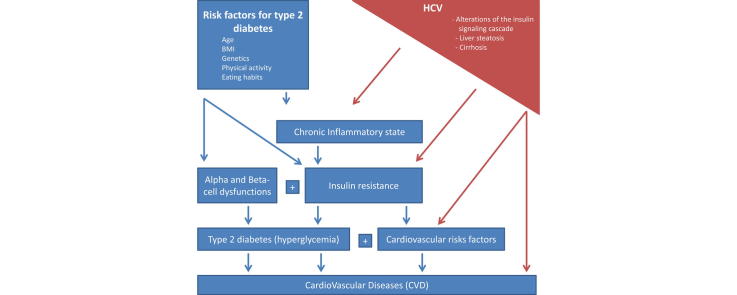
Graphical abstract
Keywords: HCV, Diabetes, Insulin resistance, Inflammation, Steatosis, Direct-acting antivirals
Abstract
The association between hepatitis C virus (HCV) infection and type 2 diabetes (T2D) has been known for over 20 years. Cross-sectional and longitudinal studies have shown a higher prevalence and incidence, respectively, of T2D in patients with chronic HCV infection. HCV induces glucose metabolism alterations mostly interfering with the insulin signaling chain in hepatocytes, although extrahepatic mechanisms seem to contribute. Both IR and T2D accelerate the histological and clinical progression of chronic hepatitis C as well as the risk of extra-hepatic complications such as nephropathy, acute coronary events and ischemic stroke. Before the availability of direct-acting antivirals (DAAs), the therapeutic choice was limited to interferon (IFN)-based therapy, which reduced the incidence of the extra-hepatic manifestations but was burdened with several contraindications and poor tolerability. A better understanding of HCV-associated glucose metabolism derangements and their reversibility is expected with the use of DAAs.
Introduction
Diabetes is one of the most prevalent non-communicable diseases throughout the world, affecting 415 million people in 2015 [1], and one in two patients does not know to suffer from diabetes. The condition is characterized by significant morbidity and mortality with a vast majority of people dying of cardiovascular complications [2].
Hepatitis C virus (HCV) infection is widespread, affecting up to 185 million people worldwide. Most patients are unaware of their infection [3] but at increased risk of liver cirrhosis, hepatocellular carcinoma (HCC) and liver-related mortality [4]. People suffering from HCV infection are also at increased risk of extrahepatic manifestations, and may develop T2D and cardiovascular complications such as nephropathy, acute coronary events and ischemic stroke, whose incidence can be reduced by antiviral treatment [5].
The scope of this review is to discuss the current level of evidence in favor of a causal association between HCV and T2D, its clinical impact, and directions for management.
Epidemiology
The epidemic of T2D is still growing particularly in developing countries with the worldwide rise in obesity. One of the epicenter of the epidemic is Asia’s large population which tends to develop diabetes at younger age and lower BMI. In Western countries the trend is stable and the aging of the population is the main risk factor for diabetes. Chronic HCV is associated with hepatic and peripheral insulin resistance (IR) and the excess diabetes risk in HCV-infected persons is hotly debated. The epidemiological and clinical interactions between HCV and insulin resistance (IR) are complex and multi-layered and have consequences for the progression of HCV, but also the response to therapy and the development of complications. Below we will detail the different facets of the epidemiological and clinical interactions between HCV and IR.
Epidemiological evidence linking HCV to IR
The prevalence of IR or type 2 diabetes (T2D) in patients infected with HCV has been shown to be high. A study assessing an outpatient clinic of a university hospital estimated that more than 30% of HCV subjects had glucose abnormalities [6]. A twofold higher prevalence has even been reported in a Taiwanese cohort when T2D was diagnosed with the use of a 75 g oral glucose tolerance test (OGTT) [7]. However, there are conflicting results, many studies focusing on the association between HCV and IR had other primary objectives when they were planned and the quality of the evidence is sometimes unsatisfactory, especially in the context of retrospective trials (Table 1).
Table 1.
Epidemiological evidences on causal association between HCV infection and Type 2 Diabetes.
| Author | Date | Country | Type of study | Methods | Diagnostic test for diabetes | N patients with HCV (characteristics) | N group of comparison (characteristics) | Results | Result | Conclusions | Biais |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Allison et al. [77] | 1994 | Taiwan | P | Case-cohort analysis from 1991 to 2001 | FPG | 21,559 | 1917 with T2D | 1.53 (95% CI: 1.29–1.81) | Positive | There is an increased risk of T2D in patients with HCV | Diagnostic of T2D is registry based |
| Fraser et al. [78] | 1996 | Israel | R | Cross-sectional study | FPG | 128 | 40 HBV+ | Positive | HCV infection and age were significant and independent predictors for developing diabetes | The HCV+ group had a higher mean age and cirrhosis was more frequent in the HCV+ group | |
| Grimbert et al. [79] | 1996 | France | R | Cross-sectional study (age gender and cirrhosis-matched) | FPG | 152 | 152 hospitalized with HBV, alcohol induced liver disease | T2D in 24% of patients with HCV and 9% in the control group | Positive | T2D is more prevalent in patients with chronic hepatitis C than in patients with other liver diseases. T2D occurs in the absence of family history and obesity in the HCV group. | Inclusion biais (hospitalized patients) |
| Mangia et al. [13] | 1998 | USA | P | Prospective cross-sectional study (age and gender-matched) | FPG | 147/385 non-cirrhotic hospitalized patients | (1) 138 chronic hepatitis (HCV/HBV/alcohol abuse) (2) 494 patients hospitalized for acute osteoarticular trauma |
Negative | The prevalence of T2D was not different among patients with HCV, HBV infection, or alcohol abuse. At multivariate analysis cirrhosis and age were the only two factors independently associated with T2D | BMI is significantly higher in the control group, the absence of a confirmatory OGTT | |
| el-Zayadi et al. [80] | 1998 | Egypt | R | Cross-sectional study | FPG | 591/150 with T2D | 223 HCV−/25 with T2D | Positive | Chronic hepatitis C patients in Egypt is three times more likely to develop T2D than HCV seronegative patients | Inclusion biais | |
| Caronia et al. [81] | 1999 | Italy | R/P | Cross-sectional study and prospective OGTT in patients with chronic HCV or HBV confirmed by biopsy | FPG, OGTT | 1151(retrospective); 197(prospective) | 181 HBV+(retrospective) 38 HBV+(prospective) |
R: 2.78 (95% CI:1.6–4.79); P: T2D in 24.4% of HCV+ patients and 7.9% in patients with HBV related cirrhosis | Positive | The study confirms an association between HCV and T2D. | Inclusion biais (BMI and hereditary for T2D not considered). T2D is associated with the occurrence of cirrhosis |
| Knobler et al. [82] | 2000 | Israel | C-C | Case-control study (age, sex, BMI and origin-matched) | FPG | 45 consecutive eligible patients without cirrhosis | 90 controls with no liver disease | 33% T2D in HCV group and 5.6% T2D in the control group | Positive | Patients with chronic HCV infection have an increased prevalence of type 2 diabetes independently of cirrhosis. | Size of control group |
| Zein et al. [83] | 2000 | USA | R | Cross-sectional study (patients with cirrhosis who underwent liver transplantation were compared to a general population) | FPG | 73 HCV+ 78 cholestatic disease 53 alcoholic disease |
General population | Before transplantation T2D in 16/64 (25%) with HCV alone T2D in 1/78 (1.3%)with cholestatic liver disease T2D in 10/ 53 (19%) with alcoholic liver disease |
Positive | The risk of diabetes is increased in patients with liver cirrhosis due to hepatitis C or alcoholic liver disease | Inclusion biais |
| Mehta et al. [10] | 2000 | USA | P | Cohort study | FPG,HbA1c, MH | 9841 | General population in the USA | 2.48 (95% CI 1.23–5.01) | Positive | Persistent HCV infection is associated with the subsequent development of T2D | No difference in the prevalence of T2D in persons with HCV antibody but not RNA. |
| Ryu et al. [84] | 2001 | Korea | P | Cross-sectional study (age, sex, BMI, cirrhosis, alcohol consumption-matched) | FPG | 404 | 627 T2D | 24% T2D in HCV group and 10.4% T2D in HBV group | Positive | Patients with chronic HCV infection have an increased prevalence of type 2 diabetes in Korean patients. Age and alcohol consumption are another risk factor for T2D in such patients. | The absence of a confirmatory oral glucose tolerance test |
| Mehta et al. [85] | 2003 | USA | P | Case-cohort analysis | FPG, MH | 1048 adults free of T2D | 548 developed T2D over 9 years of follow-up | 11.58 (95% CI 1.39–96.6) | Positive | Pre-existing HCV infection may increase the risk for T2D in persons with recognized diabetes risk factors. | The absence of a confirmatory oral glucose tolerance test |
