Abstract
Objective
To validate a symptom-based, fistula screening questionnaire and estimate obstetric fistula (OF) prevalence in rural Nepal.
Design
Cross-sectional and nested case-control study.
Setting
Sarlahi District, Nepal.
Population
Parous, reproductive-age women.
Methods
The questionnaire assessed symptoms of vesicovaginal (VVF) and rectovaginal (RVF) fistula, stress (SUI) and urge urinary (UUI) incontinence, fecal incontinence (FI), and included interviewer observations on the smell and presence of urine and/or stool. All women who screened positive for OF and a randomly-selected group of women who screened negative for OF were included in a nested case-control study (one case: four normal controls: four incontinent controls) and underwent confirmatory clinical examinations.
Main outcome measure
Clinically-confirmed OF, questionnaire sensitivity (Se) and specificity (Sp).
Results
Of the 16,893 women who completed cross-sectional screening, 68 were “screened-positive cases.” Of these, 55 (82%) “screened-positive cases,” 203 “screened-negative normal controls,” and 203 “screened-incontinent controls” participated in the case control study, which confirmed one case of VVF and one case of both VVF and RVF without any false negative cases. For VVF, the screening tool demonstrated Se 100% (95%CI: 34.2, 100), Sp 86.9% (95%CI; 83.3, 89.9), and estimated VVF prevalence as 12 per 100,000 (95%CI: 3, 43); for RVF, it demonstrated Se 100% (95%CI: 20.7, 100), Sp 99.8% (95%CI: 98.6, 100) and estimated RVF prevalence as 6 per 100,000 (95%CI: 1, 34).
Conclusions
The OF screening questionnaire demonstrated high sensitivity and specificity in this low-prevalence setting.
Keywords: vesicovaginal fistula, rectovaginal fistula, symptom-based screening questionnaire, sensitivity, specificity, rural Nepal
Tweetable abstract
Community-based obstetric fistula screening tool validation study, Nepal, n=16,893: High Se, Sp & feasibility
INTRODUCTION
Obstetric fistula (OF) is an often neglected maternal morbidity, and generally occurs in lower-resource settings among women with prolonged or obstructed labor without access to timely, quality obstetric care.1 In addition to fetal demise, prolonged pressure of the fetus’s head can restrict blood flow, causing ischemia, necrosis and formation of a hole (fistula) between the woman’s vagina and bladder/ lower urinary tract (vesicovaginal fistula (VVF)), and/or vagina and rectum (rectovaginal fistula (RVF)).2 Women with OF have constant leakage of urine and/or feces. They may also develop genital sores and dermatitis, sexual dysfunction, amenorrhea, and lower extremity neurological deficits such as weakness and foot drop.3,4 Furthermore, women are often socially stigmatised and sustain psychological trauma.5
OF is widely-cited to affect 2 to 3.5 million women globally, with 50,000 to 100,000 annual incident cases. However, these figures were derived from expert opinion or extrapolation of expert experience from hospital-based settings.6,7,8–13 Hospital-based studies likely underestimate prevalence, as women with OF often lack access to skilled care; social stigma and isolation further impedes care-seeking.14 A 2007 systematic review identified 29 studies with population-based incidence and prevalence estimates and found incidence estimates ranging from 20,000 to 30,000 annual cases with a global prevalence estimate of 654,000 cases; however, only four of these studies described their methodology for calculating these estimates.14 Demographic and Health Surveys (DHS) in Malawi, the Democratic Republic of Congo, Ethiopia, Niger, Nigeria, Pakistan, Rwanda and Uganda have included different symptom-based fistula questions to estimate population prevalence; however, as these questions have not been validated, the accuracy of these prevalence estimates is unknown.15–21
A 2013 systematic review and meta-analysis22 found only ten studies used clinically-confirmed cases to estimate community-based OF prevalence; four of these were conducted in Sub-Saharan Africa23,24 or South Asia.25,26 Subsequent to the review, two population-based cross-sectional studies, from Southern Sudan and Pakistan, used confirmatory clinical examinations. However, none of these studies used a control population to assess the validity of the screening questions used to identify cases.27,28 At present, there is no widely used, well-validated fistula screening tool. This study aimed to determine the validity of a symptom-based, fistula screening questionnaire and estimate obstetric fistula prevalence in a community-based rural setting in Nepal. A community-based cross-sectional survey (screening phase) was followed by a nested case control study with clinical examination of potential cases and non-cases (validation phase).
METHODS
Study design and site
A community-based cross-sectional survey of parous, reproductive age women was conducted in 136 communities (sectors) of Sarlahi District in rural southern Nepal which encompassed a population of approximately 80,000 to identify all households with women of child bearing age. The study was nested in a randomised controlled trial on the impact of sunflower oil massage on neonatal morbidity (L. Mullany, PI; trial registered at clinicaltrials.gov, NCT01177111). Nepal is a low-income country with a gross national income per capita of $600 in 2011. Sarlahi District is comprised of mostly rural agrarian communities with 75% of the population living below the Nepali poverty line, and (in 2010) with <20% of home births attended by skilled attendants and <10% of births occurring in a health care facility.33
A screening questionnaire was designed to assess current experience of vesicovaginal fistula (VVF) and/or rectovaginal fistula (RVF) symptoms as well as stress and urge urinary incontinence (SUI, UUI), fecal incontinence (FI), and pelvic organ prolapse (POP) [Appendix S1: Questionnaire]. Women who screened positive on the questionnaire were selected for inclusion in a nested case-control study. Two types of controls were randomly selected among women who screened negative, in a ratio of one:four:four (case: control: control). The first type included women with reported symptoms of urinary incontinence and/or fecal incontinence. The second type were women who did not have incontinence or fistula symptoms. All consenting cases and controls underwent a confirmatory clinical exam by one female urogynecologist/ female pelvic medicine and reconstructive surgeon who was masked to the screening results. In addition to fellowship training and board certification by the American Board of Obstetrics and Gynecology in Female Pelvic Medicine and Reconstructive Surgery, this urogynecologist also underwent specific training in diagnosing and caring for obstetric fistula patients in Ethiopia and Nepal.
