Abstract
Nitrogen (N), phosphorous (P) and potassium (K) are the most limiting factors in crop production. N often affects the amino acid composition of protein and in turn its nutritional quality. In Brassica plants, abundant supply of N fertilizer decreases the relative proportion of glucosinolates (GSLs), thus reducing the biological and medical values of the vegetables. Hence effort was made to evaluate the influence of different proportions of nutrient solutions containing N–P–K on the GSL profiles of rocket salad (Eruca sativa Mill.). Fifteen desulpho-(DS) GSLs were isolated and identified using liquid chromatography–mass spectrometry (LC/MS) analysis. Rocket salad plants supplied with lesser amount of N, P or higher concentrations of K showed a typical improvement in total GSL contents. In contrast, total GSL levels were less at higher N supply. Furthermore, with N concentrations above 5 mM and K concentrations less than 2.5 mM, the GSL amounts were on average 13.51 and 13.75 μmol/g dry weight (DW), respectively. Aliphatic GSLs predominated in all concentrations of NPK while indolyl GSLs made up marginally less amount of the total compositions. Five and 2 mM N and P possessed much higher levels of several types of aliphatic GSLs than other concentrations, including glucoerucin, glucoraphanin and dimeric 4-mercaptobutyl GSL. From this perspective, it is contended that supply of less N results in enhancing the metabolic pathway for the synthesis of GSLs in rocket salad.
Keywords: Eruca sativa L, NPK, Glucosinolate, Glucoraphanin, Glucoerucin, Dimeric 4-mercaptobutyl GSL, HPLC
1. Introduction
The requirements of Nitrogen–Phosphorous–Potassium (N–P–K)-containing fertilizers have been dramatically increased in the past 60 years (Burt et al., 2009). Fertilizers are important to determine the nutritional content (Martinez-Ballestra et al., 2008). Different methods were employed for the production of fertilizers which in turn improves the nutritional quality and levels of minerals, phyto-nutrients and vitamins. Several reports state the possibility of improving polyphenols, antioxidants, anthocyanins, vitamin C, and micro nutrients in vegetables and fruits by the organic fertilizers method (Peck et al., 2006) but on contrary several researchers found no effect (Hoefkens et al., 2009). Rocket salad (Eruca sativa Mill.) is a well known fresh vegetable that belonged to the Brassica family. The consumption of vegetables as fresh salad has been increased world wide because of the health consciousness. Leaves of rocket salad are mainly used as an astringent, antiphlogistic, diuretic, tonic, depurative, emollient, laxative, digestive, stimulant, and rubefacient (Yaniv et al., 1998). Similar to other Brassica vegetables, rocket salad is known for various phytochemical metabolites such as polyphenols, vitamin C and GSLs (Lazzeri et al., 2003, Kim et al., 2006, Martınez-Sanchez et al., 2008). Among the bioactive components, GSLs are mainly involved in biological activities. Based on the amino acid side chain GSLs are classified as aliphatic, indolyl, or aromatic (Verkerk et al., 2009). Generally, levels of glucoerucin were high and the amounts of 4-glucoraphanin were less in the seeds of rocket salad (Iori et al., 1999). Recent report claimed that glucosativin was the dominant GSL in both Diplotaxis and Eruca species (Bennett et al., 2006).
Since N is an integral part of the plant structure, optimum level of N is required for the development of the plant growth (Benbrook, 2009). The high supplementation of N is not as widely studied, whereas the accumulation of nitrate (NO3) in plants there has been studied extensively. The nitrate present in the plants causes methemoglobinaemia in infants and causes abnormal acid secretion in the intestine and stomach (Umar and Iqbal, 2006). Therefore, it is suggested to consume less nitrate containing vegetables (Santamaria, 2006, Powlson et al., 2008). Increasing nitrogen (N) tends to decrease total GSL content in different Brassica crops (Chen et al., 2006, Li et al., 2007), however the influence of N on GSL content in the Brassicaceae family has been widely studied (Rangkadilok et al., 2004). Therefore, this study aimed to study the effects of N–P–K nutrients on GSL concentration in rocket salad.
2. Materials and methods
2.1. Chemicals and reagents
Sinigrin (2-propenyl GSL), aryl sulfatase (Type H-1, EC 3.1.6.1) and DEAE-Sephadex A-25 were bought from Sigma–Aldrich Chemical Company (St Louis, MO, USA). Ultra-pure water was prepared by PURELAB Option-Q system (ELGA LabWater, VWS Ltd., UK). HPLC-grade acetonitrile (CH3CN) was obtained from J.T Baker chemical CO. and sodium acetate trihydrate (CH3COOHNa·3H2O) was procured from Hayashi Pure Chemical Industries, Ltd. (Osaka, Japan).
