Abstract
Cellulose producing bacterial strain was isolated from citrus fruit juice fungus. The isolated strain was identified as Gluconacetobacter sp. gel_SEA623-2 based on several morphological characteristics, biochemical tests, and 16S rRNA conducted. Culture conditions for bacterial cellulose production by SEA623-2 were screened in static trays. Conditions were extensively optimized by varying the kind of fruit juice, pH, sugar concentration, and temperature for maximum cellulose production. SEA623-2 has a high productive capacity in citrus processing medium, but not in other fruits. The optimal combination of the media constituents for bacterial cellulose production is as follows: 10% citrus juice, 10% sucrose, 1% acetic acid, and 1% ethanol at 30 °C, pH 3.5. Bacterial cellulose produced by SEA623-2 has soft physical properties, high tensile strength, and high water retention value. The cellulose produced by the selected bacteria is suitable as a cosmetic and medical material.
Keywords: Gluconacetobacter sp. gel_SEA623-2, Bacterial cellulose, Citrus, Cosmetic material
1. Introduction
Cellulose is the most abundant biological macromolecule on earth and is produced by a variety of organisms, such as bacteria (Cannon and Anderson, 1991). Unlike cellulose from wood pulp, bacterial cellulose (BC) is devoid of other contaminating polysaccharides and its isolation and purification are relatively simple, not requiring energy or chemical intensive processes. BC has large specific surface area, higher water retention value, moldability, and high tensile strength. Its diameter is generally 0.1 μm, which is 300× smaller than of wood fibrils (Yoshino et al., 1996). Recently, BC is receiving increased attention from various industries for its potential in developing biomedical materials due to its effectiveness in treating and protecting wounds under wet conditions (Sutherland, 1998). The best-known commercial applications of bacterial cellulose include the following: acoustic transducer diaphragm made of dried cellulose sheet (Nishi et al., 1990); wound dressing material (artificial skin) made of wet and purified cellulosic membrane (Fontana et al., 1990); and Nata de Coco, a traditional Philippine fermented dessert from coconut, which became very popular in Japan a few years ago (Yoshinaga et al., 1997).
BC is produced by a variety of microorganisms that belong mostly to the Gluconacetobacter genus of the Acetobacteraceae family (Jonas and Farah, 1998, Nobles et al., 2001). The members of this family are obligate aerobes that have the ability to convert ethanol to acetic acid and can grow at low pH levels (Kersters et al., 2006). Gluconacetobacter is one of the most frequently characterized acetic acid bacteria for BC production (Yeo et al., 2004). Recently, the mass production of BC by Gluconacetobacter species has been extensively studied. Gluconacetobacter has superior BC production ability than other microorganisms (Jung et al., 2005). It can be mass produced using surplus fruits as the culture medium. It is not only an environment-friendly process, but it can also add value to the final product.
The aim of this study was to screen for bacteria that are capable of producing bacterial cellulose from citrus fruit juice and to optimize conditions for bacterial cellulose production for commercialization and industrial applications.
2. Methodology
2.1. Isolation of bacteria producing cellulose from citrus fruit juice
This strain has been isolated from inoculums of a strain in citrus vinegar. It has a strong ability to produce biocellulose. Citrus juice (0.1 mL) was transferred into 0.9 mL of 0.38% NaCl. Serial dilutions from 100 to 10−6 were prepared using sterilized saline solution. An aliquot of 0.1 mL of each dilution was spread plated onto Gluconobacter oxydans (GBO) agar containing glucose (100 g/L), yeast extract (10 g/L), CaCO3 (20 g/L), and agar (15 g/L). The plates were incubated at 30 °C for 72 h. The colonies with a clear zone around were selected and transferred to tube containing 50 mL of biocellulose producing medium. A cellulose positive strain was one that produced cellulose on the liquid medium. One culture confirmed as pure was characterized and described below.
2.2. Physiological characterization of the isolated cellulose producing bacteria
Physiological characteristics of the isolates were also determined using commercially available identification systems (API 20E; bioMerieux, Kobe, Japan). All the tests were performed in duplicate. They were carried out according to the instructions in the test kit, but incubation was done at 30 °C instead of 37 °C. The results of API tests were interpreted using the ‘apiweb’ program.
