Abstract
Background
Indirect calorimetry (IC) requires a steady state (SS) protocol when determining measured resting energy expenditure (mREE). Achieving stringent criteria for a SS interval may be difficult for patients on maintenance hemodialysis (MHD) as they may become uncomfortable either due to the test itself or their health status. The study aim was to explore if a shortened SS interval was within acceptable limits for bias and precision.
Materials and Methods
For this cross-sectional, secondary analysis, adults (N=125) who received MHD thrice weekly were enrolled. The IC test was performed for a length of total time no greater than 30 consecutive minutes. SS was evaluated in accordance to 10-, 5-, 4-, 3- and 2-minute (min) time intervals. Degree of bias and level of agreement between mREE at the 10-min SS was compared to the mREE at 5-, 4-, 3- and 2-min using t-tests and Bland-Altman analysis. The a priori alpha level was set at ≤ 0.5
Results
The sample was primarily male, African American, and non-Hispanic with a mean age of 55.4 ± 12.2 years who reported being on MHD for an average of 62.4 ± 74.3 months.
None of the mREE measures were significantly different than the 10-min SS interval. Seventy-two percent of the participants were able to achieve SS at the 10-min interval, 83.2% at 5-min, 87.2% at 4-min, and 89.6% for both 3- and 2-min.
Conclusion
In patients on MHD, an abbreviated SS interval of less than 10 min (e.g., 5-min) yielded valid mREE measurements.
Introduction
Individuals diagnosed with chronic kidney disease (CKD) requiring maintenance hemodialysis (MHD) may experience hypermetabolism1–4 resulting in an increased energy expenditure (EE) that attributes to their higher risk for protein-energy wasting and poor clinical outcomes.5–8 To ensure that the practitioner prescribes an appropriate caloric recommendation for optimal health, the methods for determining EE must be standardized, reliable and accurate.
In the clinical setting, indirect calorimetry (IC) is considered the “gold standard” method for measuring resting energy expenditure (REE).9–12 Under resting conditions, oxygen consumption (VO2) and carbon dioxide production (VCO2) are measured by IC. These values, in turn, are imputed into the Weir equation13 with the intent of deriving the measured resting energy expenditure (mREE).10,12,14 In order to yield the most precise results, IC is ideally measured over a consecutive 24-hour period. However, this methodological approach is not feasible within the clinical setting.14 Thus, the concept of a steady state (SS) interval was first proposed by McClave, et al.15 These researchers documented that a shortened time interval of IC measurement [e.g., 20-, 30-, 40-minute (min)] is representative of mREE data obtained over a 24-hour period, and does reflect mREE at a baseline physiologic state.15 A SS interval is established within one-min increments, and is a time period whereby the mean VO2 and VCO2 is changed by less than a predetermined percentage range (e.g. ≤ 10%).14 The most widely accepted and stringent SS definition among mechanically ventilated patients includes an IC test that collects 10 consecutive minutes and then discards the first 5-min of data with the remaining 5-min required to achieve a coefficient of variation (CV) of VO2 and VCO2.16 To satisfy this SS criterion, it generally requires at least 20–30 minutes of continuous testing.
In some debilitated conditions such as traumatic brain injury17 and cancer,18 whereby it is difficult to achieve SS for an extended period of time, abbreviated SS intervals (≤ 5 min) have been published. Based on our own research observations, we identified that some participants diagnosed with CKD on MHD were unable to achieve a SS interval for an extended period of time (e.g., 10-min). Thus, we were uncertain whether current criteria for SS were reasonable for such a medically complex population. At the time of this investigation, there was no available literature to inform our decisions in terms of SS criteria specific to the CKD patient group. Hence, our objective was to explore whether we could shorten the SS interval, without introducing bias or error, by comparing the level of agreement between the 5-, 4-, 3-, and 2-min intervals to a standard reference interval (e.g., 10-min).
Materials and Methods
Subjects and Design
The current study was a cross-sectional, secondary analysis of data (N=125) collected from a three-year federally funded study that developed and validated a predictive energy equation for hemodialysis patients. In the original study, participants were recruited from dialysis clinics associated with nephrology teams from Rutgers University (RU), Case Western Reserve University (CWRU), and Pennsylvania State University-Hershey Medical Center (PSU-HMC). Participants were screened for eligibility if they were adults (≥ 18 years), were diagnosed with stage 5 CKD, received MHD thrice weekly for a minimum of 3 months, and were able to answer study related questions. Exclusion criteria were as follows: a hospitalization, surgical/elective procedure or any cardiac-related event within the past 30 days from enrollment; a cardiac implantable device; infection or non-healing wound; active malignancy; treated by either peritoneal dialysis, shortened daily hemodialysis, nocturnal hemodialysis or kidney transplant with prescribed immunosuppressive agents; were pregnant, lactating or < 3 months post-partum; and/or reported use of substances known to impact metabolic rate. Informed consent, which followed Institutional Review Board requirements at each research site (CWRU #08-12-37, PSU-HMC #40781EP and RU #2012001976), was obtained from those willing to participate and who met eligibility criteria.
Inter- and Intra-Rater Reliability and Training Calibration
To assure inter- and intra-rater reliability, a 2-day training/calibration session was arranged prior to recruitment and enrollment efforts launched at any of the three research sites. The primary objective of this training session was to demonstrate the correct procedures for each of the proposed measurements as well as to afford the investigators the ability to acquire hands-on skills for collecting such measurements on “control” patients. The demonstrations were digitally videotaped with the files shared so that they could be viewed at later times in case any questions were raised as to the correct technique. In addition, each of the study investigators received a study protocol manual that gave detailed procedures on the methods involved within the proposed project. Once data collection began, the lead investigators at each of the research sites monitored the study protocol closely. Over the course of the 3-year study, there were scheduled visits to the each of the research sites by the principal investigator with the intent of assuring quality control and oversight, as well as to confirm that procedures are followed and were standardized from one research site to the next. Co-investigators met weekly via teleconferencing to discuss recruitment, enrollment, and methodological procedures, and to address any issues or questions that were raised. All study equipment was calibrated and tested as per factory specifications prior to participant testing.