2.2. Plant materials and culture conditions
Rocket seeds ‘Odyssey’ were purchased from Sakata seed Company (Yokohama, Japan). The seeds were sown in a 72 hole plug tray with bed soil by supplying water at regular internals (September 3, 2010) and the seedlings were transplanted to vermiculite after two weeks (14 days after sowing, DAS). All the plants were cultivated in the completely monitored greenhouse environment at the Chungnam National University (Daejeon, Korea). Temperature, light and humidity were noted as 24.8 °C, 178.3 μmol m−2 s−1 and 11.4%, respectively. After transplanting, first week every rocket salad was treated with N–P–K, 10–1–5 mM nutrient solutions. After that for 7 weeks, each rocket salad was treated with different N–P–K molar concentrations (Table 1). Rocket salads were harvested on November 10 (69 DAS), and their leaves were washed thoroughly with water and the length and fresh weight measured(Table 2). The plants were freeze dried, after being powdered using the motor and pestle and stored in the refrigerator for the analysis of GSLs.
Table 1.
Composition of nutrient solutions for different N–P–K molar concentrations.
| Nutrients | N–P–K treatments (mM) |
||||||
|---|---|---|---|---|---|---|---|
| Control | N1/2 | N2 | P1/2 | P2 | K1/2 | K2 | |
| (10–1–5) | (5–1–5) | (20–1–5) | (10–0.5–5) | (10–2–5) | (10–1–2.5) | (10–1–10) | |
| KCl | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Ca(NO3)2 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| NH4NO3 | 2.5 | – | 7.5 | 2.5 | 2.0/5.0 | 2.5 | 2.5 |
| NH4H2PO4 | 1.0 | 1.0 | 1.0 | 0.5 | 2.0 | 1.0 | 1.0 |
| MgSO4 | 0.5 | 0.5 | – | 0.5 | 0.5 | 0.5 | 0.5 |
| MgCl2 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Na2SO4 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| NH4Cl | – | – | – | 0.5 | – | – | – |
| Micronutrients | Concentrations | |
| ppm | g/L | |
| MnCl2 | 0.50 | 1.8010 |
| H3BO3 | 0.50 | 2.8600 |
| ZnSO4 | 0.05 | 0.2220 |
| (NH4)Mo₇O24·4H2O | 0.01 | 0.0920 |
| CuSO4·5H2O | 0.02 | 0.0785 |
| Fe-EDTA | 3.00 | 22.6200 |
(5–1–5); half concentration of N from the control treatment, (20–1–5); double concentration of N from the control treatment, (10–0.5–5); half concentration of P from the control treatment, (10–2–5); double concentration of P from the control treatment, (10–1–2.5); half concentration of K from the control treatment, (10–1–10); double concentration of P from the control treatment.
Table 2.
Plant growth of rocket salad.
| Plant growth | N–P–K treatments (mM) |
||||||
|---|---|---|---|---|---|---|---|
| Control | N1/2 | N2 | P1/2 | P2 | K1/2 | K2 | |
| (10–1–5) | (5–1–5) | (20–1–5) | (10–0.5–5) | (10–2–5) | (10–1–2.5) | (10–1–10) | |
| Height (cm) | 22.1 | 26.4 | 28.5 | 23.9 | 23.8 | 27.2 | 19.5 |
| Fresh weight (g) | 27.9 | 27.6 | 19.4 | 33.1 | 23.3 | 36.6 | 17.4 |
(5–1–5); half concentration of N from the control treatment, (20–1–5); double concentration of N from the control treatment, (10–0.5–5); half concentration of P from the control treatment, (10–2–5); double concentration of P from the control treatment, (10–1–2.5); half concentration of K from the control treatment, (10–1–10); double concentration of P from the control treatment.
