Abstract
As one of the dominant plantations in north and central China, poplar was considered as the uppermost wood raw materials, however, the chemical constituents of poplar wood weren’t effectively used by high added value. Therefore, the molecules of wood extractives in Populus lasiocarpa and Populus tomentosa were extracted and studied to further utilize the bio-resources. The results showed that the LD-010, LD-021, LD-150, LD-174 wood extractives were identified as having 3, 24, 3 27 components, respectively. P. lasiocarpa wood was fit to extract 2,4-hexadiyne, 1,3,3-trimethyl-2-hydroxymethyl-3,3-dimethyl-4-(3-methylbut-2-enyl)-cyclohexene, and P. tomentosa wood was fit to extract 1,5-hexadien-3-yne, (all-E)-2,6,10,15,19,23-hexamethyl-2,6,10,14,18,22-tetracosahexaene. So the extractives of poplar wood contained rich and rare drug and biomedical activities.
Keywords: Antibacterial molecules, Wood extractives, Woody activities, Poplar, GC–MS
1. Introduction
Poplar tree, which was known as aspen and cottonwood, was a native of the Northern Hemisphere. It could grow from 15 to 50 m tall and had trunks up to 2.5 m (Heping et al., 1999). The leaves were oval to heart-shaped, and green in the spring and summer; the flowers bloomed from March to May; the fruits are small, thick-skinned capsules which contained dozens of tiny seeds covered with silky white hairs; the bark was smooth, rough, uneven, soft, wrinkled, cracked and range in color from white to dark gray (Rennenberg et al., 2010, Ashraf et al., 2013a). According to the bark color, poplar was mainly divided into four categories. White poplar, which had a high drought tolerance and diamond-shaped marks in the bark, could easily grow in different types of soil and climate conditions; lombardy poplar was long and column-like while its branches extend upward rather than outward from the trunk; eastern poplar was known as the Eastern Cottonwood, big and feature serrated edges; balsam poplar was known as black cottonwood and thrived in swampy soil (Divya, 2011, Ashraf et al., 2013b). Poplar trees were extremely desirable for homeowners looking to infuse their yards with shade and beauty. However, poplar trees are not recommended to plant close to houses or other buildings because it’s roots may damage buildings.
Poplar wood had a variety of different ways to contain snowboards, boats, boxes, pallets, paper, matches, electric guitars, harps, and violas (Daniel et al., 2012, Surhio et al., 2014). Poplar grown for 2–5 years till harvest is usually burned, as biofuel for energy instead of non-renewable energy sources such as coal reaching as high as 12 oven dry tones every year per tree (Poulomi et al., 2010, Batool et al., 2015). What’s more, poplar wood could be used for cheap plywood, matches, pallets and camembert cheese boxes (Daniel et al., 2012). Populus lasiocarpa and Populus tomentosa were the dominant species of plantation in north and central China and more than 2 Mha were planted (Yoshinori and Eckhard, 1976, Ningxia et al., 2012, Naureen et al., 2014). However, the researches on the active ingredients of poplar wood were scant. Therefore, the molecular characteristics of wood extractives were investigated and analyzed by the optimized extracting techniques so as to further utilize P. lasiocarpa and P. tomentosa wood resource.
2. Materials and methods
2.1. Materials
P. lasiocarpa wood was collected from the Zhumadian Forest Zone, Henan Province, China. P. tomentosa wood was collected from the Linyi Forest Zone, Shandong Province, China. The fresh wood was shaved, powdered and kept in vacuum. Acetic ether, methanol, benzene, petroleum ether and ethanol were of chromatographic grade and prepared for the experiments. Cotton thread and cotton bag were both extracted by benzene/ethanol solution for 12 h. The ratio Vethanol/Vbenzene was 2 double.