Measurements
As part of the original study, each participant had testing completed on a non-hemodialysis treatment day at a designated research site, which included the following: REE measurement using the IC device (i.e., metabolic cart), body composition analysis using bioelectrical impedance analysis (BIA), clinical measurements (e.g., height, weight, waist and hip circumferences, blood pressure, respiration rate, heart rate, and body temperature), collection of laboratory samples for analysis (e.g., glycosylated hemoglobin, c-reactive protein, serum creatinine, etc.), and patient physical examination and interview [e.g., Subjective Global Assessment, Multi-Pass 24-hour Dietary Recall, Nutrition-Related Quality of Life, and demographic data (e.g., age (years), sex, race, ethnicity, co-morbid conditions, etiology of CKD and dialysis vintage (length of dialysis in months)].
Measurement of REE
Following the evidence-based recommendations for conducting indirect calorimetry studies published by the Academy of Nutrition and Dietetics,19 participants were instructed to avoid vigorous physical activity for 12 hours prior to the metabolic measurements. Participants were also asked to fast for the same time period, but if such a fast was not medically feasible, a shorter 4-hour fast was deemed acceptable. To assure that most of the fasting occurred while the participant may have slept, measurements were obtained in the morning or soon after they had arisen from sleep. The IC measurements were completed in a quiet, comfortable room, after the participant had been in a recumbent position for a minimum of 20 minutes. Participants were instructed to avoid fidgeting, hyperventilation, or sleeping during the test. The measurement was initiated by placing a rigid plastic canopy over the participant’s head or by using a facemask with an air tight seal so that the expired gas could be captured and directed into the calorimeter. A 15-min interval was initially used, with the first 5-min automatically discarded. The remaining 10-min of measured values were collected so that the steady state threshold of ≤10% coefficient of variation for oxygen consumption and carbon dioxide production was achieved. If this threshold was not reached, the measurement was extended to 30-min in an attempt to reach the 10% threshold.
The IC devices (i.e., metabolic carts) used for the original study included the Quark RMR® (Cosmed, Rome, Italy) at both RU and PS-HMC and the Vmax® Encore (CareFusion, Yorba Linda, CA, USA) at CWRU. Ashcraft and Frankenfield validated the Quark RMR® for measuring REE.20 The Vmax® Encore and the Quark RMR® were also tested for reliability and found to be comparable devices.21 Minute-by-minute measurements of VCO2 (mL/min), VO2 (mL/min), and respiratory quotient (RQ;VCO2/CO2) were obtained. The metabolic cart was connected through a serial port to a personal computer, and each data set was then downloaded to into a Microsoft Excel® spreadsheet upon completion of the test for data manipulation and then uploaded into the Statistical Program for Social Sciences Version 22 (Chicago, IL) for further analysis. The Weir equation ([(3.94* VO2)+(1.11* VCO2)]*1.44)10 was used to calculate mREE.
Steady State Intervals
This study defined a SS interval as a consecutive time period in minutes during the IC test where the CV was ≤ 10% for average VO2 and VCO2. SS was measured in 10-, 5-, 4-, 3- and 2-min intervals. We used the criterion measure of a 10-min SS interval based on the work of McClave et al.15 We felt that it would be too onerous for the participants to conduct a 60-min IC test for the purpose of evaluating longer SS intervals (e.g., 20-, 30-, and 40-min). However, we wanted a reasonably precise measure of resting energy expenditure (REE) for which to index the shortened SS protocols. If SS was not achieved, then mREE could not be calculated for the desired time interval. The minute-by-minute recordings of VCO2 and VO2 were evaluated to determine if each time interval achieved SS condition (i.e. ≤10% CV over the interval). Data were validated by simultaneously monitoring the respiratory gases within context of the RQ at a physiological range of 0.8–1.3 and the participant’s total tidal volume.18,22 As described by Reeves, et al.,18 the averaged VO2, VCO2, and RQ values were recorded for each SS interval so that the mREE could be derived by the Weir Equation.13 If the participant achieved SS more than once during the entire IC test, the first time interval when SS occurred was selected for analysis. Measured REE for each SS time interval (10-, 5-, 4-, 3-, 2-min) represented the average of the mREE measurements derived by the minute-by-minute recordings.
Statistical Analysis
Level of significance was set at an alpha of ≤ 0.05. Continuous variables were tested for normality and were reported as mean ± standard deviation (SD) unless otherwise stated. Following Bland and Altman’s procedure for testing agreement, mean bias (mean difference between mREE at 10-min SS compared to each SS time interval) was tested using one sample t-test for each period.23 Bland-Altman plots were used to describe the level of agreement between mREE at 10-min SS and each shorter period measurement. Limits of agreement, set at 95% confidence interval (CI), were used to determine if the compared data were interchangeable.18,23 Current publications report that mREE values, within the MHD population, typically vary between 5–18% when compared to control subjects.2,24,25 Due to the variance in mREE, an additional limit of agreement was set at ± 10% of the 10-min SS interval mean mREE for individuals who met SS parameters for each comparable grouping. A mean difference (of mREE) close to zero suggested a good level of agreement.16–18,26
Results
Active participant recruitment/enrollment began in June 2013 with 1,123 prospective participants recruited/referred over the course of the three years. Of this total number, 726 resulted in screening failures (64.6%) with 208 participants (18.5%) consented/enrolled; however 83 of these participants (39.9%) either withdrew, did not show for research appointments, or were unable to complete indirect calorimetry (IC) testing. The higher attrition rate occurred during the winter months, and was partially attributed to inclement weather that caused the closing of university campuses. At the close of the grant cycle in August 2015, there were 125 participants measured with IC data available for the current study. The sample (N=125) was primarily male, African American, and non-Hispanic with a mean age of 55.4 ± 12.2 years (Table 1). The most frequent etiology for CKD was hypertension followed by diabetes. Dialysis vintage was reported on average of 62.4 ± 74.3 months.
