Abstract
Sporosarcina pasteurii, a common soil bacterium has been tested for microbial treatment of cement mortar. The present study also seeks to investigate the effects of growth medium, bacterial concentration and different buffers concerning the preparation of bacterial suspensions on the compressive strength of cement mortar. Two growth media, six different suspensions and two bacterial concentrations were used in the study. The influence of growth medium on calcification efficiency of S. pasteurii was insignificant. Significant improvement in the compressive as well as the tensile strength of cement mortar was observed. Microbial mineral precipitation visualized by Scanning Electron Microscopy (SEM) shows fibrous material that increased the strength of cement mortar. Formation of thin strands of fillers observed through SEM micrographs improves the pore structure, impermeability and thus the compressive as well as the tensile strengths of the cement mortar. The type of substrate and its molarity have a significant influence on the strength of cement mortar.
Keywords: Bacteria, Sporosarcina pasteurii, Cement mortar, Growth medium, Image analysis, Compressive strength
1. Introduction
Concrete is the most extensively consumed construction material on the Earth. However, it is vulnerable to deterioration, corrosion, and cracks and hence loses its strength over a period of time. The consequent damage and loss of strength requires immensely expensive remediation and repair. Many organic and inorganic treatments have been adopted but as of now, only little success has been achieved. As a result, there is an impending need for technology development and improvement to meet the demand of society. Interdisciplinary research at the confluence of microbiology and civil engineering has already proven its potential by utilizing the biological activity of living cells (Boquet et al., 1973, Stocks-Fischer et al., 1999, Newnham, 1997, Ramakrishnan et al., 2005, Bachmeier et al., 2002) for improving the quality of building materials.
One of the important characteristics of cells, which is not confined to human beings alone, is the ability of mineral precipitation for the formation of bones and teeth. Other living organisms, including some of the bacteria, also have the ability to form bones and teeth-like material such as natural pearls and shells. This phenomenon of bio-mineralization has inspired some investigators for developing innovative high-performance composites for construction applications (Ramakrishnan et al., 2005, Bachmeier et al., 2002).
The bacterial remediation technique has been proposed for repairing structures of historical importance to preserve their esthetic value, because the conventional techniques, such as epoxy injection, cannot be used to remediate cracks in these structures (Ramachandran et al., 2001, Bang and Ramakrishnan, 2001). The cracks in granite were effectively remediated by microbiologically-induced calcite precipitation (MICP) mixed with filling material such as silica fume and sand (Gollapudi et al., 1995, Zhong and Islam, 1995). Sporosarcina pasteurii, when used under favorable conditions in concrete can continuously precipitate calcite that has a coarse crystalline structure which readily adheres to concrete. In addition to the ability to grow upon itself continuously, it is highly insoluble in water. The process for system improvement for bio-remediation, identifying the primary components and interplay among different disciplines, has been published recently (DeJong et al., 2010, DeMuynck et al., 2010). Attempts have been made to make the process of calcium carbonate precipitation more efficient using different techniques such as mutating S. pasteurii to test their ability to enhance urease activity and calcite production (Achal et al., 2009), or by immobilization of S. pasteurii with polyurethane to check efficiency of the cells (Bang et al., 2001). A recent review (DeMuynck et al., 2010) has provided an in-depth comparison of different approaches with due attention to the background that accounts for the choice of the microorganism and the proposed metabolic pathways. A previous study with S. pasteurii and Pseudomonas aeruginosa showed a significant improvement (about 18%) in the compressive strength of the cement mortar (Ramachandran et al., 2001, Ramakrishnan et al., 1998). Scanning Electron Microscopy (SEM) also confirmed the role of microbiologically-induced precipitation within the mortar matrix (Ramakrishnan et al., 1999).
Ramakrishnan et al. (2003) have investigated whether the cement mortar beams supplemented with bacteria performed better, when subjected to alkaline, sulfate and freeze-thaw attacks. They studied the effect of different concentrations of bacteria on (a) the alkali aggregate reactivity of mortar beams, (b) their sulfate attack resistance, and (c) their freeze-thaw durability.
This study elucidates the role of bacterial growth media, buffers, and bacterial concentration in the enhancement of compressive strength of bacteria-modified mortar, using S. pasteurii (ATCC 6453). Besides, Echerichia coli DH5α (ATCC 53868) has been used for studying the effect of the presence of microorganisms on cement mortar. The microstructure analysis of the bacteria-modified mortar has been done using the Scanning Electron Microscopy (SEM).
2. Materials and methods
2.1. Bacterial strains
S. pasteurii (ATCC 6453) was used throughout the study as the test organism, whereas E. coli DH5α (ATCC 53868) was used as a negative control for comparing the effect of the presence of microorganisms on cement mortar.
2.2. Growth media and bacterial concentration
S. pasteurii was grown in two different media, viz. (i) ammonium-sulfate and yeast extract (NH4–YE medium) – a specific broth for S. pasteurii, and (ii) Nutrient Broth (NB medium), a general medium that stimulates cellular metabolism. E. coli DH5α cells were grown in Lysogeny Broth (LB) media. Compositions of the three media (per liter) were as follows:
NH4–YE medium: 20 g yeast extract; 10 g di-ammonium sulfate [(NH4)2SO4]; 0.13 M tris buffer (pH = 9.0); 20 g agar.