2.3. Sequence alignment and phylogenetic analysis
Genomic DNA was isolated according to the following procedure and 16S rRNA was amplified using the universal primers (Sarafin et al., 2014). Forward primer: 5′-AGAGTTTGATCCTGGC TCAGGACGQQ-3′ reverse primer: 5′-ACGGCTACCTTGTTA CGACTT-3′. PCRs were performed in 50 mmol/L KCl, 10 mmol/L Tris–HCl, pH 9.0, 0.1% Triton X-100, and 1.5 mmol/L MgCl2 with 5 U Taq DNA polymerase per reaction (Promega Corp, Madison, WI). Amplification was performed in a Biometra PCR TGRADIENT (Biometra, Göttingen, Germany) as follows: 94 °C for 0.5 min, 50 °C for 1 min, and 72 °C for 1 min, for 35 cycles. The amplified DNA was sequenced by automated DNA sequencer ABI 3037xl DNA sequencer (Applied Biosystems, Foster City, CA, USA). Sequences were compared to the non-redundant NCBI database by using BLASTn, with the default settings used to find the most similar sequence and were sorted using E score. Phylogenetic analysis and trees were constructed with MEGA6.0 (Tamura et al., 2013). Phylogenetic trees were constructed with 15 sequences using Likelihood method.
2.4. Optimization of culture conditions for bacterial cellulose production
To determine the effects of various kinds of fruit juices, sugar concentration, pH, and temperature on cellulose production, the following variables were used: fruit juice unshiu, orange, grape, apple, pear; sugar 5, 10, 20, 30 Brix; pH 2.0, 2.5, 3.0, 3.5, 4.0, 5.0; and temperature: 20, 30, 35, 40 °C. The experiments were done in triplicate.
2.5. Statistical analysis
All statistical analyses were performed with SAS for the results obtained from three replications. Means and standard deviations for each treatment were calculated. Duncan’s multiple range test (DMRT) were used for comparing differences of the mean values at 0.05 confidence level.
3. Results
3.1. Isolation of bacteria from citrus fruit juice determination of an isolated strain producing material cellulose
The aim of the study was to identify and characterize novel strains of bacteria capable of producing cellulose. Twenty microbial samples were collected from citrus fruit juice fungus. There were significant differences found in acetobacter in GBO medium. For 8 out of 20 isolates, CaCO3 clear zones appeared on agar plates containing CaCO3 medium, which were identified as members of the Acetobacteraceae family. By repeated plating onto GBO medium, pure culture of the bacterial colony was obtained. It was observed that the colony of bacterial isolates on the GBO agar was smooth, circular, convex, and cream-colored. There was only one strain named SEA623-2, screened from citrus fruit juice, which produced significant amounts of cellulose. This isolate was kept in glycerol stock solution at −70 °C as stock culture, for further studies.
3.2. Identification of bacteria by taxonomic characteristics and 16S rRNA sequence analysis
A pure culture of the bacteria was identified to be strain SEA623-2 by AP20E and 16S rRNA sequencing. Strain SEA623-2 is clearly different from all the other strains with respect to d-rhamnose utilization. Strain SEA623-2 is closely related to strain Gluconacetobacter hansenii. The only significant difference between these two strains is the presence of d-rhamnose instead of d-sucrose (Table 1).
Table 1.
Identification of endophytic bacterial isolates based on biochemical tests.
| Parameter | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| ONPG | − | − | − | − | − | − | − |
| Arginine dihydrolase | − | − | − | − | − | − | − |
| Lysine decarboxylase | − | − | − | − | − | − | − |
| Ornithine decarboxylase | − | − | − | − | − | − | − |
| Citrate | − | − | − | − | − | − | − |
| Hydrogen sulfide | − | − | − | − | − | − | − |
| Urease | − | − | − | − | − | − | − |
| Tryptophane deaminase | − | − | − | − | − | − | − |
| Indole | − | − | − | − | − | − | − |
| Sodium pyruvate | − | − | + | + | − | + | + |
| Gelatinase | − | − | − | − | − | − | − |
| d-Glucose | + | + | + | + | + | + | + |
| d-Mannitol | − | − | − | − | − | − | − |
| Inositol | − | − | − | − | − | − | − |
| d-Sorbitol | − | − | − | + | − | − | − |
| l-Rhamnose | + | − | − | + | − | − | + |
| d-Sucrose | − | + | − | + | + | − | + |
| d-Melibiose | + | + | + | + | + | + | |
| Amygdalin | − | − | − | − | − | − | − |
| d-Arabinose | + | + | + | + | + | + | + |
| NO2 production | − | − | − | − | − | + | − |
| Nitrate reduction | − | − | − | − | − | − | − |
| Cellulose production | + | − | − | − | − | − | − |
+, 100% positive; −, 100% negative.
