Abstract
Cell-to-cell communication or quorum sensing (QS) leads to biofilm formation and causing other virulence factors which are extreme problems for food safety, biofilm related infectious diseases etc. This study evaluated the anti-QS activity of the Amomum tsaoko extract (0.5–4 mg/ml) by using Chromobacterium violaceum a biosensor strain and biofilm formation by crystal violate assay. Experimental results demonstrated that the overall yield of Amomum tsao-ko extract was 11.33 ± 0.3% (w/w). MIC for Staphylococcus aureus (Gram positive), Salmonella Typhimurium and Pseudomonas aeruginosa (Gram negative) was 1, 2 and 2 mg/ml, respectively. A concentration of 4 mg/ml extract showed highest biofilm inhibition 51.96% on S. Typhimurium when 47.06%, 45.28% were shown by S. aureus, P. aeruginosa respectively. The damage of biofilm architecture was observed by Confocal Laser Scanning Microscopy (CLSM). A level of 44.59% inhibition of violacein production was demonstrated when the dose was 4 mg/ml. Swarming motility inhibition was observed in a dose dependent manner. Taken together, the treatment of A. tsaoko extract can deliver value to food product and medicine by controlling pathogenesis.
Keywords: Amomum tsaoko, Biofilm, Drug, Food spoilage, Quorum sensing
1. Introduction
Amomum tsao-ko (Zingiberaceae) is commonly known as “Black Cardamom” or “Tsaoko Amomum” which is grown in China (Guangxi, Guizhou, Yunnan), North Vietnam and some other parts of Asia (Delin and Larsen, 2000). It is widely used as Chinese Traditional Medicine in the treatment of hemorrhoids, windy, vomiting, diarrhea, malaria, throat infections, stomach disorders, dyspepsia, nausea, and abdominal pain and also in many dishes e.g. Chinese medicinal soup dish, beef herbal dish, hotpot etc. (Feng et al., 2010, Wu et al., 2012, Lim, 2013). As it has commercial value, some other fruits of this genus are being mixed with Amomum tsao-ko (A. tsaoko) as adulterants. A. tsaoko is being over-harvested and has been declared as “Near Threatened” species by the International Union for Conservation of Nature (IUCN) (Leong-Skornickova et al., 2012). In the past, lots of studies have been done on its anti-cancer, anti-inflammatory, antioxidant, anti-microbial activities and anti-Hepatitis B effect (Lim, 2013) but the anti-QS and biofilm inhibition activities have not yet been investigated.
Bacterial spoilage of food and other infections are a carefully orchestrated process controlled by QS (cell to cell communication mechanism through gene expression) – which is regulated by the activity (production/detection) of small molecules called autoinducers (AIs). When the environmental concentration of AIs (correlated to bacterial population density) reaches a certain threshold, the QS is activated. The expression of a variety of genes is modulated by the AI receptors which finally control the behavior of virulence factor expression – biofilm formation, bioluminescence, sporulation, conjugation and swarming motility (Annous et al., 2009, Skandamis and Nychas, 2012, Solano et al., 2014, Wu et al., 2014).
Biofilms are considered as sources of infections and provide safety challenges in a wide range of food factories including dairy processing, sea food processing, poultry and meat processing; hence a critical hindrance to the food preservation system. The biofilm allows bacteria to grow in a rhythmic way into an extracellular polymeric network without any environmental depression even protecting them from antimicrobials and attack from the immune system (Srey et al., 2013, Solano et al., 2014).
Considering the impact of QS and biofilm, several strategies (cleaning agents, disinfectants, CIP, ultrasonication, phages, irradiation, mixed vibriophages, scCO2, essential oil etc.) have been developed in the food factory and in drug discovery (pharmaceuticals) (Srey et al., 2013). But to minimize the food spoilage, develop new edible packaging materials, remove biofilm from membrane filter of water treatment/pipe lines and to prevent the bacterial infection in human, we have to find the natural, healthy and “generally regarded as safe” (GRAS) anti-QS and anti-biofilm agent. Traditional medicinal plants are popular for their new bioactive compounds and therapeutic values. So we have made an attempt to find the activity of A. tsao-ko which could potentially be used as a de novo natural anti QS and anti biofilm inhibitor or natural chemotherapeutic agent.
2. Materials and method
2.1. Plant material
Dried A. tsaoko (Black Cardamom) fruits were purchased from the nearest supermarket (Auchan, Wuxi, China). Fruits were authenticated by Dr. Jian Tang (Professor, Jiangnan University, Wuxi, China), a voucher with specimen No. JNU201409S05 has been deposited at herbarium of Food Nutritional and Functional Factors Research Center of Jiangnan University, Wuxi, China. Then, fruits were well cleaned and washed with water, dried in the shade at room temperature. After this period, A. tsaoko has been grinded (FW100, Tai Si Te Instrument Com. Ltd., Tianjin, China) and transformed to powder. The powders were preserved in clean, sealed plastic containers and kept away from light, heat and moisture until use.