NB medium: 3 g Nutrient Broth; 20 g urea [(NH2)2CO]; 10 g ammonium chloride [NH4Cl]; 25.2 mM sodium bicarbonate [NaHCO3].
LB medium: 10 g tryptone; 5 g yeast extract; 10 g NaCl.
S. pasteurii cells were grown in the first two media separately, whereas E. coli DH5α cells were grown in LB medium. The cells were allowed to multiply overnight with 1% inoculation from freshly-prepared primary cultures. Cells were harvested by centrifugation at 3000g for 10 min and washed twice with phosphate buffer. Cell pellets were suspended in buffer to obtain a high-density stock of cells; and the cell concentrations were determined by recording absorbance at 600 nm. The final cell concentrations for different treatments were adjusted by diluting portions of these stocks on the basis of hemocytometer counts.
Measurement of turbidity or optical density (OD) is not a direct measurement of bacterial numbers, but an indirect measurement of cell biomass that includes both living and dead cells. Hence, to quantify viable the cells, a plate-count method was used and cell counting by hemocytometer under microscope was also performed to have an accurate estimate.
2.3. Solutions for bacterial suspensions
Bacterial cells in appropriate number were suspended in water and phosphate buffered solutions containing varying concentrations of urea (CH4N2O) as a substrate for bacterial activity, and calcium chloride (CaCl2·2H2O) as a source of Ca2+ ion. A total of six solutions (S0 to S5) were used for assessing the influence of molarity of salts and buffer on the calcification efficiency of S. pasteurii. Compositions of these solutions are given in Table 1. The solutions S2 and S3, containing only the phosphate buffer in different proportions, were used as controls for highlighting the requirement of substrate and calcium source in bacterial calcification.
Table 1.
Composition of solutions used for preparing bacterial suspensions.
| Solution | Chemical composition |
||
|---|---|---|---|
| CaCl2 | Urea | Phosphate buffer | |
| S0 | – | – | – |
| S1 | 0.20 M | 0.20 M | – |
| S2 | – | – | 0.10 M, pH = 7.80 |
| S3 | – | – | 0.02 M, pH = 7.16 |
| S4 | 0.09 M | 0.07 M | 0.02 M, pH = 7.16 |
| S5 | 0.05 M | 0.03 M | 0.02 M, pH = 7.16 |
2.4. Preparation of bacterial mortar and specimens
Ordinary Portland cement Type I, conforming to ASTM (2008), was used for experiments. Locally available clean red sand conforming to ASTM (2008) was used as fine aggregate. Suspensions of bacterial cells in various solutions, as described above, were used for preparation of mortar specimens. Physical properties of the materials are given in Table 2.
Table 2.
Physical properties of materials.
| Materials | Physical properties | Value |
|---|---|---|
| OPC cement Type I |
Normal consistency | 24% |
| Initial setting time | 131 min | |
| Calcium oxide, CaO | 63.85% | |
| Silicon dioxide, SiO2 | 19.83% | |
| Aluminum oxide, Al2O3 | 5.29% | |
| Ferric oxide, Fe2O3 | 3.53% | |
| Magnesium oxide, MgO | 0.52% | |
| Sulfur trioxide, SO3 | 2.43% | |
| K2O | 0.07% | |
| Na2O | 0.21% | |
| Loss of ignition, LOI | 2.82% | |
| Fine aggregate (Coarse sand) |
Specific gravity | 2.65 |
| Fineness modulus | 1.90 | |
| Water absorption | 0.3% | |
| Clay lumps and friable particles | Nil | |
| Material finer than 75 mm | 0.72% | |
| Coal and lignite | Nil | |
| Organic impurities | Nil | |
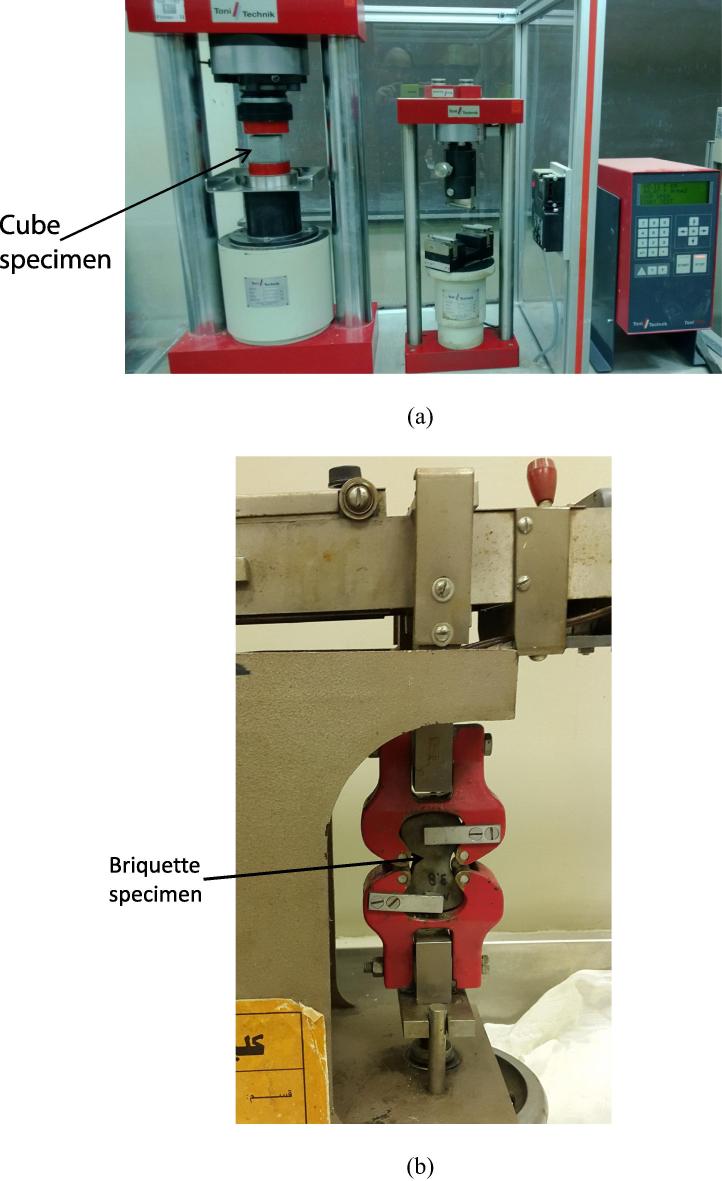