(1) Gluconacetobacter sp. gel_SEA623-2; (2) Gluconacetobacter liquefaciens; (3) G. hansenii; (4) Gluconacetobacter diazotrophicus; (5) G. oxydans; (6) Acetobacter tropicalis; (7) Acetobacter xylinum.
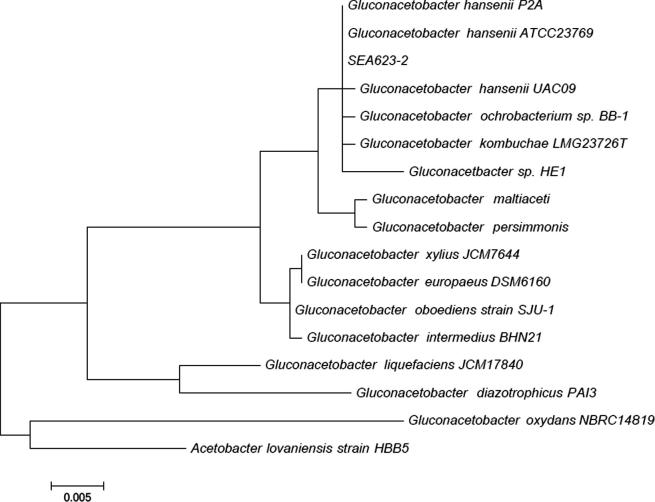
For a more accurate identification of the isolated strain, base sequences were analyzed using the 16S rRNA complete sequencing method and their similarities were examined. 16S rRNA fragment was amplified by PCR from genomic DNA using 16S rRNA universal primers. 16S rRNA sequence of strain SEA623-2 was 1320 bp, having 100% with G. hansenii. In the 16S rRNA tree (Fig. 1), Gluconacetobacter strains formed distinct lineages. Hence, the strain was identified to belong to the Gluconacetobacter sp. but the strain was not strictly the same as G. hansenii. Therefore, the SEA623-2 might be a new isolate and was named Gluconacetobacter sp. gel_SEA623-2.
Figure 1.
Phylogenetic tree of the 16S rRNA genes from bacterial cellulose producing bacteria. Sequences were aligned with clustalW and the tree constructed using the Likelihood method with MEGA6. The tree was rooted with the 16S rRNA gene sequence of SEA623-2 (not shown). Bar represents 0.05 substitution per nucleotide position. GenBank accession number is shown.
3.3. Optimization of culture conditions for bacterial cellulose production
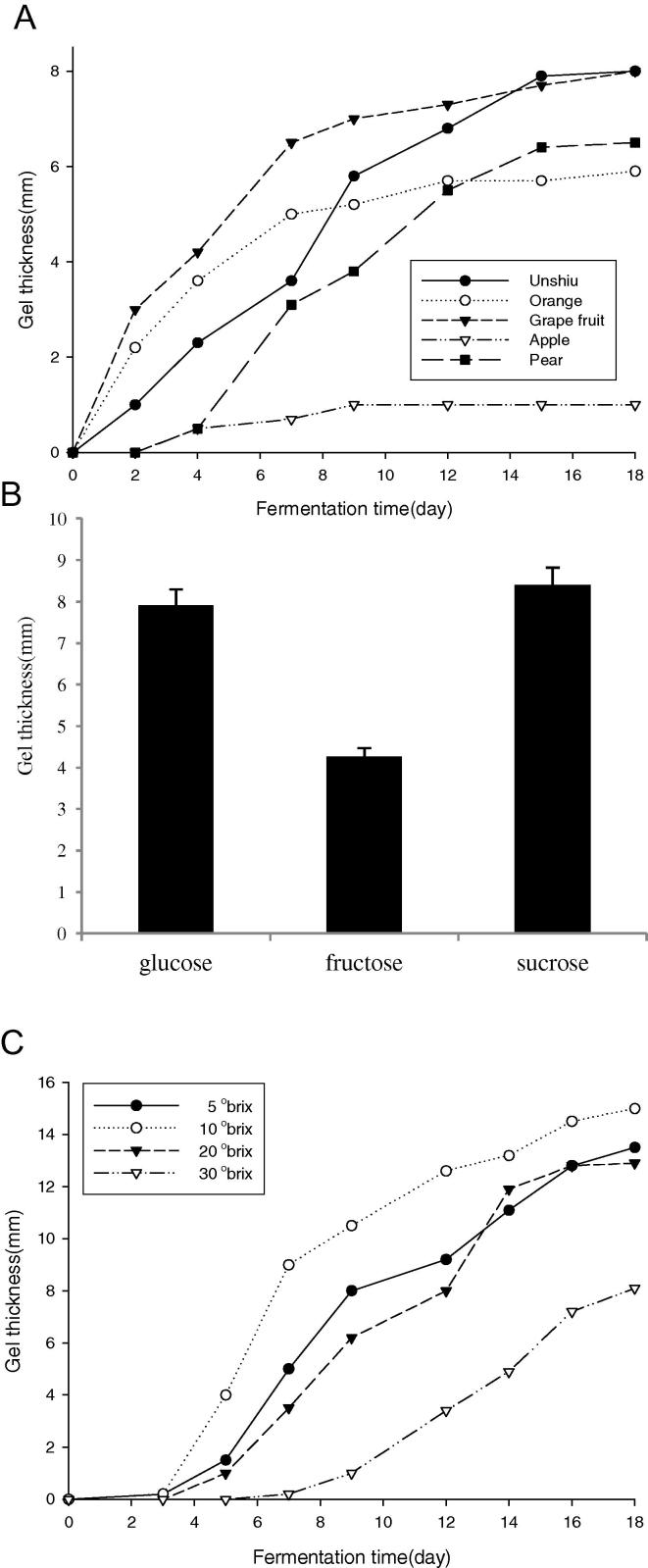
