Abstract
The objectives of this study were to explore the effect of COST (one thousand Da molecular weight chitosan oligosaccharide) on the differentiation of 3T3-L1 preadipocytes and to determine the mechanism of action. 3T3-L1 preadipocytes were used as the target cells, and the induction of the methods for the differentiation of 3T3-L1 preadipocytes was based on classic cocktails. The MTT assay was used to filtrate the concentration of COST. On the 6th day of induced-differentiation, the differentiation of 3T3-L1 cells was detected by Oil Red O staining. The expression of PPARγ and C/EBPα mRNA was determined using real-time fluorescence quantitative PCR (Q-PCR). COST inhibited 3T3-L1 preadipocyte differentiation in a dose-dependent manner and decreased lipid accumulation. At the molecular level, the expression of the transcription factors, PPARγ and C/EBPα, was reduced by COST during adipogenesis. These results indicate that COST effectively inhibited the differentiation of 3T3-L1 preadipocytes. The mechanism is related to the down-regulation expression of PPARγ and C/EBPα.
Keywords: Cost, 3T3-L1 preadipocytes, Pparγ, C/ebpα
1. Introduction
Along with economic development, the incidence of obesity is increasing rapidly. Obesity features excessive fat accumulation and storage due to an imbalance between energy intake and energy expenditure (Chen et al., 2013). Obesity is also a chronic metabolic disease that increases the risk of a number of diseases (Oliveros et al., 2014), such as type 2 diabetes, hypertension and atherosclerosis (Corvera and Gealekman, 2014). Furthermore, several studies suggest that obesity is associated with the development of cancer (McDonnell et al., 2014). The metabolism of adipose tissue plays an important role in obesity. White adipose tissue and brown adipose tissue are two distinct adipose tissues in mammals, including adult humans. The function of white adipose tissue is to store excess energy when nutrient intake exceeds energy expenditure (Bi and Li, 2013). By contrast, brown adipose tissue is involved in energy dissipation to produce heat through non-shivering thermogenesis. Cold exposure and treatment with β3-adrenergic agonists enhance brown adipose tissue activity and promote white adipose tissue browning (Palou et al., 2013). Therefore, turning white fat into brown fat has become a new strategy for the prevention and management of obesity and related diseases.
Currently, the modes of action of diet pills include blocking the intestinal digestion of fat; targeting the neurotransmission of norepinephrine, dopamine and serotonin; and inhibiting the endocannabinoid system (Carter et al., 2012). Different degrees of side effects exist for many anti-obesity medications. Orlistat is a lipase inhibitor that reduces the intestinal absorption of fat and is widely used in anti-obesity treatment. However, orlistat can cause fecal incontinence, reduce the absorption of fat-soluble vitamins and cause liver damage (Hada et al., 2015). Some anti-obesity drugs have been removed from the market because of their serious side effects.
3T3-L1 preadipocytes differentiate into mature adipocytes in vitro. The 3T3-L1 cell line is widely used as an adipocyte differentiation model system for studying the metabolic mechanisms of obesity and the molecular mechanisms of adipogenesis (Boschi et al., 2014). Adipokines are a type of bioactive polypeptide and include leptin, adiponectin and tumor necrosis factor-alpha, which are released from adipocytes. These peptides play a vital role in regulating appetite and energy expenditure and are necessary for modulating insulin sAlong with economic development, the incidence of obesity is increasing insulin sensitivity and fat metabolism (Kang et al., 2016). Obesity is often accompanied by adipocyte hyperplasia or hypertrophy and excessive lipid storage in adipose tissue (Kim et al., 2014). Therefore, the regulation of preadipocyte differentiation or the inhibition adipogenesis may provide an effective mechanism to prevent obesity and obesity-related diseases.
During differentiation, the peroxisome proliferator-activated receptor-γ (PPARγ) and CCAAT/enhancer binding protein-α (C/EBPα) are two key transcription factors (Zhang et al., 2014). PPARγ, a sub-family of nuclear hormone receptors, activates the gene expression of fatty acid-binding protein and phosphoenolpyruvate carboxykinase to promote lipid synthesis. C/EBPα proteins play a crucial role during the maturation and differentiation of adipocytes. The expression of C/EBPα generates a coordinated effect with PPARγ to induce adipogenesis (Liss et al., 2014). In this study, we elucidated the mechanism and effects of COST on adipocyte differentiation.
