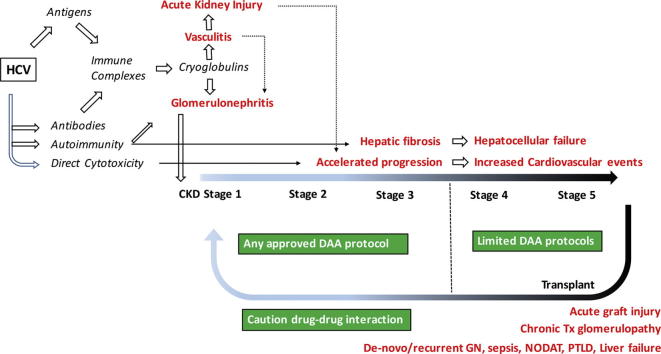
Graphical abstract
Keywords: Acute kidney injury, Chronic kidney disease, Glomerulonephritis, Dialysis, Renal transplantation, Direct-acting antivirals
Abstract
Hepatitis-C (HCV) infection can induce kidney injury, mostly due to formation of immune-complexes and cryoglobulins, and possibly to a direct cytopathic effect. It may cause acute kidney injury (AKI) as a part of systemic vasculitis, and augments the risk of AKI due to other etiologies. It is responsible for mesangiocapillary or membranous glomerulonephritis, and accelerates the progression of chronic kidney disease due to other causes. HCV infection increases cardiovascular and liver-related mortality in patients on regular dialysis. HCV-infected patients are at increased risk of acute post-transplant complications. Long-term graft survival is compromised by recurrent or de novo glomerulonephritis, or chronic transplant glomerulopathy. Patient survival is challenged by increased incidence of diabetes, sepsis, post-transplant lymphoproliferative disease, and liver failure. Effective and safe directly acting antiviral agents (DAAs) are currently available for treatment at different stages of kidney disease. However, the relative shortage of DAAs in countries where HCV is highly endemic imposes a need for treatment-prioritization, for which a scoring system is proposed in this review. It is concluded that the thoughtful use of DAAs, will result in a significant change in the epidemiology and clinical profiles of kidney disease, as well as improvement of dialysis and transplant outcomes, in endemic areas.
List of abbreviations
- AA-protein
Amyloidal-A protein
- AASLD
American Association For The Study Of Liver Disease
- ADA
American Diabetes Association
- AKD
Acute kidney disease
- AKI
Acute kidney injury
- C1q, C3a, C4, C5a, C5-9
Respective complement components
- CKD
Chronic Kidney disease
- CLD
Chronic liver disease
- CYP-450
Cytochrome P-450
- D +ve
HCV positive donor
- DAAs
Direct-acting antivirals
- DNA
Desoxyribonucleic acid
- EASL
European Association For The Study Of The Liver
- eGFR
Estimated glomerular filtration rate
- ELISA
Enzyme-linked immunosorbent assay
- ESKD
End-stage kidney disease
- FCH
Fibrosing cholestatic hepatitis
- FDA
Food and Drug Administration
- FSGS
Focal segmental glomerulosclerosis
- GN
Glomerulonephritis
- GT
Genotype
- HBV
Hepatitis B virus
- HCV
Hepatitis C virus
- HCV +ve
HCV infected
- HCV −ve
HCV non-infected
- HIV
Human Immunodeficiency virus
- IFN-α
Interferon-alpha
- IgA, IgG, IgM
Immunoglobulins A, G and M (respectively)
- κ-RF
Kappa-Rheumatoid factor
- MGN
Membranous glomerulonephritis
- MPGN
Membranoproliferative (Mesangiocapillary) glomerulonephritis
- mTOR
Mammalian target of rapamycin
- NHL
Non-Hodgkin lymphoma
- NODAT
New-onset diabetes after transplantation (Post-transplant diabetes mellitus)
- NSx
Non-structural viral protein-number (x)″
- PCRc
Polymerase chain reaction for Hepatitis C virus
- PTLD
Post-transplant lympho-proliferative disorder
- R +ve
HCV positive recipient
- RBV
Ribavirin
- RCT
Randomized controlled trial
- RDT
Regular dialysis treatment
- RF
Rheumatoid Factor
- RNA
Ribonucleic Acid
- RR
Relative Risk
- SVRx
Sustained Viral Response In (x) weeks
- USRDS
United States Renal Data System
Introduction
The kidney is an important component of the HCV clinical syndrome, besides the liver, the musculoskeletal, immune and hematopoietic systems and the skin. This notorious viral infection imposes itself as a cause of kidney disease, a major risk in dialysis wards, and a significant threat in renal transplantation. Fortunately, we are close to bringing it down to its knees, thanks to the discovery of directly acting drugs (DAAs), which will soon send this review, and many on the same topic, to the archives of medical history!
HCV as a cause of kidney disease
HCV can cause kidney disease in four ways: (a) glomerular immune complex deposition; (b) direct viral invasion of the renal parenchyma; (c) renal complications of its extrarenal (e.g. hepatic) manifestations; and (d) nephrotoxicity of drugs used for its treatment. These mechanisms often interact in the pathogenesis of several acute and chronic clinical renal syndromes.
Acute kidney disease (AKD)
HCV can cause acute kidney disease, which often progresses to acute kidney injury (AKI), in patients with acute or fulminant cryoglobulinemic vasculitis. Chronic HCV infection, per se, can be a significant risk factor for AKI in patients with dehydration, sepsis, or advanced liver injury. Finally, AKI is a potential risk in several HCV treatment protocols.
Cryoglobulinemic vasculitis
This is a systemic disease reported in <5% [1]–15% [2] of HCV-infected (HCV +ve) patients. It is rarely associated with “occult” HCV infection that can be only unveiled by nucleic acid testing in liver or bone marrow biopsy [3]. It is characterized by multi-organ involvement, mainly affecting the lungs and kidneys, skin, musculoskeletal system and peripheral nerves. The fundamental lesion is endothelial injury, small vessel necrosis, perivascular inflammation with lymphocytic and neutrophilic infiltration and luminal occlusion by cryoglobulins and fibrin thrombi.
In the kidneys, this leads to focal fibrinoid necrosis of the glomerular tufts, often with crescent formation (Fig. 1). The renal tubules are affected by ischemic and inflammatory lesions and contain hyaline and blood casts. The interstitium is edematous and infiltrated with inflammatory cells. The ureteric and bladder mucosa may display vasculitic purpuric lesions.
Fig. 1.
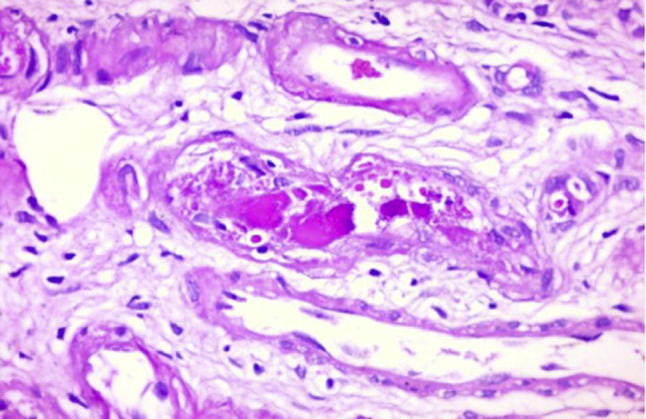
Cryoglobulinemic renal vasculitis. Renal arteriole showing endothelialitis and cryoglobulin deposits in a patient with AKI due to HCV-associated cryoglobulinemia. H&E stain. Curtesy Dr. Wesam Ismail.
The mechanism of vascular injury is typically attributed to C1q, the active complement component incorporated within the cryoglobulin complex (Fig. 2). This leads to endothelial injury by dual effects, namely, (a) activation of the complement cascade via the classical pathway; and (b) binding to endothelial complement receptors thereby localizing the injury in target capillary beds. Complement activation generates chemotactic factors, C3a and C5a, which recruit and activate pro-inflammatory leucocytes. It also leads to the formation of C5-9, the Membrane Attack Complex that may have an important role in endothelial damage.
Fig. 2.
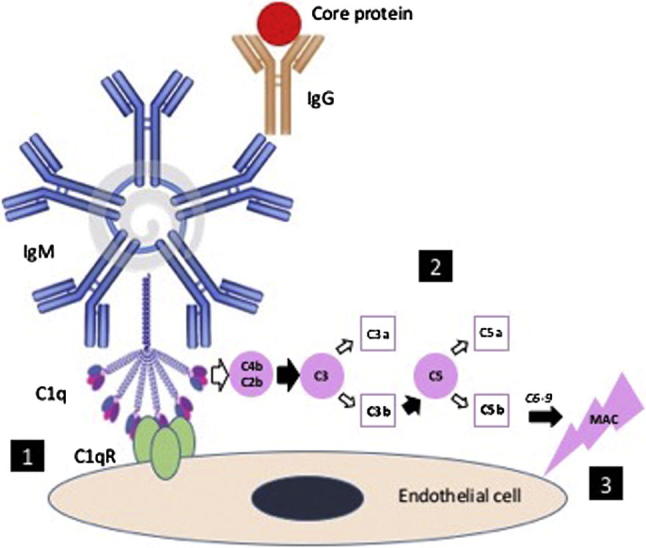
Cryoglobulin. Anti-viral core-protein IgG antibodies provoke an IgM-rheumatoid factor antibody response, which fixes and activates complement through the classical pathway [4]. C1q binding to its receptor on the endothelial cells localizes the immune complexes to target tissues such as skin, lung, nerve and kidney. C1q propagates the complement cascade leading to formation of C4b C2b complex, which is a C3 convertase. C3 is thus split into C3a and C3b, the latter being a C5 convertase that splits C5 into C5a and C5b. C3a and C5a are chemotactic; they recruit neutrophils and trigger an inflammatory response. C5b interacts with C6-C9 to form C5-9 (Membrane Attack Complex [MAC]) which, besides the inflammatory process, may be directly involved in endothelial injury.
In addition, a direct viral cytopathic effect has been proposed to participate in the pathogenesis of endothelial injury [5] on the basis of observations in human hepatic sinusoids and umbilical cord [6].
The clinical presentation ranges from isolated hematuria to acute kidney injury (AKI), sometimes associated with thrombotic microangiopathy (Fig. 3). If left untreated, the prognosis becomes extremely gloomy with regards to renal, as well as patient survival. On the other hand, successful treatment may lead to complete or partial recovery, unless the damage has already been extensive, leading to healing with focal or global sclerosis.
Fig. 3.
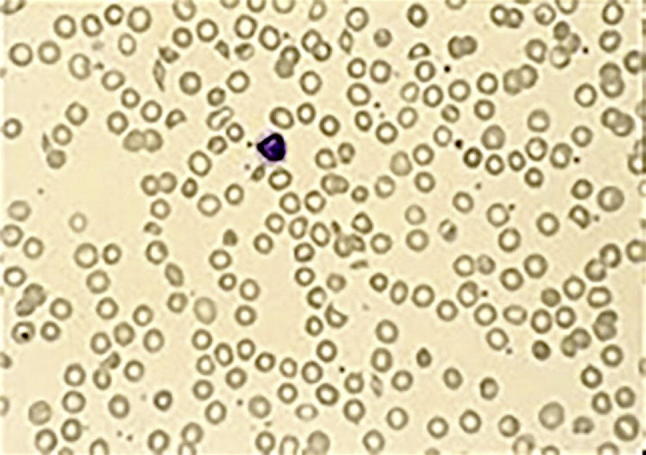
Blood smear in a patient with cryoglobulinemic vasculitis and thrombotic microangiopathy. Note the red cell fragmentation with microcytes (M) and schistocytes (S).
