Abstract
Rheumatoid arthritis (RA) is a chronic disease. It causes chronic inflammation of the joint. Recent studies suggested that interleukin 4 (IL4) contributes to susceptibility and severity of rheumatoid arthritis (RA). Especially, it was reported that promoter polymorphism (−590, T/C) of IL4 gene has been associated with susceptibility of RA. The aim of present study was to investigate whether the promoter polymorphism (−590, T/C) of IL4 gene is associated with the susceptibility of RA using meta-analysis. And in order to perform meta-analysis, comprehensive meta analysis program was used. Genetic models (co-dominant, dominant, recessive, and allele) were used to determine odds ratios (ORs), 95% confidence intervals (CIs), and P values. Nine case-control studies with case and control design were included in this meta-analysis. Overall, meta-analysis revealed a strong association with susceptibility of RA [OR = 1.303, 95% CI = 1.093–1.554, P = 0.003 in allele model (C vs. T); OR = 1.247, 95% CI = 1.054–1.474, P = 0.010 in dominant model (CC vs. CT + TT); OR = 2.148, 95% CI = 1.263–3.651, P = 0.005 in recessive model (CC + CT vs. TT)]. Our data demonstrated that promoter polymorphism (−590, T/C) of IL4 gene may be contributed to susceptibility of RA. However, more studies with a larger sample size are needed to provide more precise evidence.
Keywords: Interleukin 4, Polymorphism, Rheumatoid arthritis, Association study, Meta-analysis
1. Introduction
Rheumatoid arthritis (RA) is a systemic inflammatory disorder and the most common inflammatory arthritis (Lee and Weinblatt, 2001, Antonisamy et al., 2015). The incidence of RA in 2008 was estimated to be 42/100,000 in South Korea (Sung et al., 2013). And the annual incidence in Norway was 28.7/100,000 (Riise et al., 2000) and 44.6/100,000 in Rochester, Minnesota (Doran et al., 2002).
RA is the most common autoimmune disease. The adaptive T-cell response interactions with environmental factors such as smoking lead to a systemic autoantibody response and it results in the synovial inflammatory process (Pablos and Canete, 2013). In these inflammatory responses, many previous studies investigated the role of genetic factors. HLA has been regarded as an important genetic factor in RA risk (Ollier and MacGregor, 1995). IL27 gene polymorphism was associated with susceptibility of RA in Chinese (Yan et al., 2015). Recent meta-analysis showed the association between CCR6 polymorphism and RA (Cheng et al., 2015).
IL4 gene is located at 5q31.1 and encodes interleukin 4 (http://www.ncbi.nlm.nih.gov). IL4 improves anti-inflammatory effect and suppresses several pro-inflammatory cytokines and systemic IL4 treatment protects the cartilage and bone destruction in established murine type II collagen-induced arthritis (Joosten et al., 1999). Also, IL4 could be used for the treatment of RA in association with anti-TNF alpha or anti-IL1 beta (Meyer, 2003). Genetic polymorphism of IL4 also has an association with RA. IL4 promoter polymorphism may be a genetic risk factor for RA severity (Pawlik et al., 2005). Previous study also suggested that the promoter polymorphism may be helpful for assessing RA severity (joint erosion and anti-CCP) and TT genotype of polymorphism (−590) showed a significant decrease of IL4 level in serum (Moreno et al., 2007).
Though the roles of IL4 polymorphisms in RA have been reported in Chinese (Li et al., 2014) or White participants, the results has been controversial. In this study, we performed a meta-analysis on all eligible case-control studies to clarify the association between IL4 polymorphism (−590) and susceptibility to RA.
2. Materials and methods
2.1. Search strategy and data extraction
We firstly found the meta-analysis studies between IL4-590 polymorphism and RA. And electronic database including pubmed, embase, google of scholar, and Korean Studies Information Service System (KISS) were investigated up to June 2015. In order to select eligible studies about IL4-590 polymorphism and RA. The keywords to find eligible studies were used: “interleukin 4”, “IL4“, or “cytokine”, AND “polymorphism” or “SNP” AND “−590” or “rs2243250“ AND “RA”. The data of first author’s name, year of publication, country, sample size of RA and control, genotype frequencies of IL4-590 polymorphism in RA and control were extracted from the final selected studies.