BC production rate of SEA623-2 under static culture was measured, as presented in Fig. 2. To find a suitable carbon source for BC production in SEA623-2, cells were cultured in the medium containing various carbon sources. Each carbon source was added to the basal medium at a concentration level of 5% (w/v) for 14 days. BC production was the highest where the cells were grown in the 10% sucrose medium (Fig. 2). Among the 5 fruit juice sources examined, unshiu fruit juice is the most favorable for BC production of SEA623-2. This bacterium has a high productive capacity in a citrus processing medium, but not in other fruits.
Figure 2.
Effect of fruit juices (A), carbon (B), and sugar concentration (C) during fermentation time. Fruit juices were used as the culture broth after dilution by 10 folds. Carbon sources were added to citrus juice medium. Concentration of each carbon source was adjusted to 10° Brix. The BC was fermented by static culture of SEA623-2 at 30 °C. Change in thickness of BC during prolonged fermentation for a period of 18 days.
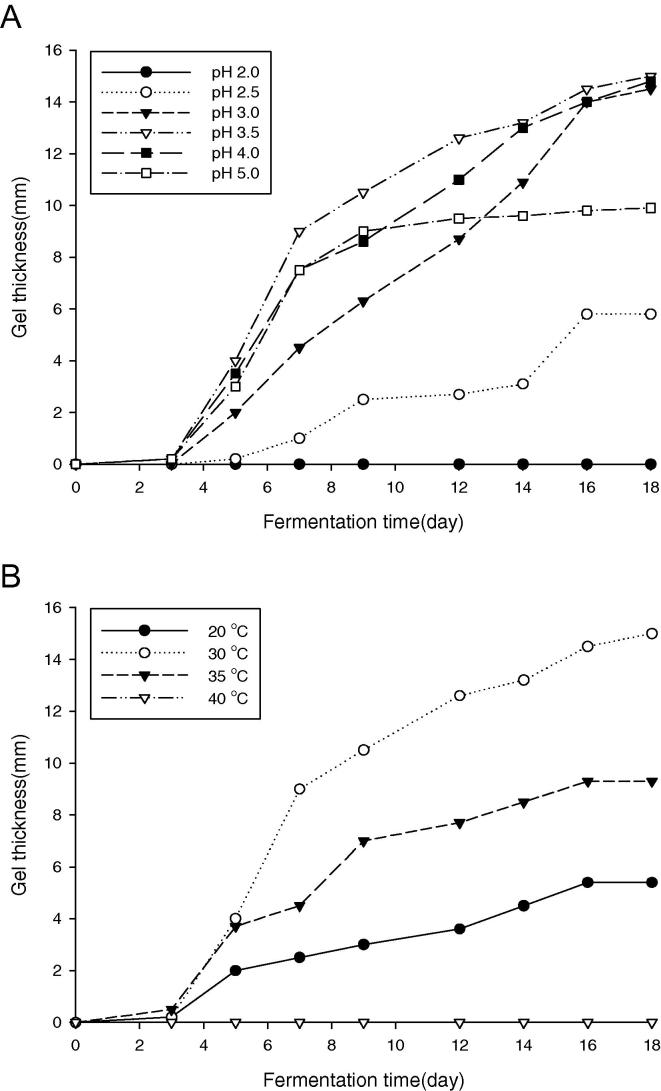
In order to investigate the effect of initial pH on BC production, SEA623-2 was cultivated in the biocellulose production medium with different pH levels (2.5–5) under static culture. The optimum pH for BC production was 3.5. The pH of the BC producing medium decreased from four relative to the culture time. The optimum temperature for BC was found to be 30 °C (Fig. 3).