A cement to sand ratio of 1:3 (w/w), and a solution to cement ratio of 0.6 (w/w) were maintained throughout the experiments. Cubes of 50 mm size were prepared for determining the compressive strength, whereas briquettes were used for determining the tensile strength of mortar. Bacterial cells at concentrations of 108 and 109 per ml in different solutions were added to the mortar. No additional food material, except that present in the diluted cultures, was supplemented in the mortar during mixing. Dry cement and sand were thoroughly mixed and then the solution containing bacteria was added. The solution without bacterial cells was used to prepare the control mortar blocks. Cubes were cast and compacted in a vibration machine, and all specimens were de-molded after 24 h of casting and then water-cured at room temperature (30 ± 2 °C) until compression and tension testing was done after 28 days. The compressive strength tests were performed on mortar cubes using 300 kN Toni Technik – Toni NORM compression testing machine according to ASTM (2007). The loading rate on the cubes was 0.72 mm/min. The tensile strength tests were performed on mortar briquettes using 1000 lbs Zero-Max tensile testing machine in accordance with ASTM (2012). The specimens under test are shown in Fig. 1. SEM examinations were made on the broken sample pieces collected from the tested mortar cubes. Three cubes were tested for each of the treatments and the controls.
Figure 1.
Testing of cement mortar specimens: (a) cube under compression test; (b) briquette under direct tension test.
The five-character specimen IDs consisted of three letters followed by two digits. The first letter ‘M’ stands for mix; the second letter represents the bacterial genotype (D: E. coli DH5α; S: S. pasteurii; N: No bacterium – control); and the third letter represents the bacterial growth medium (N: No medium; L: LB medium; B: Nutrient Broth; Y: NH4YE medium). Of the two digits, the first stands for the log value of the bacterial cell concentration; whereas the second represents the numeric in the solution ID.
2.5. Treatments
Experiments were designed to assess the effect of: bacterial growth medium, bacterial cell concentration and solutions on the compressive strength of the cement mortar. In all, eleven treatments were maintained, based on the following parameters:
-
(i)
Bacterial strain: S. pasteurii
-
(ii)
Bacterial growth media: NB and NH4–YE, as described above
-
(iii)
Bacterial cell concentrations: 108 and 109 cells/ml.
-
(iv)
Solutions used for preparing bacterial suspensions: S0–S5, as described in Table 1.
2.6. Controls
Three types of control mortar specimens were prepared:
-
(i)
Cement mortar in solutions but no bacteria (Specimens: MNN00, MNN01 and MNN02),
-
(ii)
Cement mortar with E. coli DH5α (108 and 109 cells/ml), in solution S0 (Specimens: MDL80 and MDL90); in solution S1 (Specimens: MDL81 and MDL91); and in solution S2 (Specimens: MDL82 and MDL92).
Thus, nine different control specimens were prepared for studying the influence of different chemicals used for bacterial suspension and the presence of microorganisms in mortar on its strength. The effect of dead cells of E. coli DH5α and S. pasteurii was supposed to be the same.
2.7. Image analysis
Scanning Electron Microscope (FEL-Spectra) was used for detecting changes in microstructure of the formed phases. The broken specimens, collected after the compressive strength tests, were used for preparing thin sections by grinding the solid and sound mortar chips with carborundum (silicon carbide), washed thoroughly in water and dried at a temperature of 90–100 °C (Winchell, 1949). The thin section slides were scanned by FEL-Spectra microscope. Three sections were prepared for each mix.
3. Results and discussion
The effect of varying bacterial concentrations and solutions on the compressive strength of cement–sand mortar was significant. The compressive strength of 28-days cured mortar cubes under different treatments is given in Table 3.
Table 3.