2.3. Extraction and desulphation of glucosinolates
Glucosinolates were extracted and desulfated by following the method of Kim et al., 2007 and (ISO 9167-1, 1992). Briefly, 100 mg of the freeze dried samples were mixed with 70% (v/v) methanol for 5 min. After proper mixing the samples were filtered using sterile filter paper and centrifuged in ice-cold condition at 12,000 rpm for 15 min. After centrifugation the tubes were collected very carefully and debris free supernatant was transferred into fresh tubes for the extraction of GSLs. The crude GSLs were desulfated using Sephadex A-25 previously activated with 0.5 M sodium acetate (ca. 40 mg as dry matrix) in an anion exchange column. Aryl sulfatase (E.C.3.1.6.1) (75 μl) was transferred into the column for the complete desulfation of the GSLs. After desulfation reaction for 16 h at room temperature, the desulfated GSLs were eluted with 0.5 ml (×3 times) of distilled water. The eluates were filtered through 0.45 μm Teflon PTFE syringe filter and analyzed immediately by HPLC or stored at −20 °C until further chemical analysis. Separately, 5 ml of sinigrin (5 mg/50 ml) as an external standard was desulfated with the same procedure mentioned above.
2.4. Desulpho-glucosinolate analysis using HPLC
For quantitative analysis, separation of desulpho (DS)-GSL was conducted on a C18 column (150 × 3.0 mm i.d., 3 μm, Inertsil ODS-3; GL Sciences, Tokyo, Japan) using a HPLC system equipped with a diode array detector (Agilent Technologies, CA, USA). The UV–Visible detector wavelength was set at 227 nm. The elution solvent consisted of solvent A (ultra-pure water) and solvent B (acetonitrile). The samples were run for 40 min to separate entire compounds. The gradient solvent system used for the HPLC separation is mentioned in our previous report (Chun et al., 2013). For MS analysis, the eluate was diverted to an API 4000 Q TRAP tandem mass spectrometer (Applied Biosystems, Foster City, CA, USA) equipped with electrospray ionization source in positive ion mode. The spray voltage was set to 5.5 kV and the capillary temperature was set to 550 °C. The scan of the masses ranged from m/z 100 to m/z 800.
3. Results and discussion
3.1. Identification and quantification of glucosinolates
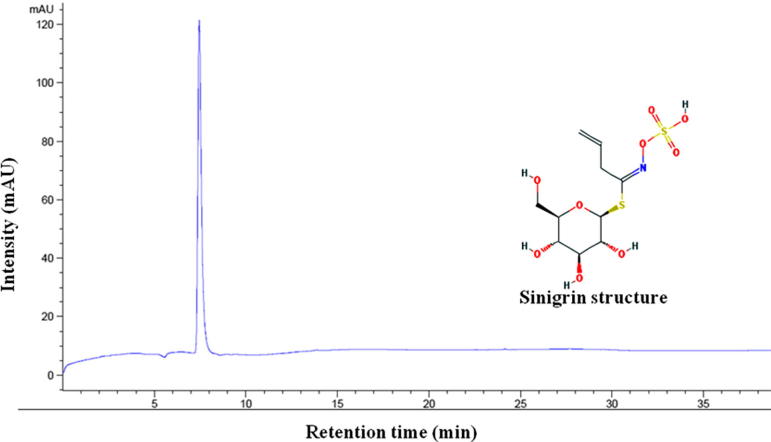
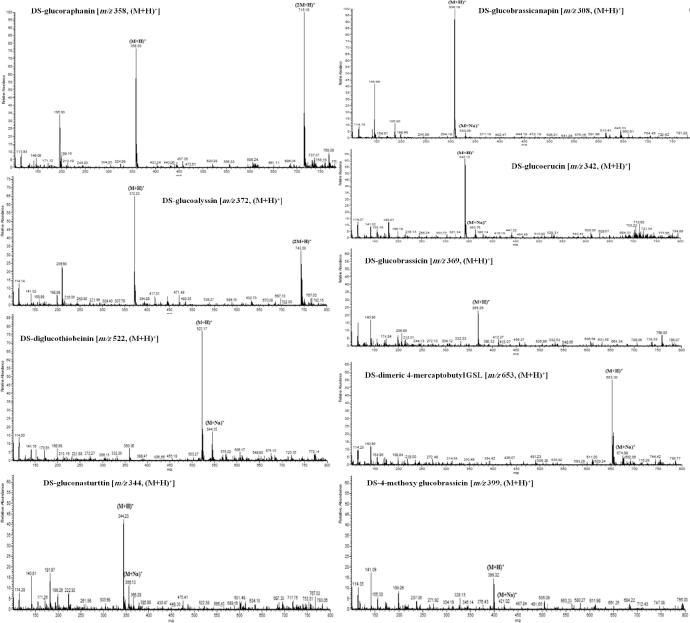
GSLs were separated based on their retention times in HPLC and compared with standard sinigrin (Figure 1, Figure 2 and Table 3). The molecular mass and chemical formula of individual GSLs were confirmed by mass spectral data using positive-ion ESI-MS mode (Barbieri et al., 2008, Lee et al., 2015). GSL identities, including chemical structures, trivial (semisystematic) names, response factor and molecular weights are listed in Fig. 3 and Table 3. Fifteen GSLs belonging to three chemical classes, including five aliphatic GSLs (sinigrin, glucoraphanin, glucoalyssin, glucoerucin and glucobrassicanapin), two indolyl GSLs (4-methoxyglucobrassicin and glucobrassicin), one aromatic GSL (gluconasturtiin), two structurally related compounds containing one inter molecular disulfide linkage (4-[β-d-glucopyranosyldisulfanyl] butyl-GSL and dimeric 4-mercaptobutyl-GSL) and five unknown GSLs were identified (Table 4). The amounts of identified GSLs were quantified with respect to sinigrin and response factor (ISO 9167-1, 1992).