| Arao et al. [86] | 2003 | Japan | R | Cross-sectional study/case control study to determine the seroprevalence of HCV infection in a cohort of 459 diabetics | FPG, MH | 707 | 159 HBV+ | 20.9% T2D in HCV group 11.9% T2D in HBV group Case-control: 10.5% HCV+ and 1.1% HBV+ in T2D cohort |
In favor | Male sex and cirrhosis were the major independent variable associated with T2D | Male sex and cirrhosis were the major independent variable associated with T2D |
| Antonelli et al. [87] | 2005 | Italy | C-C | Case-control study (population-based age-matched control group) | FPG, MH | 564 non cirrhotic | 302 individuals screened for thyroid disorders (exclusion criteria: history of alcohol abuse, drug addiction, or positivity for markers of viral hepatitis) | The RR for type 2 diabetes in NC-HCV+ patients was 1.81 (95% CI 1.15–2.89) versus control subject and 2.71 (1.08–7.07) versus NC-HBV+ patients | Positive | There is an association of T2D with HCV-related hepatitis. HCV + T2D patients have a different clinical phenotype (lower BMI, no hereditary factors) | |
| Papatheodoridis et al. [88] | 2006 | Greece | R | Cross-sectional study (controlled for HCV genotype, ethnicity, severity of liver disease and fibrosis) | FPG, MH | 260 | 174 HBV+ | 14% T2D in HCV group; 13% T2D in HBV group | Negative | T2D is strongly associated with more severe liver fibrosis. | Severity of liver disease |
| Lecube et al. [6] | 2006 | Spain | C-C | Case-control study | HOMA, OGTT | 28 | 14 HCV− | In favor | Insulin resistance mediated by proinflammatory cytokines, but not a deficit in insulin secretion is the primary pathogenic mechanism involved in the development of diabetes associated with HCV infection | Size of the 2 groups | |
| Simo et al. [89] | 2006 | Spain | P | Longitudinal (cumulative incidence of glucose abnormalities in HCV treated patients) | FPG, IGT | 234/610 screened but excluded due to the presence of T2D or diabetogenic factors | 96 SVR; 138 no SVR | 0.48 (95% CI:0.24–0.98) | Positive | The incidence of glucose abnormalities are independently related to HCV SVR, baseline triglycerides and γ-GT | |
| White et al. [8] | 2008 | M | Meta-analysis (prospective and retrospective studies) | 34 studies | R: 1.68 (95% CI 1.15–2.20) and P: 1.67 (95% CI 1.28–2.06) | Positive | Excess T2D risk with HCV infection in comparison to non-infected controls is consistent in both prospective and retrospective studies | Heterogeneity of the studies | |||
| Huang et al. [7] | 2008 | Taiwan | P | Prospective OGTT | FPG, OGTT | 683 | 515 controls age and sex matched | 27.7% Normoglycemia, 34.6% IGT and 37.8% T2D in 683 patients with HCV; | Positive | There is a 3.5-fold increase in glucose abnormalities in HCV + patients in comparison with controls when OGTT is used as a screening test | HbA1c was not measured |
| Jadoon et al. [90] | 2010 | Pakistan | R | Cross-sectional study | FPG | 3000 T2D (13.7%HCV+) | 10,000 blood donors (4.9% HCV+) | 3.03 (95% CI: 2.64–3.48) | Positive | There is a higher prevalence of HCV infection in patients with T2D | Inclusion biais |
| Elhawary et al. [91] | 2011 | Egypt | C-C | Case-control study | FPG | 289 | 289 healthy controls | 13.84% T2D in HCV group and 4.15% T2D in healthy controls | Positive | The diabetic patients in the HCV group were older, more likely to have a history of alcohol drinking than the non diabetic HCV cases | Inclusion biais |
| Soverini et al. [92] | 2011 | Italy | R | Cross-sectional design (consecutive patients in three Italian centers) | FPG | 859 with T2D | (14 HBV+/51 HCV+) | Negative | The prevalence of HBV and HCV is non-negligible in patients with T2DM and such cases may long remain undiagnosed | ||
| Naing et al. [9] | 2012 | M | Meta-analysis | 35 studies | 7.39 (95% CI: 3.82–9.38) | Positive | Among HCV-infected patients male patients with age over 40 years had an increased frequency of type 2 diabetes | Heterogeneity of the studies | |||
| Memon et al. [93] | 2013 | Pakistan | P | Case series (period of 4 months in 2009) | FPG | 361/120 with T2D (31.5%) | 2.01 (95% CI:1.15, 3.43) | Positive | Advancing age, increased weight, and HCV genotype 3 are independent predictors of type 2 diabetes in HCV seropositive patients, and there is a statistically significant association of cirrhosis observed with type 2 diabetes mellitus | The absence of a confirmatory oral glucose tolerance test. Cirrhosis can be a confounding factor | |
| Ruhl et al. [14] | 2014 | USA | P | Prospective cohort | FPG, HbA1c | 15,128 with diabetes status and HCV antibody or HCV-RNA | General population in the USA | Negative | In the U.S. population, HCV was not associated with diabetes or with IR among persons with normal glucose | Low number of HCV viremic patients, a significant proportion of sampled patients were not examined and the absence of a confirmatory oral glucose tolerance test | |
| Lin et al. [12] | 2016 | Taiwan | P | Case-cohort analysis from 1991 to 2010 | FPG | 21,559 | 1917 with T2D | 10.9% T2D in the anti-HCV seronegative group and 16.7% T2D in the anti-HCV seropositive group | Positive | Chronic HCV infection was associated with an increased risk for diabetes after adjustment for other risk predictors | Insurance registry database |
The types of studies were classified in 4 categories: prospective (P), Retrospective (R), Case-control (C-C) and Meta-analysis (M). The diagnostic tests for T2D are fasting plasma glucose (FPG), Oral glucose tolerance test with 75 g (OGTT), Glycated Hemoglobin (HbA1c), and medical or drug history (MH). N is the number of patients with HCV screened and included and the number of patients included in the control group. All the positive results are statistically significant (P < 0.05). Abbreviations: no sustained viral response (no SVR) and sustained viral response (SV), Type 2 Diabetes (T2D), Hepatitis C virus (HCV), Hepatitis B virus (HBV).
To address whether HCV infection was associated with IR, a systematic review, combining data from 34 studies and covering more than 300,000 patients, found a pooled adjusted odds ratio (OR) for T2D in HCV-infected persons of approximately 1.7 [8]. This was later confirmed by another systematic review including 35 observational studies with a pooled OR of 1.7 for T2D in HCV subjects compared to uninfected controls and a pooled OR of 1.9 when comparing to hepatitis B virus (HBV)-infected controls [9]. In the setting of the Third National Health and Nutrition Examination Survey (NHANES-III), out of 9841 subjects evaluated, in which 8.4% had T2D and 2.1% were anti-HCV-positive, the adjusted OR for T2D in subjects older than 40 years was 3.8 compared to those without HCV infection [10]. A large prospective case-cohort analysis of 1084 adults suggested that HCV increases the risk of diabetes especially in HCV patients who are a priori already at high risk of developing diabetes, i.e. because affected by severe obesity or older than 65: these persons, during FU, were 11 times more likely to develop diabetes than HCV-negative individuals [11]. A recent community-based prospective study from Taiwan showed an increased risk of developing diabetes for HCV-seropositive vs. HCV-seronegative individuals (hazard ratio 1.53, 95% confidence interval [CI] 1.29–1.81), and this is independently of age and BMI [12]. Although the overwhelming majority of evidence pleads in favor of an association between T2D and HCV, some studies have reported diverging conclusions. For instance, Mangia et al. were the first to disprove the association with a prospective study comparing the occurrence of T2D in 247 cirrhotic patients (63.5% HCV positive), 138 patients with chronic hepatitis (73.8% HCV positive) and 494 patients with an acute trauma. The multi-variated analysis found only age and cirrhosis to be associated with T2D but the control groups who were matched for gender and age but not for BMI and T2D diagnosed relied only on fasting plasma glycemia [13]. A recent cross-sectional study from the NHANES database, including 15,128 adult participants of whom 1.7% were anti-HCV positive (but only 1.1% viremic) and 10.5% were diabetic, failed to find an association between HCV and diabetes or IR (assessed by the homeostasis model assessment – HOMA-IR) [14]. The explanation for this discrepancy with the previous literature is unclear, although important limitations of the latter study include a lack of power due to the low number of HCV viremic patients, a significant proportion of sampled patients were not examined and the absence of a confirmatory OGTT.