2.2. Experiment methods
Weighed 54 powder of wood, which were about 20 g each (0.1 mg accuracy) were then parceled by the cotton bag and tied by cotton thread, and signed. Extraction was carried out in 350 ml solvents by the Foss method for 7 h. Solvents were ethanol/methanol (Vethanol/Vmethanol = 2), petroleum ether/acetic ether (Vpetroleum ether/Vacetic ether = 2), and benzene/ethanol solution, respectively. Ethanol/ methanol extraction, petroleum ether/acetic ether extraction, and benzene/ethanol extraction were done at the temperature of 75 °C, 90 °C and 95 °C, respectively. After extraction, one piece was taken out, dried in 105 °C to oven dry, and weighed. The extractives were obtained by evaporation in 60–70 °C.
Weighed 2 pieces of wood, which were 20 g each (1.0 mg accuracy), were finally parceled by cotton bag and tied by cotton thread, and signed. Ethanol/Methanol extraction and petroleum ether/acetic ether extraction (EMPA) were gradually carried out by large-caliber Soxhlet. After one step extraction, one piece was taken out, dried in 105 °C to oven dry, and weighed. The wood extractives were obtained by evaporation in 60–70 °C.
2.3. GC/MS condition
The ethanol/methanol extractives of P. lasiocarpa wood (LD-010), ethanol/methanol extractives of P. tomentosa wood (LD-021), benzene/ethanol extractives of P. tomentosa wood (LD-150), EMPA extractives of P. lasiocarpa wood (LD-174) were analyzed, respectively. Each 0.5 mg extractive was analyzed by a GC/MS-QP2010 (Shimadzu Corp., Japan). The GC/MS analysis was the same as that in the documents (Wanxi et al., 2013, Wanxi et al., 2014, Yusoff et al., 2013).
2.4. Experiment analyses
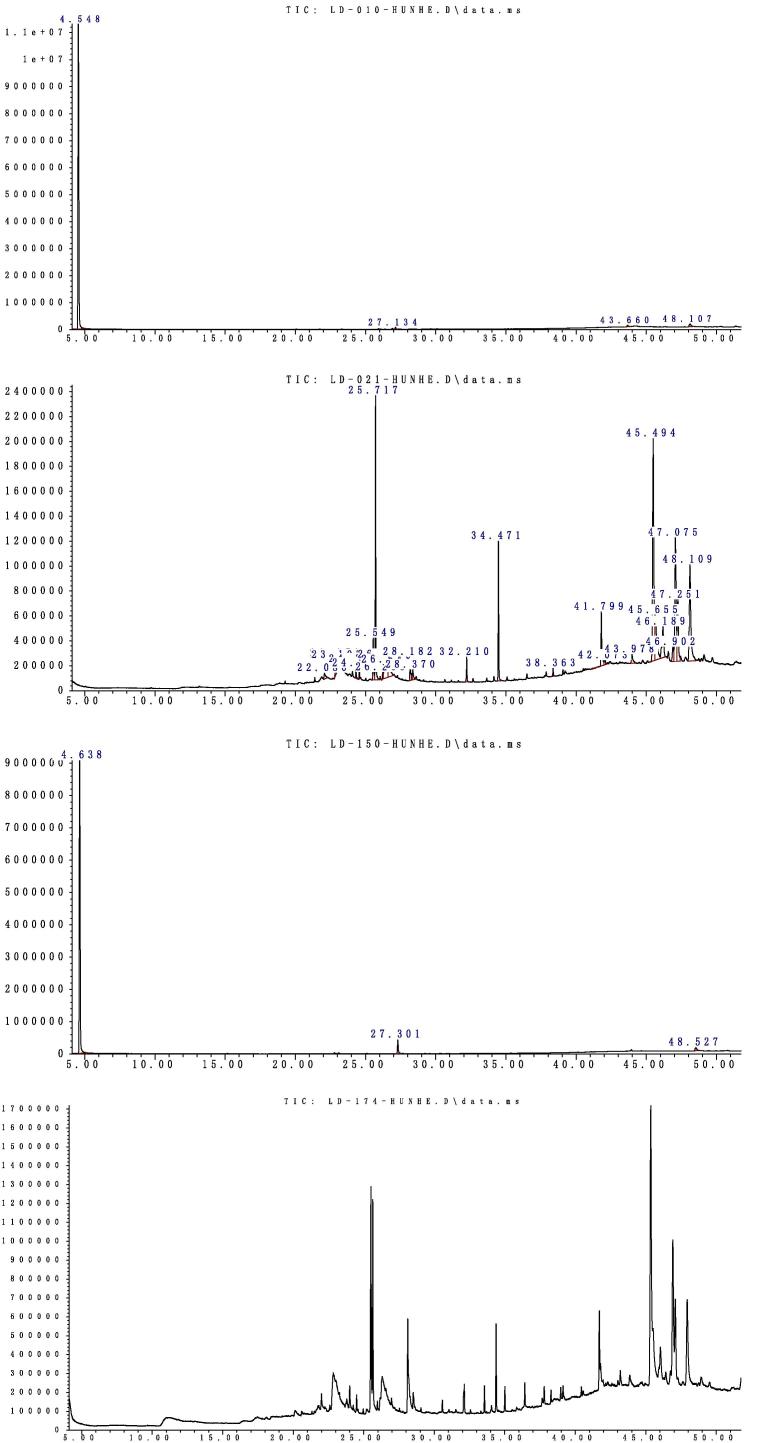
The LD-010, LD-021, LD-150, LD-174 extractives were obtained respectively. The total ion chromatograms of four extractives by GC/MS were shown in Fig. 1. Relative content of each component was counted by area normalization. The results are listed in Table 1, Table 2, Table 3, Table 4, respectively. By analyzing the MS data, the NIST standard MS map by computer, open-published books and papers, the components and their contents were identified.
Figure 1.