Figure 1.
HPLC chromatogram and chemical structure of sinigrin.
Figure 2.
HPLC chromatograms of glucosinolate (GSL) isolated from the seed sprouts of rocket salad (a) N1/2 treatment (N–P–K, 5–1–5 mM), (b) control (10–1–5), (c) N2 (20–1–5). Peak numbers refer to the GSLs listed in Table 3.
Table 3.
Identification of glucosinolate from rocket salad.
| No | RT | Trivial name | (Semisystematic name) | Molecular weighta | Response factorb |
|---|---|---|---|---|---|
| 1 | 6.14 | Glucoraphanin | (4-methylsulfinylbutyl GSL) | 357 | 1.07 |
| 2 | 7.72 | Sinigrin | (2-propenyl GSL)c | 279 | 1.00 |
| 3 | 8.53 | Glucoalyssin | (5-(methylsufinyl)pentyl) | 371 | 1.07 |
| 4 | 13.20 | Diglucothiobeinin | (4-(β-d-glucopyranosyldisulfanyl)butyl GSL) | 521 | 1.00†† |
| 5 | 16.19 | Glucobrassicanapin | (4-pentenyl GSL) | 307 | 1.15 |
| 6 | 18.65 | Unknown | Unknown | NDd | 1.00†† |
| 7 | 20.77 | Glucoerucin | (4-methylthiobutyl GSL) | 341 | 1.00†† |
| 8 | 21.90 | Unknown | Unknown | ND | 1.00†† |
| 9 | 22.77 | Glucobrassicin | (3-indolylmethyl GSL) | 368 | 0.29 |
| 10 | 24.41 | – | Dimeric 4-mercaptobutyl GSL | 652 | 1.00†† |
| 11 | 25.23 | – | 4-methoxy glucobrassicin | 398 | 0.25 |
| 12 | 26.04 | Gluconasturtiin | (2-phenylethyl) | 343 | 0.95 |
| 13 | 27.19 | Unknown | Unknown | ND | 1.00†† |
| 14 | 28.71 | Unknown | Unknown | ND | 1.00†† |
| 15 | 33.66 | Unknown | Unknown | ND | 1.00†† |
As desulfo-glucosinolates.
International Organization for Standardization (ISO 9167-11992).
External standard.
ND – Not detected.
Undecided by the ISO.
Figure 3.
Mass spectrum of glucosinolate (GSL) isolated from the seed sprouts of rocket salad.
Table 4.
Effects of nutrient solution on glucosinolate contents (μmol/g DW.) in rocket salad (n = 4).