Virological response on IR
The effect of sustained virological response (SVR) on various clinical outcomes provides another line of evidence linking HCV infection with IR [15]. SVR is associated with a reduction in HCC incidence, liver-related mortality and overall mortality [16]. A number of clinical trials concurred to demonstrate that SVR was associated also with improved IR. For instance, a longitudinal analysis of the Hepatitis C Antiviral Long-Term Treatment against Cirrhosis (HALT-C) Trial found that SVR was associated with an improvement in IR as measured by HOMA2-IR [17] and another study based on the Milan Safety Tolerability study cohort found a reduction of de novo IR development in SVR patients compared to non-SVR patients although the mean baseline and post-treatment HOMA values were similar in SVR patients [18]. Although there was no significant difference in glucose abnormalities incidence in SVR subjects enrolled in another trial of 202 patients treated for HCV in Italy [19], a recent study from Taiwan showed a reduction of diabetic complications, including renal and cardiovascular complications, after antiviral treatment [20]. Although most of these studies were performed in patients undergoing interferon (IFN)-based therapy, recent preliminary reports suggests that direct-acting antiviral (DAA) agents are associated with similar improvement of IR after 12 weeks of treatment [21] and the persistency of a lower fasting glucose levels at 24 weeks from the end of DAA [22].
Therefore, epidemiological evidence linking HCV to IR, is rather compelling, although the association seems strongest in at-risk individuals with additional risk factors such as older age and higher BMI.
T2D and HCV a two-way association [23]
Interestingly, a recent systematic review has also shown a significant association between the presence of T2D and the risk of HCV infection [24]. The review showed that patients with T2D were at an increased risk of acquiring HCV infection compared to non-T2D subjects (pooled OR = 3.50). Although the mechanism underlying this finding could not be identified in this study, the increased risk is likely to be due to the repeated, invasive medical procedures that T2D patients usually undergo, exposing them to blood borne infections if universal precautions are not strictly followed.
Clinical consequences of IR/T2D in HCV
Hepatic fibrosis and cirrhosis
Not only is there a strong epidemiological association between HCV and IR and/or diabetes but IR is strongly associated with worse outcomes and increased fibrosis progression in HCV subjects. Type 2 diabetes and IR were independent predictors of liver-related mortality in a NHANES-III study including 264 chronic HCV subjects [25], [26]. IR was shown to be an independent factor associated with fibrosis progression in HCV subjects (P = 0.03) and was associated with the stage of fibrosis (P < 0.001) [27]. IR is also associated with outcomes in cirrhotic HCV subjects as shown by a study conducted on 348 cirrhotic HCV patients that identified baseline diabetes as independently associated with survival and complications of cirrhosis, including bacterial infections and HCC (P = 0.016) [28]. This finding was confirmed in a Taiwanese study including 6251 HCV subjects where incident diabetes was a risk factor for cirrhosis and decompensated cirrhosis despite adjusting for a wide range of other factors [29]. Interestingly, DNA polymorphisms in the patatin-like phospholipase domain-containing 3 (PNPLA3) gene were the strongest predictor for advanced fibrosis in diabetic subjects although the association was much weaker in non-diabetic subjects, suggesting again a potentiation of constitutional risk factors by IR and diabetes [30]. In addition to liver-related outcomes, a systematic review of 22 observational studies confirmed that HCV was associated with increased cardiovascular diseases, especially in subgroups of patients with diabetes and arterial hypertension [31].
Hepatocellular carcinoma (HCC)
In addition to fibrosis progression and cirrhosis decompensation, diabetes is also associated with an increased risk of HCC development. An association between T2D and HCC across all aetiologies of liver disease had already been shown in previous systematic reviews by pooling case-control or cohort studies (pooled risk ratio, 2.5; 95% confidence interval, 1.9–3.2) [32]. A more recent systematic review assessing the risk of HCC specifically in HCV subjects found that T2D was associated with an increased risk of HCC in this population of patients [33]. Interestingly, steatosis, strongly associated with HCV, the metabolic syndrome and IR, was also associated with the development of HCC. The association of IR and HCC in HCV subjects was confirmed in a retrospective study from Japan including 4302 HCV positive patients with a mean follow-up of 8.1 years that identified T2D as an independent risk factor for the development of HCC, whereas the risk for HCC was reduced when mean Hemoglobin A1c level was below 7.0%, suggesting that improved control of diabetes may reduce HCC risk in these patients [34]. Thus, T2D and HCV appear to act synergistically as risk factors for HCC.
Zheng et al. have also reported a worse graft outcome in HCV-positive recipients with liver grafts from donors with diabetes. The reason of such association is still debated but could be related to pre-existing graft steatosis and fibrosis induced by IR and the occurence of post-transplantation fibrosis secondary to the HCV-recurrence [35].
SVR has been shown to significantly reduce the incidence of HCC in subjects treated with IFN-based regimens [36]. However, it has been recently shown that HCC risk somehow persists after IFN-based SVR (annual risk of 0.33% in a Veterans’ population): here, diabetes was significantly associated with post-SVR HCC, along with presence of cirrhosis, age and HCV genotype 3 [37]. In addition, recent data in 399 SVR subjects followed-up for a median of 7.8 years have shown that diabetes and cirrhosis are risk factors for HCC development despite SVR [38] and another study has shown that even in patients with early fibrosis stages (F0-F2) a pre-treatment diabetes (or even impaired glucose tolerance) is associated with HCC development after SVR (HR 3.8, 95% CI 1.4–10.1), suggesting that antiviral therapy should be initiated early, i.e. before glucose metabolic alterations occur [39].
The effect of IR on efficacy of antiviral therapy
In the era of interferon (IFN)-based therapy, IR seemed associated with lower SVR rates, regardless of viral genotype [40], [41]. Two separate systematic reviews recently addressed this question and found similar results. For instance, when pooling 14 studies involving more than 2700 subjects, HCV subjects with IR had a reduction in SVR rates of 20% compared to HCV subjects without IR and baseline HOMA-IR was strongly associated with response to IFN-based therapy [42]. Nevertheless, attempts to improve IR during IFN-based therapy using antidiabetic drugs have not demonstrated a clear improvement in SVR in the context of pioglitazone therapy [43] or metformin therapy [44]. Interestingly, statin therapy was associated with improved SVR among diabetic subjects receiving IFN-based therapy whereas insulin-dependent diabetic subjects achieved lower SVR rates underlining again the association between IR severity and outcomes in HCV [45].
Although long-term data are lacking, in contrast to the effect on IFN-α based therapy, baseline IR does not seem to affect the outcomes of DAA-based therapy. HOMA-IR scores had no effect on virological response to telaprevir-based regimens [26], [46], danoprevir monotherapy [47] or the sofosbuvir/simeprevir combination [48]. These findings suggest that the prognostic relevance of IR and diabetes in HCV therapy outcome is much reduced compared to IFN-based regimens.
Factors involved in the occurrence of insulin resistance (IR)
T2D is the result of IR and pancreatic dysfunction. The latter is not limited to pancreatic beta cells dysfunction but includes also alpha cell dysregulation. The earliest defect is IR [49]. The factor the most associated with IR is visceral fat, even though the metabolic changes related to overweight and fat disposal remain unexplained. Abbasi et al. have shown that only a quarter of the variance of IR is explained by BMI [50]. Lean individuals may be as insulin resistant as obese patients with T2D and in such cases the only clue for the clinician is fasting hyperinsulinemia [51]. The common risk factors for T2D are age, family history, BMI, sedentarity, smoking habits and the occurrence of cirrhosis.