Figure 3.
Effect of initial pH 2.5–5 (A) and temperature (20–40 °C) (B) on BC production by SEA623-2 under static culture. The culture broth contained 1.0% (v/v) ethanol, 1.0% (v/v) acetic acid, 10% citrus juice, and 10% sugar. The BC was fermented by static culture of SEA623-2 at 30 °C. Change in thickness of BC during prolonged fermentation for a period of 18 days.
In this study, the optimum culture medium conditions consist of 10% citrus juice, 10% sucrose, 1% acetic acid, and 1% ethanol in static trays.
3.4. SEM analysis
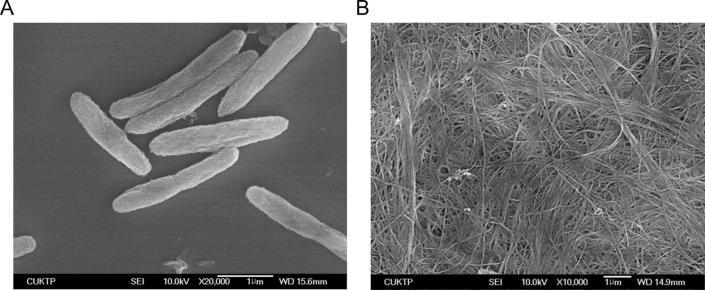
SEM showed a reticulated structure consisting of ultra fine and pure cellulose fibrils. The strands are entangled and curved resulting in reticulated and denser structure (Fig. 4) while the fibers are highly extended at static production (Watanabe et al., 1998). These fibrils assembled together and formed a porous structure with high aspect ratio. Nascent chains of BC aggregated to form subfibrils (bundles) which are then crystallized into microfibrils (ribbons) (Park et al., 2006). The thinner fiber characteristic of BC was demonstrated by SEM morphology. The thinner and longer fibers having large surface area could mean higher water holding capacity, moldability, and high tensile strength.
Figure 4.
Scanning Electron Micrograph (SEM) pictures of SEA623-2 (A) and top surface of bacterial cellulose produced by SEA623-2 (B). The samples were mounted and gold coated in preparation for SEM imaging. SEM was performed using the model JSM-6390, JEOL, Japan. SEM experiment was conducted at an accelerated voltage of 10 kV.
4. Discussion
In recent years, natural polymers such as cellulose, alginate and chitosan have been used worldwide as biomedical materials and devices, as they offer more advantages over synthetic polymers. BC has good properties such as high-burst pressure, high-water content and the ultrafine highly nanofibrous structure, foam of mimic natural extracellular matrix (ECM) for tissue engineering. BC is a promising natural polymer with many applications, especially for skin tissue repairing. The aim of this study was to clarify the usefulness of BC for use as a dressing and scaffold material (Jeong et al., 2014).
The bacteria strain capable of producing cellulose in this study was obtained from citrus fruit juice. The isolated strain exhibited a high similarity value to G. hansenii. The only significant difference between these two strains is the presence of d-rhamnose instead of d-sucrose. The strain was named Gluconacetobacter sp. gel_SEA623-2. The optimal combination of the media composition for bacterial cellulose includes the following: 10% citrus juice, 10% sucrose, 1% acetic acid, and 1% ethanol at 30 °C, pH 3.5. The BC produced by SEA623-2 in a static culture is located on the air/liquid surface, and its thickness increased with the culture time. Therefore, SEM confirmed that the BC produced by SEA623-2 was pure cellulose and free of any other impurities. All results proved that SEA623-2 could be a candidate microorganism for commercial production of BC. Further studies are planned for the development of an optimized medium and determination of optimal culture conditions for enhancement of the yield and production capacity.