| Noa | N–P–K treatments (mM) |
||||||
|---|---|---|---|---|---|---|---|
| Control | N1/2 | N2 | P1/2 | P2 | K1/2 | K2 | |
| (10–1–5) | (5–1–5) | (20–1–5) | (10–0.5–5) | (10–2–5) | (10–1–2.5) | (10–1–10) | |
| 1 | 2.09 ± 0.46 | 4.13 ± 1.02 | 1.62 ± 0.76 | 2.07 ± 1.10 | 3.25 ± 3.05 | 1.38 ± 0.43 | 3.85 ± 4.08 |
| 2 | ND | ND | ND | ND | 0.08 | 0.08 | 0.10 ± 0.02 |
| 3 | 0.09 ± 0.00 | ND | 0.08 | ND | 0.08 | 0.07 | 0.09 ± 0.01 |
| 4 | 0.50 ± 0.29 | 1.11 ± 0.25 | 0.83 ± 0.52 | 0.63 ± 0.44 | 0.37 ± 0.10 | 0.86 ± 0.47 | 0.85 ± 0.60 |
| 5 | 0.15 ± 0.07 | 0.59 ± 0.24 | 0.22 ± 0.16 | ND ± ND | 0.73 ± 0.42 | ND | ND |
| 6 | 0.12 ± 0.05 | 0.24 ± 0.03 | 0.13 ± 0.05 | 0.20 ± 0.17 | 0.15 ± 0.04 | 0.10 ± 0.05 | 0.23 ± 0.03 |
| 7 | 4.88 ± 5.35 | 11.47 ± 4.01 | 4.22 ± 2.46 | 4.04 ± 2.47 | 5.18 ± 2.94 | 4.52 ± 2.85 | 4.72 ± 3.59 |
| 8 | 0.18 ± 0.09 | 0.33 ± 0.11 | 0.11 ± 0.02 | 0.36 ± 0.31 | 0.38 ± 0.18 | 0.15 ± 0.12 | 0.40 ± 0.18 |
| 9 | 0.06 ± 0.02 | 0.08 ± 0.03 | 0.03 ± 0.01 | 0.08 ± 0.04 | 0.07 ± 0.03 | 0.03 ± 0.00 | 0.06 ± 0.02 |
| 10 | 6.37 ± 3.68 | 11.31 ± 2.25 | 4.23 ± 2.00 | 13.75 ± 10.68 | 18.61 ± 3.60 | 4.72 ± 3.69 | 10.89 ± 8.25 |
| 11 | 0.88 ± 0.32 | 1.67 ± 0.96 | 0.56 ± 0.14 | 1.16 ± 0.39 | 1.44 ± 0.55 | 0.68 ± 0.22 | 1.07 ± 0.08 |
| 12 | ND | ND | 0.15 ± 0.04 | ND | 0.17 ± 0.03 | 0.10 ± 0.04 | 0.12 ± 0.04 |
| 13 | ND | ND | 0.17 ± 0.11 | ND | 0.12 ± 0.03 | 0.12 ± 0.00 | 0.12 ± 0.02 |
| 14 | 0.40 ± 0.18 | 0.61 ± 0.08 | 0.21 ± 0.08 | 0.63 ± 0.50 | 0.50 ± 0.24 | 0.28 ± 0.16 | 0.60 ± 0.18 |
| 15 | 1.14 ± 0.66 | 1.31 ± 0.62 | 0.95 ± 0.42 | 0.88 ± 0.29 | 0.87 ± 0.32 | 0.65 ± 0.31 | 1.34 ± 0.78 |
| Total | 16.86 ± 8.84 | 32.86 ± 6.23 | 13.51 ± 6.17 | 23.80 ± 15.48 | 32.02 ± 5.46 | 13.75 ± 6.22 | 24.43 ± 15.97 |
ND – not detected.
(5–1–5); half concentration of N from the control treatment, (20–1–5); double concentration of N from the control treatment, (10–0.5–5); half concentration of P from the control treatment, (10–2–5); double concentration of P from the control treatment, (10–1–2.5); half concentration of K from the control treatment, (10–1–10); double concentration of P from the control treatment.
Numbers from Table 3.
The proportions of total GSL content and individual GSLs are shown in Table 4. Analyses of GSLs in various concentrations of nutrient solution in rocket salad showed distinct differences in profiles. Amount of GSLs was expressed in μmol/g dry weight (DW). The level of total GSLs in different NPK ratios varied nearly 2.5-fold ranging from a high of 32.86 μmol/g DW observed in 50% less concentration of N [(N1/2) (5–1–5) to a low of 13.51 μmol/g DW in 100% higher concentration of N [(N2) (20–1–5)]. An intermediate level was observed in 50% less concentration of K [(K1/2) (10–1–2.5) 13.75 μmol/g DW, whereas total GSL content in control condition (10–1–5) showed 16.86 μmol/g DW. Results also revealed significant differences in amounts of individual GSLs between different proportions of nutrient concentrations used. Two GSLs, glucoerucin and dimeric 4-mercaptobutyl GSL, were found to be the predominant in all the conditions and documented >4 μmol/g DW or 16–58% of the total GSL content, overall glucoraphanin detected 1.62–3.85 μmol/g DW. Although glucoraphanin was detected in all the conditions, the levels were comparatively higher to indolyl GSL.