To complement this survey-based approach to identifying potential cases of OF, we carried out a case-finding strategy using local research interviewers that lived within our study population to identify women suspected of having OF but who were not included in the cross-sectional screening survey. We also encouraged women that presented for clinical examination to let us know of any women in their community with OF symptoms. Surgical care for any identified cases of OF or other gynecologic pathology were offered pro bono. IRB approval was obtained both from Johns Hopkins School of Public Health (Baltimore, USA) and the Institute of Medicine, Tribhuvan University (Kathmandu, Nepal).
Fistula screening tool
The OF screening questionnaire included VVF and RVF symptom questions that were originally developed by the late Thomas Elkins (Professor, Johns Hopkins Department of Gynecology and Obstetrics), and UI, FI, POP screening questions previously validated in the U.S. and other countries. 35–38 Further face validity with modification of the questionnaire as appropriate was performed after consultation with expert fistula surgeons in the USA, Ethiopia, UK, and Nepal. The final OF screening questionnaire was further refined after discussion among all levels of study personnel, and translated into both Nepali and Maithili. These versions were back-translated and pilot-tested among the Maithili-speaking field interviewers.
VVF was defined as a positive response to either of the following two questions: 1) “Currently, does your clothing get wet with your urine during sleep?”; 2) “When you are not urinating, do you currently experience continuously dripping urine through the birth canal that you cannot stop?”. If the women answered yes to this question, they were subsequently asked if the urine leakage occurred "all day and all night". Similarly, RVF was defined as a positive response to the following question: “When you are not defecating, do you currently experience feces passing through the birth canal that you cannot stop?”
In addition, women were asked for the month and year of their last delivery, as well as for the onset of OF symptoms. The two dates were compared to determine whether the onset of symptoms corresponded with the delivery. Finally, both the VVF and RVF screening questionnaires included a subjective observation by the interviewer of wetness of the subject’s clothes due to urine/feces, or for the smell of urine/feces around the subject.
Women who screened positive for OF were all approached to be included in the nested case control study and undergo clinical examinations. Women who screened negative for OF were entered into a pool and randomly selected for the nested case control study. Specifically, two types of controls were selected: those who reported incontinence symptoms (incontinence controls) and those who had no incontinence symptoms (true controls) in a ratio of one:four:four (case: incontinence control: true control). Pelvic examinations were performed as per standard care to confirm presence or absence of pelvic fistula. Specifically, any participant found to have watery fluid in the vagina without a clear VVF also underwent additional examination procedures, including a dye test (presence of blue or orange staining on a gauze placed in the vagina after retrograde filling of the bladder with dyed blue sterile saline and/or after study participants ingested phenazopyridine, which results in excretion of orange urine from the kidneys) to confirm presence of a bladder and/or ureteral fistula. Any participant found to have stool and/or foul-smelling discharge in the vagina without a clear RVF underwent additional examination procedures including a dye test (presence of blue staining on a gauze placed in the vagina after inserting dyed blue water-soluble lubricating jelly in the rectum) and/or a flat tire test (presence of air bubbles in the vagina after pumping the rectum with air while maintaining fluid in the vagina) to confirm presence of rectal fistula. We also performed the above test(s) on participants who reported symptoms suspicious for VVF and/or RVF on the day of clinical examination if no clear fistula tract was found. Finally, at the end of each examination session, we performed the above additional tests on any participants who screened positive for OF symptoms but was not found to have a clear fistula tract on initial examination. As an additional safeguard against missing any fistula cases, our research protocol also included performing radiologic tests such as intravenous pyelogram and/or barium enema studies on participants who reported suspicious symptoms but who were still not found to have a fistula after the above examination procedures.