Compressive strength of cement mortar under different treatments.
| Mix set | Bacteria⁎ |
Suspension | Compressive strength (MPa) | ||
|---|---|---|---|---|---|
| Genotype | Medium | Concentration (cells/ml) | |||
| Control specimens | |||||
| MNN00 | – | – | – | S0 | 31.7 |
| MNN01 | – | – | – | S1 | 28.4 |
| MNN02 | – | – | – | S2 | 28.2 |
| MDL80 | DH5α | LB | 108 | S0 | 31.2 |
| MDL90 | DH5α | LB | 109 | S0 | 31.0 |
| MDL81 | DH5α | LB | 108 | S1 | 27.9 |
| MDL91 | DH5α | LB | 109 | S1 | 28.2 |
| MDL82 | DH5α | LB | 108 | S2 | 27.8 |
| MDL92 | DH5α | LB | 109 | S2 | 27.5 |
| Specimens containing S. pasteurii | |||||
| MSB91 | SP | NB | 109 | S1 | 38.8 |
| MSB92 | SP | NB | 109 | S2 | 26.8 |
| MSY91 | SP | NH4–YE | 109 | S1 | 38.9 |
| MSY92 | SP | NH4–YE | 109 | S2 | 26.9 |
| MSB81 | SP | NB | 108 | S1 | 37.0 |
| MSB82 | SP | NB | 108 | S2 | 27.0 |
| MSY81 | SP | NH4–YE | 108 | S1 | 37.4 |
| MSY82 | SP | NH4–YE | 108 | S2 | 26.6 |
| MSY83 | SP | NH4–YE | 108 | S3 | 26.5 |
| MSY84 | SP | NH4–YE | 108 | S4 | 39.6 |
| MSY85 | SP | NH4–YE | 108 | S5 | 38.7 |
SP = S. pasteurii; LB = Lysogeny Broth; NB = Nutrient Broth.
3.1. Control specimen
Calcification potential of a bacterium emanates from its ability to breakdown urea enzymatically (DeMuynck et al., 2010, Tittelboom et al., 2010). E. coli DH5α is known to have no urease activity; therefore, it was used as a control strain for exclusive assessment of the influence of experimental parameters on the activity of S. pasteurii strain harboring active urease gene (Kim and Spizizen, 1985, Stocks-Fischer et al., 1999, Tiwari et al., 2014).
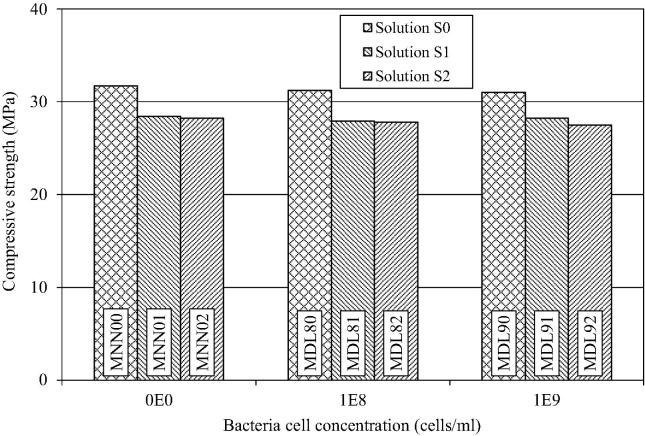
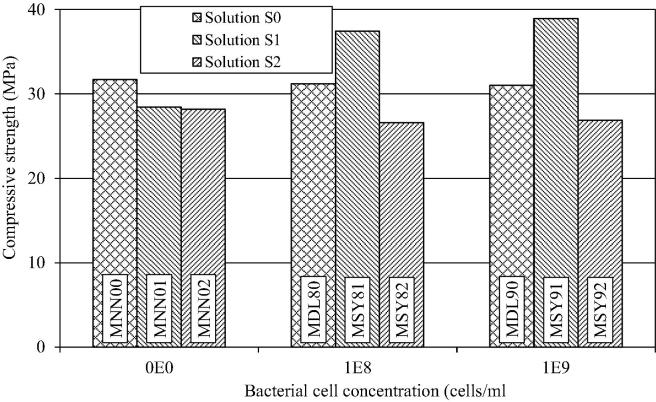
The influence of the bacterial concentration of E. coli DH5α and suspension on the compressive strength of the mortar is shown in Fig. 2, which allows for the following deductions:
-
(i)
The increase in bacterial concentration from 108 to 109 cells/ml reduced the compressive strength of the mortar cubes slightly, the difference being nominal; an average of test results of the two concentrations was used for making comparisons. In solution S0, compressive strength of the mortar with bacteria (MDL80, MDL90) was reduced by 1.9%, whereas in the presence of solutions S1 and S2, the compressive strength of mortar containing bacteria (MDL81, MDL82, MDL91, MDL92) was reduced by 1.2% and 2.0% respectively. It indicates that the addition of blank strain E. coli DH5α had almost no effect on the compressive strength of the cement mortar.
-
(ii)
The effect of solution S1 (urea: 0.2 M; CaCl2: 0.2 M) on the compressive strength of cement-mortar was assessed by comparing the compressive strength of (a) MNN00 (no bacteria; S0) with MN001 (no bacteria; S1), (b) MDL80 (bacteria: 108; S0) with MDL81 (bacteria: 108 cells/ml; S1), and (c) MDL90 (bacteria: 109 cells/ml; S0) with MDL91 (bacteria 109 cells/ml; S1). Application of solution S1 in cement mortar resulted in 10.0% average reduction in the compressive strength of mortar.