Total ion chromatograms of four extractives of Populus wood by GC/MS.
Table 1.
Compounds of LD-010 wood extractives of Populus lasiocarpa by GC/MS.
| No. | R.T. (min) | P.A. (%) | Chemical compound |
|---|---|---|---|
| 1 | 4.548 | 97.05 | 2,4-Hexadiyne |
| 2 | 27.134 | 0.47 | Dibutyl phthalate |
| 3 | 43.66 | 0.72 | Benzo[h]quinoline, 2,4-dimethyl- |
| 4 | 48.107 | 1.76 | Carbonic acid, monoamide, N-(2-ethylphenyl)-, propyl ester |
Table 2.
Compounds of LD-021 wood extractives of Populus tomentosa by GC/MS.
| No. | R.T. (min) | P.A. (%) | Chemical compound |
|---|---|---|---|
| 1 | 22.08 | 0.63 | Benzoic acid, 4-hydroxy- |
| 2 | 22.98 | 1.99 | 4-((1E)-3-hydroxy-1-propenyl)-2-methoxyphenol |
| 3 | 23.04 | 0.38 | 4-((1E)-3-hydroxy-1-propenyl)-2-methoxyphenol |
| 4 | 23.10 | 0.52 | 4-((1E)-3-hydroxy-1-propenyl)-2-methoxyphenol |
| 5 | 24.33 | 0.54 | 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester |
| 6 | 24.57 | 0.42 | 9-Octadecyne |
| 7 | 25.55 | 2.11 | n-Hexadecanoic acid |
| 8 | 25.72 | 12.09 | Dibutyl phthalate |
| 9 | 26.24 | 0.34 | 3,5-Dimethoxy-4-hydroxycinnamaldehyde |
| 10 | 26.39 | 4.17 | 2-Pentanone, 1-(2,4,6-trihydroxyphenyl) |
| 11 | 26.65 | 1.82 | 3-Phenylbicyclo(3.2.2)nona-3,6-dien-2-one |
| 12 | 28.18 | 1.20 | 9,12-Octadecadienoic acid (Z,Z)- |
| 13 | 28.37 | 0.70 | Oleic acid |
| 14 | 32.21 | 1.10 | Hexanedioic acid, bis(2-ethylhexyl) ester |
| 15 | 34.47 | 5.92 | 1,2-Benzenedicarboxylic acid, diisooctyl ester |
| 16 | 38.36 | 0.35 | Phthalic acid, hexadecyl propyl ester |
| 17 | 41.80 | 3.42 | 2,6,10,14,18,22-Tetracosahexaene, 2,6,10,15,19,23-hexamethyl-, (all-E)- |
| 18 | 42.07 | 0.43 | Thiazol-4(5H)-one, 2-(4-morpholyl)-5-(2-pyridylmethylideno)- |
| 19 | 43.98 | 1.00 | 1H-Indole, 1-methyl-2-phenyl- |
| 20 | 45.49 | 20.61 | Benzene, 2-[(tert-butyldimethylsilyl)oxy]-1-isopropyl-4-methyl- |
| 21 | 45.66 | 5.67 | 1H-Cycloprop[e]azulene, decahydro-1,1,4,7-tetramethyl-, [1aR-(1a.alpha.,4.beta.,4a.beta.,7.beta.,7a.beta.,7b.alpha.)]- |
| 22 | 46.19 | 3.88 | 1,2,5-Oxadiazol-3-amine, 4-(4-methoxyphenoxy)- |
| 23 | 46.90 | 1.32 | Benzo[b]naphtho[2,3-d]furan |
| 24 | 47.08 | 12.28 | 2,4-Cyclohexadien-1-one, 3,5-bis(1,1-dimethylethyl)-4-hydroxy- |