Analysis
Overall frequencies of women’s self-reported OF symptoms were compared to clinical exam diagnosis, and the sensitivity, specificity, and positive and negative predictive value (PPV, NPV) of the tool were calculated. Population prevalence and 95% confidence intervals were calculated using the Wilson score interval.29,30 Descriptive analyses of categorical variables used frequencies, and means and standard deviations were used for continuous data. Bivariate analyses of characteristics of VVF false positives and true negatives were conducted, and a multivariate model was used to assess which characteristics were most associated with false positive status. Additionally, a comparison of VVF screening questions was conductive by false positive or true negative status, as well as by case control status to identify which questions, or combination of questions, best classified women. Chi-square tests were performed for categorical values and Student’s t-tests were performed for continuous variables. The data were analyzed in SAS version 9.3 (Cary, NC), and all statistical tests were double-sided at the 5% level.31
RESULTS
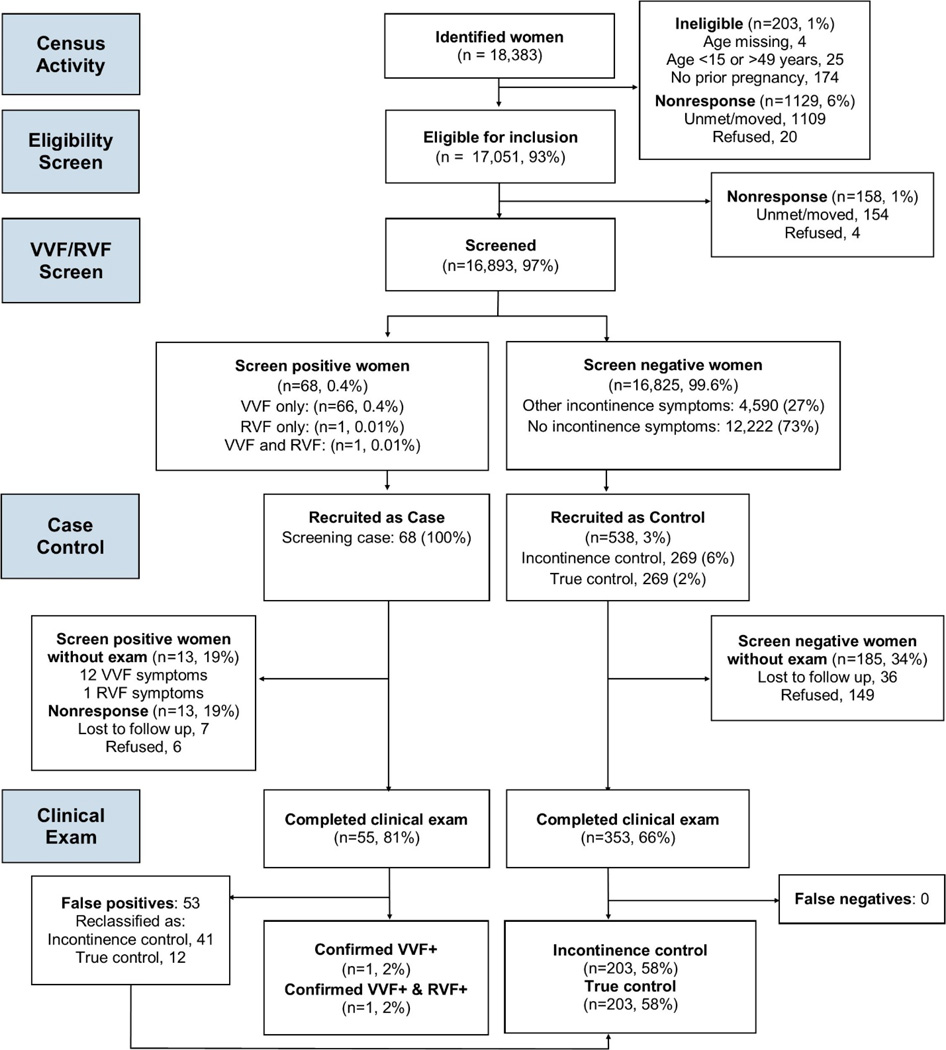
There were 18,383 parous women of reproductive age identified from the oil trial database [Figure 1]. Among these, 17,051 (93%) met the eligibility criteria and 16,893 (97%) completed the cross-sectional screening. Only four women (0.02%) refused the questionnaire, and 154 could not be found at home or had moved (0.9%). None of the women who consented to the questionnaire refused to answer a question, and the percent of women who responded “Don’t know” for any given question was low (less than 0.1%). It took 30 minutes or less to complete the screening questionnaire.
Figure 1.
Study participation and results of screening and clinical examination.
VVF: bladder/ lower urinary tract vaginal fistula. RVF: rectovaginal fistula
Women’s self-reported OF status in the VVF/RVF screen resulted in 68 “screened positive cases”: VVF only symptoms (66, 0.4%), RVF only symptoms (1, 0.01%), both RVF and VVF symptoms (1, 0.01%). All 68 “screened positive cases” were recruited for the case control study. There were 16,825 women who screened negative for OF: 4,590 (27%) with incontinence symptoms and 12,222 (73%) without incontinence symptoms. In accordance with the one:four:four (case: control: control) ratio, a total of 538 controls (269 incontinence controls and 269 non-incontinent/ true controls), were randomly selected among the screened negative women for the case-control study.
Among the screened positive cases (68), 13 women declined to participate or were not found: 12 with VVF only symptoms, and one with RVF only symptoms. Of the 55 women who underwent clinical examination (81%), one case of VVF and one case of a woman with both VVF and RVF were confirmed. Both of these cases occurred in women after obstructed labor for which they received cesarean sections, and were not associated with other potential causes of pelvic fistula such as sexual assault other pelvic injury. The remaining 53 women were false positives and reclassified as 41 incontinence controls and 12 true controls after clinical examination and detailed interview. Among the 538 screened negative controls selected to be in the case control study, 353 (66%) completed the clinical exam. There were no false negatives. After reclassifying the 53 false positives as controls, there were 203 clinically-confirmed incontinence controls and 203 non-incontinent/ true controls. The screening tool correctly identified both OF cases. No cases of VVF or RVF were found after examining the control patients (no false negatives). Using our case-finding strategy, we identified an additional two OF cases (both VVF only) based on respondent referral. Both of these women were not in our cross-sectional study and so were not screened and therefore were not eligible for the nested case control study.