-
(iii)
Similarly, the effect of using solution S2 (No substrate solution; 0.1 M phosphate buffer, pH 7.8) on the compressive strength of cement-mortar could be assessed by comparing the compressive strength of (a) MNN00 (no bacteria; S0) with MN002 (no bacteria; S2), (b) MDL80 (bacteria: 108 cells/ml; S0) with MDL82 (bacteria: 108 cells/ml; S2), and (c) MDL90 (bacteria: 109 cells/ml; S0) with MDL92 (bacteria: 109 cells/ml; S2). The addition of solution S2 in cement mortar resulted in an average reduction of 11.1% in the compressive strength of mortar.
Figure 2.
Influence of cell concentration of E. coli DH5α and solutions on compressive strength of mortar.
The observed changes in compressive strength by incorporation of E. coli DH5α were less than 2% in almost all cases and there was no consistent improvement/degradation in strength due to the addition of this bacterium. The bacterium proved ineffective in the presence of the substrate solution also. In previous studies also, E. coli has shown no calcification potential (Ghosh et al., 2005). Bachmeier et al. (2002) assessed the calcification potential of some bacteria and used E. coli (pBR322) as a control, because it has no urease activity. This suggests that the selection of an active bacterial strain is important for improving the mortar strength.
3.2. Effect of growth medium
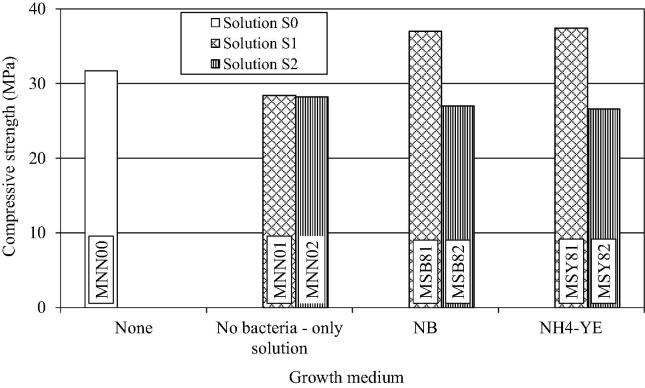
The influence of the growth medium for S. pasteurii at a cell count of 108 cell/ml on the compressive strength of mortar is shown in Fig. 3. For solution S1 (urea: 0.2 M; CaCl2: 0.2 M), improvement in the compressive strength of mortar with bacteria grown in both MSB81 and MSY81 media was closely similar (Average = 17.4%). As discussed above, addition of solutions results in 10–11% reduction in the compressive strength of mortar and therefore the gain in strength as a result of calcification was significant. When urea and calcium chloride are degraded and utilized due to bacterial activity, they no longer remain as impurities in the mortar and contribute toward calcification. Growth media were used in this study only for cultivation and maintenance of the bacterial cultures. Before adding to the mortar, the bacterial cells were pelleted out and washed with buffer, leaving very little amount of the medium to go into the mortar. Thus there was no significant carry over effect of the medium on the subsequent activity of S. pasteurii cells in the mortar. It is generally believed that the improvement in compressive strength of mortar is due to the calcite deposition on the microorganism cell surfaces and within the pores of cement–sand matrix, which plugs the pores within the mortar (Stocks-Fischer et al., 1999, Newnham, 1997, Ramakrishnan et al., 2005).
Figure 3.
Influence of growth medium for S. pasteurii on compressive strength of mortar for a concentration of 108 cells/ml.
For solution S2 (no substrate solution; 0.1 M phosphate buffer, pH 7.8), there was an average decrease of 15.5% in the compressive strength of the mortar, using bacteria grown in either of the two media possibly because of the absence of substrate and the calcium source due to which the phosphate buffer acted as an impurity.
3.3. Effect of bacterial cell concentration
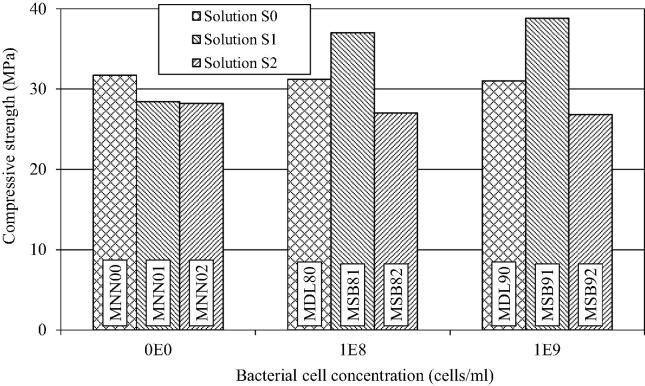
The effect of concentration of S. pasteurii cells in the solutions S1 and S2 used for making the mortar has been shown for cells grown on NB medium (Fig. 4) and for cells grown on NH4YE medium (Fig. 5).
Figure 4.
Influence of cell concentration of S. pasteurii grown in NB medium and solutions on compressive strength of mortar.
Figure 5.
Influence of cell concentration of S. pasteurii grown in NH4–YE medium and solutions on compressive strength of mortar.
