Graphical abstract
Keywords: Hepatitis C virus, Extrahepatic manifestations vasculitis, Cryoglobulins, Direct acting anti-HCV drugs
Abbreviations: ANCA, antineutrophil cytoplasmic antibody; APS, antiphospholipid syndrome; CAPS, catastrophic antiphospholipid syndrome; BAL, bronchoalveolar lavage; CRP, C reactive protein; CTD, connective tissue disease; DAA, direct acting antiviral drugs; ESR, erythrocyte sedimentation rate; GIT, gastrointestinal tract; HSP, Henoch-Schonlein Purpura; HUS, hemolytic uremic syndrome; IFN α, interferon alpha; IHD, ischemic heart disease; MOH, minister of health; MRI, magnetic resonance imaging; NHL, non Hodgkin lymphoma; PAN, polyarteritis nodosa; PCR, polymerase chain reaction; PFT, pulmonary function test; PN, peripheral neuropathy; RNA, ribonucleic acid; TIAs, transient ischemic attacks; TTP, thrombotic thrombocytopenic purpura
Abstract
Vasculitis is a remarkable presentation of the extrahepatic manifestations of HCV. According to the presence or absence of cryoglobulins it is subdivided into two main types: cryoglobulinemic vasculitis and non cryoglobulinemic vasculitis based on the attribution of vasculitis to serum cryoglobulins as a pathogenic factor. The attribution of cryoglobulinemia to HCV represents a success story in the history of immunology, microbiology, and clinical medicine. HCV can bind to and invade lymphocytes, consequently triggering an immune response through different mechanisms. The epidemiology of the disease is well described and the clinical picture describes cutaneous, pulmonary, musculoskeletal, neurological, renal, endocrine, gastrointestinal, hepatic and cardiovascular manifestations. It may also be associated with sicca symptoms, an increased risk of lymphoma and serious catastrophic events. The pathology is well characterized. A classification criteria of the syndrome that was validated in 2014 is discussed. Management of CV is decided according to the presence and severity of its clinical presentation. It is divided into asymptomatic, mild, moderate, severe and life threatening disease. Recently introduced direct antiviral agents are proving safe and effective in the management of cryoglobulinemic vasculitis, and it is advocated that the two types of vasculitis be given prioritization in the Egyptian mass campaign to eradicate HCV.
Introduction
Out of all extrahepatic manifestations of Hepatitis C virus (HCV), the vasculitic ones deserve a special consideration. They have a multisystem presentation since they involve the blood vessels. The majority of cases are attributed to the presence of cryoglobulins (CGs), a substance with unique physicochemical properties and significant morbidity and mortality. The story of unveiling the relationship of HCV and mixed Cryoglobulinemia (MC) is a remarkable story of scientific achievement, where an autoimmune disease could be attributed to an infectious agent. The final act of exploring the consequences of applying the newly introduced antiviral agents on patients with Cryoglobulinemic Vasculitis (CV) will give us an insight into the management of autoimmune phenomena when intertwined with microbial pathogenic factors.
HCV vasculitic syndromes will be discussed under two main topics based on the attribution of vasculitis to the presence or absence of serum cryoglobulins as a pathogenic factor:
-
–
HCV vasculitis in the presence of CGs or CV.
-
–
HCV vasculitis in the absence of CGs or non CV.
HCV vasculitis in the presence of CGs (CV)
CGs are immunoglobulins (Igs) that precipitate when exposed to cold and redissolve upon warming and cryoglobulinemia refers to the presence of such CGs in patient’s serum [1].
Historical background
In 1933, Wintrobe and Buell described cold precipitable proteins for the first time in a patient suffering from multiple myeloma [2]. Later on in 1947, Lerner and Watson realized that such proteins are gamma-globulins and gave them the term “CGs”. In 1962, Lospalluto and his colleagues mentioned that CGs are formed of two components: 7S and 19S that nowadays correspond to IgG and IgM respectively, and they also found Rheumatoid Factor (RF) activity of the 19S component in 1966. Meltzer and Franklin reported that palpable purpura, arthralgia and myalgia are the typical clinical manifestations related to CGs; hence, they were given the name “Meltzer triad”. They confirmed the previously mentioned components of CGs and introduced the term essential MC as their etiology was unknown to them [3].
According to the type of Igs, Brouet classified CGs into the following:
-
–
Type I CGs: composed of HCV antigens, complement components and monoclonal Igs (mIg) usually IgM or IgG and less commonly IgA or free light chains and this type is commonly seen in monoclonal gammopathies like multiple myeloma and Waldenstrom’s macroglobulinemia.
-
–
Type II CGs: formed of a mixture of polyclonal IgG and monoclonal Igs usually of IgM type with RF activity commonly seen secondary to many infections mainly HCV, Hepatitis B virus (HBV), and Human immunodeficiency virus (HIV).
-
–
Type III CGs: includes a mixture of polyclonal IgG and IgM with RF activity usually found secondary to many connective tissue diseases especially Sjogren’s syndrome, rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) [4].
Types II and III CGs are called MC that are now mainly seen secondary to HCV in 80%:90% of cases. CGs are frequently seen in HCV patients (40%:60% of cases) usually in asymptomatic form but in 1% to 5% of cases can precipitate in small and medium sized vessels of different tissues inducing CV [5].
The development of the syndrome was attributed to many risk factors including female gender, advanced age, alcohol consumption above 50 gr/day, longer duration of infection, type II MC, higher MC serum levels, clonal B cell expansion in both the blood and liver, HCV genotype 2 or 3, and extensive liver fibrosis [6], [7].
Pathogenesis of CV
Binding and invasion
HCV can directly invade lymphocytes through its E2 protein that binds to CD81 of lymphocytes facilitating its inoculation [8], [9].
Immune response
A-Molecular mimicry theory: HCV is a single stranded RNA virus so it cannot integrate into human DNA but according to molecular mimicry theory, the viral E2 protein is antigenically similar to human Igs and this stimulates anti Ig antibodies that can in turn stimulate complement cascade forming immune complex (the CG molecule) [10].
- B-Role of T lymphocytes: there are many clues in the literature arguing for a pivotal role for these cells in CV including:
-
•Biopsy from peripheral nerves and skin involved in CV revealed monocytes, memory and activated T cells but only few B cells [11].
-
•
Many studies showed CD4 Th1 predominance in CV with its proinflammatory chemokines including chemokines CXCL9, 10 and 11 especially 10 and its receptor CXCR3 as well as Macrophage Induced Protein 1 α and β (MIP1 α, MIP1 β) that together with Th1 cytokines especially Interferon γ (IFN γ) and Tumor Necrosis Factor α (TNF α) were markedly increased in nerve biopsies from HCV induced CV patients compared to neuropathies due to other causes in one study. CXCL10-mRNA is overexpressed in hepatocytes in HCV infected patients and has a role in the pathogenesis of the disease through recruitment of inflammatory cells namely T cells but not neutrophils to the site of inflammation and sera of patients with HCV related CV showed also high levels of CXCL10 that not only has a role in the pathogenesis of the disease but are also related to histological severity and lobular inflammation. Moreover, low levels of CXCL10 were associated with low viremia and hence better response to interferon treatment [11], [12], [13].
-
•
Evidence of inhibition of CD4 + CD25 + T cells (T Reg) with its known role in prevention and control of autoimmunity [14].
C-The arguments for B cells
-
•
Chronic HCV infection results in B cell invasion, chronic stimulation, activation, proliferation and clonal expansion in the liver, bone marrow and peripheral blood that is initially polyclonal then evolves into oligoclonal and finally into monoclonal expansion which is commonly seen in CV as well as monoclonal gammopathies and Non Hodgkin Lymphoma (NHL) [15].
-
•
In HCV patients, there is evidence of increased CD5+ B cells that overexpress CD81 cells which when they bind with E2 HCV proteins, can sensitize and activate B cells causing Naïve B cell proliferation, polyclonal B cell formation and importantly increased expression of activation induced deaminase that has many biological roles including mutation of B cells and lymphomagenesis by increasing expression of lymphomagenesis related genes in CD19+ lymphocytes and this among other things may explain the higher incidence of NHL among HCV induced CV patients [16], [17].