2.2. Preparation of extract and % of yield
For extraction, 100 g of powdered A. tsaoko was blended (JJ-1, JianTan city Antoulin Feng Equipment Co. Ltd, China) with 1:20 solvents (ethanol:water = 80:20) for 3 h with agitation at 45 °C. After that, the extracts were taken out and vacuum filtered using a Buchner funnel through Whatman (No. 1) filter paper. Again the same process was followed for second and third time extractions. Then, the extracts were concentrated using a vacuum rotary evaporator (BC-W203, Shanghai Biochemical Equipment Co. Ltd, China). Following vacuum drying (DZF-650 Shanghai Yi Heng Sci. Equipment Co. Ltd, China) at 40 °C, the extracts were scraped out, weighed to obtain percentage of yield and stored in desiccator.
2.3. Strains and culture conditions
Staphylococcus aureus (ATCC 6538), Salmonella Typhimurium (ATCC 50013), and Pseudomonas aeruginosa (ATCC 9027) were purchased from China General Microbiological Culture Collection Center (Beijing, China). Chromobacterium violaceum ATCC 12472 was purchased from Spanish Type Culture Collection (CECT-494), Paterna (Valencia). They were cultured as per the recommended procedure from the purchase center.
2.4. Bioassay for anti-quorum sensing
2.4.1. Preliminary screening (Disk diffusion assay)
To evaluate the anti QS capacity of the A. tsaoko extract, the qualitative disk diffusion method was used as the previously published procedure (Khan et al., 2009). An aliquot of 100 μl fresh C. violaceum culture dilution was plated on Luria–Bertani agar (CM159, Beijing Land Bridge Technology Co. Ltd, China) Petri dishes (LB broth supplemented with 1.5% bacteriological agar). Sterile paper disks (Whatman No. 40; 6.0 mm in diameter) with A. tsaoko extract (10 μl extract/disk) were plotted above the agar. The Petri dishes were incubated at 30 °C for 30 h. Results were determined by observing the growth inhibition colorless ring diameter, opaque circle around the extract loaded paper disk and around positive control (vanillin, 250 μg/ml from Sino Pharma Chemical Reagent Co., China) loaded disk. The negative control was LB broth loaded paper disk.
2.4.2. Flask incubation assay for quantification of violacein production
With slight modifications, Truchado et al.’s (2012) the previously described procedure was used to quantify the anti-QS activity of A. tsaoko extract. C. violaceum ATCC 12472 (1 × 108 CFU/ml) was inoculated in Erlenmeyer flasks containing LB supplemented with A. tsaoko extract to obtain different concentrations (0.5, 1, 2, 4 mg/ml). The flasks were incubated at 30 °C in a shaking incubator (SPH-1102, Xi’an Heb Biotechnology Co. Ltd, Shaanxi, China) for 30 h. 1 ml culture of each test tube was centrifuged at 18,630g (Scilogex D3024R, USA) for 15 min to precipitate the insoluble pigment. The pellet was resuspended in 1 ml of dimethyl sulfoxide (DMSO; Aladdin Industrial Corporation, Shanghai, China) and homogenized by vortexing. Violacein absorbance at 585 nm was determined using a UV–visible spectrophotometer (Epoch, BioTek Instruments Inc, USA). The control sample consisted of incubating the C. violaceum in LB broth (CM158, Beijing Land Bridge Technology Co. Ltd) without adding extract. Inhibition was calculated with respect to control and triplicate measurements were performed.
2.5. Minimum Inhibitory Concentration (MIC) & anti-biofilm assay
The 30% ethanol elution fraction of A. tsaoko (100 μl) which had serial dilutions (0.5, 1.0, 2.0, 3.0, 4.0 mg/ml) was added to the wells of a 24-well culture plate (Sarstedt, Newton, NC) containing 800 μl of fresh LB broth (CM158, Beijing Land Bridge Technology Co. Ltd) and 100 μl bacteria suspension (108 CFU/ml). After incubation for 24 h, growth medium in every well was observed very carefully. The well which is clearer than other wells to look at confirmed the inhibition of bacterial growth. The clear well containing concentration of extract declared the MIC value for tested bacterial strain. Anti biofilm assay was performed as previously described by Gao et al. (2015) with modification. After following MIC, suspension cultures were removed. The wells were rinsed 3 times with PBS (Sigma, China), fixed by drying for 3 h at 37 °C in incubator. Once the wells were fully dried, 1000 μl of 0.1% crystal violet (Sinopharm chemical reagent co. ltd) stain was added to each well to stain for 15 min. The excess stain was rinsed off with tap water and 1000 μl of 95% (v/v) ethanol was added to each well for 1 h to release the stain. 100 μl from each well was then transferred to a new plate for spectrophotometric (Epoch, BioTek Instruments Inc, USA) analysis (OD570 nm). For all the assays, controls without A. tsaoko fractions and without inoculation were prepared. The procedure was performed in triplicate and the mean ± SD was calculated.