COST, the chitosan depolymerization product, is a β-1,4 glycosidic linked glucosamine, with good solubility in water. It is nontoxic and is more easily degraded and absorbed compared to chitosan (Liu et al., 2013). Chitosan has been widely used in pharmaceutics. In our previous studies, we showed that nano-particle systems made from chitosan were efficient carriers for the transport of insulin through the nasal mucosa (Su et al., 2010). However, chitosan has high viscosity and is insoluble in water, but it is soluble in acidic solutions. These properties of chitosan limit its application as a biodegradable and biocompatible biopolymer in the pharmaceutical and biotechnological industries. Therefore, water-soluble chitosan is attractive because of its lower viscosity and solubility in water. Recently, a study on water-soluble chitosan microparticles loaded with insulin supported the hypothesis that insulin-loaded water-soluble chitosan microparticles are promising oral vehicle systems for insulin. In addition, several reports indicated that water-soluble chitosan microspheres reduced blood lipids and plasma viscosity in rats fed high-fat diets (Tao et al., 2013). This suggests that water-soluble chitosan microspheres are effective in improving hyperlipidemia. Simultaneously, nanoparticles composed of water-soluble chitosan were prepared, and the results of the in vitro studies indicated that the water-soluble chitosan nanoparticles could be used as a carrier system for protein drugs. Moreover, water-soluble chitosan nanoparticles are efficient at inhibiting hypercholesterolemia in rats fed high-fat diets. More intriguingly, COST possesses promising biological effects, including antidiabetic, antioxidant, anti-obesity and anti-inflammation activities (Fang et al., 2014). Therefore, studies focused on the application of COST in medicine have attracted great interest from researchers.
Our team has confirmed that the COST has high activity of anti-obesity, and can prevent hyperlipidemia and nonalcoholic fatty liver induced by high fat diet in SD rats. In the present study, different concentrations of COST were evaluated for their effects on lipogenesis in 3T3-L1 cells. Additionally, the possible mechanisms for this effect are discussed, focusing on the expression of adipogenic transcription factors, such as PPARγ and C/EBPα.
2. Material and methods
2.1. Materials
3T3-L1 fibroblasts were purchased from the Chinese Academy of Sciences cell bank. COST was obtained from Shandong Laizhou Haili Biological Products Co, Ltd. Dulbecco’s modified Eagle’s medium (DMEM), bovine calf serum (BCS) and Oil Red O were purchased from GIBCO (Burlington, ON, Canada). Fetal bovine serum (FBS) and penicillin-streptomycin were purchased from Hyclone (USA). Dexamethasone (DEX), dimethylsulfoxide (DMSO), 1-methyl-3-isobutylxanthine (IBMX), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and insulin were purchased from Sigma–Aldrich (USA). RNAiso Reagent, cDNA synthesis Kit and RT-PCR Kit were purchased from Toyo Spinning (Shanghai) Biotechnology Limited. All other chemicals and solvents were of analytical grade.
2.2. 3T3-L1 cell culture and differentiation
3T3-L1 preadipocytes were cultured at 37 °C and 5% CO2 with Dulbecco’s modified Eagle’s medium (DMEM) containing 10% (v/v) bovine calf serum (BCS) and 1% penicillin-streptomycin. After reaching 80–90% confluence, the 3T3-L1 cells were subcultured. First, the cells were flushed with a 0.25% (w/v) pancreatic enzyme solution to remove serum containing trypsin inhibitor. Then, the cells were treated with a trypsin-EDTA solution. For adipocyte differentiation, the 3T3-L1 cells were grown in 6-well plates inoculated at 2–3 × 103 cell/cm2 to confluence. At 80% confluence, the cells were differentiated with 10 μg mL−1 insulin, 1 μM dexamethasone and 0.5 mM IBMX in DMEM containing 10% (v/v) fetal bovine serum (FBS) and 1% penicillin-streptomycin. On day 2 of differentiation, the cells were treated with 1 μg mL−1 insulin in DMEM with 10% (v/v) fetal bovine serum (FBS) and 1% penicillin-streptomycin. The culture medium was changed every 2 days for 15 days.