| 25 | 47.25 | 5.72 | 5(1H)-Azulenone, 2,4,6,7,8,8a-hexahydro-3,8-dimethyl-4-(1-methylethylidene)-, (8S-cis)- |
| 26 | 48.11 | 11.42 | 4-Methylmannonic.delta.-lactone |
Table 3.
Compounds of LD-150 wood extractives of Populus lasiocarpa by GC/MS.
| No. | R.T. (min) | P.A. (%) | Chemical compound |
|---|---|---|---|
| 1 | 4.64 | 94.04 | 1,5-Hexadien-3-yne |
| 2 | 27.30 | 3.95 | Dibutyl phthalate |
| 3 | 48.53 | 2.01 | 1H-Indole, 1-methyl-2-phenyl- |
Table 4.
Compounds of LD-174 wood extractives of Populus tomentosa by GC/MS.
| No. | R.T. (min) | P.A. (%) | Chemical compound |
|---|---|---|---|
| 1 | 22.01 | 0.75 | Benzoic acid, 3-ethoxy- |
| 2 | 22.84 | 1.45 | 4-((1E)-3-hydroxy-1-propenyl)-2-methoxyphenol |
| 3 | 22.87 | 0.22 | 4-((1E)-3-hydroxy-1-propenyl)-2-methoxyphenol |
| 4 | 22.89 | 0.35 | 4-((1E)-3-hydroxy-1-propenyl)-2-methoxyphenol |
| 5 | 24.00 | 1.06 | Pentadecanoic acid |
| 6 | 24.49 | 0.49 | 9-Octadecyne |
| 7 | 25.51 | 8.82 | n-Hexadecanoic acid |
| 8 | 25.64 | 7.36 | 1,2-Benzenedicarboxylic acid, butyl 2-methylpropyl ester |
| 9 | 26.17 | 0.33 | 3,5-Dimethoxy-4-hydroxycinnamaldehyde |
| 10 | 26.31 | 4.29 | Phenol, 2,6-dimethyl-4-nitro- |
| 11 | 26.57 | 0.84 | 4-Ethoxy-2,5-dimethoxybenzaldehyde |
| 12 | 28.12 | 3.62 | 9,12-Octadecadienoic acid (Z,Z)- |
| 13 | 28.18 | 1.86 | 9,12-Octadecadienoic acid (Z,Z)- |
| 14 | 28.51 | 0.69 | Octadecanoic acid |
| 15 | 30.58 | 0.41 | Octadecane |
| 16 | 32.08 | 0.54 | Nonadecane, 9-methyl- |
| 17 | 32.13 | 0.89 | Hexanedioic acid, bis(2-ethylhexyl) ester |
| 18 | 33.56 | 0.85 | Eicosane |
| 19 | 34.38 | 2.86 | 1,2-Benzenedicarboxylic acid, diisooctyl ester |
| 20 | 35.01 | 0.83 | Eicosane |
| 21 | 36.42 | 1.02 | Eicosane |
| 22 | 37.80 | 0.65 | Eicosane |
| 23 | 38.28 | 0.33 | 2,6,10,14,18,22-Tetracosahexaene, 2,6,10,15,19,23-hexamethyl-, (all-E)- |
| 24 | 38.97 | 0.50 | 2-Ethylacridine |
| 25 | 39.13 | 0.67 | Eicosane |
| 26 | 41.71 | 3.10 | Anthracene, 9,10-dihydro-9,9,10-trimethyl- |
| 27 | 41.79 | 0.95 | 2-(Acetoxymethyl)-3-(methoxycarbonyl)biphenylene |
| 28 | 43.19 | 0.71 | Cyclotrisiloxane, hexamethyl- |
| 29 | 45.35 | 20.10 | 1,3,3-Trimethyl-2-hydroxymethyl-3,3-dimethyl-4-(3-methylbut-2-enyl)-cyclohexene |