Table 1.
Demographic and clinical characteristics of study participants (N=125)
| Demographic and clinical characteristics | n (%) | Mean (SD) |
|---|---|---|
| Gender | ||
| Male | 75 (60.0) | |
| Female | 50 (40.0) | |
| Race | ||
| Black/African American | 106 (84.8) | |
| Caucasian | 19 (15.2) | |
| Ethnicity | ||
| Non-Hispanic | 95 (76.0) | |
| Unknown | 22 (17.6) | |
| Hispanic | 8 (6.4) | |
| CKD etiology | ||
| Hypertension | 50 (40.0) | |
| Diabetes | 42 (33.6) | |
| ESRD (unspecified) | 24 (27.2) | |
| Miscellaneous | 12 (9.6) | |
| HIV/AIDS | 8 (6.4) | |
| Glomerulonephritis | 7 (5.6) | |
| Polycystic Disease | 4 (3.2) | |
| Acute Kidney Injury | 3 (2.4) | |
| Systemic Disease | 2 (1.6) | |
| Age (years) | 55.38 (12.17) | |
| Dialysis vintage (months) | 62.35 (74.30) | |
| BMI | 29.43 (6.77) | |
BMI, body mass index; CKD, Chronic Kidney Disease; ESRD, end-stage renal disease; HIV/AIDS, human immunodeficiency virus/acquired immune deficiency syndrome
Bias
The number of participants able to achieve the SS definition for the predefined time intervals varied (Table 2). Of the total sample, 72.0% were able to achieve SS at 10-min