Non-cryoglobulinemic AKI
Compared to the general population, HCV-infected patients are at many-fold risk of developing AKI of diverse, apparently unrelated etiology. In an observational, community-based study of 648 subjects with chronic HCV infection, as many as 63 patients (9.7%) experienced 124 episodes of AKD events over a period of follow-up ranging from 3 months to 6 years [7]. According to Risk-Injury-Failure-Loss of function-Endstage (RIFLE) criteria, there were 58 (46.8%) at risk, 20 (16.1%) injury, 44 (35.5%) and failure, 2 (1.6%). AKI was most frequently attributed to hypovolemia associated with excessive vomiting or diarrhea. The second common cause was bacterial infection in the lungs, urinary or gastrointestinal tract. 7.3% of patients had advanced cirrhosis, and developed AKI following an episode of hematemesis, presumably due to ischemic acute tubular necrosis. 6.5% were associated with hepatic encephalopathy including the hepatorenal syndrome. Decompensated liver disease, history of intravenous drug abuse, diabetes mellitus and high baseline serum creatinine were independent predictors of developing AKI. End-Stage Kidney Disease (ESKD) eventually developed in 17.5% of patients who developed AKI, compared to 1% of those who did not. Risk factors for ESKD were pre- existing diabetes, hypertension or CKD [7].
Treatment-induced AKI
AKI has been infrequently reported with interferon treatment in patients with cryoglobulinemic vasculitis [8], either by inducing a flare, or the induction of acute allergic intestinal nephritis [9]. The latter usually responded promptly to corticosteroids.
Kidney injury has not been attributed to any of the DAAs. However, the real-life HCV-TARGET observational study [10] has reported acute deterioration of kidney function with Sofosbuvir-based treatment protocols in 5/17 patients (29%) with eGFR <30 mL/min/1.73 sqm, 6/56 patients (11%) with eGFR 30–45 mL/min/1.73 sqm, compared to 14/1559 patients (<1%) with eGFR > 60 mL/min/1.73 sqm. We are currently investigating a few sporadic cases of acute glomerular injury during, or immediately following Sofosbuvir treatment, though the link has not been established yet (unpublished data).
Chronic kidney disease
HCV-associated chronic kidney disease may be attributed to cryoglobulinemia, viral antigen-antibody complexes and possibly a direct viral cytopathic effect.
Cryoglobulinemic glomerulonephritis
HCV infection accounts for over 90% of cases with Type II mixed essential cryoglobulinemia. The latter builds up over years of active infection, at an increment of about 3% per year [11]. The average reported incidence is 40–50% [12], with considerable variation in different cohorts. This is partly attributed to the duration effect, as well as to geographic and genetic factors.
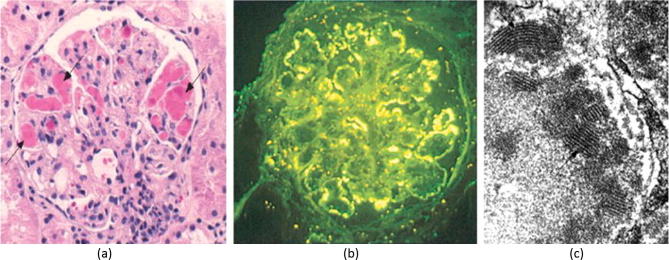
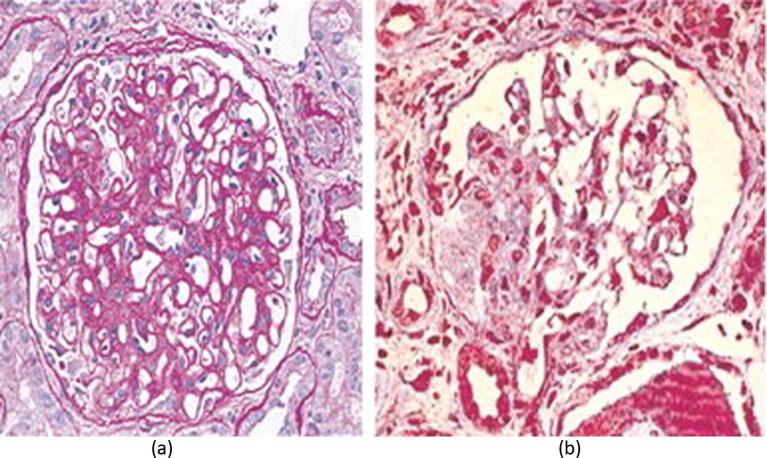
About 30% of affected patients ultimately develop mesangiocapillary glomerulonephritis. Cryoglobulins precipitate in the glomerular mesangium during their “macromolecular trafficking” owing to the affinity of the IgM kappa Rheumatoid Factor (κ-RF) to cellular fibronectin present in the mesangial matrix [13]. By virtue of their integral complement component, they attach to complement receptors and initiate a mesangial inflammation. Complement also activates the glomerular endothelium, which adheres to the circulating cryoglobulins that deposit in the capillaries providing the main histological diagnostic clue (Fig. 2, Fig. 4). Endothelial injury includes the peritubular capillaries leading to an interstitial inflammatory response, which eventually leads to fibrosis and largely accounts for impaired function.
Fig. 4.
Type I MCGN in HCV-associated cryoglobulinemia. (a) H&E stain showing capillary cryoglobulin thrombi (arrow), mesangial expansion with extrinsic inflammatory cellular infiltration; (b) Immunofluorescence showing IgM deposition along the glomerular capillary walls; c) Electron microphotograph showing dense subendothelial cryoglobulin deposits with typical finger-printing appearance. From Barsoum [5] with permission.
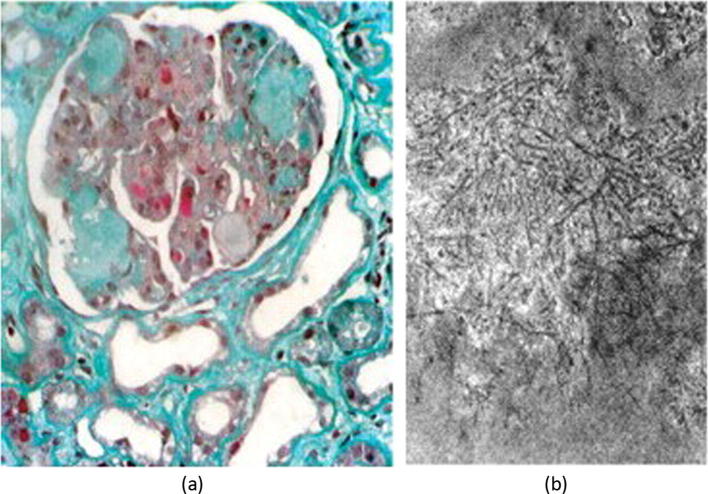
An interesting scenario has been described in patients with concomitant infection with Schistosoma mansoni and HCV, both being frequently endemic in the same geographical regions, e.g. Egypt. The glomerular lesions are characterized by a combination of mesangial expansion, amyloid deposits, and capillary cryoglobulin and fibrin deposits (Fig. 5). The amyloid component is the hallmark of this co-infection, being attributed to an imbalance in between the release and the re-uptake of AA protein by the macrophages. AA is a chemoattractant that is released as a part of the innate immune response to infection. Its biological half-life is checked by rapid re-uptake by the macrophages. The latter function is known to be downregulated by late-phase parasitic antigens as well as viral core proteins, hence the progressive accumulation of circulating AA protein, and subsequent deposition in inflamed tissues [14].
Fig. 5.
Cryoglobulinemic mesangiocapillary glomerulonephritis associated with schistosomiasis. (a) Masson trichrome stain showing typical HCV-cryoglobulinemic deposits, mesangial expansion and amyloid deposits (Class VI Schistosomal glomerulopathy [14]); (b) Electron microscopy showing randomly deposited amyloid fibrils. From Barsoum [14] with permission.
The clinical presentation of cryoglobulinemic glomerulonephritis is a combination of the Meltzer triad (comprising skin vasculitis, arthralgia and myalgia) along with manifestations of chronic kidney disease. The latter vary from asymptomatic hematuria and/or proteinuria at one end of the spectrum, to progressive renal failure on the other. Nephrotic syndrome occurs in one-fifth of cases and nephritic syndrome in one-sixth. Hypertension occurs in about 70% of patients. The diagnosis is established by associated Complement-4 (C4) consumption and strong serum rheumatoid factor (RF) reactivity, and confirmed by the detection of circulating cryoglobulins, and HCV by a polymerase chain reaction (PCRc). It is noteworthy that there is no correlation in between the extent of renal disease and severity of hepatic involvement. In a long-term follow-up study of 231 cases, the 10-year survival in patients with cryoglobulinemic glomerulonephritis was 62.1% [12].
Non-cryoglobulinemic Immune-complex-mediated glomerulonephritis
Mesangiocapillary glomerulonephritis may be associated with HCV infection despite the absence of circulating cryoglobulins, in which case HCV-IgG immune complexes are responsible for the glomerular pathology (Fig. 6). Viral non-structural protein-3 (NS3) was detected in the glomerular deposits which were linear or granular along the capillary walls and in the mesangium [15].
Fig. 6.
Non-cryoglobulinemic HCV-associated mesangiocapillary glomerulonephritis. (a) Light microscopic appearance of typical glomerular lobulation with mesangial expansion; (b) Electron microphotograph showing granular subendothelial immune complex deposits. From Barsoum [5] with permission.
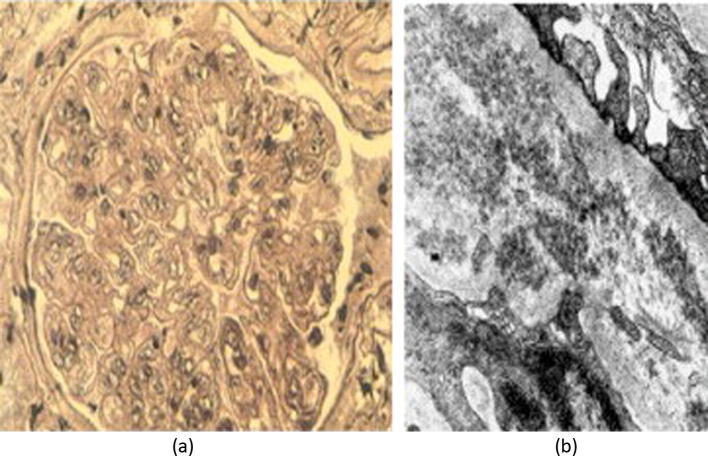
Membranous nephropathy (Fig.7a) has been associated with HCV infection on statistical [16] and immunological [17] grounds. HCV core RNA was detected in the glomerular deposits, suggestive of antigen plantation in the basement membrane. IgA nephropathy and Focal Segmental sclerosis (Fig.7b) have also been associated with HCV infection [16], yet the evidence of a direct causal relationship remains controversial.
Fig. 7.
Infrequent glomerular lesions associated with chronic HCV infection. (a) Membranous nephropathy; (b) Focal-segmental sclerosis (collapsing type in this case). From Barsoum [5] with permission.
Viral cytopathic effects
HCV antigen may be demonstrated in the glomeruli without detectable antibodies by enzyme-linked immune-sorbent assay (ELISA), or viral replication in peripheral blood by conventional PCRc. This may be partly attributed to relative insensitivity of the commonly available techniques [18]. However, it may also suggest an alternative pathogenic mechanism involving direct viral glomerular cytotoxicity [5] in analogy with the occurrence of polyarteritis nodosa in HBV infection without detectable immune complex deposition [19].
It is possible to speculate that the direct endothelial cytopathic effect [5], [6] may explain the accelerated atherosclerosis observed in HCV-infected patients [20]. The latter has been partly blamed for the relatively fast progression of CKD in HCV +ve patients, regardless of the etiology [21].