| 30 | 45.52 | 5.98 | Benzo[h]quinoline, 2,4-dimethyl- |
| 31 | 46.04 | 3.43 | Benzo[b]naphtho[2,3-d]furan |
| 32 | 46.76 | 0.72 | Cyclotrisiloxane, hexamethyl- |
| 33 | 46.92 | 10.32 | 5(1H)-Azulenone, 2,4,6,7,8,8a-hexahydro-3,8-dimethyl-4-(1-methylethylidene)-, (8S-cis)- |
| 34 | 47.09 | 5.30 | 5-Methyl-5,8-dihydro-1,4-naphthoquinone |
| 35 | 47.94 | 7.70 | 4-Dehydroxy-N-(4,5-methylenedioxy-2-nitrobenzylidene)tyramine |
3. Results and discussion
3.1. Molecular properties of poplar wood extractives
According to GC/MS result, 3 components were identified from The LD-010 wood extractives of P. lasiocarpa. Its main components were 2,4-hexadiyne (97.046%), dibutyl phthalate (0.471%), benzo[h]quinoline, 2,4-dimethyl-(0.723%), carbonic acid, monoamide, N-(2-ethyl- phenyl)-, propyl ester (1.760%).
The 24 components were identified from The LD-021 wood extractives of P. tomentosa. Its main components were benzene, 2-[(tert-butyldimethylsilyl) oxy]-1-isopropyl-4-methyl- (20.61%), 2,4-cyclohexadien-1-one, 3,5-bis(1,1-dimethylethyl)-4-hydroxy- (12.28%), dibutyl phthalate (12.09%), 4-methylmannonic.delta.-lactone (11.42%), 1,2-benzenedicarboxylic acid, diisooctyl ester (5.92%), 5(1h)-azulenone, 2,4,6,7,8,8a-hexahydro-3,8-dimethyl-4-(1-methylethylidene)-, (8s-cis)- (5.72%), 1h-cycloprop[e]azulene, decahydro-1,1,4,7-tetramethyl-, [1ar-(1a.alpha.,4.beta.,4a.beta.,7.beta.,7a.beta.,7b.alpha.)]- (5.67%), 2-pentanone, 1-(2,4,6-trihydroxyphenyl) (4.17%), 1,2,5-oxadiazol-3-amine, 4-(4-methoxyphenoxy)- (3.88%), 2,6,10,14,18,22-tetracosahexaene, 2,6,10,15,19,23-hexamethyl-, (all-e)- (3.42%), 4-((1e)-3-hydroxy-1-propenyl)-2-methoxy-phenol (2.89%), n-hexadecanoic acid (2.11%), 3-phenylbicyclo(3.2.2)nona-3,6-dien-2-one (1.82%), benzo[b] naphtho[2,3-d]furan (1.32%), 9,12-octadecadienoic acid (z,z)- (1.20%), hexanedioic acid, bis(2-ethylhexyl) ester (1.10%), 1h-indole, 1-methyl-2-phenyl- (1.00%), oleic acid (0.70%), benzoic acid, 4-hydroxy- (0.63%), 1,2-benzenedicarboxylic acid, bis(2-methylpropyl) ester (0.54%), thiazol-4(5h)-one, 2-(4-morpholyl)-5-(2-pyridylmethylideno)- (0.43%), 9-octadecyne (0.42%), phthalic acid, hexadecyl propyl ester (0.35%), 3,5-dimethoxy-4-hydroxycinnamaldehyde (0.34%).
