Graphical abstract
Keywords: HCV, Cardiovascular risk, Hearth, Atherosclerosis, Death, Fibrosis
Abstract
Chronic hepatitis C virus (HCV) infection is a systemic disease that leads to increased risks of cirrhosis and its complications, as well as extrahepatic disturbances, including immune-related disorders and metabolic alterations such as insulin resistance and steatosis. Recent accumulating evidence suggests that HCV infection can increase cardiovascular risk, and that viral eradication can improve cardiovascular outcomes in the clinical setting. These data are strengthened by evidence identifying potential mechanisms (in)directly linking HCV infection to vascular damage. However, the high prevalence of both HCV infection and cardiovascular alterations, as well as the presence of contrasting results not identifying any association between HCV infection and cardiovascular dysfunction, provides uncertainty about a direct association of HCV infection with cardiovascular risk. Further studies are needed to clarify definitively the role of HCV infection in cardiovascular alterations, as well as the impact of viral eradication on cardiovascular outcomes. These features are now more attractive, considering the availability of new, safe, and very effective interferon-free antiviral agents for the treatment of HCV infection. This review aims to discuss carefully available data on the relationship between HCV infection and cardiovascular risk.
Introduction
Infection by hepatitis C virus (HCV) accounts for a high proportion of cases of chronic liver disease worldwide, and it has spread pandemically [1]. As expected, longitudinal cohort studies observed that patients with chronic hepatitis C (CHC) have a high risk of liver-related morbidity and mortality, mostly due to the progression toward liver cirrhosis and its complications [2], [3]. Growing evidence, however, has moved HCV infection from the traditional picture of a localized, liver-focused disease, to the concept of a systemic disease capable of producing extrahepatic manifestations [4]. In this complex landscape, HCV-infected individuals have increased risks of developing immune-related disorders, such as cryoglobulinemia and its sequelae, including B cell non-Hodgkin lymphoma, and systemic autoimmune diseases, all of which arise from HCV lymphotropism [4].
Even stronger than the link between HCV infection and immune-related disorders is the association, made first from preliminary studies and then widely confirmed, between HCV infection and metabolic alterations. Different lines of evidence identified a higher prevalence of fatty liver infiltration, insulin resistance (IR), and diabetes in individuals infected with HCV, with a “protective” serum lipid profile characterized by lower low-density lipoprotein levels [5], [6], [7], [8], [9], [10], [11], [12]. These metabolic changes arise from the ability of the virus to affect liver lipid metabolism and insulin signaling, as confirmed by experimental studies and evidence that viral eradication corrects these metabolic alterations [5], [12].
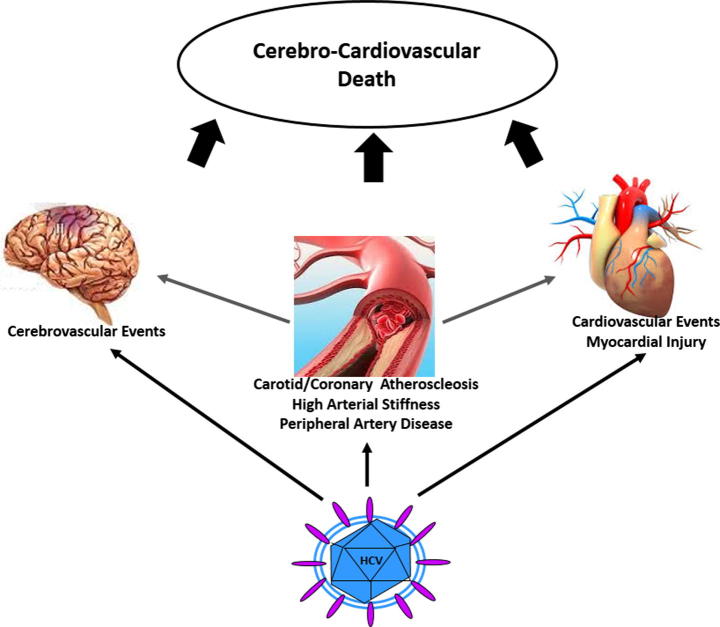
Besides these proven and well-recognized associations between HCV infection and both immune-related and metabolic disorders, recent emerging and growing evidence suggests a link between HCV infection and neurological/psychiatric disorders [13] and cardiovascular alterations [14]. However, both HCV infection and cardiovascular alterations are common conditions observed in a large proportion of the general population. It is difficult, therefore, to establish whether a simple association exists between the two conditions, or whether other pathogenic mechanisms directly or indirectly link chronic HCV infection to cardiovascular disorders. Beyond being useful from a speculative point of view, a better understanding of this topic could have very relevant clinical consequences, due to the availability of new, oral, safe, and highly effective antiviral regimens for the treatment of HCV infection [15]. In this review, we aimed to analyze the evidence linking HCV infection to a wide spectrum of cardiovascular alterations (Fig. 1), the impact of HCV eradication on these vascular disorders, and the potential mechanisms underlying these associations.
Fig. 1.
Cardiovascular alterations and HCV infection. HCV infection is associated with a wide spectrum of cardiovascular alterations, including cerebrovascular events, coronary/carotid atherosclerosis, high arterial stiffness, peripheral artery disease, myocardial injury, cardiovascular events, and CCV-related death.