In general, the compressive strength improved with increase in bacterial cell count for the solution S1 (Fig. 4). The enhancement in compressive strength for increase in concentration of bacteria from 108 to 109 cells/ml was 4.9% for bacteria grown in NB medium, and 4% for bacteria grown in NH4–YE medium (Fig. 5), showing that the medium of growth for bacteria had no significant effect on its calcification efficiency in the mortars. However, the bacterial cell count had an impact on calcification level in mortars (Pacheco-Torgal and Labrincha, 2013). Ghosh et al. (2009), using Shewanella species, found that highest compressive strength of the mortar was attained with a cell count of 105 cells/ml. However for healing of cracks, LeMetayer-Levral et al. (1999) have suggested a cell concentration of 109 cells/ml to be optimal.
The increase in the concentration of bacteria from 108 to 109 cells/ml, suspended in solution S2, caused slightly more reduction in the compressive strength which might be due to increase in the biomass.
3.4. Effect of solutions in suspension
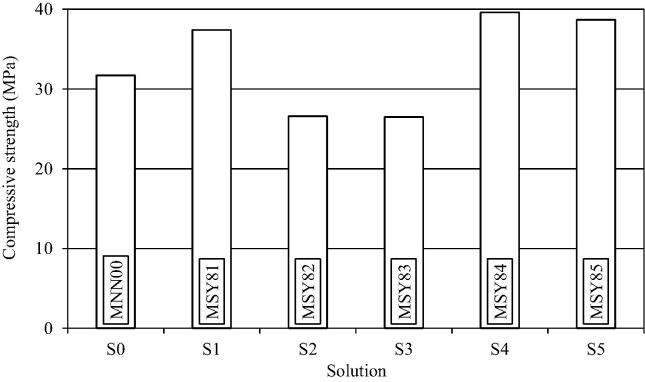
In addition to S1 and S2, three more suspensions (S3 to S5) were studied for bacterial growth in NH4–YE medium. The effect of solutions on the compressive strength of mortar has been plotted in Fig. 6 for a bacterial concentration of 108 cells/ml. All of the specimens (MSY81, MSY82, MSY83, MSY84, and MSY85) considered in figure are for S. pasteurii grown in NH4–YE medium except the control (MNN00).
Figure 6.
Influence of the solutions on compressive strength of mortar for S. pasteurii at a concentration of 108 cells/ml.
Figure shows that the maximum compressive strength of the mortar [24.9% more than the control (MNN00)] was attained for suspension S4 (specimen MSY84), containing phosphate buffer. The strength of mortar for suspensions S1 and S5 was 18.0% and 22.1% higher respectively. The reduction in the molarity of substrate solutions S4 and S5 could be one of the reasons for increase in the compressive strength of the mortar mixes using these suspensions (specimens MSY84 and MSY85). Moreover, phosphate buffers are known to enhance the activity of bacteria. With lower salt content, level of un-degraded residues acting as impurities would be low, causing little change in behavior of the cement mortar.
Microbial calcite formation is directly dependent on the availability of urea as a substrate for urease activity and Ca+2 derived from an appropriate source (DeMuynck et al., 2010). As long as the whole or the major part of these salts is utilized by microbial activity, their increasing concentration would result in a greater calcification and a corresponding increase in the mortar strength. However, the unutilized amount of salts would obviously have a negative impact.
3.5. SEM micrographs
The micrographs of the control plain cement mortar (MNN00) and of a better-performing bacteria-modified mortar (MSY84) are presented in Figure 7, Figure 8 respectively. The selection of specimen MSY84 is based on its high compressive as well as the tensile strength. The direct tensile strength of specimens MNN00 and MSY84, determined using standard briquettes (ASTM, 1985), were found to be 2.7 and 3.7 MPa respectively. Thus the compressive strength of the mix MSY84 increased by 24.9% and its tensile strength by 37%, as compared to the plain cement mortar.
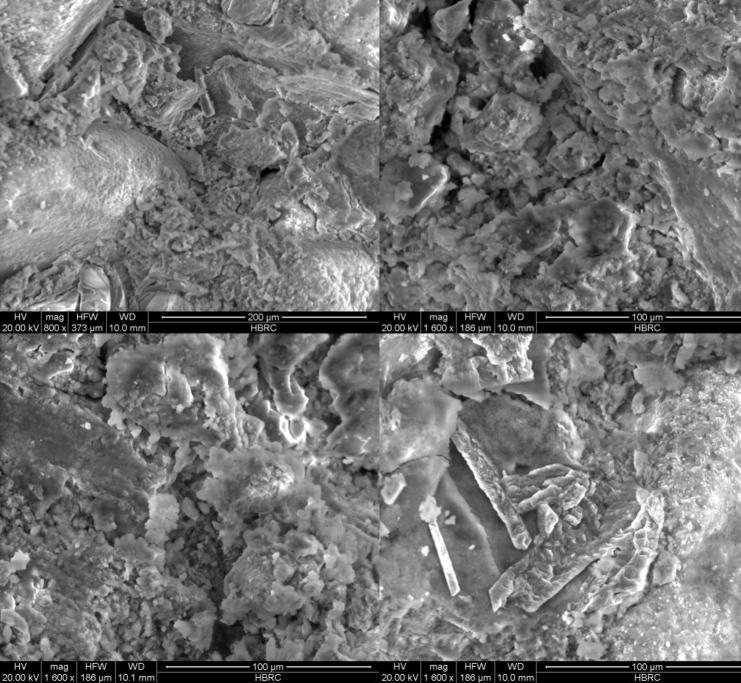
Figure 7.