The 3 components were identified from the LD-150 wood extractives of P. tomentosa. Its main components were 1,5-hexadien-3-yne (94.04%), dibutyl phthalate (3.95%), 1H-indole, 1-methyl-2-phenyl- (2.01%).
The 27 components were identified from the LD-174 wood extractives of P. lasiocarpa. Its main components were; 1,3,3-trimethyl-2-hydroxymethyl-3,3-dimethyl-4-(3-methylbut-2-enyl)-cyclohexene (20.10%), 5(1h)-azulenone, 2,4,6,7,8,8a-hexahydro-3,8-dimethyl-4-(1-methylethylidene)-, (8s-cis)- (10.32%), n-hexadecanoic acid (8.82%), 4-dehydroxy-n-(4,5-methylenedioxy-2-nitrobenzylidene)tyramine (7.70%), 1,2-benzenedicarboxylic acid, butyl 2-methylpropyl ester (7.36%), benzo[h]quinoline, 2,4-dimethyl- (5.98%), 9,12-octadecadienoic acid (z,z)- (5.49%), 5-methyl-5,8-dihydro-1,4-naphthoquinone (5.30%), phenol, 2,6-dimethyl-4-nitro- (4.30%), eicosane (4.01%), benzo[b]naphtho[2,3-d]furan (3.43%), anthracene, 9,10-dihydro-9,9,10-trimethyl- (3.10%), 1,2-benzenedicarboxylic acid, diisooctyl ester (2.86%), 4-((1e)-3-hydroxy-1-propenyl)-2-methoxyphenol (2.02%), cyclotrisiloxane, hexamethyl- (1.44%), pentadecanoic acid (1.06%), 2-(acetoxymethyl)-3-(methoxycarbonyl)biphenylene (0.95%), hexanedioic acid, bis(2-ethylhexyl) ester (0.90%), 4-ethoxy-2,5-dimethoxybenzaldehyde (0.84%), benzoic acid, 3-ethoxy- (0.75%), octadecanoic acid (0.69%), nonadecane, 9-methyl- (0.54%), 2-ethylacridine (0.5026%), 9-octadecyne (0.49%), octadecane (0.41%), 2,6,10,14,18,22-tetracosahexaene, 2,6,10,15,19,23-hexamethyl-, (all-e)- (0.33%), 3,5-dimethoxy-4-hydroxycinnamaldehyde (0.33%).