interval, 83.2% at 5-min, 87.2% at 4-min, and 89.6% for both 3- and 2-min. Participants who did not meet a 10-min SS (n=35) were compared to those who did (n=90) across demographic and clinical characteristics. No statistically significant differences were identified. Similarly, whether or not a participant achieved a 10-min SS was not significantly associated with the device used. None of the mREE mean measures were significantly different than the 10-mins SS interval (Table 2). With the exception of the 3-min SS interval, all abbreviated SS intervals were close to zero. There was no significant bias detected between the 10-min SS interval and shorter measurement periods. However, the 5-, 4-, 3-, and 2-min SS intervals did show greater variability when compared to the reference value (10-min SS)..
Table 2.
Mean mREE at Each Time interval and Comparison of Shorter Periods to the 10 Minute Reference Interval.
| Steady State Time Interval |
n with valid SS | Mean ± SD | Mean difference± SD)from 10 minute measure1 |
P value of difference |
|---|---|---|---|---|
| 10-min mREE | 90 | 1503.31 ± 321.52 | Reference | Reference |
| 5-min mREE | 104 | 1517.83 ± 336.95 | −1.67 ± 43.9 | 0.720 |
| 4-min mREE | 109 | 1531.15 ± 333.65 | −0.41± 49.5 | 0.938 |
| 3-min mREE | 112 | 1549.60 ± 331.76 | −11.54 ± 55.4 | 0.051 |
| 2-min mREE | 112 | 1529.34 ± 333.92 | 0.81 ± 67.7 | 0.910 |
For individuals who reached steady state in both measurement periods (n=90)
Agreement
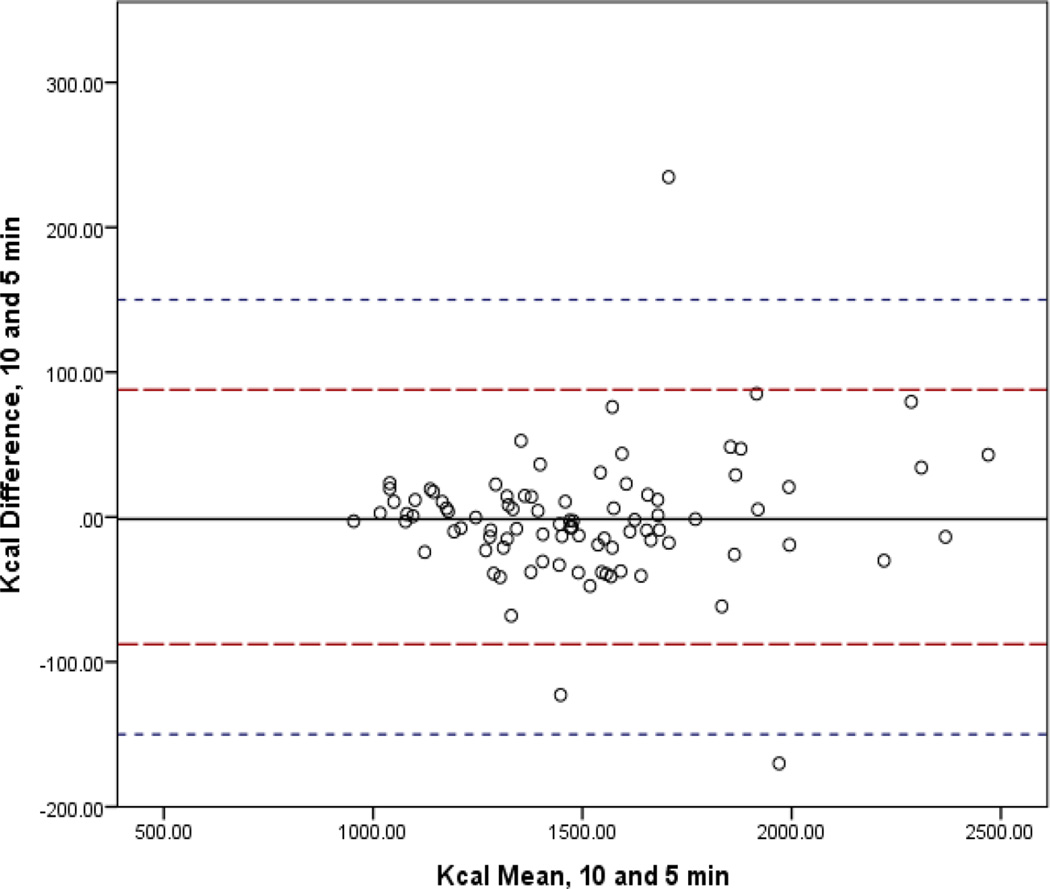
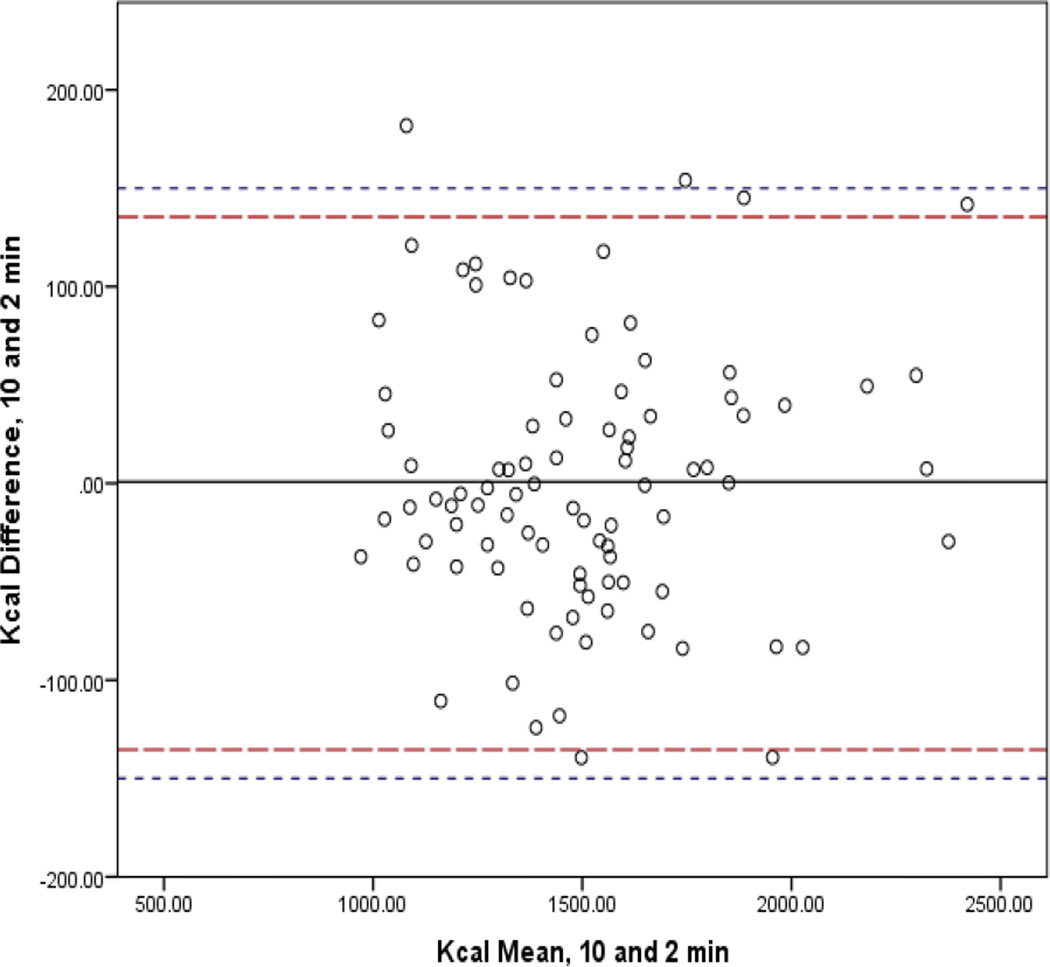
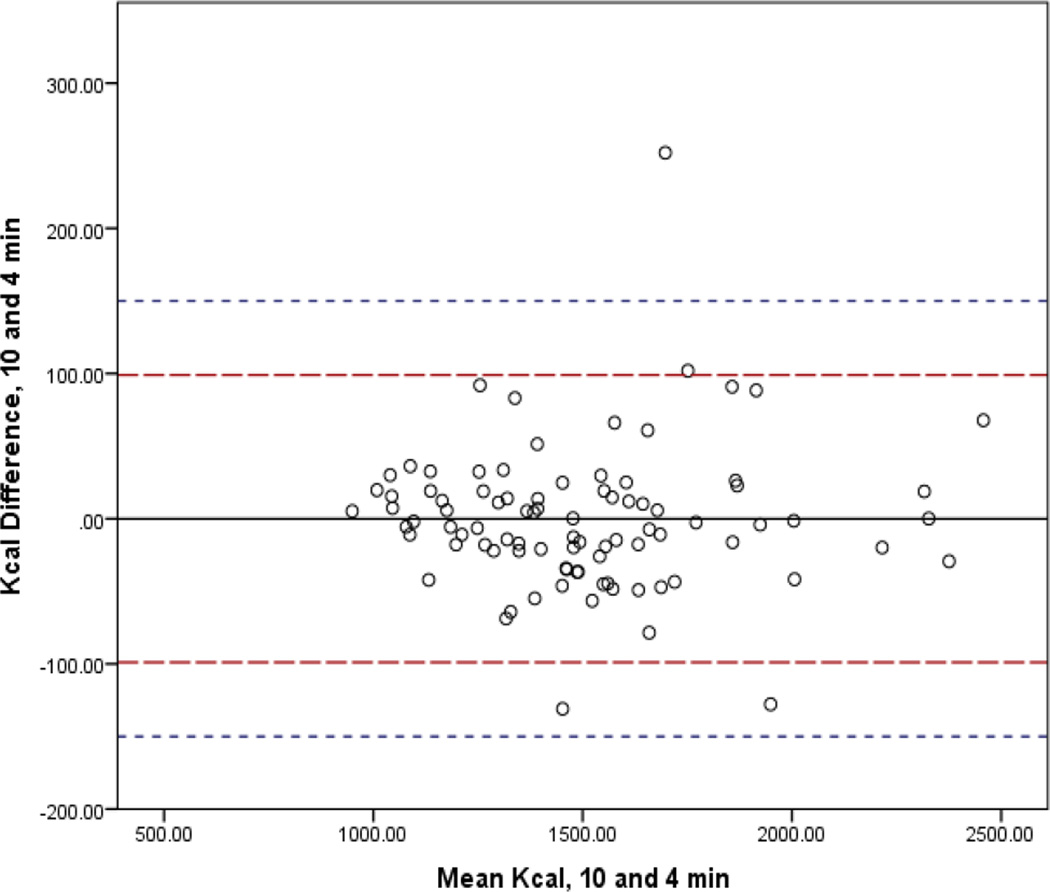
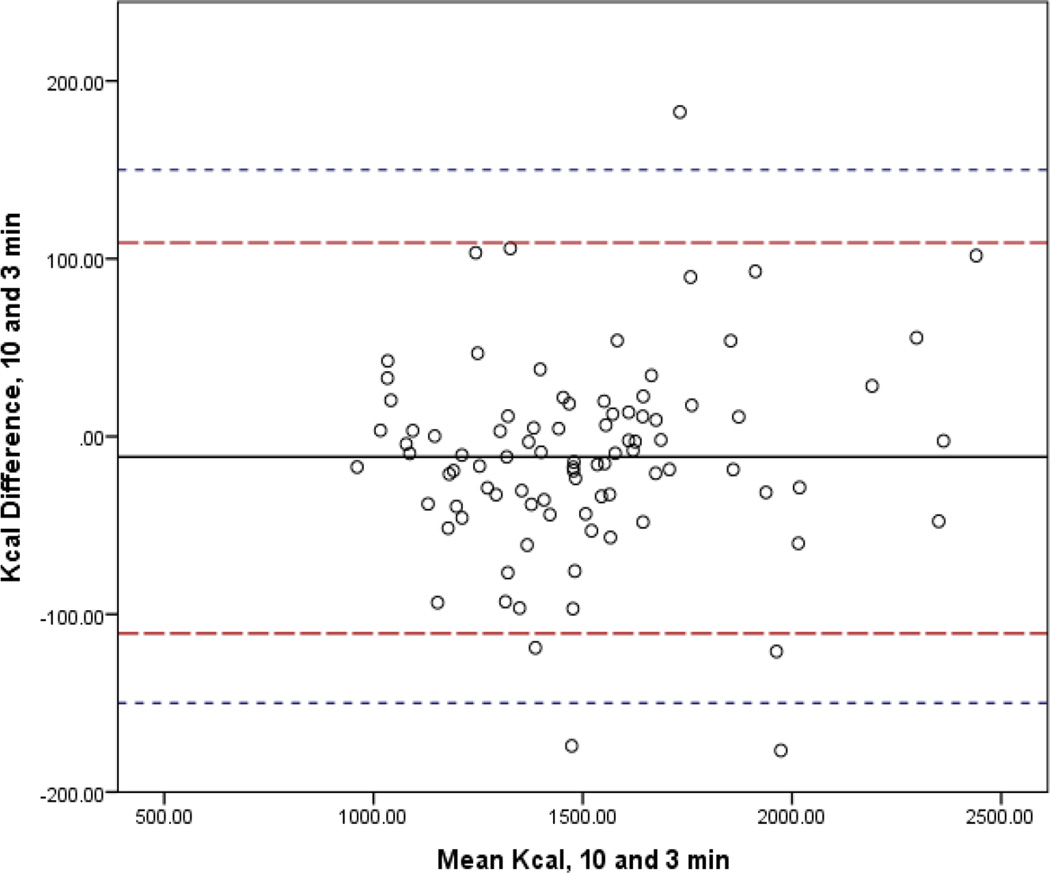
In Figures 1–4, the mean bias is represented by the solid line and 95% CI by the long dashed lines. Lines very close to zero on the Y axis indicate low bias. The short dashed line indicates the reference period (10-min) within 10% band of agreement (±150.3). For the 3-min interval, three (3.3%) participants had differences that were not within the limits of agreement. For the 5- and 2-min intervals, two participants (2.2%) fell outside the 10% band. Only one participant (1.1%) did not achieve the band of acceptability for the 4-min interval. The 95% confidence intervals of the mean difference between the interval measures increased as comparison intervals shorten.