Dialysis-related HCV infection
HCV infection is widely spread in dialysis units where hygienic measures are suboptimal. In certain units, the prevalence of infection exceeds 80% [22]. Not only does this negatively impact on patient survival [23], and subsequent transplant outcomes [24], but it also generates a reservoir that disseminates infection to the community.
HCV does not cross the dialysis membranes, so infection is invariably caused by inter-patient transmission, usually by the staff. Accordingly, transmission can be prevented by adequate staff training on universal dialysis wards hygiene rather than isolating infected patients. However, local regulations in certain countries impose isolation, which has been rewarded by significant reduction in transmission, expressed as a decline in the sero-conversion of HCV negative patients [25].
Increased mortality of HCV-infected patients on regular dialysis (Fig. 8) is mainly attributed to cardiovascular events, which reflect the chronic endothelial damage induced by the virus [6], [20]. Sepsis and liver failure also contributes to the decline in patient survival.
Fig. 8.
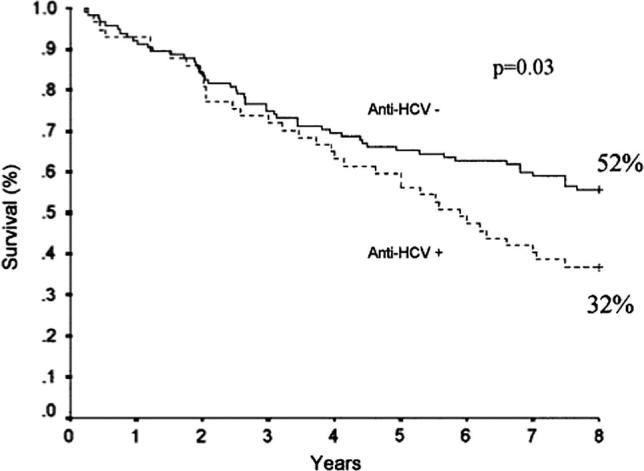
Impact of HCV infection on survival on regular hemodialysis. From Espinosa et al. [23] with permission.
HCV in renal transplantation
Donors
The prevalence of HCV infection among potential donors is expected to mirror that in the general population, with a global average of 3% [26]. This number is much higher in developing countries; it goes up to 15% in Egypt, which has the highest prevalence in the world. Owing to the risk of transmission with the graft, there is a general agreement that such donors are unsuitable for HCV non-infected (HCV −ve) recipients.
There is controversy regarding the risk of transmission to HCV +ve recipients. Since the immune response to the virus is strain-specific, at least in Chimpanzees [27], viral genotype discrepancy in between the donor and recipient constitutes a risk of superinfection, which has actually been observed in thalassemic children [28].
The same risk may be, at least theoretically, extrapolated even within the same viral genotype, owing to differences in subtypes [29] or quasispecies [30]. Owing to prolonged pressure of the immune response over many years of active infection, the virus typically undergoes limited mutations within the same genotype, yielding different strains that can co-exist within the same person. Transplanting an organ carrying such strains almost certainly exposes the recipient to a new infection to which he/she may not have an adequate immunological memory.
Available data seem to suggest a real risk in real-life challenges to these concerns [31]. In the Organ Procurement and Transplantation Network (OPTN) database (2001–2006), 6.25% of cadaver kidneys and 2.97% of living-donor kidneys were obtained from HCV-infected subjects. The outcomes were significantly inferior regarding both patient and graft survival [32]. In a large study including 2169 transplants, the relative risk of death in HCV +ve patients (R+) receiving HCV +ve donor (D+) kidneys was 2.1 when compared to R-/D- controls. Graft loss in the same study was also increased, yet it was related to the recipient’s HCV infection per se, regardless of the donor’s status [33].
The debate continues despite these limitations, since HCV +ve patient survival, in almost all relevant reports, was still significantly superior to that on dialysis [34] (Fig. 9). This seems to justify taking the risk of receiving a D+ graft rather than waiting for years on dialysis. Furthermore, the policy of using D+ kidneys avoids wasting a lot of organs that would have saved many lives [35].
Fig. 9.
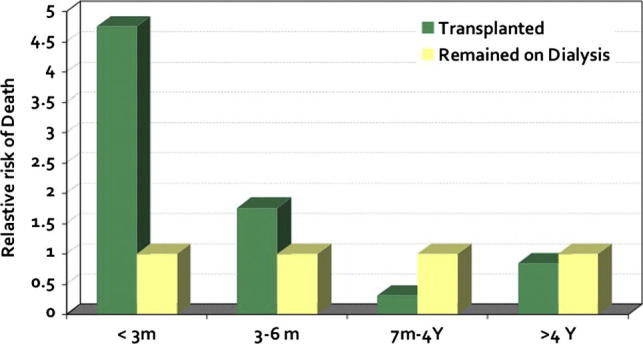
Five-year comparative relative risk of death of HCV +ve patients on hemodialysis versus kidney transplantation. Based on data from Pereira et al. [37].
Recipients
Potential transplant recipients have a much higher prevalence of HCV infection than that in the general population, ranging all the way from 3% to 80% in different countries. This reflects the frequency of exposure to contaminated dialysis, transfusions, interventions, etc. The main infection-related factors that determine eligibility to transplantation depend on the extent of liver damage, extrarenal morbidity, and the presence of cryoglobulinemia [36].
Active HCV infection carries a significant risk to patient as well as graft survival. The main adverse events are encountered either during the first 3 months or over 10 years post-transplant (Fig. 9) [37].
Early events
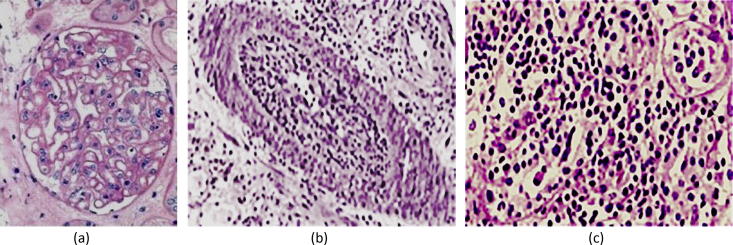
There is a significantly increased risk of acute transplant glomerulopathy (RR 6.8–8.8) and acute vascular rejection (RR 2.2) in HCV +ve recipients (Fig. 10)[38]. Either complication may be associated with thrombotic microangiopathy [39], usually in the presence of a thrombophilic environment. The latter includes congenital or acquired deficiency of coagulation inhibitors, anticardiolipin antibodies, or the use of high doses of calcineurine or mTOR inhibitors.
Fig. 10.
Early post-transplant complications in HCV +ve recipients. (a) Acute cellular rejection; (b) Acute vascular rejection; (c) acute transplant glomerulopathy. From Barsoum [5] with permission.
There is no agreement on the risk of acute cellular rejection, the RR of which was reported to vary from 0.9 to 1.3 [40].
Late complications
In a metanalysis of 6365 unique HCV +ve patients included in 8 clinical trials, the long-term patient- and graft survival were compromised (RR 1.79 and 1.56 respectively) [41]. All-cause mortality was increased, mainly due to liver or cardiovascular disease [24]. Graft loss was mainly attributed to de novo or recurrent glomerulonephritis, and to increased incidence of chronic transplant glomerulopathy [33].
In that analysis and other cohorts, the main complications reported in HCV +ve recipients were infection, post-transplant diabetes (NODAT) or lymphoproliferative disorders (PTLD), glomerulonephritis, cryoglobulinemic vasculitis and hepatocellular failure.
Infection
Infection is decidedly the second common cause of death among kidney transplant recipients at large [42]. There is controversy whether HCV +ve recipients are at a higher risk of infection-related mortality. While it was so in a small Indian cohort [43], this effect was not substantiated by USRDS data [42], nor in a large Spanish study including 4304 renal transplant recipients [44]. However, another Spanish study including 1302 kidney transplant recipients showed that while there was no difference in the overall incidence of infection in between HCV +ve and HCV −ve recipients, bacteremia and upper urinary infection were significantly more common in the former [45].
Diabetes
The reported incidence of new-onset diabetes after transplantation (NODAT) is variable owing to difference in the diagnosis, time from transplant, study population, and immunosuppressive agents used. Adopting the definitions of the American Diabetes Association (ADA) [46], and the International Consensus Guidelines on NODAT [47], Vincenti et al. reported an incidence of 20.5% within the first 6 months post-renal transplantation [48].
In a retrospective analysis of 555 kidney transplants, hepatitis C virus (HCV) infection was an independent risk factor for post-transplant diabetes. It had a negative impact on both patient and graft survival, irrespective of the time of onset and duration of diabetes [49]. A meta-analysis of 10 studies including 2502 patients showed that HCV +ve patients were nearly four times more likely to develop NODAT, compared with uninfected individuals [41]. Impaired Insulin sensitivity [50] and direct viral damage on pancreatic β cells were the proposed underlying mechanisms [51].
Post-transplant lymphoproliferative disease (PTLD)
The overall cumulative incidence of PTLD in kidney transplant is about 1.18% after 5 years [52], with mortality rates exceeding 50% [53]. A direct effect of HCV infection on the carcinogenesis of lymphoid cells has been well documented [54]. HCV +ve patients with native kidneys have a 1.26-fold increased risk of non-Hodgkin lymphoma (NHL), compared to the general population [55]. In occasional case reports, even Hodgkin lymphoma was controlled following antiviral treatment of HCV infection [56].
The potential risk PTLD in HCV +ve transplant recipients is controversial, since an intact immune system is believed to be a prerequisite for sustained B-lymphocyte proliferation [57]. Clinical data are inconsistent, showing both positive [58] and neutral [57] impacts of HCV infection. However, PTLD was reported to regress upon reduction of immunosuppression and successful control of HCV viremia [59].
Glomerulonephritis
Proteinuria
In a single-center retrospective study of 322 renal transplant recipients, positive pre-transplant serology for anti-HCV antibodies (9.6% of patients) was an independent risk (RR 5.36) for the development of significant (>1 g/24 h) proteinuria [60]. De-novo glomerular lesions were detected in 26/44 biopsies obtained from these patients. This difference was not observed in a long-term (87.73 ± 26.79 months) follow-up of 273 patients of whom 169 had anti-HCV antibodies [61]. A third study of 335 recipients showed that while mild proteinuria (<300 mg/day) occurred at a comparable frequency in HCV +ve and HCV −ve patients, moderate and severe proteinuria was significantly more common in HCV +ve patients (48.2% vs. 29.4% respectively) [62].
It is conceivable that post-transplant proteinuria is not a single entity. It can be a marker of rejection, drug toxicity, recurrence or de novo glomerulonephritis, etc. The impact of HCV in the pathogenesis of these conditions is quite variable, hence the differences in between different cohorts.
Recurrence of glomerulonephritis
HCV-associated MPGN and MGN are known to recur after transplantation [63]. In different reports, the incidence of recurrence ranged from 20% to 30% for MPGN and 3% to 7% for MGN [64]. Recurrence usually occured after the second year. Most cases were non-cryoglobulinemic. Otherwise, the clinical, laboratory and histopathological features were similar to those with native kidney disease.
De-novo glomerulonephritis
HCV seropositivity is a significant risk factor in the development of de novo glomerulonephritis. In one study, 63% of diagnostic renal allograft biopsies in HCV +ve recipients showed pathologic findings of de novo GN (45% MPGN and 18% MGN), compared to 5.8% and 7.7% respectively in HCV −ve patients [65]. Similar observations were reported in other cohorts [66], [67]. De-novo FSGS has also been reported in HCV +ve patients, yet with a similar frequency to that in HCV −ve patients [68]. It has been attributed to other factors as glomerular ischemia, drug effect, etc. However, the potential of an independent direct podocyte cytopathic effect of HCV cannot be excluded in HCV +ve patients [5].
-
•
De-novo membranoproliferative glomerulonephritis.