2.3. MTT assay
Cell viability was assessed using the MTT assay. 3T3-L1 preadipocytes were plated into 96-well plates at a density of 3 × 104 cells per well in a 100-μl cell suspension and cultured in DMEM containing 10% BCS for 24 h. The cells were then treated with 0, 1, 10, 50, 100 and 1000 μg mL−1 COST for 24 h. After 24 h, the MTT (20 μ1, 5 mg mL−1) solution was added to each well and the cells were incubated at 37 °C for 4 h. The medium was then removed and the insoluble formazan crystals were dissolved in DMSO (100 μl/well). The absorbance at 485 nm was measured using a microplate spectrophotometer (Mithras LB-940, Berthold, Germany).
2.4. Determination of lipid accumulation by Oil Red O staining
COST at a concentrations of 1, 10 and 100 μg mL−1 was added to the DMEM with 10% FBS at each of the three stages of adipocyte differentiation. After differentiation, the cells were washed twice with phosphate-buffered saline (PBS, pH = 7.4) and fixed with formaldehyde (4%, 2 ml) for 1 h at room temperature. After washing with distilled water three times, the cells were then stained with 1.2 mg mL−1 Oil Red O dye/60% isopropanol solution for 1 h. Excess Oil Red O dye was washed away with distilled water. The images of the Oil Red O stained cells were acquired using an inverted contrast phase microscope (Axiovert 40 CFL, Carl Zeiss, Jena, Germany).
2.5. RNA isolation and real-time quantitative PCR
On day 2 of differentiation, total RNA from 3T3-L1 cells was extracted with Trizol Reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. First-strand cDNA synthesis was performed with 1 μg of total RNA using a TranScriba Kit (A&A Biotechnology, Poland). Quantification of gene expression in the 3T3-L1 cells treated with COST was measured using a real-time PCR system (SmartCycler DX real-time PCR System Cepheid, USA). PCR was performed in a final volume of 20 μl, including 10 ng of sample cDNA, 5 μM of specific forward and reverse primers, and 15 μl of Real Time 2xPCR Master Mix EvaGreen. The reaction mixtures were incubated for an initial denaturation at 93 °C for 3 min, followed by 45 PCR cycles of 1 min at 93 °C, 1 min at 60 °C and 1 min at 72 °C.
PPARγ:
(+)5′-GCCCTTTGGTGACTTTATGG-3′, (−) 5′-CAGCAGGTTGTCTTGGATGT-3′;
C/EBP:
(+)5′-TTGCACCTCCACCTACATCC-3′, (−) 5′-CCACAAAGCCCAGAAACCTA-3′. The relative amount of each gene was calculated using the 2−ΔΔCt method.
2.6. Statistical analysis
The data were expressed as mean ± SD. Unpaired Student’s t-tests were used when appropriate to evaluate the differences between the two group means. Differences between the mean values of multiple groups were analyzed by one-way analysis of variance. Values of P < 0.05 were considered statistically significance.
3. Results
3.1. Induced-differentiation of 3T3-L1 preadipocytes
Lipid droplets in cells stained with Oil Red O before inducing differentiation are tiny and spindly (Fig. 1A). On day 6 of differentiation, the cells grew round and ring lipid droplets emerged (Fig. 1B). On the 14th day, the intracellular lipid droplets were plump and full and the 3T3-L1 preadipocytes differentiated into mature fat cells (Fig. 1C) (see Fig. 2).
Figue 1.
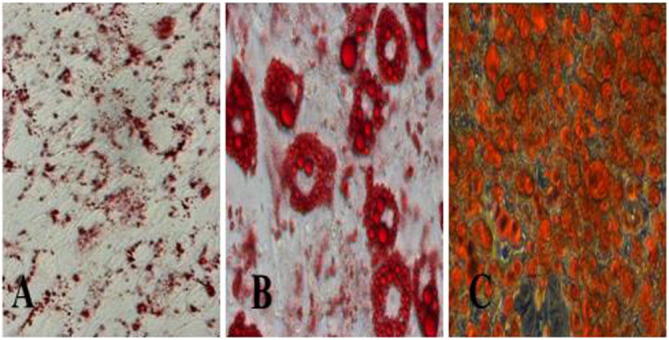
A: 0 day, B: 6 day, C: 14 day. Intracellular lipid accumulation during differentiation stained with Oil Red O, the results showed more and more fat droplets accumulation.