HCV infection and carotid atherosclerosis
Ishizaka and colleagues first reported an association between carotid atherosclerosis and HCV infection in a cohort of 1992 Japanese subjects who had undergone general health-screening tests, including ultrasonographic evaluation of the carotid arteries [16]. Patients with HCV infection had a trend toward a larger intima-media thickness (IMT) compared to controls, as well as a significantly higher prevalence of carotid plaques (64% vs. 25%). This association was maintained even after adjusting for well-known metabolic confounders [16]. Targher and colleagues confirmed the association between HCV infection and carotid atherosclerosis in a small Italian study that compared patients with chronic liver diseases of viral and nonviral causes to healthy controls [17]. The IMT of patients with CHC was smaller compared to the IMT of individuals with nonalcoholic fatty liver disease, but larger compared to the IMT of controls. Moreover, CHC was an independent risk factor for atherosclerosis. Consistent with these data, other cross-sectional studies from both Western and Asian populations reported a higher prevalence of carotid atherosclerosis in individuals infected with HCV compared to uninfected controls. This association was maintained after adjustment for cardiometabolic confounders [18], [19], [20], [21]. Similarly, a high risk of carotid atherosclerosis was reported in the setting of HCV-HIV co-infected patients [22], [23], [24].
Although the above studies found a strong and independent link between HCV infection and carotid atherosclerosis, some other reports in HCV mono-infected, HIV co-infected, and hemodialysis patients found no association between HCV infection and vascular alterations [25], [26]. Due to the presence of contrasting and inconclusive results, we recently performed a meta-analysis of nine case-control studies to examine the effect of HCV infection on carotid plaques [27]. We found a twofold higher risk of carotid plaques in individuals infected with HCV compared to uninfected controls, without significant heterogeneity among studies. Similar results were obtained when we considered IMT as the outcome, instead of carotid plaques. In addition, the impact of HCV infection on the presence of carotid plaques was directly affected by smoking status, with the effect of HCV infection being more pronounced in populations with a high smoking prevalence.
HCV infection and coronary atherosclerosis
The first evidence of an association between HCV infection and coronary atherosclerosis was from Vassalle and colleagues. These authors observed this link in a case-control study of 491 patients with angiographic documentation of coronary artery disease (CAD) (stenosis > 50%) and a control group of 195 patients admitted to the same institute for reasons other than CAD [28]. The authors found a higher prevalence of HCV infection in the CAD group compared to the controls (6.3% vs. 2.0%), which remained higher after correction for cardiometabolic risk factors [28]. Similar results were reported in a Turkish study of 139 HCV-seropositive and 225 HCV-seronegative patients, which found that HCV infection was an independent predictor of severity of coronary atherosclerosis [29]. Consistent with these data, and in the different setting of hemodialysis patients, another Turkish study of 26 HCV-infected and 26 HCV-uninfected patients suggested that HCV led to a significantly lower coronary flow reserve compared to that of noninfected individuals, as assessed by echocardiography [30]. Along this line, Butt and colleagues observed a significantly higher risk of incidental CAD in subjects with HCV infection compared to uninfected subjects in an observational cohort of more than 150,000 US subjects [31].
Conversely, Forde and colleagues, in a large cohort of adults (4809 with and 71,668 without HCV infection) followed in general practices in the UK [32] found no difference in the incidence of myocardial infarction (MI) according to HCV status. Furthermore, a study in Pomerania evaluating the risks of stroke and MI in HbsAg- or anti-HCV-positive patients compared to noninfected subjects did not report an independent effect of these infections [33].
HCV infection and cerebro-cardiovascular (CCV) events
Several case-control or cohort studies investigated the potential association between HCV infection and CCV events, defined as ischemic stroke, MI, angina, congestive heart failure, or transient ischemic attack. These studies reported contrasting results, ranging from HCV infection as a risk factor to HCV infection conferring a protective status [31], [32], [34], [35], [36], [37], [38], [39], [40], [41]. For example, considering cerebrovascular events, Liao and colleagues performed a population-based cohort study of 4094 Taiwanese adults newly diagnosed with HCV infection and 16,376 Taiwanese controls matched by age and sex. HCV infection was an independent predictor of stroke occurrence after adjustment for cardiometabolic risk factors [35]. On the other hand, in a US case-control study of 126,926 HCV-infected subjects and 126,926 controls, Butt and colleagues showed a significantly lower prevalence of stroke in individuals infected with HCV [41].
Due to the inconsistency of these results, we performed a meta-analysis of aggregate data [27]. In the eight studies evaluated, we found that HCV infection had a significant negative impact on CCV events. When we considered cerebrovascular and cardiovascular events separately, we confirmed HCV as a risk factor for both outcomes. As expected, however, we found strong heterogeneity among the studies. Moreover, the effect of HCV infection on CCV events was more pronounced in populations at increased cardiovascular risk (i.e. with a high prevalence of diabetes or hypertension).