VVF/RVF screening validation
The VVF screening tool had a sensitivity of 100% (95% CI: 34.2, 100) and specificity of 86.9% (95% CI: 83.3, 89.9) with a positive predictive value of 3.6% (95% CI: 1.0, 12.3) and negative predictive value of 100% (95% CI: 98.9, 100). The RVF screening tool had a sensitivity of 100% (95% CI: 20.7, 100) and specificity of 99.8% (95% CI: 98.6, 100) with a positive predictive value of 50% (95% CI: 9.5, 90.5) and negative predictive value of 100% (95% CI: 99.1, 100).
The prevalence of VVF was 11.8 per 100,000 reproductive age, parous women (95% CI: 3.2, 43.2). The prevalence of RVF was 5.9 per 100,000 reproductive age, parous women (95% CI: 1.0, 33.5). The prevalence for either type of obstetric fistula is estimated to be 12 per 100,000 women (95% CI: 3, 43).
Characteristics associated with VVF false positive classification
In order to improve the OF screening tool and reduce the false positive rate, an in-depth analysis of clinically confirmed VVF false positives (n=53) was conducted. There were no RVF false positives. Among VVF false positives, 77% had some type of urinary incontinence: six SUI only (11%), four UUI only (8%), 31 SUI and UUI (59%). Additionally, 11 (23%) had POP and 26 (49%) had vaginal discharge present at time of exam. Only three women (6%) were without urinary incontinence, POP, or vaginal discharge.
Compared to true negatives, VVF false positives were older (35 vs. 32 years, p=0.03), had higher gravidity (4.6 vs. 3.7, p=0.001), and more vaginal deliveries (4.3 vs. 3.5, p<0.001) [Table 1a]. Additionally, VVF false positives were less likely to have stress incontinence only (11% vs. 24%, p=0.02), but more likely to have both SUI and UUI (59% vs. 15%, p<0.001). However, only having both SUI and UUI remained significantly associated with false positive status in a multivariate model [Table 1b]. Compared to true negatives, the odds of having both SUI and UUI was more than 8 times higher among false positives (OR 8.23, 95% CI: 3.79, 17.84, p<0.001).
Table 1.
Characteristics of false positives and true negatives among eligible, screened women without fistula in the case control population (n=406)
| Characteristic | a. Bivariate Analysis | b. Model Results | ||||
|---|---|---|---|---|---|---|
| False positive (n=53) |
True negative (n=353) |
Total (n=406) |
p-value | Odds Ratio (95%CI) |
p-value | |
| Age, mean (SD) | 35.1 (7.7) | 32.3 (8.6) | 32.7 (8.5) | 0.03* | 1.00 (0.95, 1.05) | 0.88 |
| Lifetime pregnancies | 4.6 (2.0) | 3.7 (1.8) | 3.8 (1.9) | 0.001* | - | - |
| Lifetime vaginal deliveries | 4.3 (3.7) | 3.5 (1.9) | 3.6 (2.0) | <0.001** | 1.14 (0.93, 1.39) | 0.20 |
| Any lifetime c-sections | 0 (0) | 7 (2.0) | 7 (1.7) | 0.60 | - | - |
| SUI only | 6 (11.3) | 91 (26.5) | 97 (24.4) | 0.02* | 0.94 (0.34, 2.62) | 0.90 |
| UUI only | 4 (7.6) | 18 (5.2) | 22 (5.5) | 0.52 | 3.31 (0.94, 11.58) | 0.06 |
| SUI and UUI | 31 (58.5) | 53 (15.4) | 84 (21.2) | <0.001** | 8.23 (3.79, 17.84) | <0.001** |
| Prolapse | 11(20.8) | 60 (17.0) | 71 (17.5) | 0.56 | 0.79 (0.35, 1.78) | 0.57 |
| Hosmer and Lemeshow Test | - | 11.00, ~χ2 8df, p=0.20 | ||||
Abbreviations (SUI) stress urinary incontinence; (UUI) urge urinary incontinence.
Comparison of VVF screening questions
Analyses of responses to each VVF screening question by false positive/ true negative status [Table 2] and by case-control group (OF cases, incontinence controls, true controls) [Table 3] suggested high specificity of all questions in correctly classifying women (all p<0.001). The clinically-confirmed VVF cases responded positively to all of the VVF screening questions, and the interviewers reported being able to see that the cases' clothing were wet with urine. Only one of the two cases was observed to have a smell of urine around her.
Table 2.