Figure 2.
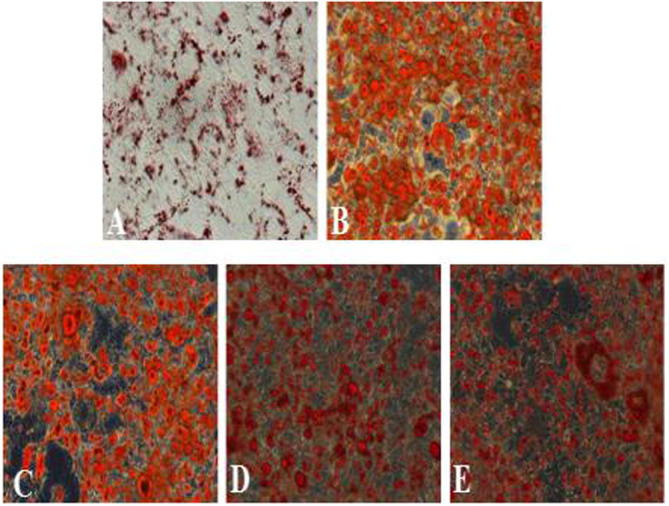
A: control, B: model, C: 1 μg mL−1, D: 10 μg mL−1, E: 100 μg mL−1. Effect of different COST concentrations on the accumulation of lipids in adipocytes. Control 3T3-L1 cells were differentiated in the absence of a differentiation inducer. During the induction of differentiation, the cells were treated with COST at concentrations of 1, 10 and 100 μg mL−1, except for the model adipocytes not exposed to COST, different concentrations COST suppressed fat accumulation of adipose cells was observed in this figure.
COST at concentrations of 1, 10, 100, 500 and 1000 μg mL−1 were used to filtrate concentrations of COST. COST at doses of 1, 10, 100, 500 and 1000 μg mL−1 reduced the viability of preadipocytes by 2.05%, 7.40%, 8.54%, 11.1% and 11.8%, respectively, compared to the control adipocytes not treated with COST (Table 1).
Table 1.
Effects of COST on the viability of 3T3-L1 cells (mean ± SD, n = 6).
| C (μg mL−1) | 1 | 10 | 100 | 500 | 1000 | 0 |
|---|---|---|---|---|---|---|
| A490nm | 0.631 ± 0.014 | 0.602 ± 0.026 | 0.595 ± 0.027 | 0.581 ± 0.042 | 0.578 ± 0.019 | 0.099 ± 0.016 |
| Survival (%) | 97.13 | 91.88 | 90.63 | 88.12 | 87.43 | 1 |
3.2. Effect of COST on the expression of PPAR γ and C/EBPα
To determine the potential intracellular mechanisms regulating the differentiation of 3T3-L1 preadipocytes, the expression of PPARγ and C/EBPα as necessary transcription factors during differentiation was monitored using quantitative real-time PCR. The expression levels of PPARγ and C/EBPα were measured after the differentiation-inducing process for 3T3-L1 cells exposed to COST at concentrations of 1, 10 and 100 μg mL−1. The results showed that COST suppressed the expression of PPARγ and C/EBPα compared to the model adipocytes not treated with COST (Fig. 3). The inhibitory effect of COST on the expression of PPARγ mRNA and C/EBPα mRNA was dose-dependent.
Figure 3.