De-novo MPGN usually occurs during the first year post-transplant. Yet it has also been reported as late as 10 years [69]. Cryoglobulins are seldom detected, presumably as a result of immunosuppression. However, serum complement C4 is usually low [36], denoting consumption in the process of immune complex deposition. The clinical, laboratory and histopathological features are similar to those associated with the primary disease in native kidneys. The pathogenic role of HCV infection is confirmed by the detection of antibodies in the glomerular deposits [9] and the response to antiviral treatment [70].
-
•
De-novo membranous nephropathy.
The reported incidence of de novo membranous nephropathy in HCV positive recipients is almost double that in HCV −ve recipients. It is usually diagnosed 2 years after transplantation. The clinical features are similar to the primary disease. Compared to the latter, anti-phospholipase A2 antibodies were not detected in most reported cases [71], and the course was more rapidly progressive, leading to graft failure in an average of 2 years [72]. These features suggest a different pathogenic mechanism, which may be related to a direct cytopathic effect of HCV on the podocytes [5].
Chronic liver disease (CLD)
HCV infection is the leading cause of CLD after kidney transplantation and is associated with increased long-term mortality [73]. In a follow-up of 42 HCV +ve recipients for a mean of 7.6 years, after transplantation, 45.2% displayed aggressive hepatitis progression. Patients who acquired HCV infection peri- or post-transplantation had an increased risk of hepatitis progression compared with those infected before transplantation [74].
The deleterious effect on hepatic pathology is generally attributed to immunosuppression. HCV viremia is consistently increased many folds after transplantation, even in those who had achieved a sustained viral response under interferon/ribavirin treatment [75]. The outcome of treatment with DAAs in this respect awaits further experience.
Ironically, the increased viral load under immunosuppression does not seem to correlate with short-term hepatic injury. In a cohort of 36 renal transplant recipients who have been infected with HCV before transplantation, 13 had progressing liver fibrosis while 23 did not, as assessed by 2 liver biopsies obtained 45 and 81 months after transplantation. There were no significant differences in the increases in serum HCV RNA or genotype distributions in “fibrosers” and “nonfibrosers” [76]. So, it seems that the altered response to infection, rather than an increase in its load, is what explains the deleterious effect of immunosuppression.
There is no consistent advantage of a particular immunosuppressive agent over another with regard to progression of hepatic fibrosis. In a large study involving 3708 HCV +ve kidney transplant recipients, neither antibody induction nor the use of corticosteroids had an effect on patient survival [77]. The use of mycophenolate mofetil was associated with both better [22] and worse [78] patient survival.
There is a theoretical advantage of cyclosporine, since it binds to cyclophylline, which is involved in viral replication [79]. However, this was not confirmed in clinical studies, that have shown no significant difference in viral replication or progression of liver fibrosis with the use of cyclosporine compared to tacrolimus [80].
Fibrosing cholestatic hepatitis (FCH)
This is a rare, severe form of liver disease characterized by cholestasis, progressive hepatic failure and death if liver transplantation is not performed. It was reported in four (1.5%) of 259 HCV-infected renal transplant recipients by the end of the first year. Liver biopsy revealed diffuse fibrosis, leukocyte infiltrates, and different degrees of cholestasis. Two patients developed subfulminant liver failure and died 2 and 3 months after biopsy, one was saved by a liver transplant, and the fourth was treated with interferon, rejected his graft and returned to dialysis [81].
Management
With the aforementioned renal impact of HCV infection, there is a strong rationale of getting rid of the virus at all stages of CKD [35]. Unfortunately, there are no prospective randomized trials that document the ultimate effect of treatment on renal or patient survival. The available data are obtained from small cohorts, treated with the old interferon/ribavirin protocols. Metanalysis of these studies has displayed conflicting results, which may be attributed to heterogeneity of patient demographics, stage and nature of kidney disease, extent of liver injury and associated extrarenal manifestations, e.g. cryoglobulinemia.
In a metanalysis of 11 studies comprising 107 patients treated with interferon with or without ribavirin, proteinuria regressed to a variable extent in those who achieved end-of-treatment viral response. A few patients relapsed when the viral clearance was un-sustained. There was no significant change in serum creatinine in all studies except 2, where the GFR was increased. No post-treatment biopsy was reported [20].
In a metanalysis of 24 prospective studies, including 529 HCV +ve patients on hemodialysis who were treated with interferon-alpha (IFN-α) it was shown that monotherapy resulted in a sustained viral response at 48 weeks (SVR48) in only 39% of cases [82]. Better outcomes (SVR48 of 50–60%) were achieved by a combination of Peg-interferon and reduced doses of ribavirin [83]. Survival was significantly improved in treatment responders, with a hazard ratio for death of 0.47 compared to untreated patients according to the DOPPs data including 4589 HCV-infected patients [84]. Despite this remarkable advantage, only 1% of patients on regular dialysis, and 3.7% of those on the transplant waiting list actually received treatment during the Interferon era [84]. This trend will undoubtedly change with the introduction of DAAs, which currently achieve a SVR12 of 100% with Paritaprevir/Ritonavir + Ombitasvir [85] or 84.6% with RBV-free Simeprevir + 1/2 dose sofosbuvir [86].
Considering the obvious independent risk of HCV infection in kidney transplant recipients, it is logical to treat all patients prior to transplantation. However, there is no randomized prospective trial to prove that the risk of HCV infection is totally eliminated by pre- transplant viral clearance. In other words, there is no evidence that previous chronic HCV infection has no long-term legacy that would still have a negative impact on patient and graft survival.
In addition to their remarkable efficacy and safety profiles, DAAs offer 2 major advantages in patients with CKD, namely a favorable pharmacokinetic profile and the lack of any immune-stimulatory effect.
DAAs are mainly metabolized in the liver. With a few exceptions, they are not retained in renal failure to any clinically significant extent. By way of contrast, 90% of an administered dose of interferon [87] and 61% of ribavirin [88] are excreted in urine. Accordingly, their adverse reactions are augmented in patients with low GFR, including the ribavirin-induced hemolytic anemia and the long list of interferon side effects [89], mostly the hematopoietic.
The immune stimulatory effect of interferon therapy is a serious threat of aggravating cryoglobulinemic syndromes [90], inducing AKI due to acute interstitial nephritis [9], and rejecting kidney transplants [91]. None of this occurs with DAAs, which extends the spectrum of their use to previously forbidden horizons.
Treatment prioritization
Despite the anticipated benefit in patients with kidney disease, the overwhelming demand imposes the necessity of prioritizing those at highest risk of death or serious morbidity. A scoring system was put together for stratifying patients accordingly, which is currently adopted by the Egyptian Ministry of Health (Table 1) [92].
Table 1.
Proposed CKD prioritization scheme for HCV treatment. From El-Fishawi et al. [92] with permission. Each patient shall be scored by adding up points in favor of treatment and subtracting points that would lower his/her priority either due to lower opportunity to benefit from treatment or lack of urgency.
| Positive points: | |
| Post-renal transplant | 5 points |
| Regular Dialysis | 5 points |
| Biopsy confirmed MCGN with hypocomplementemia | 4 points |
| Biopsy-confirmed MCGN without hypocomplementemia | 3 points |
| Nephrotic syndrome | 2 points |
| Previous treatment failure | 2 points |
| HBV/HIV/ CMV co-infection | 2 points |
| Cryoglobulinemic vasculitis | 2 points/AASLD stage |
| Stage of kidney disease (MDRD-4) | 1 point/stage |
| Stage of liver disease (Fibroscan) | 1 point/stage |
| Negative points: | |
| Age > 50 | −1 point/5 years |
| Decompensated cirrhosis | −5 points |
| Concurrent drug-drug interaction with selected Protocol | −5 points |
| Concomitant heart disease | −1 point/NYHA score |
| Concomitant pulmonary disease | −1 point/−10% FVC1 |
| Concomitant CNS disease | −1 point/10% disability |
The threshold for accepting patients for treatment shall vary according to availability.
Choice of treatment protocol
The choice of a treatment protocol out of the plethora of available DAAs (Table 2) depends on many factors. Before embarking on a particular protocol, confounding factors must be taken into consideration, including the extent of liver damage, viral genotype, previous treatment, co-morbid conditions, and concomitant regular drug administration [92].
Table 2.
Direct Antiviral Agents currently approved for clinical use.
| ID | VNSP target | Daily dose | Combinations |
||||
|---|---|---|---|---|---|---|---|
| Sofosbuvir 400 mg | Ribavirina | Peg-Interferonb | |||||
| A2 | EAS (GT-4) | ||||||
| A4 | Sofosbuvir | NS5B | 400 mg | NEUTRINO (GT-1, GT-4), (FDA approved) | |||
| B1 | Ledisprevir | NS5A | 90 mg | ION (GT-1)-(FDA approved) NAID SYNERGY (GT-4) | |||
| B2 | ION (GT-1) (FDA approved) – SOLAR-2 (GT-4) | ||||||
| C1 | OPTIMIST (GT-1) (FDA approved) | ||||||
| C2 | Simeprevir | NS3 | 150 mg | COSMOS (GT-1) | |||
| C4 | QUEST studies [FDA approved (GT-1)] | ||||||
| D1 | Study AI444040 (GT1,2,3,4) FDA-approved GT-1,GT-3 | ||||||
| D2 | Daclatasvir | NS5A | 60 mg | EMCUPc (GT1,2,3,4)-ALLY-1 (GT-1)d | |||
| D4 | COMMAND-4 | ||||||
| Paritaprevir | NS3 | 150 mg | |||||
| E1e | Ritonavir | CY450 | 100 mg | PEARL-1 (GT4) , RUBY-1 (GT1) | |||
| Ombitasvir | NS5A | 25 mg | (FDA approved) | ||||
| Paritaprevir | NS3 | 150 mg | |||||
| E2e | Ritonavir | CY450 | 100 mg | PEARL-1 (GT4), RUBY-1 (GT1) | |||
| Ombitasvir | NS5A | 25 mg | (FDA approved) | ||||
| F1 | Grazoprevir | NS3/4A | 100 mg | C-EDGE, C-SURFER (GT-1,4) | |||
| Elbasvir | NS5A | 50 mg | (FDA approved) | ||||
| F2 | Grazoprevir | NS3/4A | 100 mg | C-EDGE, C-SURFER (GT-1,4) | |||
| Elbasvir | NS5A | 50 mg | (FDA approved) | ||||
Weight-based dosage: 1000 mg [<75 kg] to 1200 mg [>75 kg]).
Alpha 2A (180 mcg) or Alpha 2B (1.5 mcg/kg).
European Multicenter Compassionate Use Program.
Fixed Ribavirin dose of 600 mg.
Dasabuvir (NS5B) added for GT-1. VNSP: Viral Nonstructural Protein; EAS: Egyptian Ancestry Study.
