biology. 2003;23:842–846. doi: 10.1161/01.ATV.0000067426.96344.32. doi:10.1161/01.ATV.0000067426.96344.32. [DOI] [PubMed] [Google Scholar]
- Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Research. 2002;30(10):e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon RM. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Research. 2009;37(3):e21. doi: 10.1093/nar/gkn1027. doi:10.1093/ nar/gkn1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez Roux AV, Ranjit N, Jenny NS, Shea S, Cushman M, Fitzpatrick A, Seeman T. Race/ethnicity and telomere length in the multiethnic study of atherosclerosis. Aging Cell. 2009;8:251–257. doi: 10.1111/j.1474-9726.2009.00470.x. doi:10.1111/j.1474-9726.2009.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury SS, Esteves K, Hatch V, Woodbury M, Borne S, Adamski A, Theall KP. Setting the trajectory: Racial disparities in newborn telomere length. The Journal of Pediatrics. 2015;166:1181–1186. doi: 10.1016/j.jpeds.2015.01.003. doi:10.1016/j.jpeds.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du M, Prescott J, Kraft P, Han J, Giovannucci E, Hankinson SE, De Vivo I. Physical activity, sedentary behavior, and leukocyte telomere length in women. American Journal of Epidemiology. 2012;175:414–422. doi: 10.1093/aje/kwr330. doi:10.1093/aje/ kwr330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbers CC, Garcia ME, Kimura M, Cummings SR, Nalls MA, Newman AB, Aviv A. Comparison between southern blots and qPCR analysis of leukocyte telomere length in the health ABC study. The Journals of Gerontology, Series A: Biological Sciences & Medical Sciences. 2014;69:527–531. doi: 10.1093/gerona/glt121. doi:10.1093/ gerona/glt121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick AL, Kronmal RA, Gardner JP, Psaty BM, Jenny NS, Tracy RP, Aviv A. Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. American Journal of Epidemiology. 2007;165(1):14–21. doi: 10.1093/aje/kwj346. doi:10.1093/aje/kwj346. [DOI] [PubMed] [Google Scholar]
- Gardner M, Bann D, Wiley L, Cooper R, Hardy R, Nitsch D, Ben-Shlomo Y. Gender and telomere length: Systematic review and meta-analysis. Experimental Gerontology. 2014;51:15–27. doi: 10.1016/j.exger.2013.12.004. doi:10.1016/j.exger.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus AT, Hicken MT, Pearson JA, Seashols SJ, Brown KL, Cruz TD. Do US black women experience stress-related accelerated biological aging? A novel theory and first population-based test of black-white differences in telomere length. Human Nature. 2010;21:19–38. doi: 10.1007/s12110-010-9078-0. doi:10.1007/s12110-010-9078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus AT, Pearson JA, Linnenbringer E, Schulz AJ, Reyes AG, Epel ES, Blackburn EH. Race-ethnicity, poverty, urban stressors, and telomere length in a Detroit community-based sample. Journal of Health and Social Behavior. 2015;56:199–224. doi: 10.1177/0022146515582100. doi:10.1177/0022146515582100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SE, Deary IJ, MacIntyre A, Lamb KJ, Radhakrishnan K, Starr JM, Shiels PG. The association between telomere length, physical health, cognitive ageing, and mortality in non-demented older people. Neuroscience Letters. 2006;406:260–264. doi: 10.1016/j.neulet.2006.07.055. doi:10.1016/j.neulet.2006.07.055. [DOI] [PubMed] [Google Scholar]
- Health and Retirement Study . 2008 telomere length data: Data description and usage (Version 1.0) Produced and distributed by the University of Michigan with funding from the National Institute on Aging; Ann Arbor, MI: 2013. [Google Scholar]
- Hunt SC, Chenz W, Gardner JP, Kimura M, Srinivasan SR, Eckfeldt JH, Aviv A. Leukocyte telomeres are longer in African Americans than in whites: The national heart, lung, and blood institute family heart study and the bogalusa heart study. Aging Cell. 2008;7:451–458. doi: 10.1111/j.1474-9726.2008.00397.x. doi:10.1111/j.1474-9726.2008.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Parks CG, DeRoo LA, Chen H, Taylor JA, Cawthon RM, Sandler DP. Obesity and weight gain in adulthood and telomere length. Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology. 2009;18:816–820. doi: 10.1158/1055-9965.EPI-08-0935. doi:10.1158/1055-9965.EPI-08-0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M, Crimmins E. Evidence of accelerated aging among African Americans and its implications for mortality. Social Science & Medicine. 