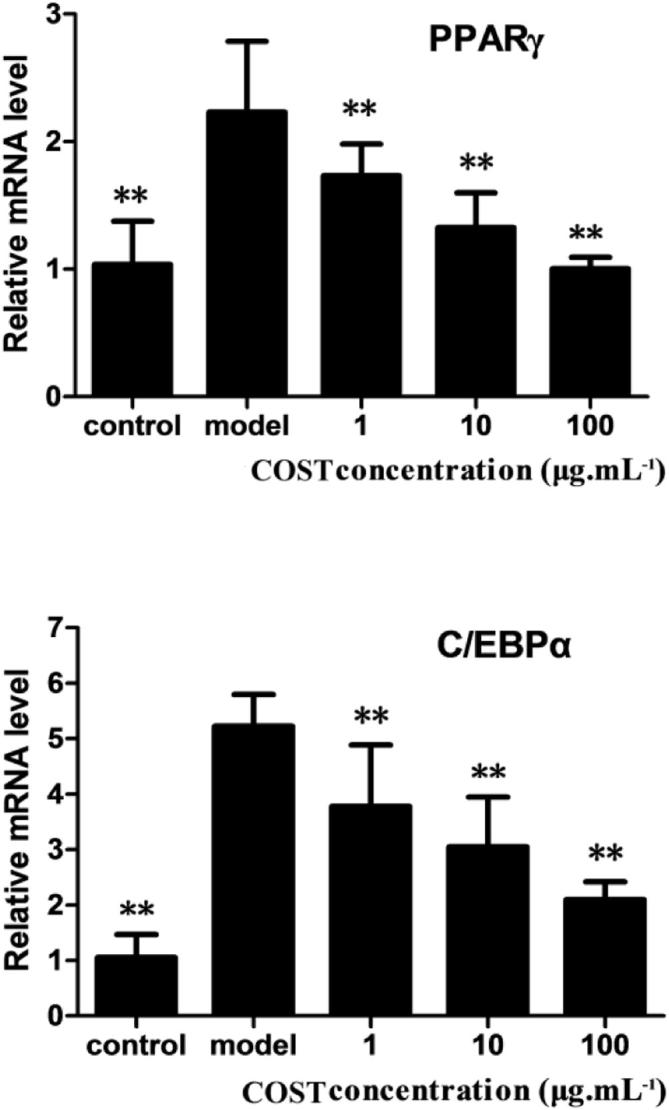
Effect of COST on the expression of PPARγ and C/EBPα. During differentiation, 3T3-L1 cells were exposed to COST at concentrations of 1, 10 and 100 μg mL−1, except for the model adipocytes not exposed to COST. Control adipocytes were not treated with a differentiation inducer. The results showed COST effectively inhibited the expression of PPARγ and C/EBPα. Data are given as mean value ± SD (n = 3), ∗∗p < 0.01 compared to the model.
Obesity is a serious global health problem that is associated with high morbidity and mortality. In the present study, 1, 10 and 100 μg mL−1 concentrations of COST suppressed lipid accumulation during differentiation. Differentiation of 3T3-L1 preadipocytes and adipogenesis is a dynamic and sophisticated process regulated by hormones, genes and signal transduction pathways. In this study, Q-PCR showed that COST downregulated the key adipogenic genes PPARγ and C/EBPα in a dose-dependent manner. The S100A16 protein is ubiquitously expressed, and S100A16 stimulates adipogenesis by increasing PPARγ promoter luciferase activity during lipogenesis (Li et al., 2013).
Fat cells are sensitive to insulin, and the decreased sensitivity of adipocytes to insulin plays a critical role in the development of type 2 diabetes. Sterol regulatory element binding protein (SREBP) is a key adipogenic transcription factor regulating the synthesis of cholesterol, fatty acids and triglycerides (Shao and Espenshade, 2014). Activation of the SREBP-1c signaling pathway promotes the synthesis of liver fat and the release of triglycerides (Lucero et al., 2015). Accumulating evidence indicates that COST has beneficial lipid-regulating effects, including decreasing plasma very-low-density lipoprotein triglyceride and increasing high-density lipoprotein cholesterol in animal models (Wang et al., 2011). This suggests that the lipid-regulating activity of COST may be associated with the expression of SREBP, PPAR and C/EBP. Cold exposure or treatment with norepinephrine can stimulate heat production as a result of increased uncoupling protein 1 (UCP1) expression (Shore et al., 2013). Therefore, mitochondria are the primary source of energy production via aerobic respiration in cells. Recent studies suggest that obesity is associated with mitochondrial dysfunction, decreased oxygen consumption rates and citrate synthase activity, which contribute to the compromised oxidative capacity of mitochondria in adipocytes from obese humans (Shore et al., 2013). This suggests that the weight-loss effects of COST may be associated with increased adipocyte mitochondrial function.