Figure 1.
Bland-Altman Analysis Comparing mREE of the Standard Reference of 10 minutes to 5 Minute Abbreviated Steady State Interval.
Legend: The mean bias is represented by the solid line and 95% CI by the long dashed lines. Lines very close to zero on the Y axis indicate low bias. The short dashed line indicates the reference period (10-min) within 10% band of agreement (±150.3).
Figure 4.
Bland-Altman Analysis Comparing mREE of the Standard Reference of 10 minutes to 2 Minute Abbreviated Steady State Interval.
Legend: The mean bias is represented by the solid line and 95% CI by the long dashed lines. Lines very close to zero on the Y axis indicate low bias. The short dashed line indicates the reference period (10-min) within 10% band of agreement (±150.3).
Discussion
Achievement of SS during IC testing assures better precision in the REE measurement. McClave and colleagues have verified that IC tests that are shorter than 24 hours (e.g., 20- to 60-min) represent true physiologic EE.15 However, when SS is unobtainable during the IC testing period, such data cannot be used for determining mREE. Too often, strict steady state criteria cited in the literature cannot be achieved by medically complex patients.17,18 These challenges, as well as the barriers related to lack of equipment and training, can lead to the impracticability of IC within the clinical care setting as well as cause practitioners to rely on predictive energy equations that may not be validated for use within their specific patient population. In our study, approximately one-fourth (28%, n=35) of the participants could not achieve the SS definition of ≤10% CV for VO2 and VCO2 for a 10-min test. Since our experience with the CKD population may have larger research and clinical implications, we sought to determine whether shorter time periods (5-, 4-, 3-, and 2-min) under the same criteria (CV ≤ 10%), which may be more achievable, would establish additional SS protocols that meet clinically acceptable standards.
According to our study sample, there were non-significant differences between the shortened SS time intervals and our standard reference criterion of 10-min. Given the inter-variability of mREE among patients on MHD (anywhere from 5–18%),1,2 we expanded our limits of agreement to +/− 10% of the 10-min mean mREE. Others, like Reeves et al.,18 employed a much tighter band of acceptability in light of their patient populations (+/− 2%) due to the consequent implications of inaccurately determining mREE among those with traumatic brain injury. All of the shortened SS intervals demonstrated bias close to zero with the exception of the 3-min SS interval, but these differences were not significant. These findings have replicated earlier investigations17, 18 among spontaneously breathing individuals diagnosed with chronic or critical illness, that abbreviated intervals for SS are likely a reasonable alternative to longer, more stringent SS definition.
As mentioned, over 25% of our sample was not able to achieve SS at a 10-min interval. Although these participants were excluded from the analysis, there were no statistically significant differences in the demographic or clinical characteristics included in the analysis and the characteristics of the total sample. We were not able to identify any differences in the gas-exchange patterns between subjects who achieved 10-min versus those that did not satisfy the established criterion. Incidentally, a greater proportion of participants in our study reached SS at the abbreviated intervals than other investigations among spontaneously breathing individuals17,18 and was comparable to studies of critically ill, ventilator-dependent patients.15,16,26 It typically takes spontaneously breathing participants time to acclimate to the IC testing and have relaxed, regular breathing patterns, whereas mechanically ventilated patients have their gas-exchange externally controlled and manipulated.12,26 Nonetheless, as shown in other ambulatory populations, reducing the SS time interval increased the proportion of participants who achieved SS, without significant differences in their mREE.17,18 We are, however, cautious in the wide application of an abbreviated SS interval (< 5 mins) into clinical practice based uniquely on our findings. In fact, our results confirm the earlier work by McClave, et al.15 Thus, clinicians can accept a mREE, among patients on MHD, for a SS interval of 5 mins with ≤10% CV when the 10 mins SS interval cannot be achieved, but using abbreviated SS time intervals (<5 mins) requires further investigation.
Even though achievement of steady state is essential for ensuring stable gas-exchange parameters, it is not enough to precisely measure REE. There are several potentially negative influencing factors on mREE, such as, the possibility of air leaks, an inaccurate calibration of the IC device, erratic breathing patterns of the participant, or abnormal levels of fractionally inspired oxygen in ventilated-dependent patients.12 Thus, monitoring RQ, which has an accepted known physiologic range, may aid in determining the validity of the IC test and its respective data.17,22 Seemingly, RQ values outside of range may be questioned and therefore, render, even if at a steady state, an IC test as invalid. During the IC test such values were analyzed, including total tidal volume, so that such SS data were valid and the REE measurements were accurately derived. Additionally, to assure higher confidence in our IC measurements, we meticulously calibrated the flow sensors and gas analyzers prior to every test obtained and adhered to evidence-based practice guidelines regarding the conditions for successful completion of IC testing.19
While there was variability in the IC device used by the research institutions (Quark RMR® vs. Vmax® Encore), there were no significant differences in the proportion of participants that were able to achieve steady state at either the reference standard (10-min) or any of the abbreviated time intervals (5-, 4-, 3-, or 2-min) by site or device. The comparability, reliability or validity of these two devices has been reported.20,21 In addition, the one research site that used the Vmax Encore® also measured the participants using a well-fitted, air tight face mask. While the canopy hood is less prone to air leaks,27,28 the participants measured via the face mask were closely monitored to assure that the gas exchange was not compromised.