3.2. Resource properties of poplar wood extractives
There were many rare components in the extractives of poplar wood. Because of its officinal value, dibutyl phthalate was a pesticide to keep internal environment homeostasis (Wanxi et al., 2014). Hexanedioic acid, bis(2-ethylhexyl) ester, which was an industrial chemical, was used in Canada in various products such as cosmetics and so on (www.chemicalsubstances.gc.ca). Phthalic acid derivatives were the main constituents of Elaeagnaceae plant which could cure chronic cardiovascular and cerebrovascular diseases and had anti-tumor, anti-inflammatory, antibacterial functions (Hao et al., 2006). The n-hexadecanoic acid might help in designing of specific inhibitors of phospholipase A(2) as anti-inflammatory agents whose binding energy was calculated by in silico method and compared with known inhibitors (Aparna et al., 2012, Qureshi et al., 2015). (Z,Z)-9,12-octadecadienoic acid had been identified as the main medical component of dried worms, and has diuretic, swelling and detoxification properties (Guo and Wei, 2006, Wanxi et al., 2013). 2,6,10,14,18,22-tetracosahexaene, 2,6,10,15,19,23-hexamethyl-, (all-E)-, which could protect liver, resist fatigue and strengthen the body’s resistance, and improve human immunity, was considered as important substances in practical and clinical uses with a huge potential in nutraceutical and pharmaceutical industries (Kim and Karadeniz, 2012). And the extractives of poplar wood were rich in drug and biomedical activities. Based on the relative content, P. lasiocarpa wood was fit to extract 2,4-hexadiyne, 1,3,3-trimethyl-2-hydroxymethyl-3,3-dimethyl-4-(3-methylbut-2-enyl)-cyclohexene, and P. tomentosa wood was fit to do (all-E)-2,6,10,15,19,23-hexamethyl-2,6,10,14,18,22-tetracosahexaene, 1,5-hexadien-3-yne.
4. Conclusion
The ethanol/methanol extractives of P. lasiocarpa wood (LD-010), ethanol/methanol extractives of P. tomentosa wood (LD-021), benzene/ethanol extractives of P. tomentosa wood (LD-150), EMPA extractives of P. lasiocarpa wood (LD-174) were identified as having 3, 24, 3 27 components, respectively. P. lasiocarpa wood was fit to extract 2,4-hexadiyne, 1,3,3-trimethyl-2-hydroxymethyl-3,3-dimethyl- 4-(3-methylbut-2-enyl)-cyclohexene, and P. tomentosa wood was fit to extract 1,5-hexadien-3-yne, (all-E)-2,6,10,15,19,23-hexamethyl-2,6,10,14,18,22-tetracosahexaene. And the extractives of poplar wood contained rich and rare drug and biomedical activities.
Acknowledgments
This work was financially supported by the Special Fund for Forest Scientific Research in the Public Welfare (No. 201504507), the National Natural Science Foundation of China (No. 31170532), and the National Forestry Technology Popularization Project (No. [2012]37; No. [2012]60; No. [2012]62).
Contributor Information
Wanxi Peng, Email: pengwanxi@163.com.
Makoto Ohkoshi, Email: mohkoshi@kpu.ac.jp.
References
- Aparna V., Dileep K.V., Mandal P.K., Karthe P., Sadasivan C., Haridas M. Anti-inflammatory property of n-hexadecanoic acid: structural evidence and kinetic assessment. Chem. Biol. Drug Des. 2012;80(3):434–439. doi: 10.1111/j.1747-0285.2012.01418.x. [DOI] [PubMed] [Google Scholar]
- Ashraf M.A., Yusoff I., Yusof I., Alias Y. Study of contaminant transport at an open tipping waste disposal site. Environ. Sci. Pollut. Res. 2013;20(7):4689–4710. doi: 10.1007/s11356-012-1423-x. [DOI] [PubMed] [Google Scholar]
- Ashraf M.A., Ullah S., Ahmad I., Qureshi A.K., Balkhair K.S., Rehman M.A. Green biocides, a promising technology: current and future applications. J. Sci. Food Agric. 2013;94(3):388–403. doi: 10.1002/jsfa.6371. [DOI] [PubMed] [Google Scholar]
- Batool S., Khalid A., Chowdury A.J.K., Sarfraz M., Balkhair K.S., Ashraf M.A. Impacts of azo dye on ammonium oxidation process and ammonia oxidizing soil bacteria. RSC Adv. 2015;5:34812–34820. [Google Scholar]
- Daniel, van der L., Safiyh T., Sean M.M., Luen-Luen L., Stephanie A.M., Denise M., Bryon S.D., Shi-You D., William S.A., Michael E.H., Susannah G.T. The metagenome of an anaerobic microbial community decomposing poplar wood chips. PLoS One. 2012;7(5):e36740. doi: 10.1371/journal.pone.0036740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divya, B. 2011. Poplar tree identification. <http://www.buzzle.com/articles/poplar-tree-identification.html>.