2.2. Inclusion criteria
Studies were included if they met the following criteria: (1) evaluated the association between the IL4 polymorphism (−590, T/C) and RA; (2) used a case-control study design; (3) contained sufficient published data for the estimation of an odds ratio (OR) with a 95% confidence interval (CI).
2.3. Statistical analysis
Hardy–Weinberg equilibrium (HWE) in the control group of include studies was evaluated by the Chi-square test. Meta-analysis and sensitivity analysis were used by the Comprehensive Meta-analysis software (Corporation, NJ, USA). The pooled p value, OR, and 95% CI were used to assess the strength of association between risk of RA and IL4-590 polymorphism (Kim et al., 2013, Seok et al., 2014). The meta-analysis was performed while omitting each study one at a time to examine the influence of each study on the pooled OR. In order to assess heterogeneity among the studies, a χ2-test-based Q statistic test and I squared test were done. The random-effects Mantel–Haenszel method was adopted if the result of the Q test was P < 0.05 or I squared value was >50%, which indicated the statistically significant heterogeneity between the studies. Otherwise, the fixed-effects Mantel–Haenszel method was adopted. And Begg’s funnel plot and Egger’s test were performed to evaluate publication bias. For sensitivity analysis, the meta-analysis was performed subtracting each study to examine the influence of each study over and over again. The P < 0.05 was regarded as statistically significant.
3. Results
The present study performed the meta-analysis to assess relationship between promoter polymorphism (−590, T/C) of IL4 gene and susceptibility of RA. Firstly, searching was conducted to find related studies from electronic databases according to search strategy. Eleven case and control studies about promoter polymorphism (−590, T/C) of IL4 gene polymorphism (Ser326Cys) and susceptibility of RA were searched (Canet et al., 2015, Cantagrel et al., 1999, Emonts et al., 2011, Hussein et al., 2013, Li et al., 2014, Nunez et al., 2008, Moreno et al., 2007, Pawlik et al., 2005, Plenge et al., 2005, Trajkov et al., 2009). Among these studies, six studies (Cantagrel et al., 1999, Pawlik et al., 2005, Moreno et al., 2007, Nunez et al., 2008, Trajkov et al., 2009, Hussein et al., 2013) were analyzed by meta-analysis in 2013 (Song et al., 2013). However, there was an error in data by the meta-analysis in 2013. Song et al., 2013 reported results of meta-analysis between promoter polymorphism (−590, T/C) of IL4 gene and susceptibility of RA. They included the data from the study (Cantagrel et al., 1999) in the meta-analysis They summarized that the genotype frequencies (C/C:C/T:T/T) of promoter polymorphism (−590, T/C) of IL4 gene in the control group were 32:36:0, but original data of the study (Cantagrel et al., 1999) is 82:36:0. In meta-analysis between specific polymorphisms and susceptibility of diseases, it was important to search eligible studies and extract exact data. So, this updated meta-analysis was performed.