Validity assessment of VVF screening questions by false positive/true negative status among eligible, screened women in the case control population (n=408)
| Responded “Yes" to VVF screening questions, n (%) | False positive (n=53) |
True negative (n=353) |
Fistula cases (n=2) |
Total (n=408) |
p- value |
|---|---|---|---|---|---|
|
VVF [2]: Currently does your clothing get wet with your urine during sleep? |
48 (90.6) |
0 (0) | 2 (100) | 50 (12.3) |
<0.001 |
|
VVF [3]: When you are not urinating, do you currently experience continuously dripping urine through the birth canal that you cannot stop? |
35 (66.0) |
0 (0) | 2 (100) | 37 (9.1) | <0.001 |
| VVF [2] or VVF [3]: VVF Case definition | 53 (100) | 0 (0) | 2 (100) | 55 (13.5) |
<0.001 |
| Asked among women who answered “Yes” to VVF [3] | n=35 | n=0 | n=2 | n=55 | |
|
VVF [3a]: Do you currently experience continuously dripping urine through the birth canal that you cannot stop all day and all night? |
23 (65.7) |
n/a | 2 (100) | 25 (67.6) |
1.00 |
|
VVF [1&4]: In which month and year did your last delivery occur? In which month and year did you begin experiencing continuously dripping urine that you cannot stop? a |
24 (68.6) |
n/a | 2 (100) | 26 (70.3) |
1.00 |
|
VVF [5] Observation: Do you think you smell the subject’s own urine around her? |
7 (13.2) | 0 (0) | 1 (50.0) |
8 (2.0) | <0.001 |
|
VVF [6] Observation: Do you think the subject’s clothing is wet with her own urine? |
15 (28.3) |
0 (0) | 2 (100) | 17 (4.2) | <0.001 |
|
VVF [7] Observation: Do you think you see wetness around the subject from her own urine? |
8 (15.1) | 0 (0) | 2 (100) | 10 (2.5) | <0.001 |
|
Most discriminating question combination analysis, n (%) a |
|||||
| 1st most discriminating: VVF [7] | 8 (15.1) | 0 (0) | 2 (100) | 10 (2.5) | <0.001 |
| + 2nd most discriminating: VVF [6] | 8 (15.1) | 0 (0) | 2 (100) | 10 (2.5) | <0.001 |
| + 3rd most discriminating: VVF [3+3a] | 6 (11.3) | 0 (0) | 2 (100) | 8 (2.0) | <0.001 |
| + 4th most discriminating: VVF [3+1&4] | 7 (13.2) | 0 (0) | 2 (100) | 9 (2.2) | <0.001 |
| + 3rd & 4th most discriminating: VVF [3+3a+1&4] | 5 (9.4) | 0 (0) | 2 (100) | 7 (1.7) | <0.001 |
|
+ 3rd, 4th & 5th most discriminating: VVF [3+3a+1&4+2] |
5 (9.4) | 0 (0) | 2 (100) | 7 (1.7) | <0.001 |
“Most discriminating” question defined as the screening question with the lowest number of false positives, which also correctly classifies all true fistula cases
Table 3.
Validity assessment of VVF screening questions by incontinence control/true control status among eligible, screened women in the case control population (n=408)
| Responded “Yes" to VVF screening questions, n (%) | Incontinence Controls (n=203) |
True controls (n=203) |
Fistula cases (n=2) |
Total (n=408) |
p- value |
|---|---|---|---|---|---|
|
VVF [2]: Currently does your clothing get wet with your urine during sleep? |
36 (17.7) | 12 (5.9) | 2 (100) | 50 (12.3) | <0.001 |
|
VVF [3]: When you are not urinating, do you currently experience continuously dripping urine through the birth canal that you cannot stop? |
28 (13.8) | 7 (3.5) | 2 (100) | 37 (9.1) | <0.001 |
| VVF [2] or VVF [3]: VVF Case definition | 41 (20.2) | 12 (5.9) | 2 (100) | 55 (13.5) | <0.001 |
| Asked among women who answered “Yes” to VVF [3] | n=41 | n=12 | n=2 | n=55 | |
|
VVF [3a]: Do you currently experience continuously dripping urine through the birth canal that you cannot stop all day and all night? |
17 (60.7) | 6 (85.7) | 2 (100) | 25 (67.6) | 0.35 |
|
VVF [1&4]: In which month and year did your last delivery occur? In which month and year did you begin experiencing continuously dripping urine that you cannot stop? a |
20 (71.4) | 4 (57.1) | 2 (100) | 26 (70.3) | 0.68 |
|
VVF [5] Observation: Do you think you smell the subject’s own urine around her? |
5 (2.5) | 2 (1.0) | 1 (50.0) | 8 (2.0) | 0.02 |
|
VVF [6] Observation: Do you think the subject’s clothing is wet with her own urine? |
11 (5.4) | 4 (2.0) | 2 (100) | 17 (4.2) | <0.001 |
|
VVF [7] Observation: Do you think you see wetness around the subject from her own urine? |
4 (2.0) | 4 (2.0) | 2 (100) | 10 (2.5) | <0.001 |
Delivery dates and dates of VVF symptom initiation were compared, and women whose reported VVF symptoms occurred after delivery were counted as a positive response.
True negatives did not respond “Yes,” to any of the VVF screening questions, nor were observed to have the smell of urine or wetness from urine [Table 2]. Of the two VVF case-defining questions, nearly all the false positives responded, “Yes,” to VVF [2], “Currently does your clothing get wet with your urine during sleep?” (48, 91%), while fewer responded, “Yes” to VVF [3], “When you are not urinating, do you currently experience continuously dripping urine through the birth canal that you cannot stop?” (35, 66%). The false positive rate was decreased to 43% (23), when women were additionally asked whether this dripping of urine occurred all day and night (VVF [3a]). The VVF screening question which had the lowest false positive response rate (8, 15%), and which did not misclassify any fistula cases, was the interviewer observation question VVF [7], “Do you think you see wetness around the subject from her own urine?” Combinations of the VVF screening questions were assessed to determine the smallest number of questions which would result in the lowest number of false positive responses, while not misclassifying any true cases. Including the interviewer observation of wetness around the women, the combination of OF screening questions that were most discriminating included VVF [3 and 3a], “Do you currently experience continuously dripping urine through the birth canal that you cannot stop all day and all night?” and VVF [1&4], which assessed whether the VVF symptoms occurred after the woman’s last delivery. The above combination resulted in only 5 false positives (9%).