SEM images of cement mortar specimen MNN00 (control).
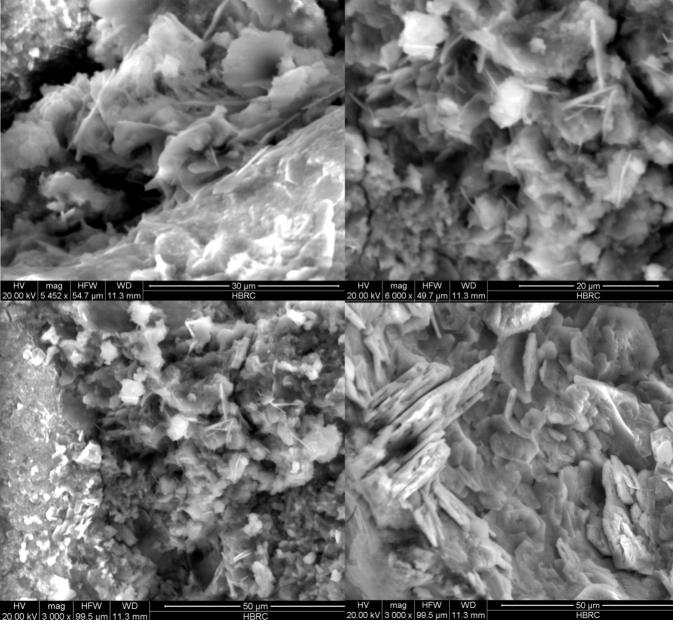
Figure 8.
SEM images of cement mortar specimen MSY84 showing filler strands of calcite formed by bacterial mineral precipitation which are magnified in the upper two images at magnifications of 5452×and 6000×.
Figure 7, Figure 8 indicate that in a mortar made with a 108 cells/ml of S. pasteurii in suspension S4, the pores are better filled with narrow strands of filler (Fig. 8); and a higher modification in pore size distribution is noticed, whereas no such filler material is observed in the micrographs of the control sample of plain mortar. This confirms the increase in compressive and direct tensile strengths of mix MSY84. The qualitative assessment of SEM images shows that the presence of narrow strands of filler is beneficial for modification of the porosity and pore-size distribution of cement mortar.
In congruence with our findings, several studies addressing the microbial calcification have shown deposition of calcite crystal in cement mortars in SEM images (DeMuynck et al., 2008, Ghosh et al., 2009), which demonstrate the real possibility of application of this technique in improving the cement mortars.
4. Conclusions
Addition of E. coli DH5α, a control bacterium, causes almost no effect on the compressive strength of the mortar, which suggests that the choice of microorganism is important for improving the compressive strength of mortars.
This study suggests that the strength of the S. pasteurii incorporated mortar increases due to mineral precipitation by the bacterial activity. Addition of S. pasteurii has a positive effect on the compressive as well as the tensile strength of the mortar. The influence of growth medium on calcification efficiency of S. pasteurii is insignificant. The type of substrate solution and its molarity have a significant influence on the strength of the mortar.
SEM examination reveals the growth of fibrous filler material within the pores due to the precipitation by S. pasteurii, which confirms the increase in strength of the mortar observed. This improvement is attained by the modification of porosity and pore size distribution of the cement mortar it generates.
Acknowledgements
This work was funded by the National Plan for Science, Technology and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia, under the Award Number (#11-BIO1959-02).
Footnotes
Peer review under responsibility of King Saud University.
References
- Achal V., Mukherjee A., Basu P.C., Reddy M.S. Strain improvement of Sporosarcina pasteurii for enhanced urease and calcite production. J. Ind. Microbiol. Biotechnol. 2009;36(7):981–988. doi: 10.1007/s10295-009-0578-z. [DOI] [PubMed] [Google Scholar]
- ASTM Standard C190 . ASTM International; West Conshohocken, PA: 1985. Method of Test for Tensile Strength of Hydraulic Cement Mortars. [Google Scholar]
- ASTM C109/C109M-07 . ASTM International; West Conshohocken, PA: 2007. Standard Test Method For Compressive Strength Of Hydraulic Cement Mortars (Using 2-in. or [50-mm] Cube Specimens) [Google Scholar]
- ASTM Standard C150 . ASTM International; West Conshohocken, PA: 2008. Specification for Portland cement. [Google Scholar]
- ASTM Standard C33 . ASTM International; West Conshohocken, PA: 2008. Specification for Concrete Aggregates. [Google Scholar]
- ASTM C 307-03 . ASTM International; West Conshohocken, PA: 2012. Standard Test Method for Tensile Strength of Chemical-Resistant Mortar, Grouts, and Monolithic Surfacings. [Google Scholar]
- Bachmeier K.L., Williams A.E., Warmington J.R., Bang S.S. Urease activity in microbiologically-induced calcite precipitation. J. Biotechnol. 2002;93(2):171–181. doi: 10.1016/s0168-1656(01)00393-5. [DOI] [PubMed] [Google Scholar]
- Bang, S.S., Ramakrishnan, V., 2001. Microbiologically-enhanced crack remediation (MECR). In: Proc. Int. Symp. Ind. Appl. Microbial Genomes. Daegu, Korea, 2001, pp. 3–13.