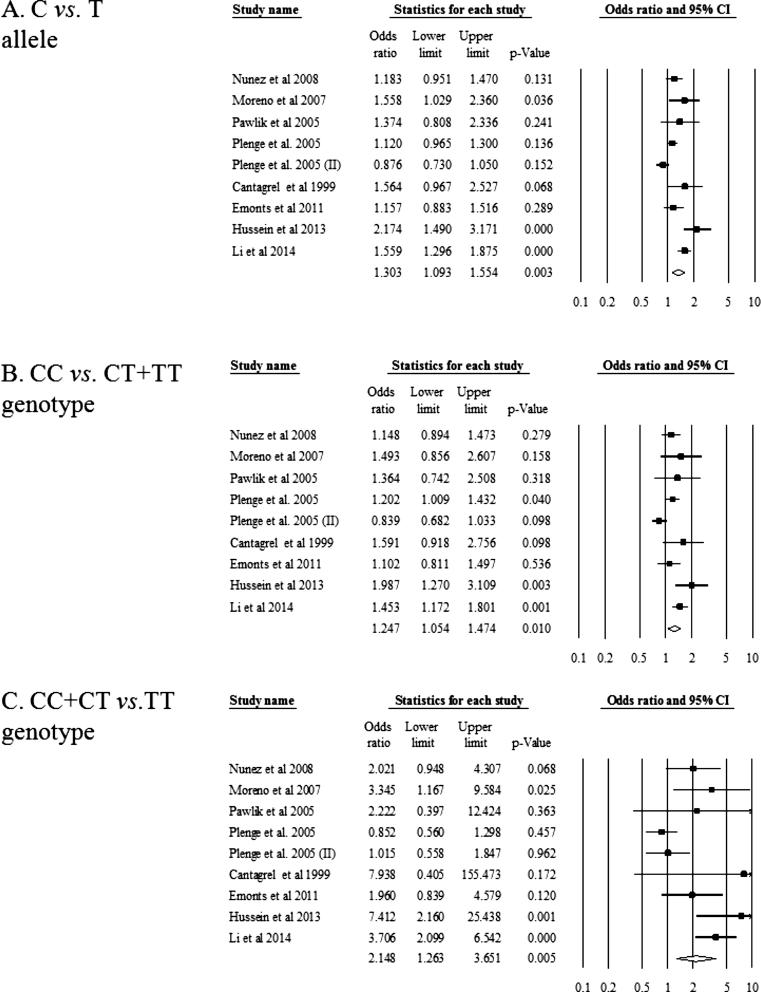
Table 1 shows characteristics of eligible studies included in the meta-analysis. The first author’s name, year of publication, country, sample size, and genotype frequencies of IL4-590 polymorphism in RA patients and controls were extracted. Among eleven studies, studies by Trajkov et al., 2009, Canet et al., 2015 were excluded in the meta-analysis because HWEs of genotype distribution of IL4-590 polymorphism in the control group was p < 0.05. Table 2 and Fig. 1 presents the results of meta-analysis between IL4-590 polymorphism and susceptibility of RA in nine studies. In all models, IL4-590 polymorphism revealed the significant association with susceptibility of RA (random model, OR = 1.303, 95% CI = 1.093–1.554, P = 0.003 in allele; random model, OR = 1.247, 95% CI = 1.054–1.474, P = 0.010 in dominant; random model, OR = 2.148, 95% CI = 1.263–3.651, P = 0.005 in recessive; Fixed model, OR = 1.128, 95% CI = 1.025–1.241, P = 0.014 in codominant 1; Random model, OR = 2.272, 95% CI = 1.313–3.931, P = 0.003 in codominant 2). Sensitivity analysis confirmed the stability of the results of meta-analysis (data not shown) and no publication bias was found in the present meta-analysis. These results indicate that promoter polymorphism (−590, T/C) of IL4 gene polymorphism is related to susceptibility of RA (Table 2 and Fig. 1).
Table 1.
Characteristics of eligible studies included in the meta-analysis.