2.6. CLSM (Confocal Laser Scanning Microscopy) observation
To observe the biofilm structure, samples were prepared according to Zhang et al. (2014a) and CLSM (Leica TCS SP2; Leica Microsystems, Heidelberg, Germany) was used. Cover slips were treated with and without A. tsaoko extract. The L13152 LIVE/DEAD® BacLight Bacterial Viability Kit (Molecular Probes, Inc., USA) was used to stain the cover slip according to the manufacturer’s instruction. Finally 3D view was performed to visualize the architecture of biofilm, surface coverage of cover slips and the density of bacteria.
2.7. Swarming motility assay
Swarming motility assay was carried out as previously described method by Kuchma et al. (2015). 25 μl of molten soft top agar (3% agar, 1% tryptone, 5% yeast extract powder, 5% sodium chloride, deionized water) was prepared containing 0.5 mg/ml, 1 mg/ml, 2 mg/ml and 4 mg/ml A. tsaoko extract. Then, it was poured immediately over the surface of a solidified LB agar plate as an overlay. The center of plate was inoculated with an overnight culture of different tested bacterium. Then plates were incubated at 37 °C. After 30 h, swimming and swarming migration zones of the bacterial cells were observed. The control plates were prepared without A. tsaoko extract. Photographs were taken using iPhone 4s from the same distance of each disk and picture contrasts were adjusted.
2.8. Statistical analysis
The results were analyzed statistically by ANOVA using IBM SPSS Statistics 20.0, OriginPro 9.0; P < 0.05 was considered to indicate statistical significance.
3. Results
3.1. % yield of Amomum tsao-ko
We selected methanol as solvent for extraction. The extract yields from the dried fruit of A. tsaoko were 11.33 ± 0.3%.
3.1.1. Preliminary screening (qualitative agar diffusion assay)
Colorless, opaque circle was observed (Fig. 1) around the extract (concentration 4 mg/ml) loaded disk. The negative control (LB broth loaded disk) did not show any inhibition when the positive control showed very clear, visible inhibition.
Figure 1.
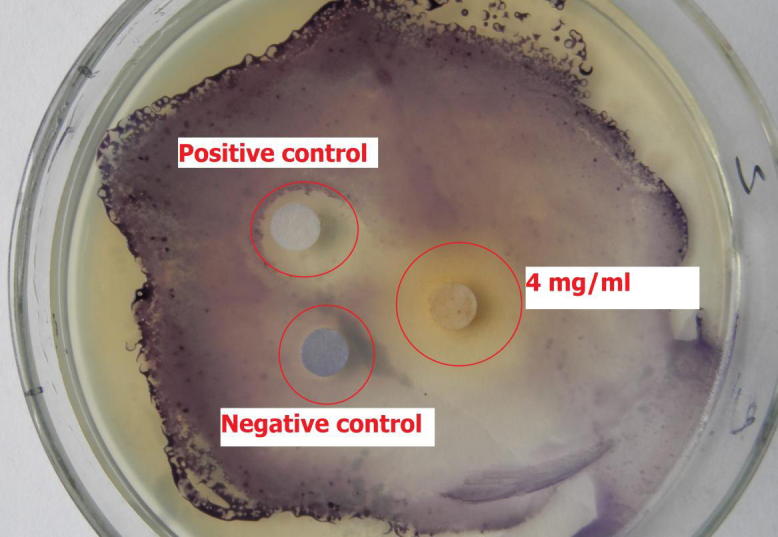
Anti-quorum sensing (anti-QS) activity by Amomum tsaoko extract against bioreporter strain CV12472, using agar disk diffusion method. Amomum tsaoko extract (4 mg/ml) and positive control (vanillin, 250 μg/ml) loaded disk are showing colorless, opaque circle. While, negative control (LB broth loaded disk) is showing no inhibition of violacein production around the disk.
3.1.2. Flask incubation assay for quantification of violacein production
Loss of purple pigmentation in C. violaceum 12472 on the presence of tested extract is an indication of QS inhibition. The inhibitory effect of the A. tsaoko extract on violacein pigment production was measured and quantified (Supplementary 1). A concentration dependent but not proportional inhibition was found (Fig. 2). A concentration of 4 mg/ml extract inhibited about 44.59% violacein production which has about 4 times higher inhibition than 2 mg/ml concentration’s inhibition (10.81% inhibition).
Figure 2.