4. Conclusions
In summary, our results suggest that COST suppresses lipid accumulation during differentiation by down-regulating the adipogenesis-related transcription factors PPARγ and C/EBPα. Glucosamine is the unit of COST, and interestingly, COST with different degrees of polymerization (DP), such as dimers, trimers, tetramers, and pentamers, have a high biological activity. Further research will focus on techniques to separate COST and obtain COST with a specific DP. To study the molecular mechanisms in greater detail, additional studies are required to determine the mechanism by which COST with a specific DP influences the expression of PPARγ and C/EBPα and whether any other pathways are involved in adipogenesis using animal models. Obesity is the risk factor of hyperlipidemia, nonalcoholic fatty liver and Type two diabetes mellitus; excessive fat accumulation is an important factor of obesity, the results from the present study identify the anti-obesity and lipid-regulating properties of COST, and the development of COST may be a promising agent for preventing obesity and obesity-related diseases.
5. Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
We are grateful to our colleagues at the Guangdong TCM Key Laboratory for Metabolic Diseases. Acknowledgements are also due to Prof. Jiao Guo for providing the platform for this research.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Jiao Guo, Email: wshxalb@163.com.
Zhengquan Su, Email: suzhq@scnu.edu.cn.
References
- Bi S., Li L. Browning of white adipose tissue: role of hypothalamic signaling. Ann. N. Y. Acad. Sci. 2013;1302:30–34. doi: 10.1111/nyas.12258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boschi F., Rizzatti V., Zamboni M., Sbarbati A. Lipid droplets fusion in adipocyte differentiated 3T3-L1 cells: a Monte Carlo simulation. Exp. Cell Res. 2014;321:201–208. doi: 10.1016/j.yexcr.2013.12.023. [DOI] [PubMed] [Google Scholar]
- Carter R., Mouralidarane A., Ray S., Soeda J., Oben J. Recent advancements in drug treatment of obesity. Clin. Med. 2012;12:456–460. doi: 10.7861/clinmedicine.12-5-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Huang G.D., Tan S.R., Guo J., Su Z.Q. The preparation of capsaicin-chitosan microspheres (CCMS) enteric coated tablets. Int. J. Mol. Sci. 2013;14:24305–24319. doi: 10.3390/ijms141224305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvera S., Gealekman O. Adipose tissue angiogenesis: impact on obesity and type-2 diabetes. Biochim. Biophys. Acta. 2014;1842:463–472. doi: 10.1016/j.bbadis.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang I.M., Yang C.H., Yang C.M. Chitosan oligosaccharides attenuate ocular inflammation in rats with experimental autoimmune anterior uveitis. Mediators Inflamm. 2014;2014:827847. doi: 10.1155/2014/827847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hada Y., Yamauchi T., Kadowaki T. The update of obesity syndrome: molecular mechanism, pathophysiology and therapies. Topics: II. recent topics on care and treatment of the obesity syndrome; 3. Pharmacotherapy of obesity. Nihon Naika Gakkai zasshi J. Jpn. Soc. Intern. Med. 2015;104:735–741. doi: 10.2169/naika.104.735. [DOI] [PubMed] [Google Scholar]
- Kang Y.E., Kim J.M., Joung K.H., Lee J.H., You B.R., Choi M.J., Ryu M.J., Ko Y.B., Lee M.A., Lee J. The roles of adipokines, proinflammatory cytokines, and adipose tissue macrophages in obesity-associated insulin resistance in modest obesity and early metabolic dysfunction. PLoS ONE. 2016;11:e0154003. doi: 10.1371/journal.pone.0154003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.O., Sakchaisri K., Asami Y., Ryoo I.J., Choo S.J., Yoo I.D., Soung N.K., Kim Y.S., Jang J.H., Kim B.Y. Illudins C2 and C3 stimulate lipolysis in 3T3-L1 adipocytes and suppress adipogenesis in 3T3-L1 preadipocytes. J. Nat. Prod. 2014;77:744–750. doi: 10.1021/np400520a. [DOI] [PubMed] [Google Scholar]