| Study | Country | RA/Control (n) | Genotyping method | RA |
Control |
RA |
Control |
HWE in control | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C/C | C/T | T/T | C/C | C/T | T/T | C | T | C | T | |||||
| Trajkov et al. (2009) | Macedonia | 85/286 | Sequencing | 47 | 37 | 1 | 95 | 187 | 4 | 131 | 39 | 377 | 195 | <0.001 |
| Nuñez et al. (2008) | Spain | 599/540 | TaqMan | 398 | 179 | 22 | 375 | 155 | 10 | 975 | 223 | 905 | 175 | 0.185 |
| Moreno et al. (2007) | Colombian | 102/102 | PCR-RFLP | 39 | 48 | 15 | 49 | 48 | 5 | 126 | 78 | 146 | 58 | 0.11 |
| Pawlik et al. (2005) | Poland | 94/102 | PCR-RFLP | 62 | 28 | 4 | 74 | 26 | 2 | 152 | 36 | 174 | 30 | 0.87 |
| Plenge et al. (2005) | Sweden | 1503/875 | Sequenom | 940 | 507 | 56 | 584 | 253 | 38 | 2387 | 619 | 1421 | 329 | 0.12 |
| Plenge et al. (2005) | North America | 835/847 | Sequenom | 594 | 219 | 22 | 571 | 254 | 22 | 1407 | 263 | 1396 | 298 | 0.38 |
| Cantagrel et al. (1999) | France | 107/68 | PCR-RFLP | 63 | 41 | 3 | 82 | 36 | 0 | 167 | 47 | 200 | 36 | 0.05 |
| Emonts et al. (2011) | Netherland | 372/460 | Sequencing | 267 | 91 | 14 | 339 | 112 | 9 | 625 | 119 | 790 | 130 | 0.94 |
| Hussein et al. (2013) | Egypt | 172/172 | PCR-RFLP | 96 | 56 | 20 | 123 | 46 | 3 | 248 | 96 | 292 | 52 | 0.58 |
| Li et al. (2014) | China | 752/798 | PCR-RFLP | 481 | 218 | 53 | 575 | 207 | 16 | 1180 | 324 | 1357 | 239 | 0.60 |
| Canet et al. (2015) | Spain | 1239/1229 | KASPar | 859 | 325 | 34 | 863 | 275 | 34 | 2043 | 393 | 2001 | 343 | 0.037 |
RA = rheumatoid arthritis, n = number of subjects.
Bold number indicates significant difference.
Table 2.
Overall analysis between IL4-590 polymorphism and susceptibility of RA in nine studies.
| Genetic comparison | Association |
Heterogeneity |
Model | Publication bias | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | P | I2 | Egger’s test | ||
| C vs. T | 1.303 (1.093–1.554) | 0.003 | <0.001 | 75.46 | Random | 0.18 |
| C/C vs. C/T + T/T | 1.247 (1.054–1.474) | 0.010 | 0.006 | 62.75 | Random | 0.22 |
| C/C + C/T vs. T/T | 2.148 (1.263–3.651) | 0.005 | <0.001 | 72.26 | Random | 0.09 |
| C/C vs. C/T | 1.128 (1.025–1.241) | 0.014 | 0.100 | 40.28 | Fixed | 0.37 |
| C/C vs. T/T | 2.272 (1.313–3.931) | 0.003 | <0.001 | 73.55 | Random | 0.10 |
Rheumatoid arthritis (RA), odds ratio (OR), confidence interval (CI), number of subjects (n).
Bold numbers indicate significant association with RA.
Figure 1.
Odds ratio and 95% CI of individual and pooled data for the IL4 polymorphism (−590) and susceptibility of RA. A: C allele vs. T allele; B: C/C genotype vs. C/T genotype + T/T genotype; C: C/C genotype + C/T genotype vs. T/T genotype.
4. Discussion
Many factors such as gene, cytokine, inflammatory mediators, adhesion molecules, and so on involve in the pathophysiology of RA. Among them, many genetic factors have been identified due to advances in genetic methods (Lee and Weinblatt, 2001). In this study, we focused on the genetic factor of RA and carried out meta-analysis to investigate the relation between IL4 polymorphism and the risk of developing RA.