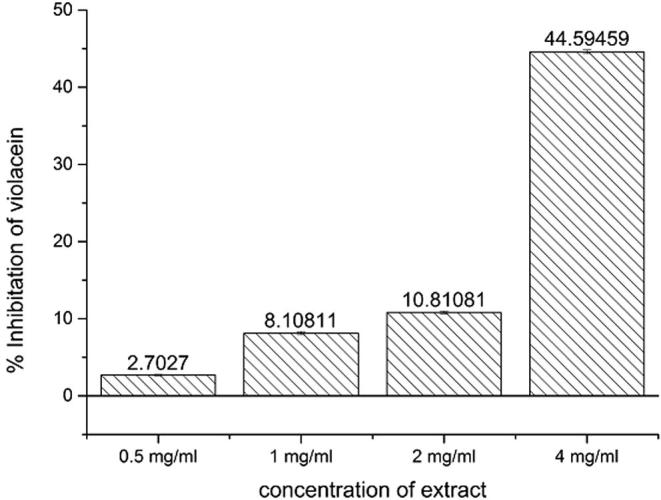
Effect of A. tsaoko extract on violacein production. Data are represented as percentage of violacein inhibition. Control (untreated) was set as 100% production. Mean values of triplicate independent experiments and SD are shown. Not significantly different at P ⩽ 0.05 of treatment.
3.2. MIC and anti-biofilm assay
The minimum concentration that causes a decline in growth of bacteria is considered to be the MIC value and in this experiment it was evaluated through careful visual inspection. Biofilm is the product of quorum sensing mechanism. In the present study, the anti-biofilm effect was determined through crystal violet assay. The results are summarized in Table 1. S. Typhimurium and P. aeruginosa presented higher MIC values than S. aureus. The obtained results for biofilm showed a concentration-dependent inhibition. The highest (51.96%) anti-biofilm activity was shown by 4 mg/ml A. tsaoko extract on S. Typhimurium.
Table 1.
Minimum Inhibitory Concentration (MIC) and % biofilm inhibition by the Amomum tsao-ko extract for Staphylococcus aureus, Salmonella Typhimurium, Pseudomonas aeruginosa.a
| Staphylococcus aureus | Salmonella Typhimurium | Pseudomonas aeruginosa | ||||
|---|---|---|---|---|---|---|
| MIC (mg/ml) | 1 |
2 |
2 |
|||
| Concentration of extract | Total biofilm | % inhibition | Total biofilm | % inhibition | Total biofilm | % inhibition |
| Control | 0.68 ± 0.06 | 0 | 1.02 ± 0.08 | 0 | 0.53 ± 0.02 | 0 |
| 0.5 mg/ml | 0.57 ± 0.02 | 16.18 | 0.97 ± 0.02 | 4.9 | 0.51 ± 0.01 | 3.77 |
| 1 mg/ml | 0.42 ± 0.02 | 38.24 | 0.89 ± 0.01 | 12.47 | 0.43 ± 0.01 | 18.87 |
| 2 mg/ml | 0.39 ± 0.03 | 42.64 | 0.65 ± 0.07 | 38.2 | 0.31 ± 0.02 | 41.51 |
| 4 mg/ml | 0.36 ± 0.02 | 47.06 | 0.49 ± 0.02 | 51.96 | 0.29 ± 0.003 | 45.28 |
Data are the mean ± SD of three independent experiments.
3.3. CLSM (Confocal Laser Scanning Microscopy) observation of biofilm
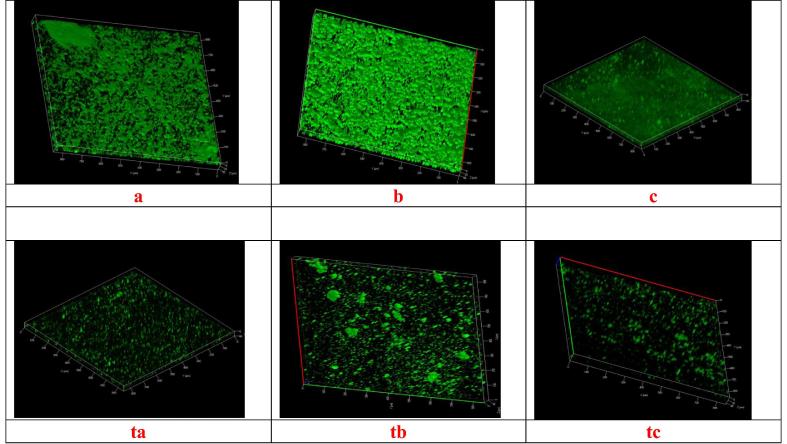
A. tsaoko extract treatment was applied on S. aureus, S. Typhimurium, P. aeruginosa biofilm. From control and treated sample observation (Fig. 3), it is clear that A. tsaoko extract has an effect on biofilm inhibition (break down the biofilm architecture, less microcolonies).
Figure 3.