Obesity, visceral fat and ectopic lipids in skeletal muscles are associated with peripheral and hepatic IR. The mechanisms are mediated through circulating free fatty acids and include generation of lipid metabolites (diacylglycerol), pro-inflammatory cytokines (tumor necrosis factor alpha [TNFα], interleukin (IL)-1beta, IL-6, monocyte-chemoattractant-protein-1 [MCP-1]) and the production of reactive oxygen species [52]. The consequences of the altered adipose tissue metabolism are that in turn it affects skeletal muscle glucose homeostasis and induces both peripheral IR [53], [54] and hepatic IR.
More recently, dysfunctions in the amino acid metabolism have also been associated with impaired insulin sensitivity and increased risk for future diabetes. Wang et al. have found that in a fasting routine examination a panel of amino acids predicted the future development of diabetes in otherwise healthy, normoglycemic individuals and that in obese individuals three amino acids predict future diabetes with a 5-fold higher risk [55]. Other studies have shown in normoglycemic women that an increase in serum branched-chain amino acid concentrations is linked to IR, independently of obesity. The postulated mechanisms could be a downregulation of genes involved in mitochondrial energy metabolism and an increased expression of adipose tissue inflammatory genes [56] even though the branched-chain amino acids have also a direct action on stimulating insulin secretion and potentially participation in early pancreatic beta cell exhaustion.
In individual at high risk of diabetes (impaired fasting or impaired glucose tolerance) lifestyle intervention can reduce the risk of developing diabetes as shown in the Da Qing study or in the Finnish Diabetes Prevention Study [57], [58]. All the interventions were characterized by modest weight loss, improved glycemic control and a reduction in the need for antidiabetic treatment. The supposed mechanism is a reduction of IR associated with preserved insulin secretory capacity due to limited beta cell dysfunction. The improvements observed in glycemia are therefore most likely to occur early in the natural history of diabetes.
Because HCV infection occurs frequently in normoglycemic individuals with unknown degrees of IR and distinct risk factor for T2D, the individual risk is difficult to establish. Moreover, there is not a proper definition of expected insulin sensitivity or reference values permitting to stratify subjects for hepatic or peripheral insulin sensitivity. A 2-step euglycemic hyperinsulinemic clamp is required to establish IR and the HOMA-IR model suffers from a lack of sensitivity on an individual base.
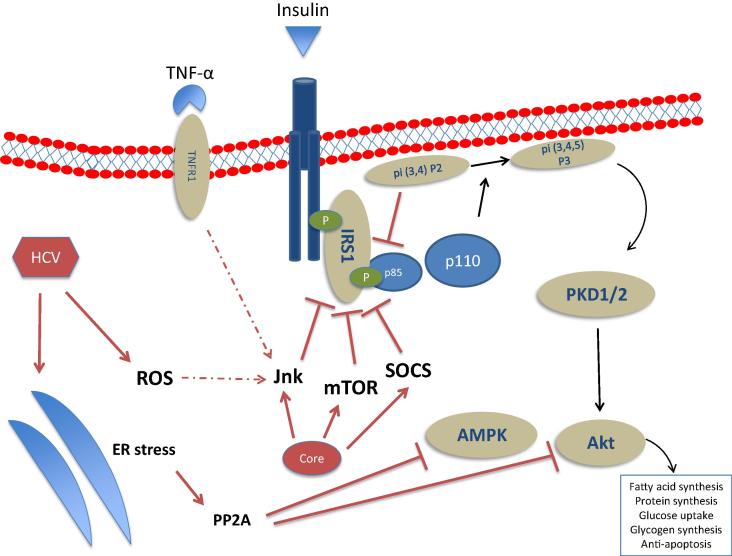
The fact that IR is increased in chronic HCV is confirmed by two studies which have shown in non-obese, non-diabetic hepatitis C patients the existence of hepatic insulin resistance and a decreased peripheral glucose uptake [59], [60]. The extrahepatic propagation of IR in hepatitis C may involve the propagation of inflammatory factors such as TNF-α, IL-8, monocyte chemoattractant protein 1 (MCP-1), IL-18, chemerin and visfatin [61], [62]. Nonetheless, HCV has been shown to directly inhibit the insulin signaling cascade in hepatocytes (Fig. 1). When liver tissue fragments taken from 42 non-obese, non-diabetic chronic hepatitis C patients and age- and BMI-matched controls were incubated with insulin, the insulin-stimulated insulin receptor substrate (IRS)-1 tyrosine phosphorylation, phosphoinositide-3-kinase activity and Akt phosphorylation were decreased in HCV infected vs. uninfected subjects [63], [64]. Experimental models have shown that the HCV core protein is sufficient to induce IR via several mechanisms acting downstream of the insulin receptor. In hepatoma cell lines, HCV core protein stimulates the proteasome degradation of −1 and −2 via the activation of the suppressor of cytokine signaling-3 [65], [66]. HCV may also activate the mTOR [67] or the protein phosphatase 2A (PP2A), an inhibitor of Akt, via an increased endoplasmic reticulum (ER) stress [68] or through a direct activation of the mTOR/S6K1 signaling pathway [69] which occurs in a PTEN-dependent manner [70].
Fig. 1.
Schematic representation of the HCV interactions (both direct and indirect) on the hepatocyte insulin signaling pathway. HCV core can directly activate inhibitors of insulin signaling: the mammalian target of rapamycin (mTOR), the suppressor of cytokine signaling (SOCS)-3, and the c-Jun N-terminal kinase (JNK). HCV increases endoplasmic reticulum (ER) stress which can lead to the activation of the protein phosphatase 2A (PP2A), an inhibitor of Akt and AMP-activated kinase (AMPK) which are key regulators of gluconeogenesis. Other abbreviations: PKD1/2: protein kinase D1/2; p85/p110: subunits p85 and p110 of phosphatidylinositol 3-kinase.
Among the indirect mechanisms, increased endoplasmic reticulum (ER) stress has also been reported to lead to the activation of PP2A by a dual mechanism involving induction of PCG1-alpha and dephosphorylation of Fox01 resulting in Akt inhibition[71]. HCV-induced liver inflammation, leads to an increased release of pro-inflammatory cytokines, such as TNF-α, which may activate stress kinases such as the c-Jun N-terminal kinase (JNK) [64], since a JNK inhibitor was able to revert the effects of the HCV core protein-mediated Ser312 phosphorylation of IRS-1 in an in vitro infection assay. Anti-TNF-α abolished IR also in HCV core transgenic mice [72]. These indirect mechanisms may also be implicated in the pathogenesis of the extrahepatic component of HCV-induced IR, as suggested by the two aforementioned studies, where a combination of euglycemic hyperinsulinemic clamp and calorimetry assay performed in HCV patients without stigmata of the metabolic syndrome pointed out a failing glucose uptake oxidation under hyperinsulinemic conditions, suggesting a HCV-associated reduced glucose uptake, mostly occurring in striated muscles [59], [60].
Conclusions
Since HCV influences the overall metabolism and favors IR by disturbing hepatic and peripheral glucose uptake there is an increased risk of diabetes in susceptible individuals. Limitations include the fact that most of the HCV infected patients studied were younger and of Caucasian or Asian descent. It is therefore expected that not all the study results show a link between HCV infection and T2D, due to preservation of the pancreatic response. Nevertheless, the actual knowledge provides sufficient evidence to clinical physicians and public-health researchers for increasing diabetes screening and prevention among HCV infected patients.
The impact of T2D and HCV infection on health expenditures is a major one. Prevention and screening should be considered as a public health priority [73]. Even though such approach is not yet supported by current guidelines, the simplicity of diabetes screening (HbA1c and fasting glycemia) in HCV infected persons invites the clinician to test regularly. On the other hand, systematic HCV screening in every diabetic patient is not realistic and would involve major costs [74], [75], [76]. Practically, to increase the standards of medical care for patients with T2D and HCV endocrinologist and gastroenterologist need to further collaborate, promptly refer hyperglycemic patients and test the cost efficacy of the existing algorithms [23].
Further research in this area should focus on one hand on the metabolic pathways linking HCV infection and diabetes: the unravelling of these mechanisms may provide insights into the pathogenesis of T2D in general. On the other hand, remaining challenges in the field consist in the management of chronic hepatitis C patients and its complications, both before and after antiviral-induced eradication.