- Guo C., Wei D. Analysis of fatty acid composition from Gryllotalpa Orientalis Burmeister by gas chromatography and its orthogonal design experiment of supercritical fluid extraction. Chin. J. Anal. Chem. 2006;34:15–18. [Google Scholar]
- Hao H., Xin Z., Biao J. Survey in study on chemical constituents from plants of Elaeagnaceae. Chin. Tradit. Herb. Drugs. 2006;37(2):307–309. [Google Scholar]
- Heping Z., James I.L.M., Lester P.S. Transpiration and water relations of poplar trees growing close to the water table. Tree Physiol. 1999;19:563–573. doi: 10.1093/treephys/19.9.563. [DOI] [PubMed] [Google Scholar]
- Kim S.K., Karadeniz F. Biological importance and applications of squalene and squalane. Adv. Food Nutr. Res. 2012;65:223–233. doi: 10.1016/B978-0-12-416003-3.00014-7. [DOI] [PubMed] [Google Scholar]
- Ningxia D., Xin L., Yun L., Shouyi C., Jinsong Z., Da H., Wenguang D., Chunkui S., Yingzhi Z., Paula M.P. Genetic transformation of Populus tomentosa to improve salt tolerance. Plant Cell Tiss. Organ. Cult. 2012;108:181–189. [Google Scholar]
- Naureen R., Tariq M., Yusoff I., Choudhury A.J.K., Ashraf M.A. Synthesis, spectroscopic and chromatographic studies of sunflower oil biodiesel using optimized base catalyzed methanolysis. Saudi J. Biol. Sci. 2014;22:322–339. doi: 10.1016/j.sjbs.2014.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulomi S., Arthur J.R., Gerald A.T. Poplar as a feedstock for biofuels: a review of compositional characteristics. Biofuels Bioprod. Bioref. 2010;4:209–226. [Google Scholar]
- Qureshi T., Memon N., Memon S.Q., Ashraf M.A. Decontamination of ofloxacin: optimization of removal process onto sawdust using response surface methodology. Desalin. Water Treat. 2015;2015:1–9. [Google Scholar]
- Rennenberg H., Wildhagen H., Ehlting B. Nitrogen nutrition of poplar trees. Plant. Biol. (Stuttg) 2010;12(2):275–291. doi: 10.1111/j.1438-8677.2009.00309.x. [DOI] [PubMed] [Google Scholar]
- Surhio M.A., Talpur F.N., Nizamani S.M., Amin F., Bong C.W., Lee C.W., Ashraf M.A., Shahid M.R. Complete degradation of dimethyl phthalate by biochemical cooperation of the Bacillus thuringiensis strain isolated from cotton field soil. RSC Adv. 2014;4:55960–55966. [Google Scholar]
- Wanxi P., Zhi L., Junbo C., Fangliang G., Xiangwei Z. Biomedical molecular characteristics of YBSJ extractives from Illicium Verum fruit. Biotechnol. Biotec. Eq. 2013;27(6):4311–4316. [Google Scholar]
- Wanxi P., Shengbo G., Dongli L., Daochun Q. Molecular characteristics of three extractives of Cinnamomum camphora leaves. Pak. J. Pharm. Sci. 2014;27 [Google Scholar]
- Yoshinori A., Eckhard W. A novel phenolic acid derivative from buds of Populus lasiocarpa. Phytochemistry. 1976;15(5):811–812. [Google Scholar]
- Yusoff I., Alias Y., Yusof M., Ashraf M.A. Assessment of pollutants migration at Ampar Tenang landfill site, Selangor, Malaysia. ScienceAsia. 2013;39:392–409. [Google Scholar]