-
•
There is also evidence of increased BAFF or B Lymphocyte Stimulator (BLyS) involved in B cell survival and activation in the sera of HCV MC +ve more than HCV MC −ve and still more than non HCV infected patients [18].
D-Role of innate immunity
Some studies reported a role for Toll Like Receptors (TLRs) especially TLR2 in CV as the study by Feldmann G and his colleagues that showed increased TLR2 expression on monocytes in MC compared to control and this may induce IL6 production that was shown in vitro to have a role in B cell proliferation and in vivo to be higher in sera of CV patients [19].Vascular cell adhesion molecule-1 (VCAM-1) may be involved in disease pathogenesis especially the severe forms of CV through mononuclear cell recruitment [20].
E-Role of host gene mutation and polymorphism
-
•
Rb gene mutation: This mutation results in decreased expression of Rb protein involved in B cell cycle inhibition whenever DNA damage occurs leading to increased mutated cells and oncogenesis [21].
-
•
Chromosomal translocation: chromosomal translocation[-t(14;18)] leads to increased expression of Bcl-2 with its antiapoptotic function on B cells resulting in overgrowth and clonality involved in MC [22].
-
•
Single nucleotide polymorphism (SNP) in BAFF gene promoter area:
-
•
This may lead to overexpression of BAFF involved in B cell survival and development in HCV induced MC [23].
-
•
SNPs on chromosome 6:
In a multicentric Genome Wide Association study (GWAS), Zignego et al. [24] detected an association between two SNPs on chromosome 6 and HCV induced MC compared to HCV without MC including: SNPs in intron of NOTCH4 gene and SNPs located between HLADRB1 and HLADQA1 segments of MHC.
F-Epigenetics
The small noncoding micro RNA (mi RNA) has posttranscriptional functions in preventing translation and inducing cleavage of complementary m-RNA. Downregulation of mi RNA 26b had been observed in HCV induced MC and NHL but this needs further investigations [25].
Epidemiology
The incidence of HCV infection in MC in the Mediterranean Basin is 90%. The incidence is reportedly 1:100,000 persons with a female-to-male ratio of 3:1 and mean age of 42–52 years [26].
Clinical picture
Initially MC as mentioned earlier affects small and medium sized vessels of different organs including the following.
Cutaneous manifestations
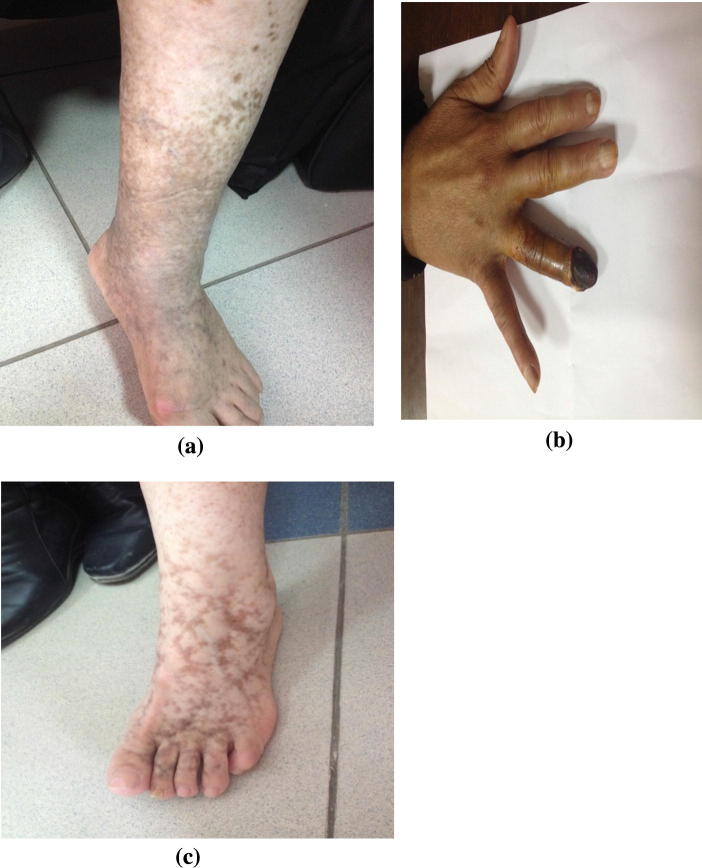
It represents the most common manifestation of the disease mainly in the form of palpable purpura and less commonly maculopapular rash that involves predominantly the lower limbs, the lower trunk, and the upper extremities to a lesser extent. These purpuric lesions are usually intermittent and sporadic but may coalesce and heal leaving hyperpigmented areas. Other less common cutaneous lesions include Raynaud’s phenomena, acral cyanosis and livedo reticularis that are commonly seen in Type I cryoglobulinemia and rarely seen in MC. A more serious severe form includes skin ulcers commonly found on lower extremities especially at the malleoli that may complicate confluent purpuric eruptions and lead in some patients to secondary infection and gangrene (Fig. 1) [27], [28].
Fig. 1.
Three cases with cutaneous manifestations of CV: a) Hyperpigmented skin lesions, b) Finger gangrene, and c) Livedo reticularis.
Musculoskeletal manifestations
The second most common manifestations (60%:90% of cases) include arthralgia usually in the form of intermittent mono-oligoarthralgia affecting mainly large joints especially the ankles and this type together with RA like polyarthritis affecting small joints that are the two most common joint manifestations of HCV. Myalgia is also frequently reported in HCV induced CV while arthritis and myositis are rarer manifestations of the disease [29].
Neurological manifestations
HCV induced CV affects mainly the peripheral nervous system in up to 50% of cases and commonly affects sensory nerves more than motor nerves. However, the mixed type had also been reported. Distribution includes polyneuropathy and mononeuritis multiplex to a lesser extent. Patients complain of paresthesia, tingling and numbness of the lower limbs that tend to exacerbate at night [30]. Less commonly, CV may affect central nervous system (CNS) usually in the form of cerebral vasculitis that can present with ischemic stroke, TIAs or cognitive impairment and can occur with high intensity white matter lesions in brain MRI [31].
Renal manifestations
This occurs in about 20%:30% of patients and carries a bad prognosis even when treated. They show a high incidence of treatment failures and relapses. It usually presents with mild proteinuria and isolated hematuria but nephrotic and nephritic syndromes and chronic renal insufficiency had also been described to a lesser extent [32]. The commonly detected histopathological pattern is Membranoproliferative Glomerulonephritis (MPGN) with immune complex deposition in the glomeruli and IgM staining in contrast to MPGN that can also be seen in HCV without cryoglobulinemia in which staining is of IgG type. Interestingly, some authors found HCV antigens in renal biopsies of patients with negative serum anti HCV antibodies and PCR denoting possible consumption of the antigen and its antibody in the immune complex [33], [34]. Other types of GN described in MC related to HCV include mesangioproliferative and focal proliferative GN in up to 20% of renal involvement.
Sicca symptoms
As noted previously, HCV is a sialotropic virus with clinical picture of dry mouth and dry eyes like that found in primary Sjogren’s syndrome having a similar histopathological picture namely focal lymphocytic infiltration, but with two important differences; infiltrates are mainly perivascular rather than pericanalicular with absence of glandular canal damage and also with CD8+ ve rather than CD4+ve predominance. Anti SSA and anti SSB antibodies may also be a point of differentiation. They tend to be positive more in the primary Sjogren’s syndrome patients [35].
Pulmonary manifestations
Subclinical alveolitis had been described in MC based on BAL findings that in rare cases may progress to clinically evident interstitial lung disease.
PFT may show evidence of small airway disease and impairment of gas exchange, with symptoms ranging from dyspnea to cough and pleurisy. Less commonly, bronchiolitis obliterans organizing pneumonia (BOOP), pulmonary hemorrhage, and pulmonary vasculitis may be found in MC [36].
Endocrine disorders
Autoimmune thyroiditis, hypothyroidism and papillary thyroid carcinoma are more common in MC patients compared to the general population. Type 2 diabetes mellitus had been also statistically linked to HCV with and without MC more than general population [37], [38].