HCV infection and other cardiovascular alterations
Some evidence links HCV infection to other cardiovascular alterations. Maruyama and colleagues reported a direct association between HCV infection and myocardial injury evaluated by electrocardiography, echocardiography or thallium-201 myocardial scintigraphy in a relatively large cohort of 217 patients with CHC [42]. Consistent with these results, an Italian study reported that 52 patients infected with HCV had higher left ventricular (LV) mass compared to 104 healthy, normotensive subjects, but similar LV mass compared to 104 individuals with hypertension [43]. A recent Australian study evaluated myocardial structure, function and tissue composition with cardiac magnetic resonance imaging in 21 patients with CHC compared to uninfected controls. Patients with CHC had significantly lower LV end-diastolic volume, reduced stroke volume, lower postcontrast myocardial T1 time and higher partition coefficient expression of myocardial fibrosis [44]. Along this line, a Japanese cohort study of 7514 individuals who underwent an annual checkup reported that HCV infection was independently associated with a higher pulse wave velocity [45]. Supporting the wide association between HCV infection and cardiovascular alterations, a Taiwanese cohort study of 7641 individuals infected with HCV and 30,564 matched controls reported a higher risk of developing peripheral artery disease in patients infected with HCV compared to uninfected controls. This risk was greater in older patients and independent of conventional risk factors [46].
HCV infection and cardiovascular mortality
Although all of the above studies suggested an increased cardiovascular risk in patients with HCV infection, these studies used surrogate endpoints for cardiovascular outcomes. The strongest outcome is represented by cardiovascular mortality. Some studies have evaluated the impact of HCV infection on cardiovascular mortality, reporting contrasting data. In 2008, Guiltinan and colleagues studied a US cohort of 10,259 HCV-infected and 10,259 uninfected matched blood donors. They were the first to report that HCV infection may increase cardiovascular mortality [47]. Similar data were later observed in a Taiwanese cohort of 18,541 anti-HCV seronegative and 1095 anti-HCV seropositive individuals [48]. However, a recent population-based cohort study of an Australian sample of 29,571 individuals receiving opioid substitution therapy reported no association between HCV infection and cardiovascular mortality [49]. Due to the uncertainty among these studies, we performed a meta-analytic evaluation of the available data. We found clear evidence that HCV infection increases the risk of cardiovascular disease-related mortality [27].
HCV infection and cardiovascular injury: potential mechanisms of virus-related damage
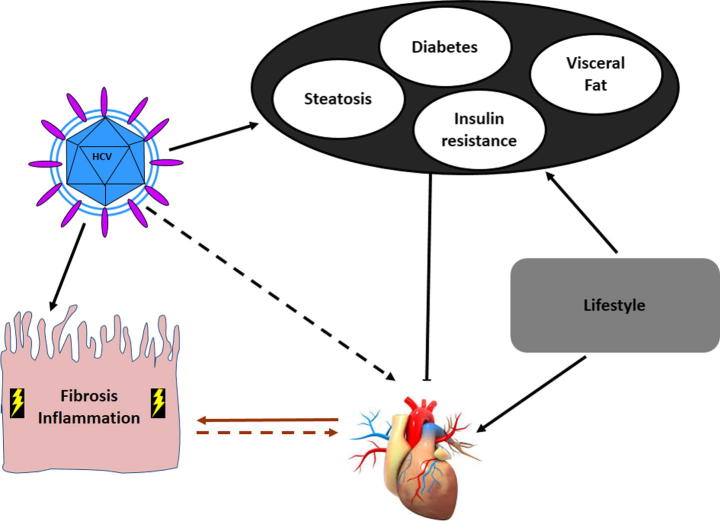
Evidence supporting the association between HCV infection and cardiovascular alterations prompts us to identify potential mechanism(s) linking these two highly prevalent conditions [50], [51]. Other than the diverse factors and metabolic comorbidities that are more prevalent in HCV-infected compared to noninfected individuals, the proinflammatory and profibrogenic HCV-related environment and a direct viral effect could be advocated as causes of cardiovascular alterations (Fig. 2).
Fig. 2.
Mechanisms potentially linking HCV infection with increased cardiovascular risk. HCV can directly and indirectly interfere with glucose and lipid metabolism, leading to IR, diabetes, fatty liver, and visceral obesity. These metabolic factors, together with lifestyle, may increase the cardiovascular risk of HCV-infected patients. In addition, HCV itself may lead to cardiovascular alterations via direct and indirect mechanism(s). On the one hand, proinflammatory and profibrogenic environments leading fibrogenesis in the liver could be systemically activated, enhancing the development of cardiovascular alterations. On the other hand, the presence of direct viral mechanisms could be hypothesized.
HCV infection has been associated with increased visceral fat [25]. Additionally, a relationship was found between HCV viral load and the visceral adiposity index [52], a marker of total body fat content and metabolic dysfunction. HCV is able to modulate lipid metabolism and insulin signaling, leading to a higher prevalence of diabetes and fatty liver infiltration in this clinical setting [5]. Consistent with this observation, recent experimental studies showed that HCV induces an increase in hepatic levels of not only triglycerides but also cholesterol esters and sphingolipids, which are potentially involved in mechanisms leading to HCV-induced steatosis and lipotoxicity [53], [54]. Among subjects infected with HCV, the presence of steatosis was found to be an independent risk factor for carotid atherosclerosis [18]. Despite the reported high prevalence of these metabolic alterations in CHC, different studies have shown that HCV-infected patients are characterized by a favorable lipoprotein profile: low LDL and VLDL cholesterol. Taken together, these observations lead us to hypothesize that although HCV-infected individuals have a similar prevalence of metabolic syndrome compared to uninfected subjects, they may have an increased cardiovascular risk due to induction of well-recognized cardiovascular risk factors (i.e. obesity, IR, type 2 diabetes, and steatosis).