Liver disease
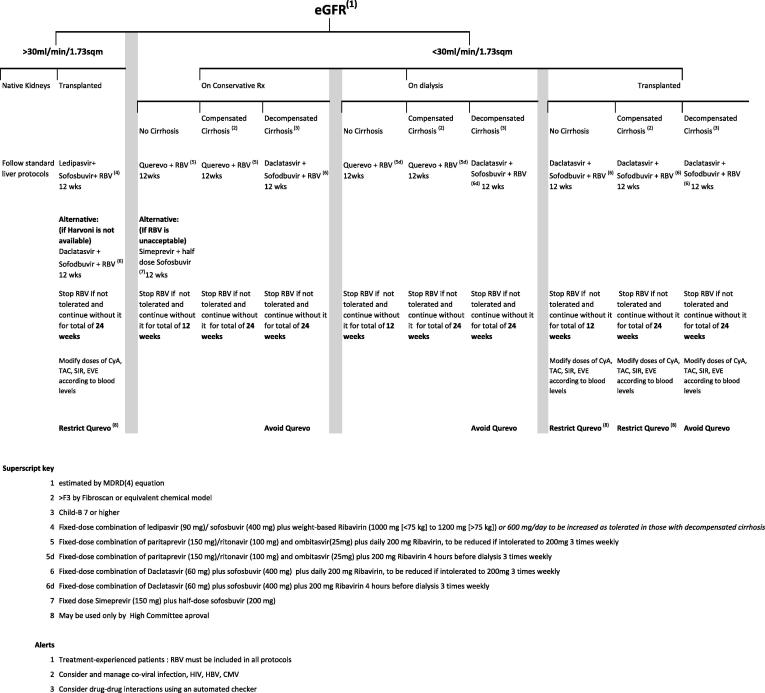
Evaluation of the extent of liver disease is essential for safe and effective DAA prescription to a patient with kidney disease, regardless of its stage (Fig. 11). Chronic hepatitis without cirrhosis permits the use of an interferon-free and ribavirin-free protocol for 12 weeks, expecting over 90% sustained viral response at 12 weeks (SVR12). The latter is accepted by the FDA as a 99% predictor of complete cure [93]. The presence of cirrhosis (Fibrosis score >3 by transient elastography – “Fibroscan”) requires the inclusion of ribavirin in most protocols. If ribavirin is contraindicated or not tolerated, DAA combination treatment must be extended for 24 weeks. Hepatocellular decompensation constitutes a significant challenge for the choice of safe antiviral treatment. Of the currently available DAAs, Daclatasvir-based therapy seems to be particularly safe and effective [94], while the Viekera family is contraindicated owing to its association with death or severe morbidity [95].
Fig. 11.
Proposed algorithm for DAA therapy in patients at different stages of CKD according to the extent of liver injury. From El-Fishawi et al. [92].
Genotype
The viral genotype is an important determinant of response to treatment. The choice of treatment protocol must be based on genotype-specific randomized controlled trials (RCTs) (Table 2). Since HCV genotypes are globally dispersed on geographical basis, it is presumed that patients in the same community share the same genotype, which justifies the adoption of country-specific guidelines. Whenever there is paucity of RCTs on a particular genotype, it makes sense to extrapolate data on pangenotypic drugs from other genotype studies [92].
Previous treatment
Treatment-experienced patients may need re-treatment because of the lack of response, treatment breakthrough, un-sustained viral response, or recurrence. Resistance to DAAs may be genotype-related, or due to nonstructural protein mutation. The latter is minimized by the use of drug combinations.
Since there is no ideal combination for drug-resistant HCV infection, it may be a better choice to wait for the emergence of new drugs or protocols in patients who are in dear need for urgent treatment [96].
In general terms, patients who have been initially treated by interferon and ribavirin are eligible for any of the selected DAA combinations. Those who fail with a sofosbuvir-based treatment may benefit from the Viekera family, and vice versa [92].
Co-morbidity
It is important to detect viral co-infection, particularly with HIV and HBV since both are associated with accelerated hepatic fibrosis. The treatment of HCV/HIV co-infection has been addressed in the ALLY-2 trial that showed 97% SVR12 following treatment with a fixed dose Sofosbuvir (400 mg/day) and a variable dose of Daclatasvir depending on the concomitant administration of anti-retroviral drugs. The standard dose is 60 mg/day, to be increased to 90 mg/day with efavirenz and nevirapine or to 30 mg/day with ritonavir-boosted protease inhibitors (e.g. the Viekera family) [97]. Other options are discussed in the recent AASLD guidelines [96]. It is noteworthy that Ribavirin is contraindicated in patients receiving the antiretroviral nucleoside analogue, didanosine, owing to the risk of mitochondrial toxicity, which leads to hepatomegaly and steatosis, pancreatitis, and lactic acidosis [98].
HBV-HCV co-infection constitutes a significant clinical challenge. Liver injury, including the risk of hepatocellular carcinoma, is augmented, even in serologically silent HBV infection (positive antibody, negative DNA). The expression of Interferon receptors is downregulated, hence the poor response to such treatment. While active HBV infection suppresses HCV replication, the extent of liver damage is even more aggressive [99]. Little is published on the renal pathology associated with this co-infection, yet patients on regular dialysis often have it, and are therefore at greatest risk of accelerated mortality. The risk depends on the dominant virus, being greater when HBV dominates. Treatment should receive top priority, combining oral drugs rather than interferon-based protocols, addressing both viruses simultaneously.
It is important to note that HCV +ve patients may be receiving treatment for other medical conditions. This has to be taken into consideration while prescribing DAAs in order to avoid drug-drug interaction than can be fatal.
Drug-drug interaction
Most of the DAAs are metabolized by the Cytochrome-P450 (CYP-450) in the liver and intestinal wall. Since this system is also involved in the metabolism of many other drugs including anticoagulants, statins, amiodarone, beta blockers, calcium channel blockers, several immunosuppressive agents and antibiotics, and many others [100], modification of CYP-450 activity by enzyme induction or inhibition can lead to critical changes in the relevant drug levels. In order to avoid such potentially serious complications, it is recommended to check for drug-drug interactions using an automated tool.
Underlying cause of CKD
It is renal function, not the renal pathology or the HCV causal role in its pathogenesis, that counts in the selection of treatment protocol. Antiviral treatment in HCV-associated diabetic nephropathy, for example, is the same as in HCV-related mesangiocapillary glomerulonephritis – unless the latter is cryoglobulinemic.
Since high-grade cryoglobulinemic vasculitis can lead to irreversible organ damage, it is recommended to control florid vasculitic manifestations without delay, even prior to institution of anti-viral treatment. Corticosteroids and other immunosuppressive agents [2] should be followed by a DAA, selected according to the criteria described in this review.
Kidney function
The kidneys are responsible for the elimination of only 1–13% of an administered dose of different DAAs, which leads to negligible retention with mild or moderate renal insufficiency. It has been determined that no dose adjustment is required with eGFR > 30 mL/min/1.73 sqm [101].
There is paucity of clinical trials on patients with eGFR < 30 mL/min/1.73 sqm (Stages IV-V CKD). Given the known risk of hemolysis with ribavirin and that of further deterioration of kidney function with full-dose sofosbuvir, these two agents must be avoided in their usual doses. The manufacturer of Ledisprevir/Sofosbuvir combination protocol (Harvoni) does not recommend its use with impaired kidney function owing to the lack of data [102].
On the other hand, the RUBY-1 trial, which is dedicated to patients with severe renal insufficiency, has shown 100% SVR-12 with the Viekera-pack (comprising paritaprevir/ritonavir + ombitasvir + dasabuvir), with or without ribavirin [85]. Although the study was conducted on patients infected with GT-1, it proves the concept of safety and efficacy with eGFR < 30 ml/min/1.73sqm, which extrapolates to other genotypes [5].
Another study has shown a SVR-12 in 13/15 patients with severe renal insufficiency with the use of simeprevir plus half-dose (200 mg) sofosbuvir for 12 weeks [86]. This interesting approach has not yet been verified in a larger sample.
Daclatasvir is a promising drug for use in patients with impaired renal function. While it is FDA-approved only for genotypes 3 and 1, it was shown to be effective in other genotypes including GT-4, when combined with Sofosbuvir and Ribavirin (e.g. Multicenter Compassionate Use Program [94]), or peg-interferon (COMMAND-4 study [103]. There are no clinical data on the use of Daclatasvir in patients with impaired renal function. However, a well-controlled study (AI444-063) of the pharmacokinetics of Daclatasvir has shown a favorable profile, even in patients with severe renal failure. The blood levels were slightly increased, yet within the desirable therapeutic range [104].
The recently FDA-approved Grazoprevir + Elbasvir combination (Zepatier) has shown remarkable efficacy as well as safety in Stages IV and V CKD, including treatment-naive as well as treatment-experienced GT-1 infected patients. In the seminal C-SURFER study, the overall SVR12 rate in such patients was 99% [105].
Dialysis
In addition to the constraints of using DAAs in CKD Stages IV/V, drug analyzability is an additional factor to consider upon selecting suitable treatment for patients on regular dialysis. Dialyzability depends on the drug’s molecular size and configuration, its protein binding, electrostatic charge and other less significant factors. It is possible to predict drug dialyzability by physical and pharmacokinetic studies, yet clinical trials remain crucial for a final conclusion.
An example of the complexity of this issue is sofosbuvir [106]. It has a relatively small molecular size that permits rapid diffusion through standard dialysis membranes. However, it is 61% protein bound, which checks its efflux. It is neutral molecule, which is activated by phosphorylation that renders it negatively charged, which repels it from the dialysis membrane. According to these opposing factors, it is imperative to design dedicated RCTs for all DAAs that can be used for patients on RDT.
Until such data is available, the only evidence-based recommendations would be the Viekera family [85] (without Dasabuvir in GT-4 according to the PEARL-1 study [107]), the Grazoprevir plus elbasvir combination in GT-1 [105] and, less confidently, the Simeprevir + 1/2 dose Sofosbuvir [86], which have yielded SVR-12 of 100%, 99% and 84.6% respectively, following 12-week treatment.
Post-transplant
Two factors must be considered while selecting antiviral treatment after kidney transplantation: graft function and drug pharmacokinetics. All rules regarding graft function in native kidneys apply to the contemporary post-transplant eGFR.
Drug pharmacokinetics is a compelling factor when cyclosporine, tacrolimus, sirolimus or everolimus is used for immunosuppression. All these agents are metabolized by CYP-450, for which most DAAs compete. This generally requires full dose treatment with DAAs and modification of immunosuppressive drug doses according to blood levels. Clinical experience has shown considerable variation in the required dose adjustments. For example, simeprevir/cyclosporine interaction is so clinically relevant that it is recommended to avoid this combination all together (EASL-B1) [101]. Simeprevir may be used with Tacrolimus or Sirolimus, yet with frequent monitoring of their blood levels. No dose adjustment is required for tacrolimus or cyclosporine use with sofosbuvir plus ribavirin, ledipasvir or daclatasvir (EASL-A2) [101].
The Viekera family is the riskiest in this context, owing to its essential component ritonavir that is meant to inhibit CYP-450 in order to sustain a therapeutic blood level of another component, paritaprevir. Using this combination requires avoidance of mTOR inhibitors all together, dose reduction of Tacrolimus to 0.5 mg weekly and Cyclosporine to one-fifth its dose (EASL-A2) [101]. Avoiding Viekera does not pause a significant challenge if the eGFR is above 30 ml/min/1.73 sqm, owing to the availability of other alternatives. Below this level, the choice becomes limited to either Simeprevir + 1/2 dose sofosbuvir on the basis of a small study, or Daclatasvir/Sofosbuvir on the basis of favorable pharmacokinetics [104].
Conclusions
HCV is far from being an innocent by-stander in patients with kidney disease. As explained in this review, it constitutes a major risk to patients’ lives at all stages of their illness. Since the damage is irreversible without treatment, every patient must be seen as a candidate for treatment. For logistic reasons, though, the lack of resources may impose treatment prioritization to those with highest expectation from treatment. Fortunately, with the recent discovery of safe and highly effective directly acting antiviral drugs, there are multiple therapeutic options that can suit different patients, taking many confounding factors into consideration. These include the CKD stage, extent of liver disease, viral strain, co-infections, previous treatment experience, current comorbidity and concomitant medical treatment. With adequate choice of a suitable protocol, cure rates over 90% are expected in most patients, with highly positive impact on survival and quality of life.