Incontinence controls were more like to respond positively to each of the VVF screening questions compared to true controls (14 to 20% vs. 4 to 6%, p<0.001) [Table 3]. While the observational questions distinguished the fistula cases from any controls, they were less useful for discriminating between incontinence controls and true controls.
DISCUSSION
Main findings
In this population-based study of reproductive-age, parous women in the Sarlahi District of rural Nepal (n=16,893 women), our OF screening questionnaire demonstrated high sensitivity and specificity for VVF and RVF, and estimated low VVF and RVF prevalence (12/100,000). Although women with OF may be socially isolated and not present for screening or care, it is notable that our case-finding strategy, which used word-of-mouth referral from local women research interviewers and women that presented for clinical examinations, resulted in only two additional cases of OF in women were the Sarlahi District not within our surveyed sectors.
Strengths and limitations
The community-based design with case-finding strategy increased our confidence in the accuracy of our OF prevalence estimate. Hospital-based designs likely underestimate OF prevalence, as women with OF often lack access to health facilities and are socially stigmatised. A 2013 systematic review and meta-analysis of obstetric fistula prevalence studies found that hospital-based OF prevalence estimates (51 per 100,000 reproductive-age women in hospital-based studies in Sub-Saharan Africa) were lower than community-based estimates (160 per 100,000 reproductive-age women in Sub-Saharan Africa, and 120 per 100,000 in South Asia).22
Although the screening questionnaire performed adequately in a low OF prevalence setting, our statistics were driven by the large number of women without OF symptoms (large number of true negatives, no false negatives). Furthermore, the screening questionnaire had a low positive predictive value (two true positives out of 55 screened positives). In other similarly low prevalence settings, performing confirmatory clinical exams on a small number of screened-positive women (in this case, 55 out of 16,893 women) would be feasible, but this may not be feasible when OF prevalence is higher. Before the questionnaire can be used, it must undergo further validation in a population with a higher suspected OF prevalence.
We demonstrated that the OF screening questionnaire was feasible to administer by women field interviewers with a high school education and understood by mostly illiterate women. Significantly this screening questionnaire did not miss any VVF or RVF cases (no false negatives). The analysis of the OF screening tool questions demonstrated that a combination of symptoms-based and observational questions may yield the most accurate VVF prevalence estimates with the lowest false positives.
Notably, the observational questions (observation of wetness, including wet clothing) generated the least number of false positive cases while correctly classifying 100% of fistula cases. These observational questions may be particularly applicable in low-literacy settings; however, using them in isolation should be cautioned as it may lead to missed OF cases as urine production varies throughout the day and incontinence barriers may mask urine wetness.
Interpretation
Although most published estimates of OF prevalence, including the recent 2013 meta-analysis22, found much higher OF prevalence than our study, most reports did not include clinical confirmation of cases or were too small to accurately estimate prevalence. This is a potentially consequential limitation of these studies in light of our finding of 53 false positive VVF cases in this low prevalence setting. Additionally, analysis of VVF false positives indicate that false positives were eight times more likely to have both SUI and UUI symptoms compared with true negatives, after controlling for age, parity, and symptoms of only SUI or UUI. Therefore, studies of OF prevalence without a confirmatory examination may result in over-estimation due to the inclusion of women with incontinence. This may be an even more significant issue as by most approximations, the prevalence of urinary incontinence in most populations is much higher than OF.
OF prevalence estimates from other population-based studies with confirmatory clinical examinations from Sub-Saharan Africa and South Asia vary widely and were significantly higher than what we found in our study: 162 per 100,000 women (95%CI: 153, 264) in Ethiopia (n=19,153)23; 95 per 100,000 women (95%CI: 2, 526) in Gambia (n=1,038)24; 30 per 100,000 women (95% CI: 10,100) in Southern Sudan (n=8865)27; 86 per 100,000 (95%CI: 2, 480) in Maharashtra, India (n=1,167)25; 260 per 100,000 (95%CI: 7, 1439) in Karnataka, India26; 390 per 100,000 women (95%CI: 220, 570) or 450 per 100,000 parous women (95%CI: 250, 650) in Pakistan (n=5,064)28. Notably, these studies used different sampling methodologies and none of the studies examined controls(women who did not report symptoms of OF). Additionally, while our estimate (12/100,000, 95%CI: 3, 43) was within the 95% confidence intervals of the estimates from Gambia and India, those studies had very wide confidence intervals, likely due to small sample sizes, whereas the large sample size used in our study (n=16,893) yielded a more precise prevalence estimate. Insights from Nepali in-country experts suggest that OF may be more common in mountainous regions of rural Nepal instead of the flat plains of the Terai region in which Sarlahi is situated. Additionally, our study population was limited to reproductive age women, and the OF screening questions only asked about current experience of OF symptoms. We would have missed women with a history of OF who underwent previous repair and older women with OF symptoms. However, using our additional case-finding strategy we only found two additional cases of OF.