Conflict of Interest
The authors have declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Biographies

Giacomo Gastaldi is a consultant in the diabetology, endocrinology, hypertension and nutrition division at the Department of Specialty Medicine of the University Hospital of Geneva, Switzerland. He is clinical lead for the use of technology in diabetes, lecturer for undergraduate medical and pharmaceutical students and an active teacher at the HES-SO in Geneva for the bachelor in nutrition. He is a committee member of the Swiss Society for Endocrinology and Diabetology. After qualifying with a degree in Medical Sciences at Geneva University in 2001, Dr Gastaldi undertook his clinical training in internal medicine and a certificate in psychosomatic and psychosocial medicine. He obtained his MD at the Geneva Faculty of Medicine in 2009 for his work on the molecular factors that control thermogenesis and their role on insulin resistance after bariatric surgery. Shortly afterward he joined the Department of Endocrinology and Diabetology at the University Hospital of Lapeyronie in Montpellier, France, for a two year fellowship. He gained scientific experience on the metabolic consequences of dysfunctional eating patterns in mice and humans as well as skills in diabetes technology. Since 2011 he is board-certified in endocrinology and diabetology. He is now pursuing his scientific work on the influence of induced thermogenesis on glycemic control and metabolism with a specific target on human insulin resistance.

Dr. Nicolas Goossens is currently a fellow in the Division of Gastroenterology and Hepatology at the Geneva University Hospital under Professor Jean-Louis Frossard. Dr Goossens earned his medical degree in 2005 from Geneva University. After training in Geneva and at the Liver Unit at the King’s College Hospital in London, UK, he received his FMH specialty title in Gastroenterology in 2013 and his subspecialty title in Hepatology in 2014. From 2014 to 2016 he did a research fellowship in the Liver Division of the Mount Sinai School of Medicine under Professors Scott Friedman and Yujin Hoshida. Dr Goossens earned his MSc in Clinical Evidence-Based Healthcare from Oxford University. His research interests have focused on the genomic aspects, prediction and prognosis of liver disease, in particular NAFLD/NASH and hepatocellular carcinoma. Dr Goossens has authored or co-authored more than 20 peer-reviewed manuscripts and reviews in the field of hepatology and gastrointestinal disease.

Dr. Sophie Clément is working at the Division of Clinical Pathology of the University Hospitals of Geneva, Switzerland. In 2005, she joined the Viropathology Unit, headed by Professor Francesco Negro, in the capacity of senior scientist in charge of supervising the different research projects of the laboratory. Dr Clément has obtained her PhD degree in Human Sciences from the University Claude Bernard in Lyon, France, in 1995. After a 2-year post-doctoral training at Northwestern University of Chicago, she joined the laboratory directed by Professor Giulio Gabbiani at the Faculty of Medicine, University of Geneva, mainly focusing her interest on myofibroblast differentiation and fibrosis. Since she joined the laboratory of Professor Negro, she is involved in projects focusing on the metabolic disorders associated with HCV infection, and more specifically on the mechanisms leading to insulin resistance and steatosis. She has published 25 peer-reviewed journal articles in the hepatology and hepatitis field as either first author or co-author.

Francesco Negro is Professor at the Departments of Specialty Medicine and of Pathology and Immunology of the University of Geneva, Switzerland. He is also Founder and Chairman of the Swiss Hepatitis C Cohort Study, and Educational Councilor of the European Association for the Study of the Liver. Professor Negro earned his medical degree in 1982 and was board-certified in Gastroenterology in 1986 at the University of Torino, Italy. He undertook post-doctoral training at the Division of Molecular Virology and Immunology, Georgetown University, USA, and at the Hepatitis Section, National Institute of Allergy and Infectious Diseases, National Institutes of Health, USA, between 1986 and 1989. Professor Negro analyzed hepatitis C virus (HCV) replication at the tissue level using several distinct approaches, establishing anatomo-clinical correlations. His studies led him to associate HCV genotype 3a with a particular form of severe liver steatosis, and to analyze the mechanisms thereof. More recently, Professor Negro’s work has focused on the pathogenesis of extrahepatic manifestations associated with HCV, and, particularly, on the mechanisms leading to glucose metabolism alterations, such as insulin resistance and diabetes, and on the epidemiology of HCV. He has participated in several clinical trials in acute and chronic HCV and has authored or co-authored more than 270 peer-reviewed manuscripts in the field of hepatology.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Cho N.H. Q&A: five questions on the 2015 IDF diabetes atlas. Diabetes Res Clin Pract. 2016;115:157–159. [Google Scholar]
- 2.Group IDFDA. Update of mortality attributable to diabetes for the IDF Diabetes Atlas: estimates for the year 2013. Diabetes Res Clin Pract 2015; 109(3): 461–5. [DOI] [PubMed]
- 3.Lavanchy D. The global burden of hepatitis C. Liver Int. 2009;29(Suppl 1):74–81. doi: 10.1111/j.1478-3231.2008.01934.x. [DOI] [PubMed] [Google Scholar]
- 4.Grebely J., Dore G.J. What is killing people with hepatitis C virus infection? Semin Liver Dis. 2011;31(4):331–339. doi: 10.1055/s-0031-1297922. [DOI] [PubMed] [Google Scholar]
- 5.Negro F., Forton D., Craxi A., Sulkowski M.S., Feld J.J., Manns M.P. Extrahepatic morbidity and mortality of chronic hepatitis C. Gastroenterology. 2015;149(6):1345–1360. doi: 10.1053/j.gastro.2015.08.035. [DOI] [PubMed] [Google Scholar]
- 6.Lecube A., Hernandez C., Genesca J., Esteban J.I., Jardi R., Simo R. High prevalence of glucose abnormalities in patients with hepatitis C virus infection: a multivariate analysis considering the liver injury. Diabetes Care. 2004;27(5):1171–1175. doi: 10.2337/diacare.27.5.1171. [DOI] [PubMed] [Google Scholar]
- 7.Huang J.F., Yu M.L., Dai C.Y., Hsieh M.Y., Hwang S.J., Hsiao P.J. Reappraisal of the characteristics of glucose abnormalities in patients with chronic hepatitis C infection. Am J Gastroenterol. 2008;103(8):1933–1940. doi: 10.1111/j.1572-0241.2008.01996.x. [DOI] [PubMed] [Google Scholar]
- 8.White D.L., Ratziu V., El-Serag H.B. Hepatitis C infection and risk of diabetes: a systematic review and meta-analysis. J Hepatol. 2008;49(5):831–844. doi: 10.1016/j.jhep.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naing C., Mak J.W., Ahmed S.I., Maung M. Relationship between hepatitis C virus infection and type 2 diabetes mellitus: meta-analysis. World J Gastroenterol. 2012;18(14):1642–1651. doi: 10.3748/wjg.v18.i14.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehta S.H., Brancati F.L., Sulkowski M.S., Strathdee S.A., Szklo M., Thomas D.L. Prevalence of type 2 diabetes mellitus among persons with hepatitis C virus infection in the United States. Ann Intern Med. 2000;133(8):592–599. doi: 10.7326/0003-4819-133-8-200010170-00009. [DOI] [PubMed] [Google Scholar]
- 11.Mehta S.H., Brancati F.L., Sulkowski M.S., Strathdee S.A., Szklo M., Thomas D.L. Prevalence of type 2 diabetes mellitus among persons with hepatitis C virus infection in the United States. Hepatology. 2001;33(6):1554. doi: 10.1053/jhep.2001.0103306le01. [DOI] [PubMed] [Google Scholar]