Gastrointestinal and hepatic manifestations
Hepatomegaly, abnormal liver function tests or abnormal liver biopsy, splenomegaly, lymphadenopathy and abdominal pain were described in up to 90%, 30%, 20% and 20% of cases respectively. Liver involvement occurs in almost 3/4 of individuals with nearly threefold increase in advanced liver fibrosis and steatosis as well as significant association with cirrhosis after adjustment for age, gender and duration of infection [39], [40].
Cardiovascular manifestations
Ischemic heart disease with coronary vasculitis, valvular heart disease with mitral valvular damage and incompetence, pericarditis, cardiomyopathies especially the hypertrophic type and congestive heart failure had been all reported in MC [41].
Catastrophic CG vasculitis
This term had been used first in catastrophic antiphospholipid syndrome (CAPS) and hence it was borrowed to refer to widespread and severe ischemic vasculitis affecting small and medium sized vessels with multiple organ involvement over a short period of time for unknown reasons including the skin, kidneys, lungs, CNS and GIT. This leads to critical life threatening manifestations as cutaneous ulcers, IHD, RPGN, critical neuropathy, stroke and intestinal mesenteric ischemia that was found in 14% of patients with CV according to one report [42].
Risk of lymphoma
Cryoglobulinemia is considered a premalignant disease due to noted B cell expansion and tendency to monoclonality that may end in B cell NHL. Risk factors of clonal B cell proliferation include longer duration of HCV infection, type II cryoglobulins, and particularly the presence of vasculitis. In a recent meta-analysis, the prevalence of HCV infection in patients with B-cell NHL was approximately 15%, much higher than the seroprevalence of HCV in the general population. Lymphoplasmocytic lymphoma, diffuse large cell lymphoma and marginal zone lymphoma are the main types of B cell lymphoma related to HCV infection [43]. More recently, Quartuccio et al. [44] documented that CV and primary Sjogren’s syndrome (pSS) were independent risk factors for lymphoma in patients with evidence of serum CGs with the highest risk found when both conditions coexisted.
Pathology
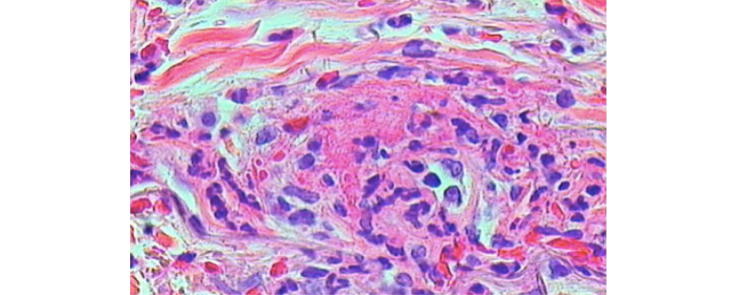
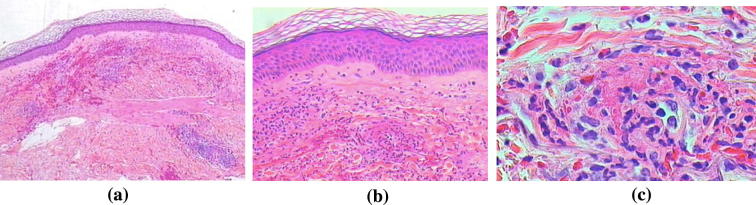
Histopathological evidence of vasculitis remains the gold standard for diagnosing CV. Biopsy is usually taken from affected organs including cutaneous lesions, nerve and renal biopsies. Bone marrow biopsy should be done to demonstrate polyclonal B cell proliferation in suspected cases with lymphoproliferative disorders [45]. Pathological features of CV differ from other types of vasculitis by nature of the vessels involved (small size vessels, i.e. arteries, capillaries, and veinules), type of inflammatory infiltrate (lymphomonocytic rather than polymorphonuclear) and site of inflammation (mainly perivascular with few wall infiltrates) [46], [47]. Skin biopsy usually reveals leukocytoclastic vasculitis (LCV) as the hallmark histopathological feature of the disease with an important finding that distinguishes it from other causes of LCV; in MC, the infiltrating cells in the perivascular area characterizing LCV are T cells and mononuclear cells and not neutrophils [27], [28] as shown in Fig. 2, Fig. 3.
Fig. 2.
Skin biopsy from a purpuric lesion shows (a) Marked extravasation of red blood cells, (b) a dense perivascular infiltrate of neutrophils and lymphocytes, and (c) thickening of blood vessel wall with fibrin deposition, a perivascular infiltrate with neutrophils show leukocytoclasia (Courtesy of Dr Hussein Hassab-ElNaby Professor of Dermatology and Dermatopathology Al-Azhar University).
Fig. 3.
Skin biopsy from a vesicular lesion shows (a) A subepidermal cleft, (b) the cleft contains neutrophils, and (c) a perivascular infiltrate of neutrophils with blood vessel wall necrosis (Courtesy of Dr Hussein Hassab-ElNaby Professor of Dermatology and Dermatopathology Al-Azhar University).
Neural biopsies show endoneurial vasculitis with vessel wall destruction, patchy focal demyelination and axonal degeneration [48]. In milder forms of PN, intravascular deposition of CGs in the vasa nervosa without frank vasculitis can be seen [49].
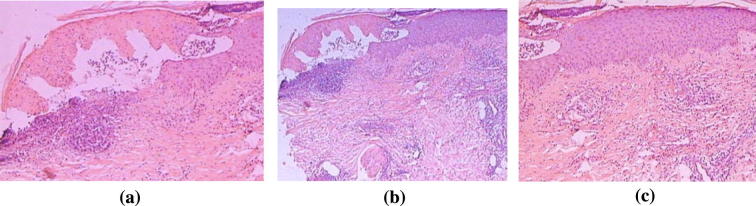
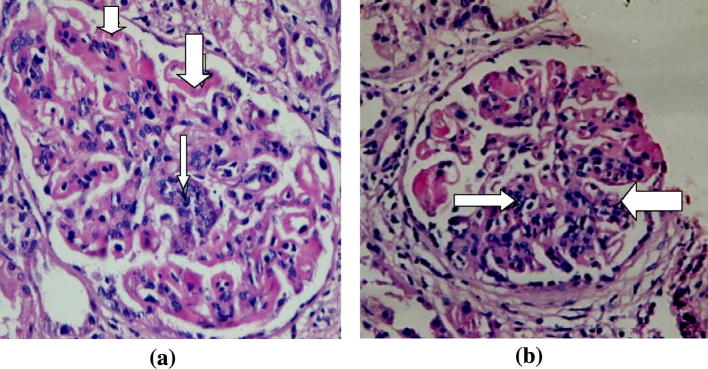
Renal biopsies are usually consistent with MPGN (Fig. 4, Fig. 5) with subendothelial Igs and complement deposition [50]. Electron microscopy reveals glomerular dense deposits with tubular, annular, or fibrillar structures [51].
Fig. 4.
A case of HCV related Glomerulonephritis (Cryoglobulinemic GN). It shows (a) irregular thickening of capillary basement membrane (GBM) with wire loop formation (thick arrows) and segmental proliferation (thin arrow). Hx and E, original magnifications × 400 and (b) wire loop formation, hyaline thrombi and segmental proliferation (arrows). Hx and E × 400. (Courtesy of Dr Sawsan Fadda Professor of Pathology Cairo University).
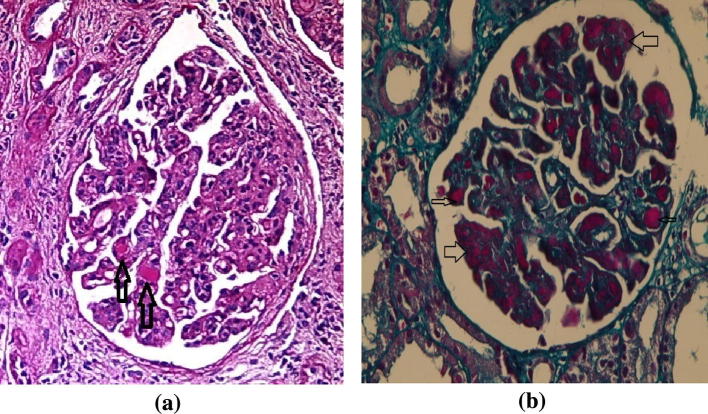
Fig. 5.