In the present study, we could find significant associations in allele, dominant, and recessive models. The results of this meta-analysis are consistent with the results of a previous meta-analysis (Canet et al., 2015). Among these, only the study by Li et al. (2014) involves Asian population (Chinese). According to NCBI database, genotype frequency in Chinese is 0.023 (C/C), 0.395 (C/T), and 0.581 (T/T) and allele frequency in Chinese is 0.221 (C) and 0.779 (T). On the other hand, genotype and allele frequencies in White are definitely different. According to NCBI database, genotype frequency in European is 0.743 (C/C), 0.239 (C/T), and 0.018 (T/T) and allele frequency in Chinese is 0.863 (C) and 0.137 (T). As shown in Table 1, genotype and allele frequencies in previous studies are similar to those in NCBI database. Interestingly, the genotypes and allele frequencies in 2014 by Li et al. (2014) are similar to those of European rather than those of Asian according to NCBI database. Previous study reported that the incidence and prevalence of RA showed different trends according to ethnicity. In Korea, the prevalence of RA in 2008 was estimated to be 0.27% (Sung et al., 2013). One study on rural African had found only 14 cases of RA in about 520,000. The prevalence of RA was 0.0026% (Brighton et al., 1988). In contrast, the prevalence of RA in a Chippewa Band (American Indian) reached to 5.3–6.8% (Harvey et al., 1981). A study in Norway reported that the prevalence of RA was 0.47% in 1994 (Riise et al., 2000). In United Kingdom, the prevalence of RA is 1.16% in women and 0.44% in men (Symmons et al., 2002). The prevalence of RA in the urbanized Chinese of Hong Kong was 0.35% (Lau et al., 1993). Thus, previous studies showed the significant geographic variations of RA occurrence and difference of prevalence according to ethnicity. These results suggest an association of RA with ethnicity and genetic factors.
5. Conclusion
In conclusion, our meta-analysis study showed that IL4 polymorphism was associated with RA. However, the limitation of our study is that there was not enough data on other ethnicities. We could not find the studies on African population and the number of studies on Asian was only one. In spite of these limitation, our meta-analysis provided evidence of the association between IL4 polymorphism and risk of RA. It is well known that early diagnosis and treatment of RA could lead to favorable outcomes. Therefore, early diagnosis and preventive treatment by detecting the susceptibility to RA in gene level would play a major role in improving the prognosis of RA. Many previous association studies revealed the association between IL4 polymorphism and RA and our present meta-analysis strengthened the relationship. If further studies will be performed in more population, promoter polymorphism (−590, T/C) of IL4 gene could be a useful marker for the early diagnosis and treatment of RA.
Author disclosure statement
No competing financial interests exist.
Acknowledgment
This work was supported by a Grant from Kyung Hee University in 2010 (KHU-20101896).
Footnotes
Peer review under responsibility of King Saud University.
References
- Antonisamy P., Duraipandiyan V., Ignacimuthu S., Kim J.-H. Anti-diarrhoeal activity of friedelin isolated from Azima tetracantha Lam. in Wistar rats. South Ind. J. Biol. Sci. 2015;1(1):34–37. [Google Scholar]
- Brighton S.W., de la Harpe A.L., van Staden D.J., Badenhorst J.H., Myers O.L. The prevalence of rheumatoid arthritis in a rural African population. J. Rheumatol. 1988;15:405–408. [PubMed] [Google Scholar]