HCV infection could lead to increased cardiovascular risk by inducing a systemic inflammatory status and oxidative stress. There is evidence that patients infected with HCV are characterized by chronic inflammation induced by innate, natural killer cell (NK)-mediated, and adaptive, T-helper-1-mediated responses [55]. This immune response can lead to lower adiponectin and higher serum tumor necrosis factor (TNF) alpha and interleukin (IL)-6 levels [56], [57], with adiponectin being inversely, and TNF alpha and IL-6 being directly, related to cardiovascular alterations. Hepatic steatosis and visceral obesity could contribute to this inflammatory status by prompting production of reactive oxygen species and inflammatory cytokines [18]. Along this line, endothelial damage directly related to the HCV infection [58], [59], or expression of a vasculitis due to mixed cryoglobulinemia [60] could further contribute to the link between HCV infection and higher cardiovascular risk.
The impact of HCV-related inflammation on cardiovascular risk could be higher in patients with more liver damage, although most studies assessing the impact of HCV infection on cardiovascular alterations have not been accounted for this feature. The few studies that have considered the severity of liver damage reported a link between it and cardiovascular injury. Among patients with biopsy-proven CHC, we found that the presence of severe liver fibrosis was an independent risk factor for carotid atherosclerosis [19]. Consistent with these data, two recent studies found a link between myocardial injury and both the severity of liver damage and necroinflammatory activity among patients infected with HCV [42], [44]. Similarly, a recent cohort study reported that HCV-infected patients with higher liver stiffness values had a greater risk of developing cardiovascular events compared to those with lower stiffness values [61]. These data suggest that the proinflammatory and profibrogenic environment leading to fibrogenesis in the liver of patients infected with HCV may also be systemically activated, further enhancing the development of cardiovascular lesions.
Finally, there are some evidences of a direct link between HCV viral load and cardiovascular alterations. An Italian study [18] showed that patients with CHC and carotid atherosclerosis had higher serum HCV-RNA levels than those without carotid atherosclerosis. Similarly, a Japanese study reported a direct link between HCV-RNA viral load and myocardial injury [42]. Consistent with these data, Boddi and colleagues found positive-strand HCV-RNA in carotid plaques from anti-HCV-positive patients but not in plaques of anti-HCV-negative patients [62]. While requiring careful interpretation, these data suggest a direct role of HCV infection in vascular damage.
Does antiviral therapy affect cardiovascular risk among HCV-infected individuals?
As HCV infection may increase cardiovascular risk, a clinically relevant issue is whether treating the infection can improve cardiovascular outcomes. A Japanese study of 200 individuals infected with HCV reported an improvement in myocardial injury in patients who achieved a sustained viral eradication after PEG-IFN plus ribavirin therapy, a transitory improvement in those who relapsed, and no improvement in nonresponders [42]. A significantly higher risk of carotid atherosclerosis was found in HCV-infected, but not in HCV-cleared patients, compared to uninfected subjects [25]. A Taiwanese community-based study of 18,541 anti-HCV seronegative and 1095 anti-HCV seropositive subjects reported an increase in mortality from circulatory diseases compared to uninfected subjects in patients with anti-HCV and detectable HCV-RNA, but not in those with undetectable HCV-RNA [48]. These two last studies provide indirect evidence of a potential positive impact of viral eradication on cardiovascular outcomes.
Another Taiwanese study of 23,665 residents reported that the risk of lethal cerebrovascular events progressively increased from patients who were anti-HCV-positive with undetectable HCV RNA, to patients with a low viral load, and further to those who were highly viremic, compared to anti-HCV-negative patients [63]. Two other Taiwanese cohort studies in individuals infected with HCV found a significant reduction in the occurrence of acute coronary syndrome, stroke, and end-stage renal disease among those who underwent PEG-IFN-based antiviral therapy compared to those who did not receive these treatments. These findings were further confirmed in a subgroup of diabetic patients [36], [39]. A preliminary study of a small group of 21 Italian HCV-infected patients with diabetes found improved fasting baseline blood glucose levels after direct antiviral agent (DAA)-based HCV eradication [64]; however, the effect of DAAs on diabetes outcomes is worthy of further investigation [65].
All of the above quoted data need to be carefully interpreted due to the different designs of the studies and the lack of data regarding outcomes of antiviral therapy. Future prospective studies should be performed to give conclusive answers to this relevant clinical area of research.
Conclusions
Growing evidence from multiple clinical and experimental studies, as well as meta-analysis results suggests that HCV infection increases cardiovascular risk in terms of atherosclerosis, myocardial injury, CCV events, and cardiovascular mortality, especially in patients at increased cardiovascular risk. Preliminary and indirect data provide evidence of a potential effect of HCV eradication on cardiovascular outcomes. Metabolic alterations related to HCV infection, the proinflammatory and profibrogenic status associated with HCV infection, and liver damage, as well as potential direct viral mechanisms could explain the reported link between HCV infection and increased cardiovascular risk. However, heterogeneity among studies in terms of design, baseline characteristics of the populations, methods used for the definition of cardiovascular outcomes and HCV infection, as well as lack of data on outcomes of antiviral therapy and on the severity of liver damage strongly limit the available evidence. Thus, further prospective data are needed to define the impact of HCV on cardiovascular outcomes and of HCV eradication on improvement of these outcomes. This issue is of particular interest in the era of safe and effective interferon-free regimens for HCV treatment. If definitively confirmed, these data could lead to the treatment of HCV infection not only to reduce risks of liver disease progression/complications, but also to reduce risks of extrahepatic (including cardiovascular) complications.