(a) Another case shows irregular thickening of GBM with formation of PAS positive hyaline thrombi (arrows). PAS stain × 400 and (b) a third case show wire loop formation (thick arrows) and hyaline thrombi (thin arrows). Masson Trichrome stain × 400 (Courtesy of Dr Sawsan Fadda Professor of Pathology Cairo University).
Differential diagnosis
The disease should be differentiated from two main sets of patients [52].
-
•
Small or medium-sized vasculitides including drug induced (hypersensitivity), IgA associated (formerly HSP), ANCA-associated vasculitis as well as vasculitis related to infections like bacterial endocarditis, poststreptococcal vasculitis and GN and vasculitis associated with CTD like SLE, RA and Sjögren’s syndrome.
-
•
Vasculitis mimickers including: Infectious like Rickettsia and malaria species as well as thromboembolic disorders as APS, TTP, HUS and atrial myxoma.
Diagnostic criteria
A recent new classification, not a diagnostic, criteria had been developed in 2011 [53] and validated in 2014 [54] for investigational and epidemiological purposes, and aims at a higher specificity. It includes three domains; questionnaire, clinical and laboratory with sensitivity of 88.5%, 95% CI (84.3% to 92.8%) and a specificity of 95.4%, 95% CI (90.9% to 99.8%) (Table 1) with the advantage of diagnosing patients with difficulties in completing a questionnaire, those who have uncommon clinical features and those with typical clinical manifestations but lack some laboratory data for any reason. This set is helpful only for patients with MC and should not be used for type I CGs. Another important thing is that positive serum CGs measured at two occasions at least 12 weeks apart is obligatory. Cases with typical clinical features of CV but with negative CGs should be followed carefully with repeated measurements for serum CGs and one last thing, patients with previous positive CGs results should be considered positive even if CGs are negative at the time of evaluation [53].
Table 1.
Preliminary classification criteria for the CV [53].
| Satisfied if at least 2 of the 3 items (questionnaire, clinical, laboratory) are positive |
| the patient must be positive for serum cryos in at least 2 determinations at ⩾ 12 week interval |
| i. Questionnaire item: (at least 2 out of the following): |
|
| ii. Clinical item: at least 3 out of the following 4 (present or past): |
|
| iii. Laboratory item: at least 2 out of the following 3 (present): |
|
The international study group recommendations (ISG-EHCV) emphasized the importance of repeated checking of serum CGs as levels of CGs may largely vary in the same patient during natural course of the disease [55].
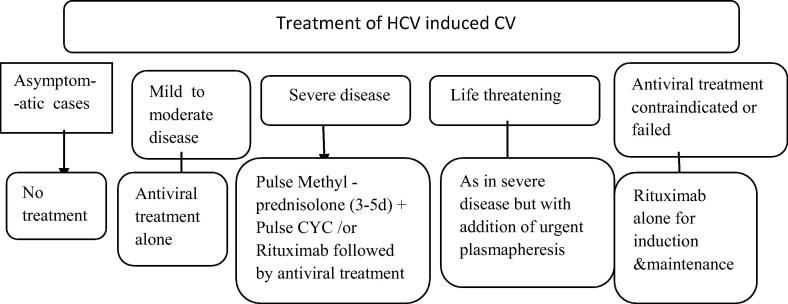
Management
We will discuss management of CV at 5 main levels of intervention (Fig. 6):
-
–
Asymptomatic CV doesn’t need therapy while symptomatic cases will be managed according to severity and target organ affection.
-
–
Mild cases presenting with joint manifestations, fatigue, myalgia and simple cutaneous manifestations as purpura usually show good response to antiviral therapy in many studies in contrast to HCV induced arthritis without CV that showed variable response to antiviral therapy (IFNα plus ribavirin) ranging from good response in some studies to bad response and even deterioration in others. However, we have to bear in mind the ability of IFNα to exacerbate autoimmune disease states [56]. The direct acting anti HCV drugs in IFNα free protocols resulted in SVR rates in many studies including cirrhotic patients and those who failed previous therapy or relapsed [57]. The success story of DAA in the management of HCV prompted their introduction into the armamentarium of the extrahepatic manifestations. The recently introduced IFNα free regimens using DAA drugs again proved safe and effective in the management of CV. In a recent work by the team of Saadoun and Cacoub [58], they successfully treated HCV induced CV cases by sofosbuvir plus ribavirin without significant harm. Currently, there is an ongoing Egyptian study entitled “The effect of Sofosbuvir on Cryoglobulinemic Syndrome” that is based on the earliest protocols sponsored by the Egyptian MOH (supported by a grant from the Science and Technology Development Fund (STDF), protocol number 15083). The early results of this study were included as part of multicenter interventional treatment trial, including 26 Egyptian and 9 Italian patients with CV, diagnosed according to the classification criteria of CV [53]. Patients were treated with Sofosbuvir following four treatment protocols: 1- Sofosbuvir + Ribavirin for 6 months, 2- Sofosbuvir + Ribavirin + Interferon for 3 months, 3- Sofosbuvir + Daclatasvir for 3 months, and 4- Sofosbuvir + Simeprevir for 3 months. Clinical assessment (according to the classification criteria of CV), cryocrit %, C4 serum level and serum level of rheumatoid factor (RF), were recorded soon before and at the end of treatment. Response was assessed as complete, partial or absent (no response). 35 patients (24 females, mean age 56 ± 11 y) were enrolled in the study. Sixteen patients showed liver cirrhosis. All patients were Child- Pugh A stage. 13,8,5,9 patients were treated by first, second, third, and fourth protocol, respectively. All patients showed a sustained viral response (SVR). All patients had purpura and all of them responded to treatment. 35 patients had fatigue and 24 patients had fibromyalgia, 34, 22 patients responded respectively. 26 patients had arthralgia and 23 patients had non erosive arthritis, 22, 20 patients responded respectively. 13 patients had Raynaudˈs phenomenon and 2 patients had necrotizing vasculitis, 12, 2 patients responded respectively after treatment. 25 patients had peripheral polyneuropathy with total response after treatment in 21 patients. One of our patients had leg ulcer and mono- neuritis multiplex with complete response and completely healed ulcer after treatment. Nephritis was diagnosed in two patients, one of whom had proteinuria and renal impairment whereas the other had proteinuria only. They showed total improvement on antiviral therapy alone. Serum level of Cryocrit (P < 0.0001), RF (P = 0.004), C4 (P = 0.003), significantly decreased after treatment [59]. However, long term effects will need a longer period of observation.
-
–
In rare cases of HCV-CV induced arthritis not responding to the above mentioned therapy, the anti-CD20 “rituximab” may be added with very good response and without deleterious HCV related morbidities [60].
-
–
More severe presentations as non-life threatening renal, neurological and cardiac affection, immunosuppressives are to be used in combination with antiviral therapy. There is no consensus, however, among experts in the field on whether to apply antiviral therapy and immune suppression sequentially or simultaneously. IV methylprednisolone 10–15 mg/kg/dose for 3–5 consecutive doses plus IV cyclophosphamide in a dose of 10–15 mg/kg can be used. Knowing HCV direct role in sustaining an in situ B-cell expansion as previously noted, another reasonable approach is to use rituximab in either immunosuppressant naive or resistant cases in a weekly dose of 375 mg/m2 for four doses followed by the antiviral treatment to hold the antigenic trigger with no HCV related morbidities. Whenever antiviral therapy failed or was contraindicated, rituximab may be used alone in the same doses mentioned before for induction followed by a maintenance dose of 200–500 mg every 6–9 months [61]. However, caution is needed in patients with high cryocrit levels to avoid worsening of vasculitis as a consequence of immune complexes formation between rituximab and IgM [62].
Fig. 6.
Management of CV. Adapted and modified from Cacoub et al. [61].