Observation of biofilm on CLSM. (a–c) Untreated biofilm of Staphylococcus aureus, Pseudomonas aeruginosa, Salmonella Typhimurium (ta-tc) treated biofilm of Staphylococcus aureus, Pseudomonas aeruginosa, Salmonella Typhimurium respectively.
3.4. Swarming motility inhibition
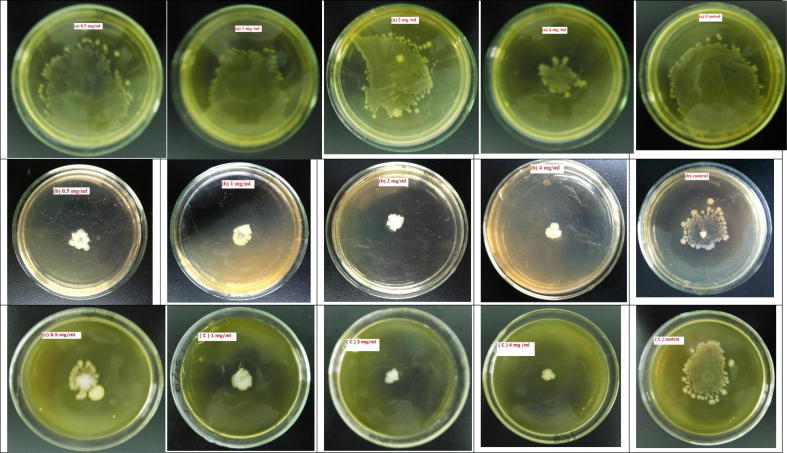
Swarming motility has been characterized as a remarkable feature for the formation of biofilm which is a flagella-dependent movement of bacterium. Fig. 4 indicats that A. tsaoko has significant influence on the swarming motility by dose dependent manners. Best anti-swarming activities were shown by 4 mg/ml extract on S. Typhimurium and S. aureus. But in the same extract’s concentration (4 mg/ml) P. aeruginosa showed less swarming inhibition activity.
Figure 4.
The migration distance of Staphylococcus aureus, Salmonella Typhimurium, Pseudomonas aeruginosa was observed while they were incubated for 30 h at different concentrations of A. tsaoko extract. Swarming motility of (a) Pseudomonas aeruginosa (b) Salmonella Typhimurium (c) Staphylococcus aureus in the presence of Amomum tsaoko extracts (left–right; 0.5, 1, 2, 4 mg/ml, control : without extract).
4. Discussion
The present study focused on the anti-QS and biofilm inhibitory effect of A. tsaoko extract. The % yield of extraction is very important on commercial prospect. Usually, plant extract contains biologically active compounds at low concentration. Minimal changes to the functional properties of the extract depend on extraction techniques. The percentage of extraction yields will increase or decrease with the ratio of solvent, temperature of extraction and extraction methods (green extractions, microwave assisted solvent extraction, ultrasound, etc.). Crude extracts of Alpinia conchigera a species from the Malaysian Ginger (Zingiberaceae) family showed 10.76% extractions yield when the ratio of powdered rhizomes and ethanol was 1:10 (w/v) (Ujang et al., 2013). This is consistent with our result. Our study showed that A. tsaoko extract, similar to Eleutherine americana Merr. (Iridaceae), Rhodomyrtus tomentosa (Aiton) Hassk. (Myrtaceae) extract (Limsuwan and Voravuthikunchai, 2008), inhibited the production of violacein both in agar diffusion assay and flask incubation assay but Boesenbergia pandurata (Roxb.) Schltr another family member of Zingiberaceae (chloroform extracted) did not show any inhibition activity. Kumar et al., (2014) observed that phenolic derivatives of ginger (member of Zingiberaceae family) can inhibit QS in pathogenic bacteria and zingerone showed 35% inhibition, [6]-gingerol showed 52.5% inhibition at concentration of 500 ppm. As an effect of A. tsaoko extract, S. Typhimurium and P. aeruginosa showed higher MIC values than S. aureus (Table 1). Yang et al. (2008) found the essential oil of A. tsaoko has the strongest bactericidal activity against S. aureus, with MIC and MBC of 0.20 g/l. This was due to that we have used the crude extract of A. tsaoko but Yang et al. (2008) used the essential oil.