Conflict of Interest
The authors have declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Biography

Dr Salvatore Petta was born in Palermo, Italy, on february 21, 1977. He obtained Degree in Medicine at the University of Palermo on July 2002, and then at the same University he became Specialist in Gastroenterology on December 2005, and Assistant Professor of Gastroenterology since April 2012.
He is a member of AISF (Italian association for the study of the liver), where he is part of a consultive board, and a member of SIGE (Italian association of Gastroenterology). His main areas of clinical and research interest are viral hepatitis and non-alcoholic fatty liver disease. He was a speaker at different national and international scientific meetings. He acts as a reviewer for the EASL International Liver Congress 2015–2017, and as Academic Editor for Plos One, Associate Editor for Liver International and Assistant Editor for Digestive and Liver Disease.
He is an author of more than 80 peer-reviewed papers published in international journals. Google Scholar: H-index 26, i10-index: 51; citation index 2751.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Hepatitis B and C in the EU neighbourhood: prevalence, burden of disease and screening policies. European Centre for Disease Prevention and Control, September 2010.
- 2.Cammà C., Di Bona D., Schepis F., Heathcote E.J., Zeuzem S., Pockros P.J. Effect of peginterferon alfa-2a on liver histology in chronic hepatitis C: a meta-analysis of individual patient data. Hepatology. 2004;39:333–342. doi: 10.1002/hep.20073. [DOI] [PubMed] [Google Scholar]
- 3.Singal A.G., Volk M.L., Jensen D., Di Bisceglie A.M., Schoenfeld P.S. A sustained viral response is associated with reduced liver-related morbidity and mortality in patients with hepatitis C virus. Clin Gastroenterol Hepatol. 2010;8:280–288. doi: 10.1016/j.cgh.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 4.Negro F., Forton D., Craxì A., Sulkowski M.S., Feld J.J., Manns M.P. Extrahepatic morbidity and mortality of chronic hepatitis C. Gastroenterology. 2015;149(6):1345–1360. doi: 10.1053/j.gastro.2015.08.035. [DOI] [PubMed] [Google Scholar]
- 5.Macaluso F.S., Maida M., Minissale M.G., Li Vigni T., Attardo S., Orlando E. Metabolic factors and chronic hepatitis C: a complex interplay. Biomed Res Int. 2013;2013:564645. doi: 10.1155/2013/564645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang M.L. Metabolic alterations and hepatitis C: from bench to bedside. World J Gastroenterol. 2016;22:1461–1476. doi: 10.3748/wjg.v22.i4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goossens N., Negro F. The impact of obesity and metabolic syndrome on chronic hepatitis C. Clin Liver Dis. 2014;18:147–156. doi: 10.1016/j.cld.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Lonardo A., Adinolfi L.E., Restivo L., Ballestri S., Romagnoli D., Baldelli E. Pathogenesis and significance of hepatitis C virus steatosis: an update on survival strategy of a successful pathogen. World J Gastroenterol. 2014;20:7089–7103. doi: 10.3748/wjg.v20.i23.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bugianesi E., Salamone F., Negro F. The interaction of metabolic factors with HCV infection: does it matter? J Hepatol. 2012;56(suppl 1):S56–S65. doi: 10.1016/S0168-8278(12)60007-5. [DOI] [PubMed] [Google Scholar]
- 10.Negro F. HCV infection and metabolic syndrome: which is the chicken and which is the egg? Gastroenterology. 2012;142:1288–1292. doi: 10.1053/j.gastro.2011.12.063. [DOI] [PubMed] [Google Scholar]
- 11.Petta S., Rosso C., Leung R., Abate M.L., Booth D., Salomone F. Effects of IL28B rs12979860 CC genotype on metabolic profile and sustained virologic response in patients with genotype 1 chronic hepatitis C. Clin Gastroenterol Hepatol. 2013;11(3):311–317. doi: 10.1016/j.cgh.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 12.Younossi Z.M., Stepanova M., Estep M., Negro F., Clark P.J., Hunt S. Dysregulation of distal cholesterol biosynthesis in association with relapse and advanced disease in CHC genotype 2 and 3 treated with sofosbuvir and ribavirin. J Hepatol. 2016;64:29–36. doi: 10.1016/j.jhep.2015.08.027. [DOI] [PubMed] [Google Scholar]
- 13.Adinolfi L.E., Nevola R., Lus G., Restivo L., Guerrera B., Romano C. Chronic hepatitis C virus infection and neurological and psychiatric disorders: an overview. World J Gastroenterol. 2015;21:2269–2280. doi: 10.3748/wjg.v21.i8.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petta S., Macaluso F.S., Craxì A. Cardiovascular diseases and HCV infection: a simple association or more? Gut. 2014;63(3):369–375. doi: 10.1136/gutjnl-2013-306102. [DOI] [PubMed] [Google Scholar]