Aliphatic GSLs were predominant in all the conditions, representing 80% of total GSL content. Dimeric 4-mercaptobutyl GSL was the most dominant (32–56% of total GSL content) followed by glucoerucin (4.04–11.47 μmol/g DW) and glucoraphanin (1.38–4.13 μmol/g DW). Results indicated that a 50% reduction of nitrogen source in the nutrient solution enhances the total GSL amount from 16.86 to 32.86 μmol/g DW, convincingly glucoraphanin level increased significantly by 111% followed by glucoerucin 19%. This tremendous increase in aliphatic GSLs in rocket salad insisted that a decrease in nitrogen availability results in triggering the metabolic pathway for the synthesis of methionine a sulfur (S) containing amino acid. Similar results were observed in broccoli Jones et al. (2007) and Dick-Hennes and Buning-Pfaue (1992). Among the GSLs in Brassica crops, glucoraphanin is considered with particular interest, for its role in curing cancer related diseases (Smith et al., 2005). Addition of a higher amount of P stimulates glucoraphanin content by 25%, likewise dimeric 4-mercaptobutyl GSL accumulation exhibited >100% compared to the control nutrient. Since glucoraphanin, glucobrassicanapin and dimeric 4-mercaptobutyl GSLs were abundant which could offer future perspectives to modify the GSL composition because these individual GSLs are in the same pathway in the biosynthesis of aliphatic GSLs (Giamoustaris and Mithen, 1996).
An important fluctuation of individual GSL was also observed in different concentrations of K. Results revealed that the addition of K played a significant improvement in glucoraphanin production which accounted 80% more than the control condition. Indolyl GSLs derived from tryptophan may be biosynthetically related with N applications. Indolyl GSLs upon degradation form different products like phytoalexins in various Brassica species, suggesting that they have a role in plant defense mechanisms (Ludwig-Muller et al., 1999, Falk et al., 2007). Gluconasturtiin, the only aromatic GSL identified, although being the less represented compound, was the only one showing some capability of discrimination between different nutrient concentrations, where its relative content did not overlap, ranging from 0.15, 0.17 and 0.12 μmol/g DW in nutrient containing N, P and K respectively.
Literature revealed limited information about the effect of NPK supply together on rocket salad. One attempt conducted to study the effect of ammonium: nitrate nutrient ratio on nitrate and GSL contents of hydroponically-grown rocket salad identified four GSLs with a higher concentration of glucoerucin (Kim et al., 2006). Nitrogen and S depletion in plants affects the metabolite profiles by activating the GSL reaction, thus N plays a role in improving primary metabolic processes such as protein synthesis (Bryant et al., 1983, Pedras et al., 2006). Therefore optimum levels of N and S subsequently enhance the GSL concentrations in Brassica vegetables.
4. Conclusion
According to this report and the above discussion, where different ratios of NPK supply enhanced both aliphatic GSL and total GSL contents, it may be concluded that understanding the role of fertilizers, and GSL profile in young plant foods (rocket salad) is crucial to design strategies that would enhance potential components used in treating cancer and their other nutritive values for the consumer, hence managing plant nutrients with other sustainable practices would be of practical interest in the food industry.
Acknowledgments
The authors thank the research fund of Chungnam National University (Daejeon, Republic of Korea) for support. The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at the King Saud University for its funding of this research through the Research Group project No (RG-1435-071).
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Mariadhas Valan Arasu, Email: mvalanarasu@gmail.com.
Sun-Ju Kim, Email: kimsunju@cnu.ac.kr.