- Canet L.M., Cáliz R., Lupiañez C.B., Canhão H., Martinez M., Escudero A., Filipescu I., Segura-Catena J., Soto-Pino M.J., Ferrer M.A., García A., Romani L., Pérez-Pampin E., González-Utrilla A., López Nevot M.Á., Collantes E., Fonseca J.E., Sainz J. Genetic variants within immune-modulating genes influence the risk of developing rheumatoid arthritis and anti-TNF drug response: a two-stage case-control study. Pharmacogenomics Gen. 2015;25(9):432–443. doi: 10.1097/FPC.0000000000000155. [DOI] [PubMed] [Google Scholar]
- Cantagrel A., Navaux F., Loubet-Lescoulié P., Nourhashemi F., Enault G., Abbal M., Constantin A., Laroche M., Mazières B. Interleukin-1beta, interleukin-1 receptor antagonist, interleukin-4, and interleukin-10 gene polymorphisms: relationship to occurrence and severity of rheumatoid arthritis. Arthritis Rheum. 1999;42(6):1093–1100. doi: 10.1002/1529-0131(199906)42:6<1093::AID-ANR5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Cheng P., Zhang Y., Huang H., Zhang W., Yang Q., Guo F., Chen A. Association between CCR6 and rheumatoid arthritis: a meta-analysis. Int. J. Clin. Exp. Med. 2015;8:5388–5396. [PMC free article] [PubMed] [Google Scholar]
- Doran M.F., Pond G.R., Crowson C.S., O’Fallon W.M., Gabriel S.E. Trends in incidence and mortality in rheumatoid arthritis in Rochester, Minnesota, over a forty-year period. Arthritis Rheum. 2002;46:625–631. doi: 10.1002/art.509. [DOI] [PubMed] [Google Scholar]
- Emonts M., Hazes M.J.M.W., Houwing-Duistermaat J.J., van der Gaast-de Jongh C.E., de Vogel L., Han H.K.H., Wouters J.M.G.W., Laman J.D., Dolhain R.J.E.M. Polymorphisms in genes controlling inflammation and tissue repair in rheumatoid arthritis: a case control study. BMC Med. Genet. 2011;12:36. doi: 10.1186/1471-2350-12-36. 1471-2350-12-36 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey J., Lotze M., Stevens M.B., Lambert G., Jacobson D. Rheumatoid arthritis in a Chippewa Band. I. Pilot screening study of disease prevalence. Arthritis Rheumatol. 1981;24:717–721. doi: 10.1002/art.1780240515. [DOI] [PubMed] [Google Scholar]
- Hussein Y.M., El-Shal A.S., Rezk N.A., Abdel Galil S.M., Alzahrani S.S. Influence of interleukin-4 gene polymorphisms and interleukin-4 serum level on susceptibility and severity of rheumatoid arthritis in Egyptian population. Cytokine. 2013;61:849–855. doi: 10.1016/j.cyto.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Joosten L.A., Lubberts E., Helsen M.M., Saxne T., Coenen-de Roo C.J., Heinegård D., van den Berg W.B. Protection against cartilage and bone destruction by systemic interleukin-4 treatment in established murine type II collagen-induced arthritis. Arthritis Res. 1999;1:81–91. doi: 10.1186/ar14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.-K., Lee H., Kim H.-J. A polymorphism in DMT1 is associated with lead-related hypertensive status. Mol. Cell Toxicol. 2013;9:415–420. [Google Scholar]
- Lau E., Symmons D., Bankhead C., MacGregor A., Donnan S., Silman A. Low prevalence of rheumatoid arthritis in the urbanized Chinese of Hong Kong. J. Rheumatol. 1993;20:1133–1137. [PubMed] [Google Scholar]
- Lee D.M., Weinblatt M.E. Rheumatoid arthritis. Lancet. 2001;358:903–911. doi: 10.1016/S0140-6736(01)06075-5. [DOI] [PubMed] [Google Scholar]
- Li X., Chai W., Ni M., Xu M., Lian Z., Shi L., Bai Y., Wang Y. The effects of gene polymorphisms in interleukin-4 and interleukin-6 on the susceptibility of rheumatoid arthritis in a Chinese population. Biomed. Res. Int. 2014;265435 doi: 10.1155/2014/265435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer O. Role of TNF-alpha and cytokines in the physiopathology of rheumatoid arthritis [Therapeutic perspectives] Bull. Acad. Natl. Med. 2003;187:935–954. discussion 954–935. [PubMed] [Google Scholar]