For fulminant or catastrophic presentations as RPGN, GIT vasculitis, peripheral necrosis of the extremities, pulmonary alveolar hemorrhage and/or CNS involvement, plasmapheresis is essential to remove circulating CGs. Double-filtration plasmapheresis is advised for its selectivity to remove CGs from plasma while preserving essential components. Three sessions weekly for six to nine sessions are the usual protocol needed with simultaneous immunosuppressive therapy. It should be administered following the same regimens noted above to prevent rebound increase in serum CGs that may occur after discontinuation of plasmapheresis and this again to be followed by antiviral as previously discussed [63], [64] (see Fig. 7, Fig. 8).
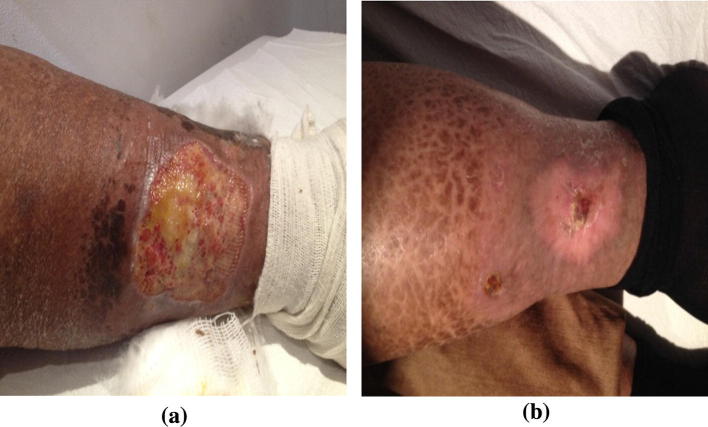
Fig. 7.
One of our patients with CV and leg ulcer before (a) and after (b) treatment with sofosbuvir, Ribavirin, and IFN combination.
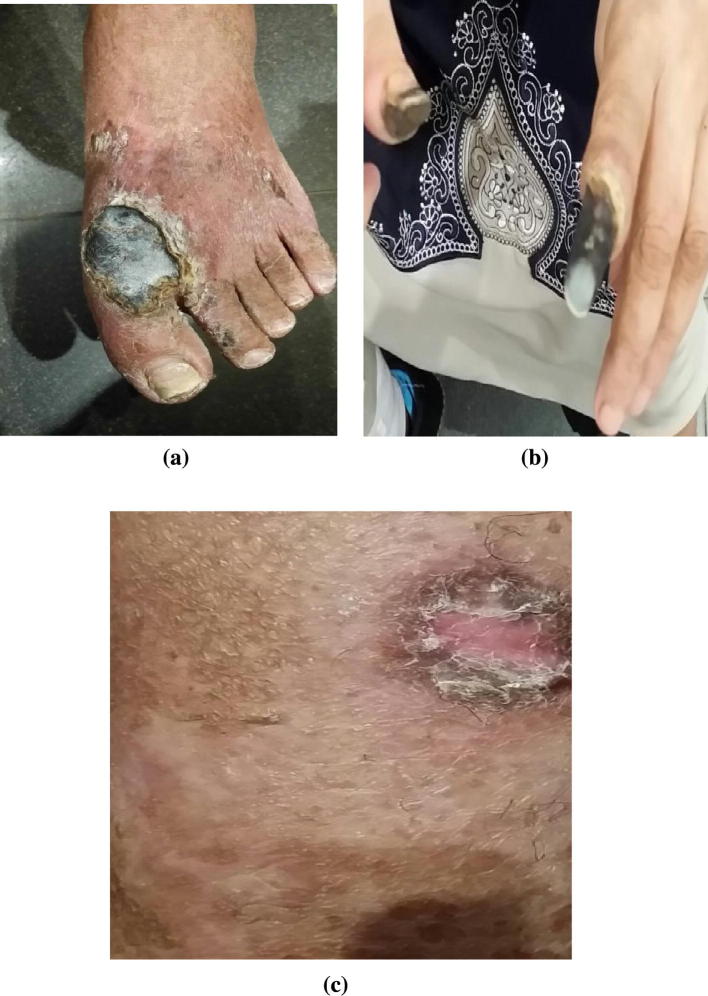
Fig. 8.
Vasculitic lesions in one of our patients with HCV related PAN showing: (a) Foot necrotizing ulcers, (b) Acral gangrene, and (c) Healed necrotizing ulcers.
Prognosis
Compared to other systemic vasculitides, CV shows two distinctive symptoms, namely B-cell NHL and chronic hepatitis, while B-cell NHL affects a minority of patients, usually as a late complication. Ages older than 60 years at diagnosis and renal involvement carry the worst prognosis [7], [65]. The overall 5 years survival after the diagnosis of vasculitis ranges from 50% to 90% in case of renal involvement.
Causes of death
The most frequent causes of death are renal involvement and widespread vasculitis most often involving the GIT. Other frequent causes of death include hepatic involvement and sepsis but in a significant number of cases, the cause remains unknown [66] (Table 2).
Table 2.
Causes of CV related mortality [60].
| Organ involvement | % |
|---|---|
| Hepatic (cirrhosis, HRS, liver failure) | 31 |
| HCC | 4 |
| Sepsis | 25 |
| Heart failure | 8 |
| Lymphoproliferative disorders | 6 |
| Neoplasm | 2 |
| CNS vasculitis | 8 |
| Gastrointestinal vasculitis | 2 |
| Lung vasculitis | 2 |
| Hyperviscosity syndrome | 7 |
| Multi-organ failure | 2 |
HCV vasculitis in the absence of CGs or non CV
In the literature, few studies discussed systemic vasculitis induced by HCV without evidence of cryoglobulinemia, most prescribed is HCV related PAN.
HCV related PAN
PAN is a medium sized necrotizing vasculitis classically related to HBV, but to a lesser extent can be found in HCV infected patients.
Patients with HCV related PAN differ from those related to CV in many respects. Clinically, more frequently reported life threatening vasculitis, severe multifocal sensorimotor mononeuropathies versus distal moderate sensory polyneuropathies, malignant hypertension, cerebral angiitis, ischemic abdominal pain, kidney and liver microaneurysms but lower rates of arthralgias, purpura and activity of chronic hepatitis distinguished this form from CV.
Laboratory-wise, there is evidence of higher acute phase reactants like ESR and CRP and more reported renal insufficiency. Histopathologically HCV related PAN affects medium sized arteries to a higher extent with necrotizing vasculitis rather than immune complex disease suggesting cell-mediated inflammation while mononuclear cell infiltrate in perivascular areas is more frequently reported in CV [67], [68].
In case CV mass campaign on a national level is to be implemented and when prioritization is to be respected, it was recommended that cases with CV as well as HCV related PAN should be among the most important HCV infected patients in whom viral clearance is warranted and this was mentioned in a recent prioritization study by an Egyptian group [69].
Conclusions
Vasculitis is a remarkable extrahepatic presentation of HCV. It includes 2 types: cryoglobulinemic and non-cryoglobulinemic vasculitis based on the presence or absence of cold-precipitable antibodies called cryoglobulins in patients’ sera. The epidemiology, pathogenesis, and clinical manifestations are discussed. The success of the new direct antiviral drugs in the management of HCV led to their introduction as therapeutic agents in the extrahepatic presentations including vasculitis. The early results are encouraging; however, long-term follow-up is needed to evaluate their efficacy and safety in this regard.