References
- Barbieri G., Pernice R., Maggio A., Pascale S.D., Fogliano V. Glucosinolates profile of Brassica rapa L. Subsp. Sylvestris L. Janch. var. esculenta Hort. Food Chem. 2008;107:1687–1691. [Google Scholar]
- Benbrook C. The impacts of yield on nutritional quality: lessons from organic farming. Hort. Sci. 2009;44(1):12–14. [Google Scholar]
- Bennett R.N., Rosa E.A.S., Mellon F.A., Kroon P.A. Ontogenic profiling of glucosinolates, flavonoids, and other secondary metabolites in Eruca sativa (salad rocket), Diplotaxis erucoides (wall rocket), Diplotaxis tenuifolia (wild rocket) and Bunias orientalis (Turkish rocket) J. Agric. Food Chem. 2006;54:4005–4015. doi: 10.1021/jf052756t. [DOI] [PubMed] [Google Scholar]
- Bryant J.P., Chapin F.S., Klein D.R. Carbon/nutrient balance of boreal plants in relation to vertebrate herbivory. Oikos. 1983;40:357–368. [Google Scholar]
- Burt R., Chataway J., Cotter J., Darcy-Vrillon B., Debailleul G., Grundy A. Changes in agriculture and food production in North America and Europe. In: McIntyre B.D., Herren H.R., Wakhungu J., Watson R.T., editors. Agriculture at a Crossroads. International Assessment of Agricultural Knowledge, Science and Technology for Development. Island Press; Washington, DC: 2009. pp. 20–78. [Google Scholar]
- Chen X.-J., Zhu Z.-J., Ni X.-L., Qian Q.-Q. Effect of nitrogen and sulfur supply on glucosinolates in Brassica campestris ssp. Chinensis. Agric. Sci. China. 2006;5:603–608. [Google Scholar]
- Chun J.-H., Arasu M.V., Lim Y.-P., Kim S.-J. Variation of major glucosinolates in different varieties and lines of rocket salad. Hort. Environ. Biotechnol. 2013;54(3):206–213. [Google Scholar]
- Dick-Hennes E., Buning-Pfaue H. Glucosinolates. In: Schreier P., Winterhalter P., editors. Progress in Flavour Precursor Studies. Allured Publishing Corp.; USA: 1992. pp. 185–188. [Google Scholar]
- Falk K.L., Tokuhisa J.G., Gershenzon J. The effect of sulfur nutrition on plant glucosinolate content: physiology and molecular mechanisms. Plant Biol. 2007;9:573–581. doi: 10.1055/s-2007-965431. [DOI] [PubMed] [Google Scholar]
- Giamoustaris A., Mithen R. Genetic of aliphatic glucosinolates. Side-chain modification in Brassica oleracea. Theor. Appl. Genet. 1996;93:1006–1010. doi: 10.1007/BF00224105. [DOI] [PubMed] [Google Scholar]
- Hoefkens C., Vandekinderen I., De Meulenaer B., Devlieghere F., Baert K., Sioen I., Henauw S.D., Verbeke W., Camp J.V. A literature-based comparison of nutrient and contaminant contents between organic and conventional vegetables and potatoes. Brit. Food J. 2009;111:1078–1097. [Google Scholar]
- International Standards Organization (ISO) ISO; Geneva, Switzerland: 1992. Rapeseed: Determination of Glucosinolates Content. Part 1. Method using High Performance Liquid Chromatography, ISO 9167-1 (E) pp. 1–9. [Google Scholar]
- Iori R., Bernardi R., Gueyrard D., Rollin P., Palmieri S. Formation of glucoraphanin by chemoselective oxidation of natural glucoerucin: a chemoenzymatic route to sulphoraphane. Bioorg. Med. Chem. Lett. 1999;9:1047–1048. doi: 10.1016/s0960-894x(99)00136-5. [DOI] [PubMed] [Google Scholar]
- Jones R.B., Imsic M., Franz P., Tomkins B.T. High nitrogen during growth reduced glucoraphanin and flavonol content in broccoli (Brassica oleracea var. italica) heads. Aust. J. Exp. Agric. 2007;47(12):1498–1505. [Google Scholar]
- Kim S.J., Kawaharada C., Ishii G. Effect of ammonium: nitrate nutrient ratio on nitrate and glucosinolate contents of hydroponically-grown salad rocket (Eruca sativa Mill.) Soil Sci. Plant Nutr. 2006;52:387–393. [Google Scholar]
- Kim S.J., Kawaharada C., Jin S., Hashimoto M., Ishii G., Yamauchi H. Structural elucidation of 4-(cystein-S-yl) butyl glucosinolate from the leaves of Eruca sativa. Biosci. Biotechnol. Biochem. 2007;71:114–121. doi: 10.1271/bbb.60400. [DOI] [PubMed] [Google Scholar]