- Moreno O., González C.I., Saaibi D.L., Otero W., Badillo R., Martín J., Ramírez G. Polymorphisms in the IL4 and IL4RA genes in Colombian patients with rheumatoid arthritis. J. Rheumatol. 2007;34:36–42. [PubMed] [Google Scholar]
- Nunez C., Santiago J.L., Varadé J., de la Calle H., Figueredo M.A., Fernandez-Gutierrez B., de la Concha E.G., Urcelay E., Martínez A. IL4 in the 5q31 context: association studies of type 1 diabetes and rheumatoid arthritis in the Spanish population. Immunogenetics. 2008;60:19–23. doi: 10.1007/s00251-007-0265-z. [DOI] [PubMed] [Google Scholar]
- Ollier W.E., MacGregor A. Genetic epidemiology of rheumatoid disease. Br. Med. Bull. 1995;51:267–285. doi: 10.1093/oxfordjournals.bmb.a072960. [DOI] [PubMed] [Google Scholar]
- Pablos J.L., Canete J.D. Immunopathology of rheumatoid arthritis. Curr. Top. Med. Chem. 2013;13:705–711. doi: 10.2174/1568026611313060003. [DOI] [PubMed] [Google Scholar]
- Pawlik A., Nieto A., Matarán L., Balsa A., Pascual-Salcedo D., Martín J. The -590 IL-4 promoter polymorphism in patients with rheumatoid arthritis. Rheumatol. Int. 2005;26:48–51. doi: 10.1007/s00296-004-0539-9. [DOI] [PubMed] [Google Scholar]
- Plenge R.M., Padyukov L., Remmers E.F., Purcell S., Lee A.T., Karlson E.W., Wolfe F., Kastner D.L., Alfredsson L., Altshuler D., Gregersen P.K., Klareskog L., Rioux J.D. Replication of putative candidate-gene associations with rheumatoid arthritis in >4000 samples from North America and Sweden: association of susceptibility with PTPN22, CTLA4, and PADI4. Am. J. Hum. Genet. 2005;77:1044–1060. doi: 10.1086/498651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riise T., Jacobsen B.K., Gran J.T. Incidence and prevalence of rheumatoid arthritis in the county of Troms, northern Norway. J. Rheumatol. 2000;27:1386–1389. [PubMed] [Google Scholar]
- Seok H., Kim S.K., Yoo K.H., Lee B.C., Kim Y.O., Chung J.H. Association of BID SNPs (rs8190315 and rs2072392) and clinical features of benign prostate hyperplasia in Korean population. J. Exercise Rehabil. 2014;10:383–388. doi: 10.12965/jer.140168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song G.G., Bae S.C., Kim J.H., Lee Y.H. Interleukin-4, interleukin-4 receptor, and interleukin-18 polymorphisms and rheumatoid arthritis: a meta-analysis. Immunol. Invest. 2013;42:455–469. doi: 10.3109/08820139.2013.804084. [DOI] [PubMed] [Google Scholar]
- Sung Y.K., Cho S.K., Choi C.B., Bae S.C. Prevalence and incidence of rheumatoid arthritis in South Korea. Rheumatol. Int. 2013;33:1525–1532. doi: 10.1007/s00296-012-2590-2. [DOI] [PubMed] [Google Scholar]
- Symmons D., Turner G., Webb R., Asten P., Barrett E., Lunt M., Scott D., Silman A. The prevalence of rheumatoid arthritis in the United Kingdom: new estimates for a new century. Rheumatology (Oxford) 2002;41:793–800. doi: 10.1093/rheumatology/41.7.793. [DOI] [PubMed] [Google Scholar]
- Trajkov D., Mishevska-Perchinkova S., Karadzova-Stojanoska A., Petlichkovski A., Strezova A., Spiroski M. Association of 22 cytokine gene polymorphisms with rheumatoid arthritis in population of ethnic Macedonians. Clin. Rheumatol. 2009;28:1291–1300. doi: 10.1007/s10067-009-1238-4. [DOI] [PubMed] [Google Scholar]
- Yan J., Shang X., Rong X. Relationship between IL-27 gene polymorphism and susceptibility of rheumatoid arthritis in Chinese Han population. Int. J. Clin. Exp. Med. 2015;8:6262–6266. [PMC free article] [PubMed] [Google Scholar]