- 12.Lin Y.J., Shaw T.G., Yang H.I., Lu S.N., Jen C.L., Wang L.Y. Chronic hepatitis C virus infection and the risk for diabetes: a community-based prospective study. Liver Int. 2016 doi: 10.1111/liv.13194. [DOI] [PubMed] [Google Scholar]
- 13.Mangia A., Schiavone G., Lezzi G., Marmo R., Bruno F., Villani M.R. HCV and diabetes mellitus: evidence for a negative association. Am J Gastroenterol. 1998;93(12):2363–2367. doi: 10.1111/j.1572-0241.1998.00688.x. [DOI] [PubMed] [Google Scholar]
- 14.Ruhl C.E., Menke A., Cowie C.C., Everhart J.E. Relationship of hepatitis C virus infection with diabetes in the U.S. population. Hepatology. 2014;60(4):1139–1149. doi: 10.1002/hep.27047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vanni E., Bugianesi E., Saracco G. Treatment of type 2 diabetes mellitus by viral eradication in chronic hepatitis C: Myth or reality? Dig Liver Dis. 2016;48(2):105–111. doi: 10.1016/j.dld.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 16.Smith-Palmer J., Cerri K., Valentine W. Achieving sustained virologic response in hepatitis C: a systematic review of the clinical, economic and quality of life benefits. BMC Infect Dis. 2015;15:19. doi: 10.1186/s12879-015-0748-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delgado-Borrego A., Jordan S.H., Negre B., Healey D., Lin W., Kamegaya Y. Reduction of insulin resistance with effective clearance of hepatitis C infection: results from the HALT-C trial. Clin Gastroenterol Hepatol. 2010;8(5):458–462. doi: 10.1016/j.cgh.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aghemo A., Prati G.M., Rumi M.G., Soffredini R., D’Ambrosio R., Orsi E. Sustained virological response prevents the development of insulin resistance in patients with chronic hepatitis C. Hepatology. 2012;56(5):1681–1687. doi: 10.1002/hep.25867. [DOI] [PubMed] [Google Scholar]
- 19.Giordanino C., Bugianesi E., Smedile A., Ciancio A., Abate M.L., Olivero A. Incidence of type 2 diabetes mellitus and glucose abnormalities in patients with chronic hepatitis C infection by response to treatment: results of a cohort study. Am J Gastroenterol. 2008;103(10):2481–2487. doi: 10.1111/j.1572-0241.2008.02002.x. [DOI] [PubMed] [Google Scholar]
- 20.Hsu Y.C., Lin J.T., Ho H.J., Kao Y.H., Huang Y.T., Hsiao N.W. Antiviral treatment for hepatitis C virus infection is associated with improved renal and cardiovascular outcomes in diabetic patients. Hepatology. 2014;59(4):1293–1302. doi: 10.1002/hep.26892. [DOI] [PubMed] [Google Scholar]
- 21.Pavone P., Tieghi T., d’Ettorre G., Lichtner M., Marocco R., Mezzaroma I. Rapid decline of fasting glucose in HCV diabetic patients treated with direct-acting antiviral agents. Clin Microbiol Infect. 2016;22(5) doi: 10.1016/j.cmi.2015.12.030. 462 e1–3. [DOI] [PubMed] [Google Scholar]
- 22.Fabrizio C., Procopio A., Scudeller L., Dell’Acqua R., Bruno G., Milano E. HCV and diabetes: towards a ’sustained’ glycaemic improvement after treatment with DAAs? Clin Microbiol Infect. 2016 doi: 10.1016/j.cmi.2016.09.021. [DOI] [PubMed] [Google Scholar]
- 23.Hammerstad S.S., Grock S.F., Lee H.J., Hasham A., Sundaram N., Tomer Y. Diabetes and hepatitis C: a two-way association. Front Endocrinol (Lausanne) 2015;6:134. doi: 10.3389/fendo.2015.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo X., Jin M., Yang M., Liu K., Li J.W. Type 2 diabetes mellitus and the risk of hepatitis C virus infection: a systematic review. Sci Rep. 2013;3:2981. doi: 10.1038/srep02981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stepanova M., Rafiq N., Younossi Z.M. Components of metabolic syndrome are independent predictors of mortality in patients with chronic liver disease: a population-based study. Gut. 2010;59(10):1410–1415. doi: 10.1136/gut.2010.213553. [DOI] [PubMed] [Google Scholar]
- 26.Younossi Z., Negro F., Serfaty L., Pol S., Diago M., Zeuzem S. Homeostasis model assessment of insulin resistance does not seem to predict response to telaprevir in chronic hepatitis C in the REALIZE trial. Hepatology. 2013;58(6):1897–1906. doi: 10.1002/hep.26437. [DOI] [PubMed] [Google Scholar]
- 27.Hui J.M., Sud A., Farrell G.C., Bandara P., Byth K., Kench J.G. Insulin resistance is associated with chronic hepatitis C virus infection and fibrosis progression [corrected] Gastroenterology. 2003;125(6):1695–1704. doi: 10.1053/j.gastro.2003.08.032. [DOI] [PubMed] [Google Scholar]
- 28.Elkrief L., Chouinard P., Bendersky N., Hajage D., Larroque B., Babany G. Diabetes mellitus is an independent prognostic factor for major liver-related outcomes in patients with cirrhosis and chronic hepatitis C. Hepatology. 2014;60(3):823–831. doi: 10.1002/hep.27228. [DOI] [PubMed] [Google Scholar]
- 29.Huang Y.W., Yang S.S., Fu S.C., Wang T.C., Hsu C.K., Chen D.S. Increased risk of cirrhosis and its decompensation in chronic hepatitis C patients with new-onset diabetes: a nationwide cohort study. Hepatology. 2014;60(3):807–814. doi: 10.1002/hep.27212. [DOI] [PubMed] [Google Scholar]
- 30.Huang C.F., Dai C.Y., Yeh M.L., Huang C.I., Tai C.M., Hsieh M.H. Association of diabetes and PNPLA3 genetic variants with disease severity of patients with chronic hepatitis C virus infection. J Hepatol. 2015;62(3):512–518. doi: 10.1016/j.jhep.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 31.Petta S., Maida M., Macaluso F.S., Barbara M., Licata A., Craxi A. Hepatitis C virus infection is associated with increased cardiovascular mortality: a meta-analysis of observational studies. Gastroenterology. 2016;150(1):145–155. doi: 10.1053/j.gastro.2015.09.007. e4; quiz e15–6. [DOI] [PubMed] [Google Scholar]
- 32.El-Serag H.B., Hampel H., Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol. 2006;4(3):369–380. doi: 10.1016/j.cgh.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 33.Dyal H.K., Aguilar M., Bartos G., Holt E.W., Bhuket T., Liu B. Diabetes mellitus increases risk of hepatocellular carcinoma in chronic hepatitis C virus patients: a systematic review. Dig Dis Sci. 2016;61(2):636–645. doi: 10.1007/s10620-015-3983-3. [DOI] [PubMed] [Google Scholar]
- 34.Arase Y., Kobayashi M., Suzuki F., Suzuki Y., Kawamura Y., Akuta N. Effect of type 2 diabetes on risk for malignancies includes hepatocellular carcinoma in chronic hepatitis C. Hepatology. 2013;57(3):964–973. doi: 10.1002/hep.26087. [DOI] [PubMed] [Google Scholar]
- 35.Zheng J., Xiang J., Zhou J., Li Z., Hu Z., Lo C.M. Liver grafts for transplantation from donors with diabetes: an analysis of the Scientific Registry of Transplant Recipients database. PLoS ONE. 2014;9(5):e98104. doi: 10.1371/journal.pone.0098104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der Meer A.J., Veldt B.J., Feld J.J., Wedemeyer H., Dufour J.F., Lammert F. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308(24):2584–2593. doi: 10.1001/jama.2012.144878. [DOI] [PubMed] [Google Scholar]
- 37.El-Serag H.B., Kanwal F., Richardson P., Kramer J. Risk of hepatocellular carcinoma after sustained virological response in Veterans with hepatitis C virus infection. Hepatology. 2016;64(1):130–137. doi: 10.1002/hep.28535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hedenstierna M., Nangarhari A., Weiland O., Aleman S. Diabetes and cirrhosis are risk factors for hepatocellular carcinoma after successful treatment of chronic hepatitis C. Clin Infect Dis. 2016;63(6):723–729. doi: 10.1093/cid/ciw362. [DOI] [PubMed] [Google Scholar]