While exact estimates may be unclear, it is probable that OF significantly impacts millions of women in parts of Sub-Saharan Africa and South Asia. Population based prevention and treatment strategies would significantly improve the quality of life of affected individuals and their communities. As most of the populations at risk for OF live in rural areas, sending skilled personal to remote places to perform physical examinations require significant resources, which is often prohibitive in resource-limited nations. This underscores the need for a validated questionnaire that can accurately diagnose women with OF and be easily administered to a large population. Without an accurate measure of OF incidence it is not possible to know if health care resources are adequately or effectively allocated. There are currently no published, rigorously studied, OF screening tools. A 2013 study in Nigeria found that the DHS fistula module had a 92% sensitivity, 83% specificity among a sample of 268 women who presented to facilities based on perceived fistula-like symptoms. The authors clearly noted however that this could not be considered a validation study due to the sample restrictions.32
CONCLUSION
The OF screening questionnaire was feasible to administer by women field interviewers with a high school education and demonstrated excellent sensitivity and specificity in this population of women with low fistula prevalence. The low OF prevalence in this study suggests that higher prevalence estimates from Sub-Saharan African or hospital-based studies are not generalizable to our population in rural South Asia. Findings from analysis of the false positive population in our study further questions previous population-based estimates as these estimates may capture women with incontinence. A useful OF screening tool needs to distinguish fistula symptoms from incontinence symptoms. We found that a combination of symptoms-based and observational questions may yield the most accurate VVF prevalence estimates. The RVF case-defining question appeared to effectively identify RVF cases, as there were no RVF false positives. However, the low OF population prevalence in this setting prohibited meaningful assessment of the tool’s positive and negative predictive values. Follow-up studies in higher prevalence settings are needed to further validate the tool.
Acknowledgments
FUNDING
This work was funded by the Maren Foundation and the Johns Hopkins Center for Global Health, and the National Institute of Child Health and Development, United States National Institutes of Health R01HD060712.
Footnotes
CONTRIBUTORS
CCGC spearheaded the project and was responsible for all stages of project completion including the IRB application, research design, implementation, analysis, and manuscript writing. DB was instrumental in the data analysis and manuscript writing. SKK, MS, JK, JMT, and LCM contributed to the study design, project implementation, data analysis, and manuscript writing. EMK and SCL were instrumental in the study design, data collection and entry, and contributed to manuscript writing.
DISCLOSURE OF INTERESTS
None declared. The ICMJE disclosure forms are available as online supporting information.
ETHICS COMMITTEE APPROVAL
IRB approval was obtained both from Johns Hopkins School of Public Health (Baltimore, MD, USA, IRB No: 00002572) and the Institute of Medicine, Tribhuvan University (Kathmandu, Nepal) on 27 January 2010.
References
- 1.Wall LL, Arrowsmith SD, Briggs ND, Browning A, Lassey A. The obstetric vesicovaginal fistula in the developing world. Obstet Gynecol Surv. 2005;60(7 Suppl 1):S3–S51. doi: 10.1097/00006254-200507001-00002. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed S, Genadry R, Stanton C, Lalonde AB. Dead women walking: neglected millions with obstetric fistula. Int J Gynaecol Obstet. 2007;99(Suppl 1):S1–S3. doi: 10.1016/j.ijgo.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 3.Miller S, Lester F, Webster M, Cowan B. Obstetric fistula: a preventable tragedy. J Midwifery Womens Health. 2005;50(4):286–294. doi: 10.1016/j.jmwh.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Wall LL. Obstetric vesicovaginal fistula as an international public-health problem. Lancet. 2006;368(9542):1201–1209. doi: 10.1016/S0140-6736(06)69476-2. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed S, Holtz SA. Social and economic consequences of obstetric fistula: life changed forever? Int J Gynaecol Obstet. 2007;99(Suppl 1):S10–S15. doi: 10.1016/j.ijgo.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 6.WHO. Obstetric fistula: Guiding principles for clinical management and programme development. Geneva: World Health Organization; 2006. [DOI] [PubMed] [Google Scholar]
- 7.Stanton C, Holtz SA, Ahmed S. Challenges in measuring obstetric fistula. Int J Gynaecol Obstet. 2007;99(Suppl 1):S4–S9. doi: 10.1016/j.ijgo.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 8.Muleta M, Rasmussen S, Kiserud T. Obstetric fistula in 14,928 Ethiopian women. Acta Obstet Gynecol Scand. 2010;89(7):945–951. doi: 10.3109/00016341003801698. [DOI] [PubMed] [Google Scholar]