Conflict of Interest
The authors have declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Biographies

Rashad Barsoum Emeritus Professor and former chairman of Internal Medicine and chief of Nephrology at Cairo University. He authored 44 chapters on kidney diseases in 25 international textbooks. He published ≈200 papers with over 2500 citations. He served on the editorial boards of 18 and reviewer for 35 international journals. He holds the Egyptian First Class Order of Arts and Sciences and was the recipient of the Egyptian Nile Award and State Appreciation Prize, the ISN Roscoe Robinson Award, the International Award of the USA National Kidney Foundation Tarek Suhaimat Award of the ASNRT, Pioneer Award of the ISN, and many others.

Emad Adel William Graduated from Faculty of Medicine, Ain shams University, Egypt, in 1997, obtained his Master’s degree in Internal Medicine in 2004, and the Doctorate degree from the same University in 2011. He currently holds an academic position as a research lecturer at the National Research Center.
He is also a Clinical Consultant and Head of the Clinical Research Unit at the Cairo Kidney Center, Egypt. His main clinical expertise is in Clinical Nephrology, Dialysis and Transplantation, and his main research interest is renal transplantation.

Soha S. Khalil Graduated from Faculty of Pharmacy, Cairo University in 1993, and completed her Good Clinical Practice qualification in 2006. She obtained her Master of Business Administration from Edinburgh Business School, Heriot-Watt University in 2015.
She served as a Clinical Pharmacist at 2 leading hospitals in Cairo until 2001. She switched to an academic-support career in 2003, when she joined the Cairo Kidney Center as a scientific and research coordinator. In addition, she served in the administration of the Fellowship and Sister Center programs of the International Society of Nephrology and the Membership Examination of the Royal Colleges of Physicians in Egypt.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Ferri C., Mascia M.T. Cryoglobulinemic vasculitis. Curr Opin Rheumatol. 2006;18(1):54–63. doi: 10.1097/01.bor.0000198002.42826.c2. [DOI] [PubMed] [Google Scholar]
- 2.Dammacco F., Domenico Sansonno D. Therapy for hepatitis C virus-related cryoglobulinemic vasculitis. N Engl J Med. 2013;369(11):1035–1045. doi: 10.1056/NEJMra1208642. [DOI] [PubMed] [Google Scholar]
- 3.Casato M., Lilli D., Donato G., Granata M., Conti V., Del Giudice G. Occult hepatitis C virus infection in type II mixed cryoglobulinaemia. J Viral Hepat. 2003;10(6):455–459. doi: 10.1046/j.1365-2893.2003.00462.x. [DOI] [PubMed] [Google Scholar]
- 4.Lauletta G., Russi R., Conteduca V., Sansonno L. Hepatitis C virus infection and mixed cryoglobulinemia. Clin Dev Immunol. 2012:502156. doi: 10.1155/2012/502156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barsoum R.S. Hepatitis C virus: from entry to renal injury—facts and potentials. Nephrol Dial Transplant. 2007;22:1840–1848. doi: 10.1093/ndt/gfm205. [DOI] [PubMed] [Google Scholar]
- 6.Balasubramanian A., Munshi N., Koziel M.J., Hu Z., Liang T.J., Groopman J.E. Structural proteins of hepatitis C virus induce interleukin 8 production and apoptosis in human endothelial cells. J Gen Virol. 2005;86:3291–3301. doi: 10.1099/vir.0.81056-0. [DOI] [PubMed] [Google Scholar]
- 7.Satapathy S.K., Lingisetty C.S., Williams S.E. Acute kidney dysfunction in patients with chronic hepatitis C virus infection: analysis of viral and non-viral factors. J Clin Exp Hepatol. 2014;4(1):8–13. doi: 10.1016/j.jceh.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fabrizi F., Aghemo A., Fogazzi G.B., Moroni G., Passerini P., D’Ambrosio R. Acute tubular necrosis following interferon-based therapy for hepatitis C: case study with literature review. Kidney Blood Press Res. 2013;38(1):52–60. doi: 10.1159/000355753. [DOI] [PubMed] [Google Scholar]
- 9.Averbuch S.D., Austin H.A., 3rd, Sherwin S.A., Antonovych T., Bunn P.A., Jr., Longo D.L. Acute interstitial nephritis with the nephrotic syndrome following recombinant leukocyte A interferon therapy for mycosis fungoides. N Engl J Med. 1984;310(1):32–35. doi: 10.1056/NEJM198401053100107. [DOI] [PubMed] [Google Scholar]
- 10.Hepatitis C Therapeutic Registry and Research Network (HCV-TARGET). <http://www.hcvtarget.org/> [accessed 15th Sep 2015].
- 11.Sansonno D., Carbone A., De Re V., Dammacco F. Hepatitis C virus infection, cryoglobulinaemia, and beyond. Rheumatology (Oxford) 2007;46(4):572–578. doi: 10.1093/rheumatology/kel425. [DOI] [PubMed] [Google Scholar]
- 12.Ferri C., Sebastiani M., Giuggioli D., Cazzato M., Longombardo G., Antonelli A. Mixed cryoglobulinemia: demographic, clinical, and serologic features and survival in 231 patients. Semin Arthritis Rheum. 2004;33(6):355–7410. doi: 10.1016/j.semarthrit.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Fornasieri A., Armelloni S., Bernasconi P., Li M., de Septis C.P., Sinico R.A. High binding of immunoglobulin M kappa rheumatoid factor from type II cryoglobulins to cellular fibronectin: a mechanism for induction of in situ immune complex glomerulonephritis? Am J Kidney Dis. 1996;27(4):476–483. doi: 10.1016/s0272-6386(96)90156-0. [DOI] [PubMed] [Google Scholar]
- 14.Baroum R. The changing face of schistosomal glomerulopathy. Kidney Int. 2004;66(6):2472–2484. doi: 10.1111/j.1523-1755.2004.66042.x. [DOI] [PubMed] [Google Scholar]
- 15.Cao Y., Zhang Y., Wang S., Zou W. Detection of the hepatitis C virus antigen in kidney tissue from infected patients with various glomerulonephritis. Nephrol Dial Transplant. 2009;24(9):2745–2751. doi: 10.1093/ndt/gfp167. [DOI] [PubMed] [Google Scholar]
- 16.Sabry A.A., Sobh M.A., Irving W.L., Grabowska A., Wagner B.E., Fox S. A comprehensive study of the association between hepatitis C virus and glomerulopathy. Nephrol Dial Transplant. 2002;17(2):239–245. doi: 10.1093/ndt/17.2.239. [DOI] [PubMed] [Google Scholar]
- 17.Okada K., Takishita Y., Shimomura H., Tsuji T., Miyamura T., Kuhara T. Detection of hepatitis C virus core protein in the glomeruli of patients with membranous glomerulonephritis. Clin Nephrol. 1996;45(2):71–76. [PubMed] [Google Scholar]
- 18.Fabrizi F., Messa P., Martin P. Novel evidence on hepatitis C virus–associated glomerular disease. Kidney Int. 2014;86(3):466–469. doi: 10.1038/ki.2014.181. [DOI] [PubMed] [Google Scholar]
- 19.Cacoub P., Maisonobe T., Thibault V., Gatel A., Servan J., Musset L. Systemic vasculitis in patients with hepatitis C. J Rheumatol. 2001;28(1):109–118. [PubMed] [Google Scholar]
- 20.Perico N., Cattaneo D., Bikbov B., Remuzzi G. Hepatitis C infection and chronic renal diseases. Clin J Am Soc Nephrol. 2009;4(1):207–220. doi: 10.2215/CJN.03710708. [DOI] [PubMed] [Google Scholar]
- 21.Tsui J.I., Vittinghoff E., Shlipak M.G., Bertenthal D., Inadomi J., Rodriguez R.A. Association of hepatitis C seropositivity with increased risk for developing end-stage renal disease. Arch Int Med. 2007;167(12):1271–1276. doi: 10.1001/archinte.167.12.1271. [DOI] [PubMed] [Google Scholar]
- 22.Covic A., Iancu L., Apetrei C., Scripcaru D., Volovat C., Mititiuc I. Hepatitis virus infection in haemodialysis patients from Moldavia. Nephrol Dial Transplant. 1999;14(1):40–45. doi: 10.1093/ndt/14.1.40. [DOI] [PubMed] [Google Scholar]
- 23.Espinosa M., Martin-Malo A., Alvarez de Lara M.A., Aljama P. Risk of death and liver cirrhosis in anti-HCV +ve long-term haemodialysis patients. Nephrol Dial Transplant. 2001;16(8):1669–1674. doi: 10.1093/ndt/16.8.1669. [DOI] [PubMed] [Google Scholar]
- 24.Fabrizi F., Martin P., Dixit V., Messa P. Meta-analysis of observational studies: Hepatitis C and survival after renal transplant. J Viral Hepat. 2014;21(5):314–324. doi: 10.1111/jvh.12148. [DOI] [PubMed] [Google Scholar]
- 25.Yang C.S., Chang H.H., Chou C.C., Peng S.J. Isolation effectively prevents the transmission of hepatitis C virus in the hemodialysis unit. J Formos Med Assoc. 2003;102(2):79–85. [PubMed] [Google Scholar]
- 26.World Health Organization. Hepatitis C. <http://www.who.int/csr/disease/hepatitis/whocdscsrlyo2003/en/index4.html> [last accessed 30th March 2016].