- Lazzeri L., Baruzzi G., Malaguti L., Antoniacci L. Replacing methyl bromide in annual strawberry production with glucosinolate-containing green manure crops. Pest Manag. Sci. 2003;59:983–990. doi: 10.1002/ps.726. [DOI] [PubMed] [Google Scholar]
- Lee D.S., Jeon D.S., Park S.G., Arasu M.V., Al-Dhabi N.A., Kim S.C., Kim S.J. Effect of cold storage on the contents of glucosinolates in Chinese cabbage (Brassica rapa L. ssp. pekinensis) South Ind. J. Biol. Sci. 2015;1(1):38–42. [Google Scholar]
- Li S., Schonhof I., Krumbein A., Li L., Stutzel H., Schreiner M. Glucosinolate concentration in turnip (Brassica rapa ssp. rapifera L.) roots as affected by nitrogen and sulfur supply. J. Agric. Food Chem. 2007;55:8452–8457. doi: 10.1021/jf070816k. [DOI] [PubMed] [Google Scholar]
- Ludwig-Muller J., Pieper K., Ruppel M., Cohen J.D., Epstein E., Kiddle G., Bennett R. Indole glucosinolate and auxin biosynthesis in Arabidopsis thaliana (L.) Heynh. glucosinolates mutants and the development of clubroot disease. Planta. 1999;208:409–419. doi: 10.1007/s004250050576. [DOI] [PubMed] [Google Scholar]
- Martinez-Ballestra M.C., Lopez-Perez L., Hernandez M., Lopez-Berenguer C., Fernandez-Garcia N., Carvajal M. Agricultural practices for enhanced human health. Phytochem. Rev. 2008;7:251–260. [Google Scholar]
- Martınez-Sanchez A., Gil-Izquierdo A., Gil M.I., Ferreres F. A comparative study of flavonoids compounds, vitamin C, and antioxidant properties of baby leaf Brassicaceae species. J. Agric. Food Chem. 2008;56:2330–2340. doi: 10.1021/jf072975+. [DOI] [PubMed] [Google Scholar]
- Peck G.M., Andrews P.K., Reganold J.P., Fellman J.K. Apple orchard productivity and fruit quality under organic, conventional, and integrated management. Hort. Sci. 2006;41(1):99–107. [Google Scholar]
- Pedras M.S., Sarwar M.G., Suchy M., Aadio A.M. The phytoalexins from cauliflower, caulilexins A, B and C: isolation, structure determination, syntheses and antifungal activity. Phytochem. 2006;67:1503–1509. doi: 10.1016/j.phytochem.2006.05.020. [DOI] [PubMed] [Google Scholar]
- Powlson D.S., Addiscott T.M., Benjamin N., Cassman K.G., De Kok T.M., Van Grinsven H., L’Hirondel J.-L., Avery A.A., Van Kessel C. When does nitrate become a risk for humans. J. Environ. Qual. 2008;37:291–295. doi: 10.2134/jeq2007.0177. [DOI] [PubMed] [Google Scholar]
- Rangkadilok N., Nicolas M.E., Bennett R.N., Eagling D.R., Premier R.R., Taylor P.W.J. The effect of sulfur fertilizer on glucoraphanin levels in broccoli (B. oleracea L. var italica) at different growth stages. J. Agric. Food Chem. 2004;52(9):2632–2639. doi: 10.1021/jf030655u. [DOI] [PubMed] [Google Scholar]
- Santamaria P. Nitrate in vegetables: toxicity, content, intake and EC regulation. J. Sci. Food Agric. 2006;86:10–17. [Google Scholar]
- Smith T.K., Lund E.K., Clarke R.G., Bennet R.N., Johnson I.T. Effects of Brussels sprout juice on the cell cycle and adhesion of human colorectal carcinoma cells (HT29) in vitro. J. Agric. Food Chem. 2005;53:3895–3901. doi: 10.1021/jf048025v. [DOI] [PubMed] [Google Scholar]
- Umar A.S., Iqbal M. Nitrate accumulation in plants, factors affecting the process, and human health implications: a review. Agron. Sustainable Dev. 2006;27:45–57. [Google Scholar]
- Verkerk R., Schreiner M., Krumbein A., Ciska E., Holst B., Rowland I., de Schrijver R., Hansen M., Gerhauser C., Mithen R., Dekker M. Glucosinolates in Brassica vegetables: the influence of the food supply chain on intake, bioavailability and human health. Mol. Nutr. Food Res. 2009;53(2):219–265. doi: 10.1002/mnfr.200800065. [DOI] [PubMed] [Google Scholar]
- Yaniv Z., Schafferman D., Amar Z. Tradition, uses and biodiversity of rocket (Eruca sativa, Brassicaceae) in Israel. Econ. Bot. 1998;52:394–400. [Google Scholar]