Conflict of Interest
The authors have declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Biographies

Prof. Gaafar Ragab graduated from the Faculty of Medicine, Cairo University, 1976, where he got his degrees in Internal Medicine: MSc, 1980, and PhD, 1985. He served his faculty as a Resident, Assistant Lecturer (1980), Lecturer (1985), Assistant Professor (1990), and Professor (1995) till now. He spent a sabbatical year 1989 at the UAB, USA in the department of Clinical Immunology and Rheumatology. He headed the Clinical Immunology & Rheumatology Unit affiliated to the Internal Medicine Department 2010–2015. He was Chief of the Internal Medicine Department’s Research Committee 1995–2013. He is Fellow of the American College of Rheumatology (ACR) since 1989. He is a Co-founder of the Egyptian Society of Immunology and Rheumatology (EGYSIR) which he headed as its President 2010–2013. He was chosen as President of the Egyptian League Against Rheumatism (ELAR) annual meeting in Alexandria 2013. He is member of the Advisory Board of the Egyptian Society of Internal Medicine (ESIM) and its Journal and served as President of ESIM annual meeting, Cairo, 2014. He is the associate editor of the Journal of Advanced Research (JARE), the interdisciplinary publication of Cairo University, in charge of its medical branch. He won the prize of honour, Cairo University, for the great efforts in international publications, for the Year 2008. Member of the Egyptian National Committee for the management of Hepatitis C Virus (the extrahepatic manifestation) as well as the International Study Group of Extrahepatic Manifestations Related to Hepatitis C Virus infection (ISG-EHCV).

Dr Mohamed Ahmed Hussein got his degrees in Internal Medicine: M.B., B.CH (2002), M.Sc. (2008), M.D. (2012), Cairo University. He served his faculty as Assistant lecturer (2009) and lecturer (2012 till now) of Internal Medicine, Cairo University. He is interested in vasculitis and works in Duplex Ultrasonography laboratory at Kasr Alainy Medical School, Cairo University 2014 till now. He attended as a consultant in clinical and ultrasonography in Arthritis Clinic, University of Udine, Italy, 2014. He received a course on Musculoskeletal Ultrasound certified by Spanish Society of Rheumatology (2010). He is member of the Egyptian Society of Immunology and Rheumatology (EGYSIR), Egyptian League against Rheumatism (ELAR), Egyptian National Committee for the management of Hepatitis C Virus (the extrahepatic manifestations) and Egyptian Society of Internal Medicine.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Vermeersch P., Gijbels K., Mariën G., Lunn R., Egner W., White P. A critical appraisal of current practice in the detection, analysis, and reporting of cryoglobulins. Clin Chem. 2008;54(1):39–43. doi: 10.1373/clinchem.2007.090134. [DOI] [PubMed] [Google Scholar]
- 2.Wintrobe M., Buell M. Hyperproteinemia associated with multiple myeloma. With report of a case in which an extraordinary hyperproteinemia was associated with thrombosis of the retinal veins and symptoms suggesting Raynaud’s disease. Bull Johns Hopkins Hosp. 1933;52:156–165. [Google Scholar]
- 3.Meltzer M., Franklin E.C., Elias K., McCluskey R.T., Cooper N. Cryoglobuline-mia—a clinical and laboratory study. II. Cryoglobulins with rheumatoid factor activity. Am J Med. 1966;40(6):837–856. doi: 10.1016/0002-9343(66)90200-2. [DOI] [PubMed] [Google Scholar]
- 4.Brouet J.C., Clouvel J.P., Danon F., Klein M., Seligmann M. Biologic and clinical significance of cryoglobulins. Am J Med. 1974;57:775–788. doi: 10.1016/0002-9343(74)90852-3. [DOI] [PubMed] [Google Scholar]
- 5.Zignego A.L., Giannini C., Gragnani L. HCV and lymphoproliferation. Clin Dev Immunol. 2012;2012:980942. doi: 10.1155/2012/980942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vallat L., Benhamou Y., Gutierrez M., Ghillani P., Hercher C., Thibault V. Clonal B cell populations in the blood and liver of patients with chronic hepatitis C virus infection. Arthritis Rheum. 2004;50:3668–3678. doi: 10.1002/art.20594. [DOI] [PubMed] [Google Scholar]
- 7.Ferri C., Sebastiani M., Giuggioli D., Cazzato M., Longombardo G., Antonelli A. Mixed cryoglobulinemia: demographic, clinical, and serologic features and survival in 231 patients. Semin Arthritis Rheum. 2004;33(6):355–374. doi: 10.1016/j.semarthrit.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Rosa D., Saletti G., De Gregorio E., Zorat F., Comar C., D’Oro U. Activation of naive B lymphocytes via CD81, a pathogenetic mechanism for hepatitis C virus-associated B lymphocyte disorders. Proc Natl Acad Sci USA. 2005;102(51):18544–18549. doi: 10.1073/pnas.0509402102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Machida K., Cheng K.T., Pavio N., Sung V.M., Lai M.M. Hepatitis C virus E2-CD81 interaction induces hypermutation of the immunoglobulin gene in B cells. J Virol. 2005;79(13):8079–8089. doi: 10.1128/JVI.79.13.8079-8089.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu Y.W., Rocheleau L., Larke B., Chui L., Lee B., Ma M. Immunoglobulin mimicry by Hepatitis C Virus envelope protein E2. Virology. 2005;332(2):538–549. doi: 10.1016/j.virol.2004.11.041. [DOI] [PubMed] [Google Scholar]
- 11.Saadoun D., Bieche I., Maisonobe T., Asselah T., Laurendeau I., Piette J.C. Involvement of chemokines and type 1 cytokines in the pathogenesis of hepatitis C virus-associated mixed cryoglobulinemia vasculitis neuropathy. Arthritis Rheum. 2005;52(9):2917–2925. doi: 10.1002/art.21270. [DOI] [PubMed] [Google Scholar]
- 12.Antonelli A., Ferri C., Fallahi P., Ferrari S.M., Frascerra S., Sebastiani M. High values of CXCL10 serum levels in patients with hepatitis C associated mixed cryoglobulinemia in presence or absence of autoimmune thyroiditis. Cytokine. 2008;42(1):137–143. doi: 10.1016/j.cyto.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Diago M., Castellano G., Garcia-Samaniego J., Perez C., Fernandez I., Romero M. Association of pretreatment serum interferon gamma inducible protein 10 levels with sustained virological response to peginterferon plus ribavirin therapy in genotype 1 infected patients with chronic hepatitis C. Gut. 2006;55(3):374–379. doi: 10.1136/gut.2005.074062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyer O., Saadoun D., Abriol J., Dodille M., Piette J.C., Cacoub P. CD4+CD25+ regulatory T-cell deficiency in patients with hepatitis C-mixed cryoglobulinemia vasculitis. Blood. 2004;103(9):3428–3430. doi: 10.1182/blood-2003-07-2598. [DOI] [PubMed] [Google Scholar]
- 15.Sansonno D., Lauletta G., De Re V., Tucci F.A., Gatti P., Racanelli V. Intrahepatic B cell clonal expansions and extrahepatic manifestations of chronic HCV infection. Eur J Immunol. 2004;34(1):126–136. doi: 10.1002/eji.200324328. [DOI] [PubMed] [Google Scholar]
- 16.Zuckerman E., Slobodin G., Kessel A., Sabo E., Yeshurun D., Halas K. Peripheral B-cell CD5 expansion and CD81 overexpression and their association with disease severity and autoimmune markers in chronic hepatitis C virus infection. Clin Exp Immunol. 2002;128(2):353–358. doi: 10.1046/j.1365-2249.2002.01844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ito M., Murakami K., Suzuki T., Mochida K., Suzuki M., Ikebuchi K. Enhanced expression of lymphomagenesis-related genes in peripheral blood B cells of chronic hepatitis C patients. Clin Immunol. 2010;135(3):459–465. doi: 10.1016/j.clim.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Gragnani L., Piluso A., Giannini C., Caini P., Fognani E., Monti M. Genetic determinants in hepatitis C virus-associated mixed cryoglobulinemia: role of polymorphic variants of BAFF promoter and Fcgamma receptors. Arthritis Rheum. 2011;63(5):1446–1451. doi: 10.1002/art.30274. [DOI] [PubMed] [Google Scholar]