- 15.EASL Recommendations on Treatment of Hepatitis C J Hepatol. 2015;63(1):199–236. doi: 10.1016/j.jhep.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 16.Ishizaka Y., Ishizaka N., Takahashi E., Unuma T., Tooda E., Hashimoto H. Association between hepatitis C virus core protein and carotid atherosclerosis. Circ J. 2003;67:26–30. doi: 10.1253/circj.67.26. [DOI] [PubMed] [Google Scholar]
- 17.Targher G., Bertolini L., Padovani R., Rodella S., Arcaro G., Day C. Differences and similarities in early atherosclerosis between patients with non-alcoholic steatohepatitis and chronic hepatitis B and C. J Hepatol. 2007;46:1126–1132. doi: 10.1016/j.jhep.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 18.Adinolfi L.E., Restivo L., Zampino R., Guerrera B., Lonardo A., Ruggiero L. Chronic HCV infection is a risk of atherosclerosis. Role of HCV and HCV-related steatosis. Atherosclerosis. 2012;221:496–502. doi: 10.1016/j.atherosclerosis.2012.01.051. [DOI] [PubMed] [Google Scholar]
- 19.Petta S., Torres D., Fazio G., Cammà C., Cabibi D., Di Marco V. Carotid atherosclerosis and chronic hepatitis C: a prospective study of risk associations. Hepatology. 2012;55:1317–1323. doi: 10.1002/hep.25508. [DOI] [PubMed] [Google Scholar]
- 20.Bilora F., Campagnolo E., Rinaldi R., Rossato A., Arzenton M., Petrobelli F. Carotid and femoral atherosclerosis in chronic hepatitis C: a 5-year follow-up. Angiology. 2008–2009;59:717–720. doi: 10.1177/0003319707311536. [DOI] [PubMed] [Google Scholar]
- 21.Ishizaka N., Ishizaka Y., Takahashi E., Ei Tooda, Hashimoto H., Nagai R. Association between hepatitis C virus seropositivity, carotid-artery plaque, and intima-media thickening. Lancet. 2002;359(9301):133–135. doi: 10.1016/s0140-6736(02)07339-7. [DOI] [PubMed] [Google Scholar]
- 22.Sosner P., Wangermez M., Chagneau-Derrode C., Le Moal G., Silvain C. Atherosclerosis risk in HIV-infected patients: the influence of hepatitis C virus co-infection. Atherosclerosis. 2012;222:274–277. doi: 10.1016/j.atherosclerosis.2012.02.027. [DOI] [PubMed] [Google Scholar]
- 23.Kakinami L., Block R.C., Adams M.J., Cohn S.E., Maliakkal B., Fisher S.G. Risk of cardiovascular disease in HIV, hepatitis C, or HIV/hepatitis C patients compared to the general population. Int J Clin Pract. 2013;67:6–13. doi: 10.1111/j.1742-1241.2012.02953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tien P.C., Schneider M.F., Cole S.R., Cohen M.H., Glesby M.J., Lazar J. Association of hepatitis C virus and HIV infection with subclinical atherosclerosis in the women’s interagency HIV study. AIDS. 2009;23:1781–1784. doi: 10.1097/QAD.0b013e32832d7aa8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mostafa A., Mohamed M.K., Saeed M., Hasan A., Fontanet A., Godsland I. Hepatitis C infection and clearance: impact on atherosclerosis and cardiometabolic risk factors. Gut. 2010;59:1135–1140. doi: 10.1136/gut.2009.202317. [DOI] [PubMed] [Google Scholar]
- 26.Caliskan Y., Oflaz H., Pusuroglu H., Boz H., Yazici H., Tamer S. Hepatitis C virus infection in hemodialysis patients is not associated with insulin resistance, inflammation and atherosclerosis. Clin Nephrol. 2009;71:147–157. doi: 10.5414/cnp71147. [DOI] [PubMed] [Google Scholar]
- 27.Petta S., Maida M., Macaluso F.S., Barbara M., Licata A., Craxì A. Hepatitis C virus infection is associated with increased cardiovascular mortality: a meta-analysis of observational studies. Gastroenterology. 2015 doi: 10.1053/j.gastro.2015.09.007. pii: S0016-5085(15)01322-0. [DOI] [PubMed] [Google Scholar]
- 28.Vassalle C., Masini S., Bianchi F., Zucchelli G.C. Evidence for association between hepatitis C virus seropositivity and coronary artery disease. Heart. 2004;90:565–566. doi: 10.1136/hrt.2003.018937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alyan O., Kacmaz F., Ozdemir O., Deveci B., Astan R., Celebi A.S. Hepatitis C infection is associated with increased coronary artery atherosclerosis defined by modified Reardon severity score system. Circ J. 2008;72:1960–1965. doi: 10.1253/circj.cj-08-0459. [DOI] [PubMed] [Google Scholar]
- 30.Yelken B., Gorgulu N., Caliskan Y., Elitok A., Cimen A.O., Yazici H. vol. 41. 2009. Association between chronic hepatitis C infection and coronary flow reserve in dialysis patients with failed renal allografts; pp. 1519–1523. (Transplant Proc). [DOI] [PubMed] [Google Scholar]