- 9.Waaldijk K, Armiya’u Y. The obstetric fistula: a major public health problem still unsolved. Int Urogynecol J. 1993;4:126–128. [Google Scholar]
- 10.Ampofo EK, Omotara BA, Otu T, Uchebo G. Risk factors of vesico-vaginal fistulae in Maiduguri, Nigeria: a case-control study. Trop Doct. 1990;20(3):138–139. doi: 10.1177/004947559002000320. [DOI] [PubMed] [Google Scholar]
- 11.Singh S, Chandhiok N, Singh Dhillon B. Obstetric fistula in India: current scenario. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(12):1403–1405. doi: 10.1007/s00192-009-1000-0. [DOI] [PubMed] [Google Scholar]
- 12.Raut V, Bhattacharya M. Vesical fistulae--an experience from a developing country. J Postgrad Med. 1993;39(1):20–21. [PubMed] [Google Scholar]
- 13.Melah GS, Massa AA, Yahaya UR, Bukar M, Kizaya DD, El-Nafaty AU. Risk factors for obstetric fistulae in north-eastern Nigeria. J Obstet Gynaecol. 2007;27(8):819–823. doi: 10.1080/01443610701709825. [DOI] [PubMed] [Google Scholar]
- 14.Stanton C, Holtz SA, Ahmed S. Challenges in measuring obstetric fistula. Int J Gynaecol Obstet. 2007;99(Suppl 1):S4–S9. doi: 10.1016/j.ijgo.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 15.Johnson K. Incontinence in Malawi: analysis of a proxy measure of vaginal fistula in a national survey. Int J Gynaecol Obstet. 2007;99(Suppl 1):S122–S129. doi: 10.1016/j.ijgo.2007.06.033. [DOI] [PubMed] [Google Scholar]
- 16.Ethiopia Central Statistical Agency, ICF International. Calverton, Maryland, USA. CSA and ICF International; 2012. 2011 Ethiopia Demographic and Health Survey: Key Findings. [Google Scholar]
- 17.National Statistical Office (NSO), ICF Macro. Zomba, Malawi, and Calverton, Maryland, USA. NSO and ICF Macro; 2011. Malawi Demographic and Health Survey 2010. [Google Scholar]
- 18.National Population Commission (NPC) [Nigeria] and ICF Macro. Abuja, Nigeria. National Population Commission and ICF Macro; 2009. Nigeria Demographic and Health Survey 2008. [Google Scholar]
- 19.National Institute of Population Studies (NIPS) [Pakistan] and ICF International. NIPS and ICF International. Islamabad, Pakistan, and Calverton, Maryland, USA: 2013. Pakistan Demographic and Health Survey 2012-13. [Google Scholar]
- 20.National Institute of Statistics of Rwanda (NISR) [Rwanda], Ministry of Health (MOH) [Rwanda], and ICF International. NISR, MOH, and ICF International. Calverton, Maryland, USA: 2012. Rwanda Demographic and Health Survey 2010. [Google Scholar]
- 21.Uganda Bureau of Statistics (UBOS) and ICF International Inc. ICF International Inc. Kampala, Uganda UBOS and Calverton, Maryland: 2012. Uganda Demographic and Health Survey 2011. [Google Scholar]
- 22.Adler AJ, Ronsmans C, Calvert C, Filippi V. Estimating the prevalence of obstetric fistula: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2013;13 doi: 10.1186/1471-2393-13-246. 246,2393-13-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muleta M, Fantahun M, Tafesse B, Hamlin EC, Kennedy RC. Obstetric fistula in rural Ethiopia. East Afr Med J. 2007;84(11):525–533. doi: 10.4314/eamj.v84i11.9572. [DOI] [PubMed] [Google Scholar]
- 24.Walraven G, Scherf C, West B, Ekpo G, Paine K, Coleman R, et al. The burden of reproductive-organ disease in rural women in The Gambia, West Africa. Lancet. 2001;357(9263):1161–1167. doi: 10.1016/S0140-6736(00)04333-6. [DOI] [PubMed] [Google Scholar]
- 25.Kulkarni R. Magnitude and determinants of chronic obstetric morbidities in Nasik District in Maharashtra. Indian Council for Medical Research. 2007 doi: 10.4103/0971-5916.169219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhatia JC, Cleland J, Bhagavan L, Rao NS. Levels and determinants of gynecological morbidity in a district of south India. Stud Fam Plann. 1997;28(2):95–103. [PubMed] [Google Scholar]
- 27.Adler AJ, Fox S, Campbell OM, Kuper H. Obstetric fistula in Southern Sudan: situational analysis and Key Informant Method to estimate prevalence. BMC Pregnancy Childbirth. 2013;13 doi: 10.1186/1471-2393-13-64. 64,2393-13-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jokhio AH, Rizvi RM, Rizvi J, MacArthur C. Prevalence of obstetric fistula: a population-based study in rural Pakistan. BJOG. 2014;121(8):1039–1046. doi: 10.1111/1471-0528.12739. [DOI] [PubMed] [Google Scholar]
- 29.Brown LD, Cai TT, DasGupta A. Interval estimation for a binomial proportion. Statistical Science. 2001;16(2):101–133. [Google Scholar]
- 30.Brown LD, Cai TT, DasGupta A. Confidence intervals for a binomial proportion and asymptotic expansions. Annals of Statistics. 2002;30(1):160–201. [Google Scholar]
- 31.SAS software Version 9.3 of the SAS System for Microsoft Windows. Cary NC: SAS Institute Inc.; 2013. [Google Scholar]
- 32.Tuncalp O, Isah A, Landry E, Stanton CK. Community-based screening for obstetric fistula in Nigeria: a novel approach. BMC Pregnancy Childbirth. 2014;14 doi: 10.1186/1471-2393-14-44. 44,2393-14-44. [DOI] [PMC free article] [PubMed] [Google Scholar]