- 19.Feldmann G., Nischalke H.D., Nattermann J., Banas B., Berg T., Teschendorf C. Induction of interleukin-6 by hepatitis C virus core protein in hepatitis C-associated mixed cryoglobulinemia and B-cell non-Hodgkin’s lymphoma. Clin Cancer Res. 2006;12(15):44918. doi: 10.1158/1078-0432.CCR-06-0154. [DOI] [PubMed] [Google Scholar]
- 20.Kaplanski G., Maisonobe T., Marin V., Grès S., Robitail S., Farnarier C. Vascular cell adhesion molecule-1 (VCAM-1) plays a central role in the pathogenesis of severe forms of vasculitis due to hepatitis Cassociated mixed cryoglobulinemia. J Hepatol. 2005;42(3):334–340. doi: 10.1016/j.jhep.2004.11.034. [DOI] [PubMed] [Google Scholar]
- 21.Machida K., Liu J.C., McNamara G., Levine A., Duan L., Lai M.M.C. Hepatitis C virus causes uncoupling of mitotic checkpoint and chromosomal polyploidy through the Rb pathway. J Virol. 2009;83(23):12590–12600. doi: 10.1128/JVI.02643-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zignego A.L., Ferri C., Giannelli F., Giannini C., Caini P., Monti M. Prevalence of bcl-2 rearrangement in patients with hepatitis C virus-related mixed cryoglobulinemia with or without B-cell lymphomas. Ann Intern Med. 2002;137:571–580. doi: 10.7326/0003-4819-137-7-200210010-00008. [DOI] [PubMed] [Google Scholar]
- 23.Novak A.J., Grote D.M., Ziesmer S.C., Kline M.P., Manske M.K., Slager S. Elevated serum B-lymphocyte stimulator levels in patients with familial lymphoproliferative disorders. J Clin Oncol. 2006;24(6):983–987. doi: 10.1200/JCO.2005.02.7938. [DOI] [PubMed] [Google Scholar]
- 24.Zignego A.L., Wojcik G.L., Cacoub P., Visentini M., Casato M., Mangia A. Genome-wide association study of hepatitis C virus- and cryoglobulin related vasculitis. Genes Immun. 2014;15(7):500–505. doi: 10.1038/gene.2014.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peveling-Oberhag J., Crisman G., Schmidt A., Döring C., Lucioni M., Arcaini L. Dysregulation of global microRNA expression in splenic marginal zone lymphoma and influence of chronic hepatitis C virus infection. Leukemia. 2012;26(7):1654–1662. doi: 10.1038/leu.2012.29. [DOI] [PubMed] [Google Scholar]
- 26.Ferri C., Ramos-Casals M., Zignego A.L., Arcaini L., Roccatello D., Antonelli A. International diagnostic guidelines for patients with HCV-related extrahepatic manifestations. A multidisciplinary expert statement. Autoimmun Rev. 2016;15(12):1145–1160. doi: 10.1016/j.autrev.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 27.Monti G., Galli M., Invernizzi F., Pioltelli P., Saccardo F., Monteverde A. Cryoglobulinaemias: a multi-centre study of the early clinical and laboratory manifestations of primary and secondary disease. GISC. Italian Group for the Study of Cryoglobulinaemias. QJM. Feb 1995;88(2):115–126. [PubMed] [Google Scholar]
- 28.Cohen S.J., Pittelkow M.R., Su W.P. Cutaneous manifestations of cryoglobulinemia: clinical and histopathologic study of seventy-two patients. J Am Acad Dermatol. Jul 1991;25(1):21–27. doi: 10.1016/0190-9622(91)70168-2. [DOI] [PubMed] [Google Scholar]
- 29.Ramos-Casals M., Trejo O., García-Carrasco M., Cervera R., Font J. Mixed cryoglobulinemia: new concepts. Lupus. 2000;9(2):83–91. doi: 10.1191/096120300678828127. [DOI] [PubMed] [Google Scholar]
- 30.Authier F.J., Bassez G., Payan C., Guillevin L., Pawlotsky J.M., Degos J.D. Detectio n of genomic viral RNA in nerve and muscle of patients with HCV neuropathy. Neurology. 2003;60:808–812. doi: 10.1212/01.wnl.0000044399.71601.ea. [DOI] [PubMed] [Google Scholar]
- 31.Dawson T.M., Starkebaum G. Isolated central nervous system vasculitis associated with hepatitis C infection. J Rheumatol. 1999;26:2273–2276. [PubMed] [Google Scholar]
- 32.Meyers C.M., Seeff L.B., Stehman-Breen C.O., Hoofnagle J.H. Hepatitis C and renal disease: an update. Am J Kidney Dis. 2003;42(4):631–657. doi: 10.1016/s0272-6386(03)00828-x. [DOI] [PubMed] [Google Scholar]
- 33.Bataille S., Kaplanski G., Boucraut J., Halfon P., Camus C., Daniel L. Membranoproliferative glomerulonephritis and mixed cryoglobulinemia after hepatitis C virus infection secondary to glomerular NS3 viral antigen deposits. Am J Nephrol. 2012;35:134–140. doi: 10.1159/000335375. [DOI] [PubMed] [Google Scholar]
- 34.Kong D., Wu D., Wang T., Li T., Xu S., Chen F. Detection of viral antigens in renal tissue of glomerulonephritis patients without serological evidence of hepatitis B virus and hepatitis C virus infection. Int J Infect Dis. 2013;17(7) doi: 10.1016/j.ijid.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 35.Cacoub P., Ratziu V., Myers R.P., Ghillani P., Piette J.C., Moussalli J. Impact of treatment on extra hepatic manifestations in patients with chronic hepatitis C. J Hepatol. 2002;36(6):812–818. doi: 10.1016/s0168-8278(02)00067-3. [DOI] [PubMed] [Google Scholar]
- 36.Manganelli P., Salaffi F., Subiaco S., Carotti M., Cervini C., Consigli G. Bronchoalveolar lavage in mixed cryoglobulinaemia associated with hepatitis C virus. Br J Rheumatol. 1996;35:978–982. doi: 10.1093/rheumatology/35.10.978. [DOI] [PubMed] [Google Scholar]
- 37.Antonelli A., Ferri C., Fallahi P., Giuggioli D., Nesti C., Longombardo G. Thyroid involvement in patients with overt HCV-related mixed cryoglobulinaemia. QJM. 2004;97:499–506. doi: 10.1093/qjmed/hch088. [DOI] [PubMed] [Google Scholar]
- 38.Antonelli A., Ferri C., Fallahi P., Sebastiani M., Nesti C., Barani L. Type 2 diabetes in hepatitis C-related mixed cryoglobulinaemia patients. Rheumatology (Oxford) 2004;43:238–240. doi: 10.1093/rheumatology/keh011. [DOI] [PubMed] [Google Scholar]
- 39.Kayali Z., Buckwold V.E., Zimmermann B., Schmidt W.N. Hepatitis C, cryoglobulinemia, and cirrhosis: a metaanalysis. Hepatology. 2002;36:978–985. doi: 10.1053/jhep.2002.35620. [DOI] [PubMed] [Google Scholar]
- 40.Remoroza R, Bonkovsky H. Extrahepatic manifestations of chronic Hepatitis C. The HCV Advocate Medical Writer’s circle, HCV support project; August, 2003.
- 41.Antonelli A., Ferri C., Ferrari S.M., Ghiri E., Goglia F., Pampana A. Serum levels of proinflammatory cytokines interleukin-1β, interleukin-6, and tumor necrosis factor α in mixed cryoglobulinemia. Arthritis Rheum. 2009;60(12):3841–3847. doi: 10.1002/art.25003. [DOI] [PubMed] [Google Scholar]
- 42.Ferri C., Mascia M.T. Cryoglobulinemic vasculitis. Curr Opin Rheumatol. 2006;18(1):54–63. doi: 10.1097/01.bor.0000198002.42826.c2. [DOI] [PubMed] [Google Scholar]
- 43.Vallisa D., Berte R., Rocca A., Civardi G., Giangregorio F., Ferrari B. Association between hepatitis C virus and non-Hodgkin’s lymphoma, and effects of viral infection on histologic subtype and clinical course. Am J Med. 1999;106(5):556–560. doi: 10.1016/s0002-9343(99)00069-8. [DOI] [PubMed] [Google Scholar]
- 44.Quartuccio L, Corazza L, Ramos-Casals M, Retamozo S, Ragab GM, Ferraccioli G, et al. Cryoglobulinemic vasculitis and primary sjogren’s syndrome are independent risk factors for lymphoma in a large worldwide population of patients with positive serum cryoglobulins. Medical or Research Professionals/Clinicians. Topic area: Clinical topics by disease. Specific topic: 18. Vasculitis.EULAR15-3300.