- 31.Butt A.A., Xiaoqiang W., Budoff M., Leaf D., Kuller L.H., Justice A.C. Hepatitis C virus infection and the risk of coronary disease. Clin Infect Dis. 2009;49:225–232. doi: 10.1086/599371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Forde K.A., Haynes K., Troxel A.B., Trooskin S., Osterman M.T., Kimmel S.E. Risk of myocardial infarction associated with chronic hepatitis C virus infection: a population-based cohort study. J Viral Hepat. 2012;19:271–277. doi: 10.1111/j.1365-2893.2011.01545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Momiyama Y., Ohmori R., Kato R., Taniguchi H., Nakamura H., Ohsuzu F. Lack of any association between persistent hepatitis B or C virus infection and coronary artery disease. Atherosclerosis. 2005;181:211–213. doi: 10.1016/j.atherosclerosis.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 34.Arcari C.M., Nelson K.E., Netski D.M., Nieto F.J., Gaydos C.A. No association between hepatitis C virus seropositivity and acute myocardial infarction. Clin Infect Dis. 2006;15(43):e53–e56. doi: 10.1086/507031. 1–5. [DOI] [PubMed] [Google Scholar]
- 35.Liao C.C., Su T.C., Sung F.C., Chou W.H., Chen T.L. Does hepatitis C virus infection increase risk for stroke? A population-based cohort study. PLoS ONE. 2012;7:e31527. doi: 10.1371/journal.pone.0031527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsu C.S., Kao J.H., Chao Y.C., Lin H.H., Fan Y.C., Huang C.J. Interferon-based therapy reduces risk of stroke in chronic hepatitis C patients: a population-based cohort study in Taiwan. Aliment Pharmacol Ther. 2013;38:415–423. doi: 10.1111/apt.12391. [DOI] [PubMed] [Google Scholar]
- 37.Adinolfi L.E., Restivo L., Guerrera B., Sellitto A., Ciervo A., Iuliano N. Chronic HCV infection is a risk factor of ischemic stroke. Atherosclerosis. 2013;231(1):22–26. doi: 10.1016/j.atherosclerosis.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 38.Enger C., Forssen U.M., Bennett D., Theodore D., Shantakumar S., McAfee A. Thromboembolic events among patients with hepatitis C virus infection and cirrhosis: a matched-cohort study. Adv Therapy. 2014;31(8):891–903. doi: 10.1007/s12325-014-0138-4. [DOI] [PubMed] [Google Scholar]
- 39.Hsu Y., Lin J., Ho H., Kao Y., Huang Y., Hsiao N. Antiviral treatment for hepatitis C virus infection is associated with improved renal and cardiovascular outcomes in diabetic patients. Hepatology. 2014;59:1293–1302. doi: 10.1002/hep.26892. [DOI] [PubMed] [Google Scholar]
- 40.Pothineni N.V., Delongchamp R., Vallurupalli S., Ding Z., Dai Y., Hagedorn C.H. Impact of hepatitis C seropositivity on the risk of coronary heart disease events. Am J Cardiol. 2014;114(12):1841–1845. doi: 10.1016/j.amjcard.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Butt A.A., Khan U.A., McGinnis K.A., Skanderson M., Kent Kwoh C. Co-morbid medical and psychiatric illness and substance abuse in HCV-infected and uninfected veterans. J Viral Hepat. 2007;14(12):890–896. doi: 10.1111/j.1365-2893.2007.00885.x. [DOI] [PubMed] [Google Scholar]
- 42.Maruyama S., Koda M., Oyake N., Sato H., Fujii Y., Horie Y. Myocardial injury in patients with chronic hepatitis C infection. J Hepatol. 2013;58:11–15. doi: 10.1016/j.jhep.2012.07.045. [DOI] [PubMed] [Google Scholar]
- 43.Perticone M., Miceli S., Maio R., Caroleo B., Sciacqua A., Tassone E.J. Chronic HCV infection increases cardiac left ventricular mass index in normotensive patients. J Hepatol. 2014;61(4):755–760. doi: 10.1016/j.jhep.2014.05.032. [DOI] [PubMed] [Google Scholar]
- 44.Ngu P.J., Butler M., Pham A., Roberts S.K., Taylor A.J. Cardiac remodeling identified by cardiovascular magnetic resonance in patients with hepatitis C infection and liver disease. Int J Cardiovasc Imaging. 2016;32(4):629–636. doi: 10.1007/s10554-015-0824-6. [DOI] [PubMed] [Google Scholar]
- 45.Tomiyama H., Arai T., Hirose K., Hori S., Yamamoto Y., Yamashina A. Hepatitis C virus seropositivity, but not hepatitis B virus carrier or seropositivity, associated with increased pulse wave velocity. Atherosclerosis. 2003;166:401–403. doi: 10.1016/s0021-9150(02)00388-x. [DOI] [PubMed] [Google Scholar]
- 46.Hsu Y.H., Muo C.H., Liu C.Y., Tsai W.C., Hsu C.C., Sung F.C. Hepatitis C virus infection increases the risk of developing peripheral arterial disease: a 9-year population-based cohort study. J Hepatol. 2015;62(3):519–525. doi: 10.1016/j.jhep.2014.09.022. [DOI] [PubMed] [Google Scholar]
- 47.Guiltinan A.M., Kaidarova Z., Custer B., Orland J., Strollo A., Cyrus S. Increased all-cause, liver, and cardiac mortality among hepatitis C virus-seropositive blood donors. Am J Epidemiol. 2008;167:743–750. doi: 10.1093/aje/kwm370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee M.H., Yang H.I., Lu S.N., Jen C.L., You S.L., Wang L.Y. Chronic hepatitis C virus infection increases mortality from hepatic and extrahepatic diseases: a community-based long-term prospective study. J Infect Dis. 2012;206:469–477. doi: 10.1093/infdis/jis385. [DOI] [PubMed] [Google Scholar]