In light of our findings, there are some limitations that warrant careful interpretation. Our sample was not a generalizable sample of patients on MHD. Younger, male individuals (which predominate in our sample) may have had a very different experience achieving steady state than females or older persons. Thus, this study should be repeated with a more diverse population to make sure that key demographic or clinical characteristics do not preclude the accurate REE measurement of patients with CKD on MHD and to increase confidence that our results are reproducible. While we were not able to detect any substantive differences in the IC devices (Quark RMR® vs. Vmax® Encore) used or the procedures employed (canopy hood vs. facemask), greater standardization of IC testing protocols would only strengthen our confidence with the findings disseminated. Despite these limitations we are the first team that has studied the ability to achieve a precise measure of REE while reducing or abbreviating the SS interval time in the MHD population. In summary, we conclude that reducing the time period of SS to less than 10 minutes (e.g., 5-min) may be a reasonably precise alternative for measuring REE. Future studies could further evaluate the ability of an abbreviated SS interval at minimizing patient burden and reduce overall research time dedicated to obtaining such data.
Figure 2.
Bland-Altman Analysis Comparing mREE of the Standard Reference of 10 minutes to 4 Minute Abbreviated Steady State Interval.
Legend: The mean bias is represented by the solid line and 95% CI by the long dashed lines. Lines very close to zero on the Y axis indicate low bias. The short dashed line indicates the reference period (10-min) within 10% band of agreement (±150.3).
Figure 3.
Bland-Altman Analysis Comparing mREE of the Standard Reference of 10 minutes to 3 Minute Abbreviated Steady State Interval.
Legend: The mean bias is represented by the solid line and 95% CI by the long dashed lines. Lines very close to zero on the Y axis indicate low bias. The short dashed line indicates the reference period (10-min) within 10% band of agreement (±150.3).
Clinical Relevancy Statement.
Obtaining precise measurements for energy expenditure is critical to the management and treatment of patients diagnosed with stage 5 chronic kidney disease on maintenance hemodialysis. Due to the medical complexity of their condition, it is difficult to achieve current steady state definitions for determining the resting energy expenditure using indirect calorimetry among this vulnerable population, thereby resulting in potentially erroneous values. Once established that an abbreviated steady state protocol is a valid measure of resting energy expenditure, patient burden and staffing time may be reduced by being able to shorten the overall indirect calorimetry testing time.
Acknowledgments
The authors thank the collaborating clinical managers and dietitians at Fresenius Medical Care facilities in Newark, NJ area, Centers for Dialysis Care in Cleveland, OH area, and Penn State-Hershey Medical Center, in Hershey, PA. Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Numbers 1R15DK090593-01A1, 6R15DK090593-02, and 3R15DK090593-02S1. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contributor Information
Laura A. Olejnik, Rutgers University, School of Health Related Professions, Newark, NJ.
Emily N. Peters, Rutgers University, School of Health Related Professions, Newark, NJ.
J. Scott Parrott, Rutgers University, School of Health Related Professions, Newark, NJ.
Andrea F. Marcus, Rutgers University, School of Health Related Professions, Newark, NJ.
Rebecca A. Brody, Rutgers University, School of Health Related Professions, Newark, NJ.
Rosa K. Hand, Case Western Reserve University, School of Medicine, Cleveland, OH.
Justin J. Fiutem, Case Western Reserve University, School of Medicine, Cleveland, OH.
Laura D. Byham-Gray, Rutgers University, School of Health Related Professions, Newark, NJ.
References
- 1.Ikizler TA, Wingard RL, Sun M, Harvell J, Parker RA, Hakim RM. Increased energy expenditure in hemodialysis patients. J Am Soc Nephrol. 1996;7(12):2646–2653. doi: 10.1681/ASN.V7122646. [DOI] [PubMed] [Google Scholar]
- 2.Neyra R, Chen KY, Sun M, Shyr Y, Hakim RM, Ikizler TA. Increased resting energy expenditure in patients with end-stage renal disease. J Paren Enteral Nutr. 2003;27(1):36–42. doi: 10.1177/014860710302700136. [DOI] [PubMed] [Google Scholar]
- 3.Cuppari L, deCarvalho AB, Avesani CM, Kamimura MA, Dos Santos LRR, Draibe SA. Increased resting energy expenditure in hemodialysis patients with severe hyperparathyroidism. J Am Soc Nephrol. 2004;15:2933–2939. doi: 10.1097/01.ASN.0000141961.49723.BC. [DOI] [PubMed] [Google Scholar]
- 4.Cuppari L, Avesani CM. Energy requirements in patients with chronic kidney disease. J Renal Nutr. 2004;14(3):121–126. doi: 10.1053/j.jrn.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Fouque D, Kalantar-Zadeh K, Kopple J, Cano N, Chauveau P, Cuppari L, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney. Int. 2008;73(4):391–398. doi: 10.1038/sj.ki.5002585. [DOI] [PubMed] [Google Scholar]