The butanol fractions of both leaf and stem/root of Quercus cerris L. (Fagaceae) exhibited the biofilm inhibition of S. aureus about 63 ± 10% and 74 ± 4% respectively at a test dose of 200 mg/ml (Hobby et al., 2012). This concentration is higher than that in our study. An interesting mechanism which interferes with biofilm formation in S. aureus involves the heptapeptide RIP (receptor-interacting protein) kinases. This peptide inhibits biofilm formation of S. aureus in vivo, possibly by blocking the agr (accessory gene regulation)-dependent QS system (Landini et al., 2010). Segev-Zarko et al. (2015) suggested that decreased biofilm growth is due to the peptide’s ability to coat either the biomaterial surface or the bacterium itself. Rosa rugosa tea polyphenol damaged the biofilm architecture of Escherichia coli K-12 and P. aeruginosa PAO1 about 67.02% and 72.90% respectively (Zhang et al., 2014a). Our CLSM observation (Fig. 3) is also similar to them. Flagellar-mediated swimming motility is associated with biofilm formation by instigating the cell-to-surface attachment, and plays an important role in the virulence of pathogens (Wu et al., 2014). A. tsaoko extract reduced the swarming motility zone of S. Typhimurium, S. aureus and P. aeruginosa (Fig. 4). It might be due to the effect of phytochemicals of A. tsaoko on flagella-related processes, namely, flagella biosynthesis, rotation, rafting and chemotaxis, etc. Interestingly Tremblay and Deziel (2008) reported that swarming motility assays of P. aeruginosa are influenced by incubation temperature, %agar, pH, and drying time under laminar flow. In this study, we used molten soft top agar for all experiments about swarming motility.
Through a phytochemical investigation on A. tsaoko extract by Zhang et al. (2014b), eight main chemical components (sitosterol, daucosterol, meso-hannokinol, quercetin, epicatechin, quercetin-7-O-β-glucoside, quercetin-3-O-β-glucoside, and catechol) were isolated. GC/MS analysis showed seventy-three compounds mainly containing 1,8-cineole (45.24%), ρ-propylbenzaldehyde (6.04%), geraniol (5.11%), geranial (4.52%), α-terpineol (3.59%) and α-phellandrene (3.07%) from the essential oil of A. tsaoko fruit. Now it is well established that most of these compounds have antimicrobial (more or less) and even antifungal activity (Martin et al., 2006, Yang et al., 2008, Yang et al., 2010, Zhang et al., 2014b). Interestingly, studies associated with some natural products have shown that plant extracts with poor antimicrobial properties could also be effective against bacterial biofilms (Upadhyay, 2014). Plant extract (major or minor components) inhibits prokaryotic and eukaryotic microorganisms with different action mechanisms (Evren and Yurt Erkan, 2015). Without further molecular analysis, it is hard to explain which components are responsible for such inhibition mechanism.
In clinical and foodborne pathogenesis biofilm associated infection is known as a trigger to chronic diseases, food spoilage and leads to billions of dollars in healthcare, food industry cost. Even dairy spoilage and refrigerated food spoilage were also created by the bacterial biofilm related enzyme (Teh et al., 2014, Mizan et al., 2015). Developed studies are demonstrating that there is a biological rationale between QS and biofilm which work on a coordinate manner leading to spoilage (Bai and Vittal, 2014). On the other hand, biofilm formation, swarming motility, cellulose synthesis etc are also regulated by newly found second messengers c-di-GMP in Gram positive and Gram negative bacteria. Some constituent/constituents of A. tsaoko extract may accelerate the activity of proteins with EAL (Simm et al., 2004) or HD-GYP domains (Ryan et al., 2006) resulting in the degradation of c-di-GMP; some enzymes can also react with this plant extract and reduce the activity of c-di-GPM. If there is a similarity between the QS signals (AHL) and chemical structure of QSI, it will show anti-QS activity because they have the ability to degrade the signal receptor (LuxR/LuxR). So the tested A. tsaoko extract (major and minor components, tsaokoaryline – a cytotoxic diarylheptanoid [7-(4-hydroxyl-3 -methoxyphenyl)-1-(4 hydroxyphenyl)-hepta-4E,6E-dien-3-one]) (Moon et al., 2005) may affect the gene expression pathway and inhibit biofilm formation, swarming motility, violacein production etc.
5. Conclusion
Future study is necessary to unveil the detailed mechanism of A. tsaoko extract (isolating single compound from crude extract) on c-di-GPM level and mode of actions with proteolytic activity, bioluminescence inhibition etc to confirm the realistic action on vivo research considering symbiotic, synergistic, antagonistic growth inhibition. In this study, we demonstrated the anti QS and anti biofilm activity of A. tsaoko extract on Gram positive and Gram negative bacterium by well established methods. These anti-QS and biofilm inhibition evidence can be an alternative to combat food spoilage, on antibiotic therapy and many other fields where natural compounds are required for human health safety. The results of this study are a benchmark for the application of A. tsaoko extract as a de novo anti-QS and anti-biofilm agent. It can be expected more with its marvelous functions.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Acknowledgements
The authors gratefully acknowledge the financial support provided by Project 31201433 of the National Natural Science Foundation of PR China, the Project BK2012555 of Jiangsu Provincial Natural Science Foundation. And we are grateful to Prof. Dr Syed A. Morshed, Mount Sinai School of Medicine, NY 10468, USA for his critical comments and suggestions to improve the manuscript.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.sjbs.2015.09.034.