- 27.Farci P., Alter H.J., Govindarajan S., Wong D.C., Engle R., Lesniewski R.R. Lack of protective immunity against reinfection with hepatitis C virus. Science. 1992;258(5079):135–140. doi: 10.1126/science.1279801. [DOI] [PubMed] [Google Scholar]
- 28.Lai M.E., Mazzoleni A.P., Argiolu F., De Virgilis S., Balestrieri A., Purcell R.H. Hepatitis C virus in multiple episodes of acute hepatitis in polytransfused thalassaemic children. Lancet. 1994;343(8894):388–390. doi: 10.1016/s0140-6736(94)91224-6. [DOI] [PubMed] [Google Scholar]
- 29.Smith D.B., Bukh J., Kuiken C., Muerhoff A.S., Rice C.M., Stapleton J.T. Expanded classification of Hepatitis C virus into 7 genotypes and 67 subtypes: updated criteria and genotype assignment web resource. Hepatology. 2014;59(1):318–327. doi: 10.1002/hep.26744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jardim A.C., Bittar C., Matos R.P., Yamasaki L.H., Silva R.A., Pinho J.R. Analysis of HCV quasispecies dynamic under selective pressure of combined therapy. BMC Infect Dis. 2013;13:61. doi: 10.1186/1471-2334-13-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bucci J.R., Lentine K.L., Agodoa L.Y., Peters T.G., Schnitzler M.A., Abbott K.C. Outcomes associated with recipient and donor hepatitis C serology status after kidney transplantation in the United States: analysis of the USRDS/UNOS database. Clin Transpl. 2004:51–61. [PubMed] [Google Scholar]
- 32.Maluf D.G., Archer K.J., Mas V.R. Kidney grafts from HCV +ve donors: advantages and disadvantages. Transplant Proc. 2010;42(7):2436–2446. doi: 10.1016/j.transproceed.2010.04.056. [DOI] [PubMed] [Google Scholar]
- 33.Singh N., Neidlinger N., Djamali A., Leverson G., Voss B., Sollinger H.W. The impact of hepatitis C virus donor and recipient status on long-term kidney transplant outcomes: University of Wisconsin experience. Clin Transplant. 2012;26(5):684–693. doi: 10.1111/j.1399-0012.2011.01583.x. [DOI] [PubMed] [Google Scholar]
- 34.Domínguez-Gil B., Andrés A., Campistol J.M., Morales J.M. Should we be using kidneys from hepatitis C virus-infected donors? Curr Opin Nephrol Hypertens. 2011;20(6):599–604. doi: 10.1097/MNH.0b013e32834bba37. [DOI] [PubMed] [Google Scholar]
- 35.Kidney Disease: Improving Global Outcomes (KDIGO) KDIGO clinical practice guidelines for the prevention, evaluation and treatment of hepatitis C in chronic kidney disease. Kidney Int Suppl. 2008;109:S1–S99. doi: 10.1038/ki.2008.81. [DOI] [PubMed] [Google Scholar]
- 36.Cruzado J.M., Gil-Vernet S., Ercilla G., Seron D., Carrera M., Bas J. Hepatitis C virus- associated membranoproliferative glomerulonephritis in renal allografts. J Am Soc Nephrol. 1996;7(11):2469–2475. doi: 10.1681/ASN.V7112469. [DOI] [PubMed] [Google Scholar]
- 37.Pereira B.J., Natov S.N., Bouthot B.A., Murthy B.V., Ruthazer R., Schmid C.H. Effects of hepatitis C infection and renal transplantation on survival in end-stage renal disease. The New England Organ Bank Hepatitis C Study Group. Kidney Int. 1998;53(5):1374–1381. doi: 10.1046/j.1523-1755.1998.00883.x. [DOI] [PubMed] [Google Scholar]
- 38.Cosio F.G., Sedmak D.D., Henry M.L., Al Haddad C., Falkenhain M.E., Elkhammas E.A. The high prevalence of severe early posttransplant renal allograft pathology in hepatitis C positive recipients. Transplantation. 1996;62(8):1054–1059. doi: 10.1097/00007890-199610270-00004. [DOI] [PubMed] [Google Scholar]
- 39.Baid S., Pascual M., Williams W.W., Jr., Tolkoff-Rubin N., Johnson S.M., Collins B. Renal thrombotic microangiopathy associated with anticardiolipin antibodies in hepatitis C-positive renal allograft recipients. J Am Soc Nephrol. 1999;10(1):146–153. doi: 10.1681/ASN.V101146. [DOI] [PubMed] [Google Scholar]
- 40.Forman J.P., Tolkoff-Rubin N., Pascual M., Lin J. Hepatitis C, acute humoral rejection, and renal allograft survival. J Am Soc Nephrol. 2004;15:3249–3255. doi: 10.1097/01.ASN.0000145896.16153.43. [DOI] [PubMed] [Google Scholar]
- 41.Fabrizi F., Martin P., Dixit V., Bunnapradist S., Kanwal F., Dulai G. Post-transplant diabetes mellitus and HCV seropositive status after renal transplantation: meta- analysis of clinical studies. Am J Transplant. 2005;5(10):2433. doi: 10.1111/j.1600-6143.2005.01040.x. [DOI] [PubMed] [Google Scholar]
- 42.2013 USRDS Annual Data Report Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Am J Kid Dis. 2014;63(1) doi: 10.1053/j.ajkd.2011.11.015. Supplement: e1–e478. [DOI] [PubMed] [Google Scholar]
- 43.Narula A.S., Hooda A., Anand A.C., Patrikar S. Impact of hepatitis C virus infection in renal transplant recipients. Indian J Gastroenterol. 2005;24(4):151–154. [PubMed] [Google Scholar]
- 44.Morales J.M., Marcén R., Andres A., Domínguez-Gil B., Campistol J.M., Gallego R. Renal transplantation in patients with hepatitis C virus antibody. A long national experience. NDT Plus. 2010;3(Suppl_2):ii41–ii46. doi: 10.1093/ndtplus/sfq070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.López-Medrano F., Fernández-Ruiz M., Morales J.M., San-Juan R., Cervera C., Carratalá J. Spanish Network for the Research of Infection in Transplantation/Network of Research in Infectious Diseases (RESITRA/REIPI) Study Group. Impact of hepatitis C virus infection on the risk of infectious complications after kidney transplantation: data from the RESITRA/REIPI cohort. Transplantation. 2011;92(5):543–549. doi: 10.1097/TP.0b013e318225dbae. [DOI] [PubMed] [Google Scholar]
- 46.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26(1):S5–S20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 47.Davidson J., Wilkinson A., Dantal J., Dotta F., Haller H., Hernández D. International expert panel. New-onset diabetes after transplantation: 2003 International Consensus Guidelines. Proceedings of an international expert panel meeting. Barcelona, Spain, 19 February 2003. Transplantation. 2003;75(10 Suppl):SS3–SS24. doi: 10.1097/01.TP.0000069952.49242.3E. [DOI] [PubMed] [Google Scholar]
- 48.Vincenti F., Friman S., Scheuermann E., Rostaing L., Jenssen T., Campistol J.M. DIRECT (Diabetes Incidence after Renal Transplantation: Neoral C Monitoring Versus Tacrolimus) Investigators. Results of an international, randomized trial comparing glucose metabolism disorders and outcome with cyclosporine versus tacrolimus. Am J Transplant. 2007;7(6):1506–1514. doi: 10.1111/j.1600-6143.2007.01749.x. [DOI] [PubMed] [Google Scholar]
- 49.Demirci M.S., Toz H., Yilmaz F., Ertilav M., Asci G., Ozkahya M., Zeytinoglu A. Risk factors and consequences of post-transplant diabetes mellitus. Clin Transplant. 2010;24(5):E170–E177. doi: 10.1111/j.1399-0012.2010.01247.x. [DOI] [PubMed] [Google Scholar]
- 50.Baid-Agrawal S., Frei U., Reinke P., Schindler R., Kopp M.A., Martus P. Impaired insulin sensitivity as an underlying mechanism linking hepatitis C and posttransplant diabetes mellitus in kidney recipients. Am J Transplant. 2009;9(12):2777–2784. doi: 10.1111/j.1600-6143.2009.02843.x. [DOI] [PubMed] [Google Scholar]
- 51.Masini M., Campani D., Boggi U., Menicagli M., Funel N., Pollera M. Hepatitis C virus infection and human pancreatic β-Cell dysfunction. Diabetes Care. 2005;28(4):940–941. doi: 10.2337/diacare.28.4.940. [DOI] [PubMed] [Google Scholar]
- 52.Caillard S., Lelong C., Pessione F. Moulin B;French PTLD Working Group. Post- transplant lymphoproliferative disorders occurring after renal transplantation in adults: report of 230 cases from the French Registry. Am J Transplant. 2006;6(11):2735–2742. doi: 10.1111/j.1600-6143.2006.01540.x. [DOI] [PubMed] [Google Scholar]
- 53.Bakker N.A., van Imhoff G.W., Verschuuren E.A., van Son W.J. Presentation and early detection of post-transplant lymphoproliferative disorder after solid organ transplantation. Transpl Int. 2007;20(3):207–218. doi: 10.1111/j.1432-2277.2006.00416.x. [DOI] [PubMed] [Google Scholar]
- 54.Kondo Y., Shimosegawa T. Direct effect of hepatitis C virus on the lymphoid cells. World J Gastroenterol. 2013;19(44):7889–7895. doi: 10.3748/wjg.v19.i44.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Omland L.H., Jepsen P., Krarup H., Christensen P.B., Weis N., Nielsen L. DANVIR cohort study. Liver cancer and non-Hodgkin lymphoma in hepatitis C virus-infected patients: results from the DANVIR cohort study. Int J Cancer. 2012;130(10):2310–2317. doi: 10.1002/ijc.26283. [DOI] [PubMed] [Google Scholar]
- 56.Takahashi K., Nishida N., Kawabata H., Haga H., Chiba T. Regression of Hodgkin lymphoma in response to antiviral therapy for hepatitis C virus infection. Intern Med. 2012;51(19):2745–2747. doi: 10.2169/internalmedicine.51.8404. [DOI] [PubMed] [Google Scholar]
- 57.Morton L.M., Landgren O., Chatterjee N., Castenson D., Parsons R., Hoover R.N. Hepatitis C virus infection and risk of posttransplantation lymphoproliferative disorder among solid organ transplant recipients. Blood. 2007;110(13):4599–4605. doi: 10.1182/blood-2007-07-101956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burra P., Buda A., Livi U., Rigotti P., Zanus G., Calabrese F. Occurrence of post- transplant lymphoproliferative disorders among over thousand adult recipients: any role for hepatitis C infection? Eur J Gastroenterol Hepatol. 2006;18(10):1065–1070. doi: 10.1097/01.meg.0000231752.50587.ae. [DOI] [PubMed] [Google Scholar]
- 59.Aravindan A.N., Moger V., Sakhuja V., Kohli H.S., Varma N., Jha V. Hepatitis C virus related lymphoproliferative disorder in a renal transplant recipient. Int Urol Nephrol. 2006;38:355–357. doi: 10.1007/s11255-006-0050-1. [DOI] [PubMed] [Google Scholar]
- 60.Hestin D., Guillemin F., Castin N., Le Faou A., Champigneulles J., Kessler M. Pre- transplant hepatitis C virus infection: a predictor of proteinuria after renal transplantation. Transplantation. 1998;65:741–744. doi: 10.1097/00007890-199803150-00024. [DOI] [PubMed] [Google Scholar]
- 61.Sabry A., Hassan R., Mahmoud I., Hamed M., Sobh M. Proteinuria after kidney transplantation: its relation to hepatitis C virus and graft outcome. Iran J Kidney Dis. 2007;1(2):88–97. [PubMed] [Google Scholar]
- 62.Gentil M.A., Rocha J.L., Rodríguez-Algarra G., Pereira P., López R., Bernal G. Impaired kidney transplant survival in patients with antibodies to hepatitis C virus. Nephrol Dial Transplant. 1999;14(10):2455–2460. doi: 10.1093/ndt/14.10.2455. [DOI] [PubMed] [Google Scholar]
- 63.Brunkhorst R., Kliem V., Koch K.M. Recurrence of membranoproliferative glomerulonephritis after renal transplantation in a patient with chronic hepatitis C. Nephron. 1996;72(3):465–467. doi: 10.1159/000188914. [DOI] [PubMed] [Google Scholar]
- 64.McKay D.B., Milford E.L., Sayegh M. Clinical aspects of renal transplantation. In: Brenner B.M., Rector F.C., editors. The Kidney. W.B. Saunders Company; PA: 1996. pp. 2625–2628. [Google Scholar]
- 65.Cruzado J.M., Carrera M., Torras J., Grinyó J.M. Hepatitis C virus infection and de novo glomerular lesions in renal allografts. Am J Transplant. 2001;1(2):171–178. [PubMed] [Google Scholar]
- 66.Ozdemir B.H., Ozdemir F.N., Sezer S., Colak T., Haberal M. De novo glomerulonephritis in renal allografts with hepatitis C virus infection. Transplant Proc. 2006;38(2):492–495. doi: 10.1016/j.transproceed.2005.12.109. [DOI] [PubMed] [Google Scholar]