- 49.Vajdic C.M., Pour S.M., Olivier J., Swart A., O’Connell D.L., Falster M.O. The impact of blood-borne viruses on cause-specific mortality among opioid dependent people: an Australian population-based cohort study. Drug Alcohol Depend. 2015;152:264–271. doi: 10.1016/j.drugalcdep.2015.03.026. [DOI] [PubMed] [Google Scholar]
- 50.Adinolfi L.E., Zampino R., Restivo L., Lonardo A., Guerrera B., Marrone A. Chronic hepatitis C virus infection and atherosclerosis: clinical impact and mechanisms. World J Gastroenterol. 2014;20:3410–3417. doi: 10.3748/wjg.v20.i13.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zampino R., Marrone A., Restivo L., Guerrera B., Sellitto A., Rinaldi L. Chronic HCV infection and inflammation: clinical impact on hepatic and extra-hepatic manifestations. World J Hepatol. 2013;5:528–540. doi: 10.4254/wjh.v5.i10.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petta S., Amato M., Cabibi D., Cammà C., Di Marco V., Giordano C. Visceral adiposity index is associated with histological findings and high viral load in patients with chronic hepatitis C due to genotype 1. Hepatology. 2010;52:1543–1552. doi: 10.1002/hep.23859. [DOI] [PubMed] [Google Scholar]
- 53.Branche E., Conzelmann S., Parisot C., Bedert L., Lévy P.L., Bartosch B. Hepatitis C virus increases occludin expression via the upregulation of adipose differentiation-related protein. PLoS ONE. 2016;11(1):e0146000. doi: 10.1371/journal.pone.0146000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Loizides-Mangold U., Clément S., Alfonso-Garcia A., Branche E., Conzelmann S., Parisot C. HCV 3a core protein increases lipid droplet cholesteryl ester content via a mechanism dependent on sphingolipid biosynthesis. PLoS ONE. 2014;9(12):e115309. doi: 10.1371/journal.pone.0115309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rehermann B. Pathogenesis of chronic viral hepatitis: differential roles of T cells and NK cells. Nat Med. 2013;19:859–868. doi: 10.1038/nm.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Petit J.M., Minello A., Jooste V., Bour J.B., Galland F., Duvillard L. Decreased plasma adiponectin concentrations are closely related to steatosis in hepatitis C virus-infected patients. J Clin Endocrinol Metab. 2005;90:2240–2243. doi: 10.1210/jc.2004-1266. [DOI] [PubMed] [Google Scholar]
- 57.Cua I.H., Hui J.M., Bandara P., Kench J.G., Farrell G.C., McCaughan G.W. Insulin resistance and liver injury in hepatitis C is not associated with virus-specific changes in adipocytokines. Hepatology. 2007;46:66–73. doi: 10.1002/hep.21703. [DOI] [PubMed] [Google Scholar]
- 58.Serres L., Vasseur P., Tougeron D., Gand E., Chagneau-Derrode C., Charier F. Cardiovascular events in chronic hepatitis C: prognostic value of liver stiffness evolution. Eur J Gastroenterol Hepatol. 2015;27(11):1286–1292. doi: 10.1097/MEG.0000000000000453. [DOI] [PubMed] [Google Scholar]
- 59.González-Reimers E., Quintero-Platt G., Martín-González C., Pérez-Hernández O., Romero-Acevedo L., Santolaria-Fernández F. Thrombin activation and liver inflammation in advanced hepatitis C virus infection. World J Gastroenterol. 2016;22:4427–4437. doi: 10.3748/wjg.v22.i18.4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Urbaczek A.C., Ribeiro L.C., Ximenes V.F., Afonso A., Nogueira C.T., Generoso W.C. Inflammatory response of endothelial cells to hepatitis C virus recombinant envelope glycoprotein 2 protein exposure. Mem Inst Oswaldo Cruz. 2014;109(6):748–756. doi: 10.1590/0074-0276140090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cacoub P., Gragnani L., Comarmond C., Zignego A.L. Extrahepatic manifestations of chronic hepatitis C virus infection. Dig Liver Dis. 2014;46(suppl 5):S165–S173. doi: 10.1016/j.dld.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 62.Boddi M., Abbate R., Chellini B., Giusti B., Giannini C., Pratesi G. Hepatitis C virus RNA localization in human carotid plaques. J Clin Virol. 2010;47:72–75. doi: 10.1016/j.jcv.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 63.Lee M.H., Yang H.I., Wang C.H., Jen C.L., Yeh S.H., Liu C.J. Hepatitis C virus infection and increased risk of cerebrovascular disease. Stroke. 2010;41:2894–2900. doi: 10.1161/STROKEAHA.110.598136. [DOI] [PubMed] [Google Scholar]
- 64.Pavone P., Tieghi T., d’Ettorre G., Lichtner M., Marocco R., Mezzaroma I. Rapid decline of fasting glucose in HCV diabetic patients treated with direct-acting antiviral agents. Clin Microbiol Infect. 2016 doi: 10.1016/j.cmi.2015.12.030. pii: S1198-743X(16)00030-6. [DOI] [PubMed] [Google Scholar]
- 65.Vanni E., Bugianesi E., Saracco G. Treatment of type 2 diabetes mellitus by viral eradication in chronic hepatitis C: myth or reality? Dig Liver Dis. 2016;48:105–111. doi: 10.1016/j.dld.2015.10.016. [DOI] [PubMed] [Google Scholar]