Appendix A. Supplementary data
Flask incubation assay for quantification of violacein production. Left–right (containing 4, 2, 1, 0.5, 0(control) mg/ml A. tsaoko extract).
References
- Annous B.A., Fratamico P.M., Smith J.L. Quorum sensing in biofilms: why bacteria behave the way they do. J. Food Sci. 2009;74(1):R24–R37. doi: 10.1111/j.1750-3841.2008.01022.x. [DOI] [PubMed] [Google Scholar]
- Bai A.J., Vittal R.R. Quorum sensing inhibitory and anti-biofilm activity of essential oils and their in vivo efficacy in food systems. Food Biotechnol. 2014;28:269–292. [Google Scholar]
- Delin W., Larsen K. Zingiberaceae. Flora China. 2000;24:322–377. [Google Scholar]
- Evren E., Yurt Erkan Y.E. In vitro effects on biofilm viability and antibacterial and anti adherent activities of silymarin. Folia Microbiol. (Praha) 2015;60(4):351–356. doi: 10.1007/s12223-015-0399-6. [DOI] [PubMed] [Google Scholar]
- Feng X., Jiang Z.T., Wang Y., Li R. Composition comparison of essential oils extracted by hydrodistillation and microwave-assisted hydrodistillation from Amomum tsao-ko in China. J. Essent. Oil Bear. Plants. 2010;13:286–291. [Google Scholar]
- Gao T., Foulston L., Chai Y., Wang Q., Losick R. Alternative modes of biofilm formation by plant-associated Bacillus cereus. Microbiologyopen. 2015;4(3):452–464. doi: 10.1002/mbo3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobby G.H., Quave C.L., Nelson K., Compadre C.M., Beenken K.E., Smeltzer M.S. Quercus cerris extracts limit Staphylococcus aureus biofilm formation. J. Ethnopharmacol. 2012;144(3):812–815. doi: 10.1016/j.jep.2012.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M.S., Zahin M., Hasan V.S., Husain F.M., Ahmad I. Inhibition of quorum sensing regulated bacterial functions by plant essential oils with special reference to clove oil. Lett. Appl. Microbiol. 2009;49(3):354–360. doi: 10.1111/j.1472-765X.2009.02666.x. [DOI] [PubMed] [Google Scholar]
- Kumar N.V., Murthy P.S., Manjunatha J.R., Bettadaiah B.K. Synthesis and quorum sensing inhibitory activity of key phenolic compounds of ginger and their derivatives. Food Chem. 2014;15(159):451–457. doi: 10.1016/j.foodchem.2014.03.039. [DOI] [PubMed] [Google Scholar]
- Kuchma N.J.D., Filkins L.M., Snavely E.A., Armitage J.P., O’Toolea G.A. Cyclic Di-GMP-mediated repression of swarming motility by Pseudomonas aeruginosa PA14 requires the motab stator. J. Bacteriol. 2015;197(3):420–430. doi: 10.1128/JB.02130-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landini P., Antoniani D., Burgess J.G., Nijland R. Molecular mechanisms of compounds affecting bacterial biofilm formation and dispersal. Appl. Microbiol. Biotechnol. 2010;86(3):813–823. doi: 10.1007/s00253-010-2468-8. [DOI] [PubMed] [Google Scholar]
- Leong-Skornickova, J., Tran, H.D., Newman, M., Lamxay, V., Bouamanivong, S., 2012. Amomum tsao-ko. The IUCN Red List of Threatened Species.
- Lim, T.K., 2013. Amomum tsao-ko. Handbook of Herbs and Spices, Second ed. pp. 813–817.