- 39.Huang C.F., Yeh M.L., Huang C.Y., Tsai P.C., Ko Y.M., Chen K.Y. Pretreatment glucose status determines HCC development in HCV patients with mild liver disease after curative antiviral therapy. Medicine (Baltimore) 2016;95(27):e4157. doi: 10.1097/MD.0000000000004157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khattab M., Eslam M., Sharwae M.A., Shatat M., Ali A., Hamdy L. Insulin resistance predicts rapid virologic response to peginterferon/ribavirin combination therapy in hepatitis C genotype 4 patients. Am J Gastroenterol. 2010;105(9):1970–1977. doi: 10.1038/ajg.2010.110. [DOI] [PubMed] [Google Scholar]
- 41.Romero-Gomez M., Fernandez-Rodriguez C.M., Andrade R.J., Diago M., Alonso S., Planas R. Effect of sustained virological response to treatment on the incidence of abnormal glucose values in chronic hepatitis C. J Hepatol. 2008;48(5):721–727. doi: 10.1016/j.jhep.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 42.Deltenre P., Louvet A., Lemoine M., Mourad A., Fartoux L., Moreno C. Impact of insulin resistance on sustained response in HCV patients treated with pegylated interferon and ribavirin: a meta-analysis. J Hepatol. 2011;55(6):1187–1194. doi: 10.1016/j.jhep.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 43.Harrison S.A., Hamzeh F.M., Han J., Pandya P.K., Sheikh M.Y., Vierling J.M. Chronic hepatitis C genotype 1 patients with insulin resistance treated with pioglitazone and peginterferon alpha-2a plus ribavirin. Hepatology. 2012;56(2):464–473. doi: 10.1002/hep.25661. [DOI] [PubMed] [Google Scholar]
- 44.Romero-Gomez M., Diago M., Andrade R.J., Calleja J.L., Salmeron J., Fernandez-Rodriguez C.M. Treatment of insulin resistance with metformin in naive genotype 1 chronic hepatitis C patients receiving peginterferon alfa-2a plus ribavirin. Hepatology. 2009;50(6):1702–1708. doi: 10.1002/hep.23206. [DOI] [PubMed] [Google Scholar]
- 45.Rao G.A., Pandya P.K. Statin therapy improves sustained virologic response among diabetic patients with chronic hepatitis C. Gastroenterology. 2011;140(1):144–152. doi: 10.1053/j.gastro.2010.08.055. [DOI] [PubMed] [Google Scholar]
- 46.Serfaty L., Forns X., Goeser T., Ferenci P., Nevens F., Carosi G. Insulin resistance and response to telaprevir plus peginterferon alpha and ribavirin in treatment-naive patients infected with HCV genotype 1. Gut. 2012;61(10):1473–1480. doi: 10.1136/gutjnl-2011-300749. [DOI] [PubMed] [Google Scholar]
- 47.Moucari R., Forestier N., Larrey D., Guyader D., Couzigou P., Benhamou Y. Danoprevir, an HCV NS3/4A protease inhibitor, improves insulin sensitivity in patients with genotype 1 chronic hepatitis C. Gut. 2010;59(12):1694–1698. doi: 10.1136/gut.2010.219089. [DOI] [PubMed] [Google Scholar]
- 48.Willemse S.B., Baak L.C., Kuiken S.D., van der Sluys Veer A., Lettinga K.D., van der Meer J.T. Sofosbuvir plus simeprevir for the treatment of HCV genotype 4 patients with advanced fibrosis or compensated cirrhosis is highly efficacious in real life. J Viral Hepat. 2016 doi: 10.1111/jvh.12567. [DOI] [PubMed] [Google Scholar]
- 49.Tabak A.G., Jokela M., Akbaraly T.N., Brunner E.J., Kivimaki M., Witte D.R. Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: an analysis from the Whitehall II study. Lancet. 2009;373(9682):2215–2221. doi: 10.1016/S0140-6736(09)60619-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abbasi F., Brown B.W., Jr., Lamendola C., McLaughlin T., Reaven G.M. Relationship between obesity, insulin resistance, and coronary heart disease risk. J Am Coll Cardiol. 2002;40(5):937–943. doi: 10.1016/s0735-1097(02)02051-x. [DOI] [PubMed] [Google Scholar]
- 51.Hollenbeck C., Reaven G.M. Variations in insulin-stimulated glucose uptake in healthy individuals with normal glucose tolerance. J Clin Endocrinol Metab. 1987;64(6):1169–1173. doi: 10.1210/jcem-64-6-1169. [DOI] [PubMed] [Google Scholar]
- 52.Boden G. Obesity, insulin resistance and free fatty acids. Curr Opin Endocrinol Diabetes Obes. 2011;18(2):139–143. doi: 10.1097/MED.0b013e3283444b09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kahn S.E., Hull R.L., Utzschneider K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444(7121):840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 54.Guilherme A., Virbasius J.V., Puri V., Czech M.P. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9(5):367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chang I.C., Huang S.F., Chen P.J., Chen C.L., Wu C.C., Tsai C.C. The hepatitis viral status in patients with hepatocellular carcinoma: a study of 3843 patients from Taiwan liver cancer network. Medicine (Baltimore) 2016;95(15):e3284. doi: 10.1097/MD.0000000000003284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wiklund P., Zhang X., Pekkala S., Autio R., Kong L., Yang Y. Insulin resistance is associated with altered amino acid metabolism and adipose tissue dysfunction in normoglycemic women. Sci Rep. 2016;6:24540. doi: 10.1038/srep24540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li G., Hu Y., Yang W., Jiang Y., Wang J., Xiao J. Effects of insulin resistance and insulin secretion on the efficacy of interventions to retard development of type 2 diabetes mellitus: the DA Qing IGT and diabetes study. Diabetes Res Clin Pract. 2002;58(3):193–200. doi: 10.1016/s0168-8227(02)00175-4. [DOI] [PubMed] [Google Scholar]
- 58.Uusitupa M., Lindi V., Louheranta A., Salopuro T., Lindstrom J., Tuomilehto J. Long-term improvement in insulin sensitivity by changing lifestyles of people with impaired glucose tolerance: 4-year results from the Finnish diabetes prevention study. Diabetes. 2003;52(10):2532–2538. doi: 10.2337/diabetes.52.10.2532. [DOI] [PubMed] [Google Scholar]
- 59.Vanni E., Abate M.L., Gentilcore E., Hickman I., Gambino R., Cassader M. Sites and mechanisms of insulin resistance in nonobese, nondiabetic patients with chronic hepatitis C. Hepatology. 2009;50(3):697–706. doi: 10.1002/hep.23031. [DOI] [PubMed] [Google Scholar]
- 60.Milner K.L., van der Poorten D., Trenell M., Jenkins A.B., Xu A., Smythe G. Chronic hepatitis C is associated with peripheral rather than hepatic insulin resistance. Gastroenterology. 2010;138(3):932–941. doi: 10.1053/j.gastro.2009.11.050. e1–3. [DOI] [PubMed] [Google Scholar]
- 61.Mitsuyoshi H., Itoh Y., Sumida Y., Minami M., Yasui K., Nakashima T. Evidence of oxidative stress as a cofactor in the development of insulin resistance in patients with chronic hepatitis C. Hepatol Res. 2008;38(4):348–353. doi: 10.1111/j.1872-034X.2007.00280.x. [DOI] [PubMed] [Google Scholar]
- 62.Knobler H., Zhornicky T., Sandler A., Haran N., Ashur Y., Schattner A. Tumor necrosis factor-alpha-induced insulin resistance may mediate the hepatitis C virus-diabetes association. Am J Gastroenterol. 2003;98(12):2751–2756. doi: 10.1111/j.1572-0241.2003.08728.x. [DOI] [PubMed] [Google Scholar]
- 63.Aytug S., Reich D., Sapiro L.E., Bernstein D., Begum N. Impaired IRS-1/PI3-kinase signaling in patients with HCV: a mechanism for increased prevalence of type 2 diabetes. Hepatology. 2003;38(6):1384–1392. doi: 10.1016/j.hep.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 64.Banerjee S., Saito K., Ait-Goughoulte M., Meyer K., Ray R.B., Ray R. Hepatitis C virus core protein upregulates serine phosphorylation of insulin receptor substrate-1 and impairs the downstream akt/protein kinase B signaling pathway for insulin resistance. J Viro. 2008;82(6):2606–2612. doi: 10.1128/JVI.01672-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kawaguchi T., Yoshida T., Harada M., Hisamoto T., Nagao Y., Ide T. Hepatitis C virus down-regulates insulin receptor substrates 1 and 2 through up-regulation of suppressor of cytokine signaling 3. Am J Pathol. 2004;165(5):1499–1508. doi: 10.1016/S0002-9440(10)63408-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pascarella S., Clement S., Guilloux K., Conzelmann S., Penin F., Negro F. Effects of hepatitis C virus on suppressor of cytokine signaling mRNA levels: comparison between different genotypes and core protein sequence analysis. J Med Virol. 2011;83(6):1005–1015. doi: 10.1002/jmv.22072. [DOI] [PubMed] [Google Scholar]