2014;118(C):27–32. doi: 10.1016/j.socscimed.2014.07.022. doi:10.1016/j.socscimed.2014.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow AT, Roth SM. Physical activity and telomere biology: Exploring the link with aging-related disease prevention. Journal of Aging Research. 2011;2011 doi: 10.4061/2011/790378. Article 790378. doi:10.4061/2011/790378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markides KS, Coreil J. The health of hispanics in the southwestern united states: An epidemiologic paradox. Public Health Reports. 1986;101:253–265. [PMC free article] [PubMed] [Google Scholar]
- Martin-Ruiz C, Jagger C, Kingston A, Collerton J, Catt M, Davies K, von Zglinicki T. Assessment of a large panel of candidate biomarkers of ageing in the newcastle 85+ study. Mechanisms of Ageing and Development. 2011;132:496–502. doi: 10.1016/j.mad.2011.08.001. doi:10.1016/j.mad.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Mitchell C, Hobcraft J, McLanahan SS, Siegel SR, Berg A, Brooks-Gunn J, Notterman D. Social disadvantage, genetic sensitivity, and children’s telomere length. Proceedings of the National Academy of Sciences. 2014;111:5944–5949. doi: 10.1073/pnas.1404293111. doi:10.1073/pnas.1404293111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham BL, Adler N, Gregorich S, Rehkopf D, Lin J, Blackburn EH, Epel ES. Socioeconomic status, health behavior, and leukocyte telomere length in the national health and nutrition examination survey, 1999-2002. Social Science & Medicine. 2013;85:1–8. doi: 10.1016/j.socscimed.2013.02.023. doi:10.1016/j.socscimed.2013.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rewak M, Buka S, Prescott J, De Vivo I, Loucks EB, Kawachi I, Kubzansky LD. Race-related health disparities and biological aging: Does rate of telomere shortening differ across blacks and whites? Biological Psychology. 2014;99:92–99. doi: 10.1016/j.biopsycho.2014.03.007. doi:10.1016/j.biopsycho.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders JL, Fitzpatrick AL, Boudreau RM, Arnold AM, Aviv A, Kimura M, Newman AB. Leukocyte telomere length is associated with non-invasively measured age-related disease: The cardiovascular health study. The Journals of Gerontology, Series A: Biological Sciences & Medical Sciences. 2012;67:409–416. doi: 10.1093/gerona/glr173. doi:10.1093/gerona/glr173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels PG, McGlynn LM, MacIntyre A, Johnson PC, Batty GD, Burns H, Packard CJ. Accelerated telomere attrition is associated with relative household income, diet and inflammation in the pSoBid cohort. PLoS ONE. 2011;6(7):e22521. doi: 10.1371/journal.pone.0022521. doi:10.1371/journal.pone.0022521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telomere Diagnostics Available from http://www.telomehealth.com. [Google Scholar]
- Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, Spector TD. Obesity, cigarette smoking, and telomere length in women. The Lancet. 2005;366:662–664. doi: 10.1016/S0140-6736(05)66630-5. doi:10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- von Zglinicki T. Oxidative stress shortens telomeres. Trends in Biochemical Sciences. 2002;27:339–344. doi: 10.1016/s0968-0004(02)02110-2. doi:10.1016/S0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- Willeit P, Willeit J, Brandstatter A, Ehrlenbach S, Mayr A, Gasperi A, Kiechl S. Cellular aging reflected by leukocyte telomere length predicts advanced atherosclerosis and cardiovascular disease risk. Arteriosclerosis, Thrombosis, and Vascular Biology. 2010;30:1649–1656. doi: 10.1161/ATVBAHA.110.205492. doi:10.1161/ATVBAHA.110.205492. [DOI] [PubMed] [Google Scholar]
- Worthman CM, Costello EJ. Tracking biocultural pathways in population health: The value of biomarkers. Annals of Human Biology. 2009;36:281–297. doi: 10.1080/03014460902832934. doi:10.1080/03014460902832934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Wang X, Gutin B, Davis CL, Keeton D, Thomas J, Dong Y. Leukocyte telomere length in healthy Caucasian and African-American adolescents: Relationships with race, sex, adiposity, adipokines, and physical activity. The Journal of Pediatrics. 2011;158:215–220. doi: 10.1016/j.jpeds.2010.08.007. doi:10.1016/j.jpeds.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]