- Li D., Zhang R., Zhu W., Xue Y., Zhang Y., Huang Q., Liu M., Liu Y. S100A16 inhibits osteogenesis but stimulates adipogenesis. Mol. Biol. Rep. 2013;40:3465–3473. doi: 10.1007/s11033-012-2413-2. [DOI] [PubMed] [Google Scholar]
- Liss A., Ooi C.H., Zjablovskaja P., Benoukraf T., Radomska H.S., Ju C., Wu M., Balastik M., Delwel R., Brdicka T. The gene signature in CCAAT-enhancer-binding protein alpha dysfunctional acute myeloid leukemia predicts responsiveness to histone deacetylase inhibitors. Haematologica. 2014;99:697–705. doi: 10.3324/haematol.2013.093278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B., Wang X., Pang C., Luo J., Luo Y., Sun R. Preparation and antimicrobial property of chitosan oligosaccharide derivative/rectorite nanocomposite. Carbohydr. Polym. 2013;92:1078–1085. doi: 10.1016/j.carbpol.2012.10.060. [DOI] [PubMed] [Google Scholar]
- Lucero D., Miksztowicz V., Macri V., Lopez G.H., Friedman S., Berg G., Zago V., Schreier L. Overproduction of altered VLDL in an insulin-resistance rat model: influence of SREBP-1c and PPAR-alpha. Clinica e investigacion en arteriosclerosis: publicacion oficial de la Sociedad Espanola de Arteriosclerosis. 2015;27:167–174. doi: 10.1016/j.arteri.2014.11.002. [DOI] [PubMed] [Google Scholar]
- McDonnell D.P., Park S., Goulet M.T., Jasper J., Wardell S.E., Chang C.Y., Norris J.D., Guyton J.R., Nelson E.R. Obesity, cholesterol metabolism, and breast cancer pathogenesis. Cancer Res. 2014;74:4976–4982. doi: 10.1158/0008-5472.CAN-14-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveros E., Somers V.K., Sochor O., Goel K., Lopez-Jimenez F. The concept of normal weight obesity. Prog. Cardiovasc. Dis. 2014;56:426–433. doi: 10.1016/j.pcad.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Palou A., Pico C., Bonet M.L. Nutritional potential of metabolic remodelling of white adipose tissue. Curr. Opin. Clin. Nutr. Metab. Care. 2013;16:650–656. doi: 10.1097/MCO.0b013e328365980f. [DOI] [PubMed] [Google Scholar]
- Shao W., Espenshade P.J. Sterol regulatory element-binding protein (SREBP) cleavage regulates Golgi-to-endoplasmic reticulum recycling of SREBP cleavage-activating protein (SCAP) J. Biol. Chem. 2014;289:7547–7557. doi: 10.1074/jbc.M113.545699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore A.M., Karamitri A., Kemp P., Speakman J.R., Graham N.S., Lomax M.A. Cold-induced changes in gene expression in brown adipose tissue, white adipose tissue and liver. PLoS ONE. 2013;8:e68933. doi: 10.1371/journal.pone.0068933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z.Q., Wu S.H., Zhang H.L., Feng Y.F. Development and validation of an improved Bradford method for determination of insulin from chitosan nanoparticulate systems. Pharm. Biol. 2010;48:966–973. doi: 10.3109/13880200903325615. [DOI] [PubMed] [Google Scholar]
- Tao Y., Zhang H.L., Hu Y.M., Wan S., Su Z.Q. Preparation of chitosan and water-soluble chitosan microspheres via spray-drying method to lower blood lipids in rats fed with high-fat diets. Int. J. Mol. Sci. 2013;14:4174–4184. doi: 10.3390/ijms14024174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Han J., Yu Y., Li X., Wang Y., Tian H., Guo S., Jin S., Luo T., Qin S. Chitosan oligosaccharide decreases very-low-density lipoprotein triglyceride and increases high-density lipoprotein cholesterol in high-fat-diet-fed rats. Exp. Biol. Med. 2011;236:1064–1069. doi: 10.1258/ebm.2011.011032. [DOI] [PubMed] [Google Scholar]
- Zhang T., Yamamoto N., Yamashita Y., Ashida H. The chalcones cardamonin and flavokawain B inhibit the differentiation of preadipocytes to adipocytes by activating ERK. Arch. Biochem. Biophys. 2014;554:44–54. doi: 10.1016/j.abb.2014.05.008. [DOI] [PubMed] [Google Scholar]