- 45.Mukhtyar C., Guillevin L., Cid M.C., Dasgupta B., de Groot K., Gross W. EULAR recommendations for the management of primary small and medium vessel vasculitis for the European Vasculitis Study Group. Ann Rheum Dis. 2009;68:310–317. doi: 10.1136/ard.2008.088096. [DOI] [PubMed] [Google Scholar]
- 46.Cacoub P., Saadoun D., Limal N., Leger J.M., Maisonobe T. Hepatitis C virus infection and mixed cryoglobulinaemia vasculitis: a review of neurological complications. AIDS. 2005;19(3) doi: 10.1097/01.aids.0000192081.33938.2f. S128-34. [DOI] [PubMed] [Google Scholar]
- 47.Cacoub P., Lidove O., Maisonobe T., Duhaut P., Thibault V., Ghillani P. Interferon-alpha and ribavirin treatment in patients with hepatitis C virus-related systemic vasculitis. Arthritis Rheum. 2002;46:3317–3326. doi: 10.1002/art.10699. [DOI] [PubMed] [Google Scholar]
- 48.Tredici G., Petruccioli M.G., Cavaletti G., Marmiroli P., Crespi V., Pioltelli P. Sural nerve bioptic findings in essential cryoglobulinemic patients with and without peripheral neuropathy. Clin Neuropathol. 1992;11(3):121–127. [PubMed] [Google Scholar]
- 49.Garcia-Bragado F., Fernandez J.M., Navarro C., Villar M., Bonaventura I. Peripheral neuropathy in essential mixed cryoglobulinemia. Arch Neurol. 1988;45(11):1210–1214. doi: 10.1001/archneur.1988.00520350048015. [DOI] [PubMed] [Google Scholar]
- 50.Beddhu S., Bastacky S., Johnson J.P. The clinical and morphologic spectrum of renal cryoglobulinemia. Medicine (Baltimore) 2002;81(5):398–409. doi: 10.1097/00005792-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 51.Ben-Bassat M., Boner G., Rosenfeld J., Pick A.I., Kahana M., Hazaz B. The clinicopathologic features of cryoglobulinemic nephropathy. Am J Clin Pathol. 1983;79(2):147–156. doi: 10.1093/ajcp/79.2.147. [DOI] [PubMed] [Google Scholar]
- 52.Suresh E. Diagnostic approach to patients with suspected vasculitis. Postgrad Med J. 2006;82(970):483–488. doi: 10.1136/pgmj.2005.042648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Vita S., Soldano F., Isola M., Monti G., Gabrielli A., Tzioufas A. Preliminary classification criteria for the cryoglobulinaemic vasculitis. Ann Rheum Dis. 2011;70:1183–1190. doi: 10.1136/ard.2011.150755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Quartuccio L., Isola M., Corazza L., Ramos-Casals M., Retamozo S., Ragab G.M. Validation of the classification criteria for cryoglobulinaemic vasculitis. Rheumatology. 2014;53(12):2209–2213. doi: 10.1093/rheumatology/keu271. [DOI] [PubMed] [Google Scholar]
- 55.Rapposelli D. Highlights from easl 2014. Gastroenterol Hepatol (N Y) 2014;10(5):312–313. [PMC free article] [PubMed] [Google Scholar]
- 56.Vassilopoulos D., Calabrese L.H. Management of rheumatic disease with comorbid HBV or HCV infection. Nat Rev Rheumatol. 2012;8:348–357. doi: 10.1038/nrrheum.2012.63. [DOI] [PubMed] [Google Scholar]
- 57.Racanelli V., Sansonno D., Piccoli C., D’Amore F.P., Tucci F.A., Dammacco F. Molecular characterization of B cell clonal expansions in the liver of chronically hepatitis C virus-infected patients. J Immunol. 2001;167:21–29. doi: 10.4049/jimmunol.167.1.21. [DOI] [PubMed] [Google Scholar]
- 58.Saadoun D., Thibault V., Pialoux G., Elkrief L., Mallet M., Musset L. All oral therapy (Sofosbuvir-Ribavirin) combination in severe HCV-mixed cryoglobulinemia vasculitis. The VASCULVADIC study. J Hepatol. 2015;62:S640. doi: 10.1016/j.jhep.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 59.Hegazy M.T., Hussein M.A., Quartuccio L., Fawzy M., Zoheir N., Ellawindi M.I. Treatment of Cryoglobulinemic Vasculitis with Sofosbuvir in Four Combination Protocols [abstract]. Arthritis Rheumatol. 2016;68(suppl 10) <http://acrabstracts.org/abstract/treatment-of-cryoglobulinemic-vasculitis-with-sofosbuvir-in-four-combination-protocols> [Google Scholar]
- 60.De Vita S., Quartuccio L., Isola M., Mazzaro C., caini P., Lenzi M. controlled trial of rituximab for treatment of severe cryoglobulinemic vasculitis. Arthritis Rheum. 2012;64:843–853. doi: 10.1002/art.34331. [DOI] [PubMed] [Google Scholar]
- 61.Cacoub P., Terrier B., Saadoun D. Hepatitis C virus-induced vasculitis: therapeutic options. Ann Rheum Dis. 2014;73:24–30. doi: 10.1136/annrheumdis-2013-203883. [DOI] [PubMed] [Google Scholar]
- 62.Sene D., Ghillani-Dalbin P., Amoura Z., Musset L., Cacoub P. Rituximab may form a complex with IgM kappa mixed cryoglobulin and induce severe systemic reactions in patients with hepatitis C virus-induced vasculitis. Arthritis Rheum. 2009;60:3848–3855. doi: 10.1002/art.25000. [DOI] [PubMed] [Google Scholar]
- 63.Ramunni A., Brescia P. Double filtration plasmapheresis: an effective treatment of cryoglobulinemia. In: Dammacco F., editor. HCV infection and cryoglobulinemia. Springer; New York: 2012. pp. 337–341. [Google Scholar]
- 64.Hausfater P., Cacoub P., Assogba U., Lebon P., Piette J.C. Plasma exchange and interferon-alpha pharmacokinetics in patients with hepatitis C virus-associated systemic vasculitis. Nephron. 2002;91:627–630. doi: 10.1159/000065023. [DOI] [PubMed] [Google Scholar]
- 65.Tarantino A., Campise M., Banfi G., Confalonieri R., Bucci A., Montoli A. Long-term predictors of survival in essential mixed cryoglobulinemic glomerulonephritis. Kidney Int. 1995;47:618–623. doi: 10.1038/ki.1995.78. [DOI] [PubMed] [Google Scholar]
- 66.Monti G., Saccardo F., Pioltelli P., Rinaldi G. The natural history of cryoglobulinemia: symptoms at onset and during follow-up. A report by the Italian Group for the Study of Cryoglobulinemias (GISC) Clin Exp Rheumatol. 1995;13(13) S129-33. [PubMed] [Google Scholar]
- 67.Saadoun D., Terrier B., Semoun O., Sene D., Maisonobe T., Musset L. HCV- associated polyarteritis nodosa. Arthritis Care Res (Hoboken) 2011;63(3):427–435. doi: 10.1002/acr.20381. [DOI] [PubMed] [Google Scholar]
- 68.Costedoat-Chalumeau N., Cacoub P., Maisonobe T., Thibault V., Cluzel P., Gatfosse M. Renal microaneurysms in three cases of hepatitis C virus related vasculitis. Rheumatology. 2002;41(6):708–710. doi: 10.1093/rheumatology/41.6.708. [DOI] [PubMed] [Google Scholar]
- 69.El-Fishawy H., Saadi G., Hassaballa M., Hussein M., Doss W., Ragab G. Antiviral treatment prioritization in HCV-infected patients with extrahepatic manifestations – an Egyptian perspective. J Adv Res. 2016;7:391–402. doi: 10.1016/j.jare.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]