- 6.Carrero JJ, Stenvinkel P, Cuppari L, Ikizler TA, Kalantar-Zadeh K, Kaysen G, et al. Etiology of the protein-energy wasting syndrome in chronic kidney disease: A consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM) J Ren Nutr. 2013;23(2):77–90. doi: 10.1053/j.jrn.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Pifer T, McCullough KP, Fort FK, Goodkin DA, Maroni BJ, Held PJ, et al. Mortality risk in hemodialysis patients and changes in nutritional indicators: Dialysis Outcomes Practice Patterns Study (DOPPS) Kidney. Int. 2002;62:2238–2245. doi: 10.1046/j.1523-1755.2002.00658.x. [DOI] [PubMed] [Google Scholar]
- 8.Lopes AA, Bragg-Gresham JL, Elder SJ, Ginsberg N, Goodkin DA, Pifer T, et al. Independent and joint associations of nutritional status indicators with mortality risk among chronic hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS) J Renal Nutr. 2009;20(4):224–234. doi: 10.1053/j.jrn.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Compher C, Frankenfield D, Keim N, Roth-Yousey L. Best practice methods to apply to measurement of resting metabolic rate in adults: a systematic review. J Am Diet Assoc. 2006;106(6):881–903. doi: 10.1016/j.jada.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 10.Haugen HA, Chan LN, Li F. Indirect calorimetry: a practical guide for clinicians. Nutr Clin Pract. 2007;22(4):377–388. doi: 10.1177/0115426507022004377. [DOI] [PubMed] [Google Scholar]
- 11.Haugen HA, Melanson EL, Tran ZV, Kearney JT, Hill JO. Variability of measured resting metabolic rate. Am J Clin Nutr. 2003;78(6):1141–1145. doi: 10.1093/ajcn/78.6.1141. [DOI] [PubMed] [Google Scholar]
- 12.Fullmer S, Benson-Davies S, Earthman CP, Frankenfield DC, Gradwell E, Lee PS, et al. Evidence analysis library review of best practices for performing indirect calorimetry in healthy and non-critically ill individuals. J Acad Nutr Diet. 2015;115(9):1417.e2–1446.e2. doi: 10.1016/j.jand.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. Nutrition. 1990;6(3):213–221. [PubMed] [Google Scholar]
- 14.Frankenfield DC. On heat, respiration, and calorimetry. Nutrition. 2010;26(10):939–950. doi: 10.1016/j.nut.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 15.McClave SA, Spain DA, Skolnick JL, Lowen CC, Kieber MJ, Wickerham PS, et al. Achievement of steady state optimizes results when performing indirect calorimetry. J Paren Enteral Nutr. 2003;27(1):16–20. doi: 10.1177/014860710302700116. [DOI] [PubMed] [Google Scholar]
- 16.Frankenfield DC, Sarson GY, Blosser SA, Cooney RN, Smith JS. Validation of a 5-minute steady state indirect calorimetry protocol for resting energy expenditure in critically ill patients. J Am Coll Nutr. 1996;15(4):397–402. doi: 10.1080/07315724.1996.10718615. [DOI] [PubMed] [Google Scholar]
- 17.McEvoy C, Cooke SR, Young IS. A reduced abbreviated indirect calorimetry protocol is clinically acceptable for use in spontaneously breathing patients with traumatic brain injury. Nutr Clin Pract. 2009;24(4):513–519. doi: 10.1177/0884533609335308. [DOI] [PubMed] [Google Scholar]
- 18.Reeves MM, Davies PS, Bauer J, Battistutta D. Reducing the time period of steady state does not affect the accuracy of energy expenditure measurements by indirect calorimetry. J Appl Physiol. 2004;97(1):130–134. doi: 10.1152/japplphysiol.01212.2003. [DOI] [PubMed] [Google Scholar]
- 19.Academy of Nutrition and Dietetics. Measuring resting metabolic rate in the critically ill Evidence Analysis Library Guideline 2013. [accessed 29 February 2016]; Internet: http://www.andeal.org/topic.cfm?menu=5299. [Google Scholar]
- 20.Ashcraft CM, Frankenfield DC. Validity test of a new open-circuit indirect calorimeter. J Paren Enteral Nutr. 2015;39(6):738–742. doi: 10.1177/0148607114526242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frankenfield DC, Ashcraft CM, Wood C, Chinchilli VM. Validation of an indirect calorimeter using n-of-1 methodology. Clin Nutr. 2016;35(1):163–168. doi: 10.1016/j.clnu.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 22.McClave SA, Snider HI. Use of indirect calorimetry in clinical nutrition. Nutr Clin Pract. 1992;7:207–221. doi: 10.1177/0115426592007005207. [DOI] [PubMed] [Google Scholar]
- 23.Myles PS, Cui J. Using the Bland-Altman method to measure agreement with repeated measures. Br J Anaesth. 2007;99(3):309–311. doi: 10.1093/bja/aem214. [DOI] [PubMed] [Google Scholar]
- 24.Kamimura MA, Draibe SA, Avesani CM, Canziani ME, Colugnati FA, Cuppari L. Resting energy expenditure and its determinants in hemodialysis patients. Eur J Clin Nutr. 2007;61(3):362–367. doi: 10.1038/sj.ejcn.1602516. [DOI] [PubMed] [Google Scholar]
- 25.Ikizler TA, Wingard RL, Sun M, Harvell J, Parker RA, Hakim RM. Increased energy expenditure in hemodialysis patients. J Am Soc Nephrol. 1996;7(12):2646–2653. doi: 10.1681/ASN.V7122646. [DOI] [PubMed] [Google Scholar]
- 26.Petros S, Engelmann L. Validity of an abbreviated indirect calorimetry protocol for measurement of resting energy expenditure in mechanically ventilated and spontaneously breathing critically ill patients. Intensive Care. Med. 2001;27(7):1164–1168. doi: 10.1007/s001340100941. [DOI] [PubMed] [Google Scholar]
- 27.Feurer I, Mullin JL. Bedside measurement of resting energy expenditure and respiratory quotient via indirect calorimetry. Nutr Clin Pract. 1986;1:43–49. [Google Scholar]
- 28.Weissman C, Askanazi J, Milic-Emili J, Kinney JM. Effect of respiratory apparatus on respiration. J Applied Physiol. 1984;57(2):475–480. doi: 10.1152/jappl.1984.57.2.475. [DOI] [PubMed] [Google Scholar]