- Bang S.S., Galinat J.K., Ramakrishnan V. Calcite precipitation induced by polyurethane-immobilized Sporosarcina pasteurii. Enzyme Microb. Technol. 2001;28(4–5):404–409. doi: 10.1016/s0141-0229(00)00348-3. [DOI] [PubMed] [Google Scholar]
- Boquet E., Boronat A., Ramoscor A. Production of calcite (calcium–carbonate) crystals by soil bacteria is a general phenomenon. Nature. 1973;246(5434):527–529. [Google Scholar]
- DeJong J.T., Mortensen B.M., Martinez B.C., Nelson D.C. Bio-mediated soil improvement. Ecol. Eng. 2010;36(2):197–210. [Google Scholar]
- DeMuynck W., Debrouwer D., Belie N.D., Verstraete W. Bacterial carbonate precipitation improves the durability of cementitious materials. Cem. Concr. Res. 2008;38(7):1005–1014. [Google Scholar]
- DeMuynck W., DeBelie N., Verstraete W. Microbial carbonate precipitation in construction materials: a review. Ecol. Eng. 2010;36(2):118–136. [Google Scholar]
- Ghosh P., Mandal S., Chattopadhyay B.D., Pal S. Use of microorganism to improve the strength of cement mortar. Cem. Concr. Res. 2005;35(10):1980–1983. [Google Scholar]
- Ghosh S., Biswas M., Chattopadhyay B.D., Mandal S. Microbial activity on the microstructure of bacteria modified mortar. Cem. Concr. Compos. 2009;31(2):93–98. [Google Scholar]
- Gollapudi U.K., Knutson C.L., Bang S.S., Islam M.R. A new method for controlling leaching through permeable channels. Chemosphere. 1995;30(4):695–705. [Google Scholar]
- Kim S.-D., Spizizen J. Molecular cloning and expression of Bacillus pasteurii urease gee in Echerichia coli. Korean J. Appl. Microbiol. 1985;13(3):297–302. [Google Scholar]
- LeMetayer-Levrel G., Castanier S., Orial G., Loubiere J.F., Perthuisot J.P. Applications of bacterial carbonatogenesis to the protection and regeneration of limestones in buildings and historic patrimony. Sediment. Geol. 1999;126(1–4):25–34. [Google Scholar]
- Newnham R.E. Molecular mechanisms in smart materials. MRS Bull. 1997;22(5):20–34. [Google Scholar]
- Pacheco-Torgal F., Labrincha J.A. Biotech cementitious materials: some aspects of an innovative approach for concrete with enhanced durability. Constr. Build. Mater. 2013;40:1136–1141. [Google Scholar]
- Ramachandran S.K., Ramakrishnan V., Bang S.S. Remediation of concrete using microorganisms. ACI Mater. J. 2001;98(1):3–9. [Google Scholar]
- Ramakrishnan, V., Bang, S.S., Deo, K.S., 1998. A novel technique for repairing cracks in high performance concrete using bacteria. In: Proc. Int. Conf. High Performance, High Strength Concrete 1998, Perth, Australia, pp. 597–617.
- Ramakrishnan, V., Deo, K.S., Duke, E.F., Bang, S.S., 1999. SEM investigation of microbial calcite precipitation in cement. In: Proc. Int. Conf. Cement Microscopy, Las Vegas, 1999, pp. 406–414.
- Ramakrishnan, V., Bang, S.S., Srinivasan, N., Ramesh, K.P., 2003. Durability of cement mortar made with different concentrations of bacteria. In: Proc. 25th Int. Conf. Cement Microscopy, Richmond, Virginia, 2003.
- Ramakrishnan V., Panchalan R.K., Bang S.S. SP-225. American Concrete Institute; 2005. Bacterial concrete – a concrete for the future; pp. 37–54. (Serviceability of Concrete). [Google Scholar]
- Stocks-Fischer S., Galinat J.K., Bang S.S. Microbiological precipitation of CaCO3. Soil Biol. Biochem. 1999;31(11):1563–1571. [Google Scholar]
- Tittelboom K.V., DeBelie N., DeMuynk W., Verstrete W. Use of bacteria to repair cracks in concrete. Cem. Concr. Res. 2010;40(1):157–166. [Google Scholar]
- Tiwari P.K., Joshi K., Rehman R., Bhardwaj V., Shamsuddin K.V., Sivasubbu S., Scaria V. Draft genome sequence of urease-producing Sporosarcina pasteurii with potential application in biocement production. Genome A. 2014;2(1) doi: 10.1128/genomeA.01256-13. e01256-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winchell N.A., editor. Elements of Optical Mineralogy. 5th ed. Wiley; New York: 1949. [Google Scholar]
- Zhong, L., Islam, M.R., 1995. A new microbial plugging process and its impact on fracture remediation. In: Proc. Soc. Petroleum Engineers 70th Ann. Tech. Conf. Dallas, Texas, 1995, pp. 703–715.