Acknowledgement
This work was carried out with the support of “Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ01093402),” Rural Development Administration, Republic of Korea.
Footnotes
Peer review under responsibility of King Saud University.
References
- Cannon R.E., Anderson S.M. Biogenesis of bacterial cellulose. Crit. Rev. Microbiol. 1991;17:435–447. doi: 10.3109/10408419109115207. [DOI] [PubMed] [Google Scholar]
- Fontana J.D., deSousa A.M., Fontana C.K., Torriani I.L., Moresch J.C., Gallotti B.J. Acetobacter cellulose pellicle as a temporary skin substitute. Appl. Biochem. Biotechnol. 1990;25:253–264. doi: 10.1007/BF02920250. [DOI] [PubMed] [Google Scholar]
- Jeong S.I., Jeong J.O., Choi J.B., Shin Y.M., Park J.S., Gwon H.J., Nho Y.C., An S.J., Park M.Y., Lim Y.M. Development and characterization of heparin immobilized bacterial cellulose (BC) for bone tissue engineering using gamma-irradiation. Tissue Eng. Regener. Med. 2014;11:56–63. [Google Scholar]
- Jonas R., Fara L.F. Production and application of microbial cellulose. Polym. Degrad. Stab. 1998;59:101–106. [Google Scholar]
- Jung J.Y., Park J.K., Chang H.N. Bacterial cellulose production by Gluconacetobacter hansenii in an agitated culture without living noncellulose producing cells. Enzyme Microb. Technol. 2005;37:347–354. [Google Scholar]
- Kersters K., Lisdiyanti P., Komagata K., Swings J. The family Acetobacteraceae: the genera Acetobacter, Acidomonas, Asaia, Gluconacetobacter, Gluconobacter, and Kozakia. Prokaryotes. 2006;5:163–200. [Google Scholar]
- Nishi Y., Uryu M., Yamanaka S., Watanabe K., Kitamura N., Iguchi M., Mitsuhashi S. The structure and mechanical properties of sheets prepared from bacterial cellulose. 2. Improvement of the mechanical-properties of sheets and their applicability to diaphragms of electroacoustic transducers. J. Mater. Sci. 1990;25:2997–3001. [Google Scholar]
- Nobles D.R., Romanovicz D.K., Brown R.M., Jr. Cellulose in cyanobacteria. Origin of vascular plant cellulose synthase? Plant Physiol. 2001;127:529–542. [PMC free article] [PubMed] [Google Scholar]
- Park J.K., Khan T., Jung J.Y. Structural studies of the glucuronic acid oligomers produced by Gluconacetobacter hansenii strain. Carbohydr. Polym. 2006;63:482–486. [Google Scholar]
- Sarafin Y., Donio M.B.S., Velmurugan S., Michaelbabu M., Citarasu T. Kocuria marina BS-15 a biosurfactant producing halophilic bacteria isolated from solar salt works in India. Saudi J. Biol. Sci. 2014;21:511–519. doi: 10.1016/j.sjbs.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland I.W. Novel and established applications of microbial polysaccharides. Trends Biotechnol. 1998;16:41–46. doi: 10.1016/S0167-7799(97)01139-6. [DOI] [PubMed] [Google Scholar]
- Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013;12:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K., Tabuchi M., Morinaga Y., Yoshinaga F. Structural features and properties of bacterial cellulose produced in agitated culture. Cellulose. 1998;5:187–200. [Google Scholar]
- Yeo S.H., Lee O.S., Lee I.S., Kim H.S., Yu T.S., Jeong Y.J. Gluconacetobacter persimmonis sp. nov., isolated from Korean traditional persimmon vinegar. J. Microbiol. Biotechnol. 2004;14:276–283. [Google Scholar]
- Yoshinaga F., Tonouchi N., Watanabe K. Research progress in production of bacterial cellulose by aeration and agitation culture and its application as a new industrial material. Biosci. Biotechnol., Biochem. 1997;61:219–224. [Google Scholar]
- Yoshino T., Asakura T., Toda K. Cellulose production by Acetobacter pasteurianus on silicone membrane. J. Ferment. Bioeng. 1996;81:32–36. [Google Scholar]