- 67.Ponticelli C., Moroni G., Glassock R.J. De novo glomerular diseases after renal transplantation. Clin J Am Soc Nephrol. 2014;9(8):1479–1487. doi: 10.2215/CJN.12571213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pouteil-Noble C., Maiza H., Dijoud F., MacGregor B. Glomerular disease associated with hepatitis C virus infection in native kidneys. Nephrol Dial Transplant. 2000;15(Suppl 8):28–33. doi: 10.1093/ndt/15.suppl_8.28. [DOI] [PubMed] [Google Scholar]
- 69.Roth D., Cirocco R., Zucker K., Ruiz P., Viciana A., Burke G. De novo membranoproliferative glomerulonephritis in hepatitis C virus-infected renal allograft recipients. Transplantation. 1995;59(12):1676–1682. doi: 10.1097/00007890-199506270-00006. [DOI] [PubMed] [Google Scholar]
- 70.Zeman M., Campbell P., Bain V.G. Hepatitis C eradication and improvement of cryoglobulinemia-associated rash and membranoproliferative glomerulonephritis with interferon and ribavirin after kidney transplantation. Can J Gastroenterol. 2006;20(6):427–431. doi: 10.1155/2006/301232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Larsen C.P., Walker P.D. Phospholipase A2 receptor (PLA2R) staining is useful in the determination of de novo versus recurrent membranous glomerulopathy. Transplantation. 2013;95(10):1259–1262. doi: 10.1097/TP.0b013e31828a947b. [DOI] [PubMed] [Google Scholar]
- 72.Morales J.M., Pascual-Capdevila J., Campistol J.M., Fernandez-Zatarain G., Muñoz M.A., Andres A. Membranous glomerulonephritis associated with hepatitis C virus infection in renal transplant patients. Transplantation. 1997;63(11):1634–1639. doi: 10.1097/00007890-199706150-00017. [DOI] [PubMed] [Google Scholar]
- 73.Baid-Agrawal S., Pascual M., Moradpour D., Frei U., Tolkoff-Rubin N. Hepatitis C virus infection in haemodialysis and kidney transplant patients. Rev Med Virol. 2008;18(2):97–115. doi: 10.1002/rmv.565. [DOI] [PubMed] [Google Scholar]
- 74.Delladetsima I., Psichogiou M., Sypsa V., Sakellariou S., Hatzakis A., Boletis N.J. Time of acquisition of HCV infection in renal transplant recipients: a major prognostic factor for disease progression. Clin Transplant. 2013;27(1):72–79. doi: 10.1111/ctr.12012. [DOI] [PubMed] [Google Scholar]
- 75.Melon S., Galarraga M.C., Villar M., Laures A., Boga J.A., de Oña M. Hepatitis C virus reactivation in anti-hepatitic C virus-positive renal transplant recipients. Transplant Proc. 2005;37(5):2083–2085. doi: 10.1016/j.transproceed.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 76.Izopet J., Rostaing L., Sandres K., Cisterne J.M., Pasquier C., Rumeau J.L. Longitudinal analysis of hepatitis C virus replication and liver fibrosis progression in renal transplant recipients. J Infect Dis. 2000;181(1):852–858. doi: 10.1086/315355. [DOI] [PubMed] [Google Scholar]
- 77.Luan F.L., Schaubel D.E., Zhang H., Jia X., Pelletier S.J., Port F.K. Impact of immunosuppressive regimen on survival of kidney transplant recipients with hepatitis C. Transplantation. 2008;85(11):1601–1606. doi: 10.1097/TP.0b013e3181722f3a. [DOI] [PubMed] [Google Scholar]
- 78.Berenguer M., Crippin J., Gish R., Bass N., Bostrom A., Netto G. A model to predict severe HCV-related disease following liver transplantation. Hepatology. 2003;38(1):34–41. doi: 10.1053/jhep.2003.50278. [DOI] [PubMed] [Google Scholar]
- 79.Fernandes F., Ansari I.U., Striker R. Cyclosporine inhibits a direct interaction between cyclophilins and hepatitis C NS5A. PLoS ONE. 2010;5(3):e9815. doi: 10.1371/journal.pone.0009815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kahraman A., Witzke O., Scherag A., Pütter C., Miller M., Dechêne A. Impact of immunosuppressive therapy on hepatitis C infection after renal transplantation. Clin Nephrol. 2011;75(1):16–25. [PubMed] [Google Scholar]
- 81.Munoz De Bustillo E., Ibarrola C., Colina F., Castellano G., Fuertes A., Andrés A. Fibrosingcholestatic hepatitis in hepatitis C virus-infected renal transplant recipients. J Am Soc Nephrol. 1998;9(6):1109–1113. doi: 10.1681/ASN.V961109. [DOI] [PubMed] [Google Scholar]
- 82.Fabrizi F., Dixit V., Messa P., Martin P. Interferon monotherapy of chronic hepatitis C in dialysis patients: meta-analysis of clinical trials. J Viral Hepat. 2008;15(2):79–88. doi: 10.1111/j.1365-2893.2007.00907.x. [DOI] [PubMed] [Google Scholar]
- 83.Gordon C.E., Uhlig K., Lau J., Schmid C.H., Levey A.S., Wong J.B. Interferon treatment in hemodialysis patients with chronic hepatitis C virus infection: a systematic review of the literature and meta-analysis of treatment efficacy and harms. Am J Kidney Dis. 2008;51(2):263–277. doi: 10.1053/j.ajkd.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 84.Goodkin D.A., Bieber B., Gillespie B., Robinson B.M., Jadoul M. Hepatitis C infection is very rarely treated among haemodialysis patients. Am J Nephrol. 2013;35(5):405–412. doi: 10.1159/000355615. [DOI] [PubMed] [Google Scholar]
- 85.Pockros P.J., Reddy K.R., Mantry P.S., Cohen E., Bennett M., Sulkowski M.S. Safety of ombitasvir/paritaprevir/ritonavir plus dasabuvir for treating HCV GT1 infection in patients with severe renal impairment or end-stage renal disease: The RUBY-I study. J Hepatol. 2015;62(S2):S257. [Google Scholar]
- 86.Bhamidimarri K.R., Czul F., Peyton A., Levy C., Hernandez M., Jeffers L. Safety, efficacy and tolerability of half-dose sofosbuvir plus simeprevir in treatment of Hepatitis C in patients with end-stage renal disease. J Hepatol. 2015;63:763–765. doi: 10.1016/j.jhep.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 87.Scott G.M. Interferon: pharmacokinetics and toxicity. Philos Trans R Soc Lond B Biol Sci. 1982;299(299):91–107. doi: 10.1098/rstb.1982.0109. [DOI] [PubMed] [Google Scholar]
- 88.University of Liverpool: Ribavirin PK fact sheet <http://www.hivdruginteractions.org/data/FactSheetImages/FactSheet_DrugID_112.pdf> [last accessed 16th October 2015].
- 89.Casanovas Taltavull T., Baliellas Comellas C., Cruzado Garrit J.M. Results of hepatitis C virus treatment in patients on hemodialysis: data from published meta-analyses in 2008. Transplant Proc. 2009;41(6):2082–2084. doi: 10.1016/j.transproceed.2009.06.140. [DOI] [PubMed] [Google Scholar]
- 90.Beuthien W., Mellinghoff H., Kempis J. Vasculitic complications of interferon-alpha treatment for chronic hepatitis C virus infection: case report and review of the literature. Clin Rheumatol. 2005;24(5):507–515. doi: 10.1007/s10067-005-1093-x. [DOI] [PubMed] [Google Scholar]
- 91.Rostaing L., Modesto A., Baron E., Cisterne J.M., Chabannier M.H., Durand D. Acute renal failure in kidney transplant patients treated with interferon alpha 2b for chronic hepatitis C. Nephron. 1996;74(3):512–516. doi: 10.1159/000189444. [DOI] [PubMed] [Google Scholar]
- 92.El-Fishawy H., Saadi G., Hassaballa M., Hussein M., Doss W., Ragab G. Antiviral treatment prioritization in HCV-infected patients with extrahepatic manifestations – an Egyptian perspective. J Adv Res. 2016;7(3):391–402. doi: 10.1016/j.jare.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.CATIE: Treatment update 198. The changing role of SVR12 in clinical trials of HCV drugs <http://www.catie.ca/en/treatmentupdate/treatmentupdate-198/hepatitis-c-virus/changing-role-svr12-clinical-trials-hcv-d> [accessed 16 March 2016].
- 94.Welzel T.M., Herzer K., Ferenci P., Petersen J., Gschwantler M., Cornberg M. Daclatasvir Plus Sofosbuvir with or without ribavirin for the treatment of HCV in patients with severe liver disease: interim results of a multicenter compassionate use program. J Hepatol. 2015;62(S2):S619–S620. [Google Scholar]
- 95.FDA Drug Safety Communication: FDA warns of serious liver injury risk with hepatitis C treatments Viekira Pak and Technivie. <http://www.fda.gov/Drugs/DrugSafety/ucm468634.htm> [accessed 16 March 2016].
- 96.AASLD/IDSA HCV Guidance Panel Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62(3):932–954. doi: 10.1002/hep.27950. [DOI] [PubMed] [Google Scholar]
- 97.Wyles D.L., Ruane P.J., Sulkowski M.S., Dieterich D., Luetkemeyer A., Morgan T.R. For the ALLY-2 Investigators. Daclatasvir plus sofosbuvir for HCV in patients coinfected with HIV-1. N Engl J Med. 2015;373(8):714–725. doi: 10.1056/NEJMoa1503153. [DOI] [PubMed] [Google Scholar]
- 98.Fleischer R., Boxwell D., Sherman K.E. Nucleoside analogues and mitochondrial toxicity. Clin Infect Dis. 2004;38(8):e79–e80. doi: 10.1086/383151. Epub 2004 Mar 26. [DOI] [PubMed] [Google Scholar]
- 99.Weltman M.D., Brotodihardjo A., Crewe E.B., Farrell G.C., Bilous M., Grierson J.M. Coinfection with hepatitis B and C or B, C and delta viruses results in severe chronic liver disease and responds poorly to interferon-alpha treatment. J Viral Hepat. 1995;2(1):39–45. doi: 10.1111/j.1365-2893.1995.tb00070.x. [DOI] [PubMed] [Google Scholar]
- 100.Lynch T., Price P. The effect of cytochrome P450 metabolism on drug response, interactions, and adverse effects. Am Fam Physician. 2007;76(3):391–396. [PubMed] [Google Scholar]
- 101.European Association for Study of Liver (EASL) Recommendations on Treatment of Hepatitis C 2015. J Hepatol. 2015;63(1):199–236. doi: 10.1016/j.jhep.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 102.Full prescribing information for HARVONI. FDA-approved patient labeling Issued: March 2015. Last reviewed on RxList: 4/7/2015. Reference ID: 3642283. U.S. <www.gilead.com>.
- 103.Hézode C., Alric L., Brown A., Hassanein T., Rizzetto M., Buti M. COMMAND-4 study team. Randomized controlled trial of the NS5A inhibitor daclatasvir plus peginterferon and ribavirin for HCV genotype-4 (COMMAND-4) Antivir Ther. 2015;21(3):195–205. doi: 10.3851/IMP2985. [DOI] [PubMed] [Google Scholar]
- 104.Garimella T., Wang R., Luo W.L., Hwang C., Sherman D., Kandoussi H. Single-dose pharmacokinetics and safety of daclatasvir in subjects with renal function impairment. Antivir Ther. 2015;20(5):535–543. doi: 10.3851/IMP2941. [DOI] [PubMed] [Google Scholar]
- 105.Roth D., Nelson D.R., Bruchfeld A., Liapakis A., Silva M., Monsour H., Jr. Grazoprevir plus elbasvir in treatment-naive and treatment-experienced patients with hepatitis C virus genotype 1 infection and stage 4–5 chronic kidney disease (the C-SURFER study): a combination phase 3 study. Lancet. 2015;386(10003):1537–1545. doi: 10.1016/S0140-6736(15)00349-9. [DOI] [PubMed] [Google Scholar]
- 106.Kirby B.J., Symonds W.T., Kearney B.P., Mathias A.A. Pharmacokinetic, Pharmacodynamic, and Drug-Interaction Profile of the Hepatitis C Virus NS5B Polymerase Inhibitor Sofosbuvir. Clin Pharmacokinet. 2015;54(7):677–690. doi: 10.1007/s40262-015-0261-7. [DOI] [PubMed] [Google Scholar]
- 107.Hézode C., Asselah T., Reddy K.R., Hassanein T., Berenguer M., Fleischer-Stepniewska K. Ombitasvir plus paritaprevir plus ritonavir with or without ribavirin in treatment-naive and treatment-experienced patients with genotype 4 chronic hepatitis C virus infection (PEARL-I): a randomised, open-label trial. Lancet. 2015;385(9986):2502–2509. doi: 10.1016/S0140-6736(15)60159-3. [DOI] [PubMed] [Google Scholar]