- 67.Pazienza V., Clement S., Pugnale P., Conzelman S., Foti M., Mangia A. The hepatitis C virus core protein of genotypes 3a and 1b downregulates insulin receptor substrate 1 through genotype-specific mechanisms. Hepatology. 2007;45(5):1164–1171. doi: 10.1002/hep.21634. [DOI] [PubMed] [Google Scholar]
- 68.Christen V., Treves S., Duong F.H., Heim M.H. Activation of endoplasmic reticulum stress response by hepatitis viruses up-regulates protein phosphatase 2A. Hepatology. 2007;46(2):558–565. doi: 10.1002/hep.21611. [DOI] [PubMed] [Google Scholar]
- 69.Bose S.K., Shrivastava S., Meyer K., Ray R.B., Ray R. Hepatitis C virus activates the mTOR/S6K1 signaling pathway in inhibiting IRS-1 function for insulin resistance. J Virol. 2012;86(11):6315–6322. doi: 10.1128/JVI.00050-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gao T.T., Qin Z.L., Ren H., Zhao P., Qi Z.T. Inhibition of IRS-1 by hepatitis C virus infection leads to insulin resistance in a PTEN-dependent manner. Virol J. 2015;12:12. doi: 10.1186/s12985-015-0241-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bernsmeier C., Calabrese D., Heim M.H., Duong H.T. Hepatitis C virus dysregulates glucose homeostasis by a dual mechanism involving induction of PGC1alpha and dephosphorylation of FoxO1. J Viral Hepat. 2014;21(1):9–18. doi: 10.1111/jvh.12208. [DOI] [PubMed] [Google Scholar]
- 72.Shintani Y., Fujie H., Miyoshi H., Tsutsumi T., Tsukamoto K., Kimura S. Hepatitis C virus infection and diabetes: direct involvement of the virus in the development of insulin resistance. Gastroenterology. 2004;126(3):840–848. doi: 10.1053/j.gastro.2003.11.056. [DOI] [PubMed] [Google Scholar]
- 73.da Rocha Fernandes J., Ogurtsova K., Linnenkamp U., Guariguata L., Seuring T., Zhang P. IDF diabetes atlas estimates of 2014 global health expenditures on diabetes. Diabetes Res Clin Pract. 2016;117:48–54. doi: 10.1016/j.diabres.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 74.Alter H.J. The hepatitis C virus and its relationship to the clinical spectrum of NANB hepatitis. J Gastroenterol Hepatol. 1990;5(Suppl 1):78–94. doi: 10.1111/j.1440-1746.1990.tb01783.x. [DOI] [PubMed] [Google Scholar]
- 75.Villano S.A., Vlahov D., Nelson K.E., Lyles C.M., Cohn S., Thomas D.L. Incidence and risk factors for hepatitis C among injection drug users in Baltimore, Maryland. J Clin Microbiol. 1997;35(12):3274–3277. doi: 10.1128/jcm.35.12.3274-3277.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Karmochkine M., Carrat F., Dos Santos O., Cacoub P., Raguin G. A case-control study of risk factors for hepatitis C infection in patients with unexplained routes of infection. J Viral Hepat. 2006;13(11):775–782. doi: 10.1111/j.1365-2893.2006.00742.x. [DOI] [PubMed] [Google Scholar]
- 77.Allison M.E., Wreghitt T., Palmer C.R., Alexander G.J. Evidence for a link between hepatitis C virus infection and diabetes mellitus in a cirrhotic population. J Hepatol. 1994;21(6):1135–1139. doi: 10.1016/s0168-8278(05)80631-2. [DOI] [PubMed] [Google Scholar]
- 78.Fraser G.M., Harman I., Meller N., Niv Y., Porath A. Diabetes mellitus is associated with chronic hepatitis C but not chronic hepatitis B infection. Isr J Med Sci. 1996;32(7):526–530. [PubMed] [Google Scholar]
- 79.Grimbert S., Valensi P., Levy-Marchal C., Perret G., Richardet J.P., Raffoux C. High prevalence of diabetes mellitus in patients with chronic hepatitis C. A case-control study. Gastroenterol Clin Biol. 1996;20(6–7):544–548. [PubMed] [Google Scholar]
- 80.el-Zayadi A.R., Selim O.E., Hamdy H., Dabbous H., Ahdy A., Moniem S.A. Association of chronic hepatitis C infection and diabetes mellitus. Trop Gastroenterol. 1998;19(4):141–144. [PubMed] [Google Scholar]
- 81.Caronia S., Taylor K., Pagliaro L., Carr C., Palazzo U., Petrik J. Further evidence for an association between non-insulin-dependent diabetes mellitus and chronic hepatitis C virus infection. Hepatology. 1999;30(4):1059–1063. doi: 10.1002/hep.510300416. [DOI] [PubMed] [Google Scholar]
- 82.Knobler H., Schihmanter R., Zifroni A., Fenakel G., Schattner A. Increased risk of type 2 diabetes in noncirrhotic patients with chronic hepatitis C virus infection. Mayo Clin Proc. 2000;75(4):355–359. doi: 10.4065/75.4.355. [DOI] [PubMed] [Google Scholar]
- 83.Zein N.N., Abdulkarim A.S., Wiesner R.H., Egan K.S., Persing D.H. Prevalence of diabetes mellitus in patients with end-stage liver cirrhosis due to hepatitis C, alcohol, or cholestatic disease. J Hepatol. 2000;32(2):209–217. doi: 10.1016/s0168-8278(00)80065-3. [DOI] [PubMed] [Google Scholar]
- 84.Ryu J.K., Lee S.B., Hong S.J., Lee S. Association of chronic hepatitis C virus infection and diabetes mellitus in Korean patients. Korean J Int Med. 2001;16(1):18–23. doi: 10.3904/kjim.2001.16.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mehta S.H., Brancati F.L., Strathdee S.A., Pankow J.S., Netski D., Coresh J. Hepatitis C virus infection and incident type 2 diabetes. Hepatology. 2003;38(1):50–56. doi: 10.1053/jhep.2003.50291. [DOI] [PubMed] [Google Scholar]
- 86.Arao M., Murase K., Kusakabe A., Yoshioka K., Fukuzawa Y., Ishikawa T. Prevalence of diabetes mellitus in Japanese patients infected chronically with hepatitis C virus. J Gastroenterol. 2003;38(4):355–360. doi: 10.1007/s005350300063. [DOI] [PubMed] [Google Scholar]
- 87.Antonelli A., Ferri C., Fallahi P., Pampana A., Ferrari S.M., Goglia F. Hepatitis C virus infection: evidence for an association with type 2 diabetes. Diabetes Care. 2005;28(10):2548–2550. doi: 10.2337/diacare.28.10.2548. [DOI] [PubMed] [Google Scholar]
- 88.Papatheodoridis G.V., Chrysanthos N., Savvas S., Sevastianos V., Kafiri G., Petraki K. Diabetes mellitus in chronic hepatitis B and C: prevalence and potential association with the extent of liver fibrosis. J Viral Hepat. 2006;13(5):303–310. doi: 10.1111/j.1365-2893.2005.00677.x. [DOI] [PubMed] [Google Scholar]
- 89.Simo R., Lecube A., Genesca J., Esteban J.I., Hernandez C. Sustained virological response correlates with reduction in the incidence of glucose abnormalities in patients with chronic hepatitis C virus infection. Diabetes Care. 2006;29(11):2462–2466. doi: 10.2337/dc06-0456. [DOI] [PubMed] [Google Scholar]
- 90.Jadoon N.A., Shahzad M.A., Yaqoob R., Hussain M., Ali N. Seroprevalence of hepatitis C in type 2 diabetes: evidence for a positive association. Virol J. 2010;7:304. doi: 10.1186/1743-422X-7-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Elhawary E.I., Mahmoud G.F., El-Daly M.A., Mekky F.A., Esmat G.G., Abdel-Hamid M. Association of HCV with diabetes mellitus: an Egyptian case-control study. Virol J. 2011;8:367. doi: 10.1186/1743-422X-8-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Soverini V., Persico M., Bugianesi E., Forlani G., Salamone F., Massarone M. HBV and HCV infection in type 2 diabetes mellitus: a survey in three diabetes units in different Italian areas. Acta Diabetol. 2011;48(4):337–343. doi: 10.1007/s00592-011-0293-x. [DOI] [PubMed] [Google Scholar]
- 93.Memon M.S., Arain Z.I., Naz F., Zaki M., Kumar S., Burney A.A. Prevalence of type 2 diabetes mellitus in hepatitis C virus infected population: a Southeast Asian study. J Diabetes Res. 2013;2013:539361. doi: 10.1155/2013/539361. [DOI] [PMC free article] [PubMed] [Google Scholar]