- Limsuwan S., Voravuthikunchai S.P. Boesenbergia pandurata (Roxb.) Schltr., Eleutherine americana Merr. and Rhodomyrtus tomentosa (Aiton) Hassk. as antibiofilm producing and antiquorum sensing in Streptococcus pyogenes. FEMS Immunol. Med. Microbiol. 2008;53:429–436. doi: 10.1111/j.1574-695X.2008.00445.x. [DOI] [PubMed] [Google Scholar]
- Martin T.S., Kikuzaki H., Hisamoto M., Nakatani N. Constituents of Amomum tsao-ko and their radical scavenging and antioxidant activities. J. Am. Oil Chem. Soc. 2006;77:667–673. [Google Scholar]
- Mizan M.F.R., Jahid I.K., Ha S.-D. Microbial biofilms in seafood: a food-hygiene challenge. Food Microbiol. 2015;49:41–55. doi: 10.1016/j.fm.2015.01.009. [DOI] [PubMed] [Google Scholar]
- Moon S.S., Cho S.C., Lee J.Y. Tsaokoarylone, a cyto-toxic diarylheptanoid from Amomum tsao-ko fruits. Bull. Korean Chem. Soc. 2005;26(3):447–450. [Google Scholar]
- Ryan R.P., Fouhy Y., Lucey J.F., Crossman L.C., Spiro S., He Y.W., Zhang L.H., Heeb S., Cámara M., Williams P., Dow J.M. Cell-cell signaling in Xanthomonas campestris involves an HD-GYP domain protein that functions in cyclic di-GMP turnover. Proc. Natl. Acad. Sci. U.S.A. 2006;103:6712–6717. doi: 10.1073/pnas.0600345103. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Segev-Zarko L., Saar-Dover R., Brumfeld V., Mangoni M.L., Shai Y. Mechanisms of biofilm inhibition and degradation by antimicrobial peptides. Biochem. J. 2015;468(2):259–270. doi: 10.1042/BJ20141251. [DOI] [PubMed] [Google Scholar]
- Simm R., Morr M., Kader A., Nimtz M., Römling U. GGDEF and EAL domains inversely regulate cyclic di-GMP levels and transition from sessility to motility. Mol. Microbiol. 2004;53:1123–1134. doi: 10.1111/j.1365-2958.2004.04206.x. [DOI] [PubMed] [Google Scholar]
- Skandamis P.N., Nychas G.J. Quorum sensing in the context of food microbiology. Appl. Environ. Microbiol. 2012;78:5473–5482. doi: 10.1128/AEM.00468-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano C., Echeverz M., Lasa I. Biofilm dispersion and quorum sensing. Curr. Opin. Microbiol. 2014;18:96–104. doi: 10.1016/j.mib.2014.02.008. [DOI] [PubMed] [Google Scholar]
- Srey S., Jahid I.K., Ha S.-D. Biofilm formation in food industries: a food safety concern. Food Control. 2013;31:572–585. [Google Scholar]
- Teh K.H., Flint S., Palmer J., Andrewes P., Bremer P., Lindsay D. Biofilm − an unrecognised source of spoilage enzymes in dairy products? Int. Dairy J. 2014;34:32–40. [Google Scholar]
- Tremblay J., Deziel E. Improving the reproducibility of Pseudomonas aeruginosa swarming motility assays. J. Basic Microbiol. 2008;48:509–515. doi: 10.1002/jobm.200800030. [DOI] [PubMed] [Google Scholar]
- Truchado P., Gimenez-Bastida J.A., Larrosa M., Castro-Ibanez I., Espin J.C., Tomas-Barberan F.A., Garcia-Conesa M.T., Allende A. Inhibition of quorum sensing (QS) in Yersinia enterocolitica by an orange extract rich in glycosylated flavanones. J. Agric. Food Chem. 2012;12(60):8885–8894. doi: 10.1021/jf301365a. [DOI] [PubMed] [Google Scholar]
- Ujang Z.B., Subramaniam T., Diah M.M., Wahid H.B., Abdullah B.B., Rashid A.A., Appleton D. Bioguided fractionation and purification of natural bioactives obtained from Alpinia conchigera water extract with melanin inhibition activity. J. Biomater. Nanobiotechnol. 2013;4(3):265–272. [Google Scholar]
- Upadhyay, A., 2014. Investigating the potential of plant-derived antimicrobials and probiotic bacteria for controlling Listeria monocytogenes (Doctoral Dissertations). Paper 326.
- Wu H., Moser C., Wang H.Z., Hoiby N., Song Z.J. Strategies for combating bacterial biofilm infections. Int. J. Oral Sci. 2014;7(1):1–7. doi: 10.1038/ijos.2014.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M., Guo P., Tsui S.W., Chen H., Zhao Z. An ethnobotanical survey of medicinal spices used in Chinese hotpot. Food Res. Int. 2012;48:226–232. [Google Scholar]
- Yang Y., Yan R.W., Cai X.Q., Zheng Z.L., Zou G.L. Chemical composition and antimicrobial activity of the essential oil of Amomum tsao-ko. J. Sci. Food Agric. 2008;88:2111–2116. [Google Scholar]
- Yang Y., Yue Y., Runwei Y., Guolin Z. Cytotoxic, apoptotic and antioxidant activity of the essential oil of Amomum tsao-ko. Bioresour. Technol. 2010;101:4205–4211. doi: 10.1016/j.biortech.2009.12.131. [DOI] [PubMed] [Google Scholar]
- Zhang J., Rui X., Wanga L., Guana Y., Sunc X., Dong M. Polyphenolic extract from Rosa rugosa tea inhibits bacterial quorum sensing and biofilm formation. Food Control. 2014;42:125–131. [Google Scholar]
- Zhang T.T., Lu C.L., Jiang J.G. Bioactivity evaluation of ingredients identified from the fruits of Amomum tsaoko Crevost et Lemaire, a Chinese spice. Food Funct. 2014;5:1747–1754. doi: 10.1039/c4fo00169a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flask incubation assay for quantification of violacein production. Left–right (containing 4, 2, 1, 0.5, 0(control) mg/ml A. tsaoko extract).