Abstract
Objective:
The Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) study is the first stroke prevention trial to include protocol-driven intensive management of multiple risk factors. In this prespecified analysis, we aimed to investigate the relationship between risk factor control during follow-up and outcome of patients in the medical arm of SAMMPRIS.
Methods:
Data from SAMMPRIS participants in the medical arm (n = 227) were analyzed. Risk factors were recorded at baseline, 30 days, 4 months, and then every 4 months for a mean follow-up of 32 months. For each patient, values for all risk factor measures were averaged and dichotomized as in or out of target.
Results:
Participants who were out of target for systolic blood pressure and physical activity, as well as those with higher mean low-density lipoprotein cholesterol and non–high-density lipoprotein, were more likely to have a recurrent vascular event (stroke, myocardial infarction, or vascular death) at 3 years compared to those who had good risk factor control. In the multivariable analysis, greater physical activity decreased the likelihood of a recurrent stroke, myocardial infarction, or vascular death (odds ratio 0.6, confidence interval 0.4–0.8).
Conclusions:
Raised blood pressure, cholesterol, and physical inactivity should be aggressively treated in patients with intracranial atherosclerosis to prevent future vascular events. Physical activity, which has not received attention in stroke prevention trials, was the strongest predictor of a good outcome in the medical arm in SAMMPRIS.
ClinicalTrials.gov identifier:
Intracranial atherosclerotic stenosis (ICAS) is an important cause of stroke worldwide1 that is associated with a particularly high risk of recurrent stroke.2,3 Post hoc analyses from the Warfarin Aspirin Symptomatic Intracranial Disease (WASID) trial, in which patients with symptomatic 50%–99% intracranial stenosis were treated with standard of care risk factor management and randomized to either warfarin or aspirin, suggested that poorly controlled blood pressure (BP)4 and elevated cholesterol5 during follow-up are important risk factors for recurrent stroke and other vascular events. These findings led to the incorporation of intensive risk factor management in the design of the Stenting and Aggressive Medical Management for Prevention of Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) trial.6 The final results of SAMMPRIS showed that multimodal aggressive medical management alone was superior to the combination of angioplasty and stenting plus aggressive medical management in patients with severe ICAS.7 A prespecified analysis of the SAMMPRIS trial was to investigate the relationship between control of risk factors achieved with intensive risk factor management protocols and outcome of patients in the medical arm of the trial. We now report the results of this analysis.
METHODS
Standard protocol approvals, registrations, and patient consents.
The SAMMPRIS protocol was approved by the site institutional review boards, US Food and Drug Administration, and Data and Safety Monitoring Board appointed by the NIH. Informed consent was obtained from each participant.
ClinicalTrials.gov identifier: NCT00576693.
Trial design.
The overall design of SAMMPRIS has been described previously.8 In brief, SAMMPRIS was an NIH-funded, investigator-initiated and designed phase III randomized trial in which 451 patients were randomized at 50 sites in the United States to aggressive medical therapy alone or percutaneous transluminal angioplasty and stenting (with the Wingspan stent system) plus aggressive medical therapy. The main eligibility criteria were TIA or nondisabling stroke within 30 days caused by 70%–99% stenosis of a major intracranial artery (middle cerebral artery, carotid, vertebral, or basilar).
The primary outcomes in SAMMPRIS were any stroke or death within 30 days after enrollment, any stroke or death within 30 days after a revascularization procedure of the qualifying lesion during follow-up, and ischemic stroke in the territory of the qualifying artery beyond 30 days. The combination of any ischemic stroke, myocardial infarction (MI), or vascular death was a secondary outcome in SAMMPRIS, but is the primary outcome in this prespecified analysis because intensive risk factor control affects all of these important vascular events. Details regarding the adjudication of adverse events (ischemic stroke, MI, vascular death) are described elsewhere.8 In brief, study neurologists who were not masked to treatment assignment evaluated patients with a potential adverse event as soon as possible after the event. Patients with neurologic events that were potentially difficult to classify (a TIA lasting >1 hour or mild ischemic stroke [an increase in the patient's NIH Stroke Scale score of <4 from study entry]) were evaluated by a second neurologist who was masked to treatment. Both neurologists' assessments were sent for central adjudication. All adverse events (neurologic or cardiac) were centrally adjudicated by neurology or cardiology adjudicators who were masked to treatment assignment.
Aggressive medical management.
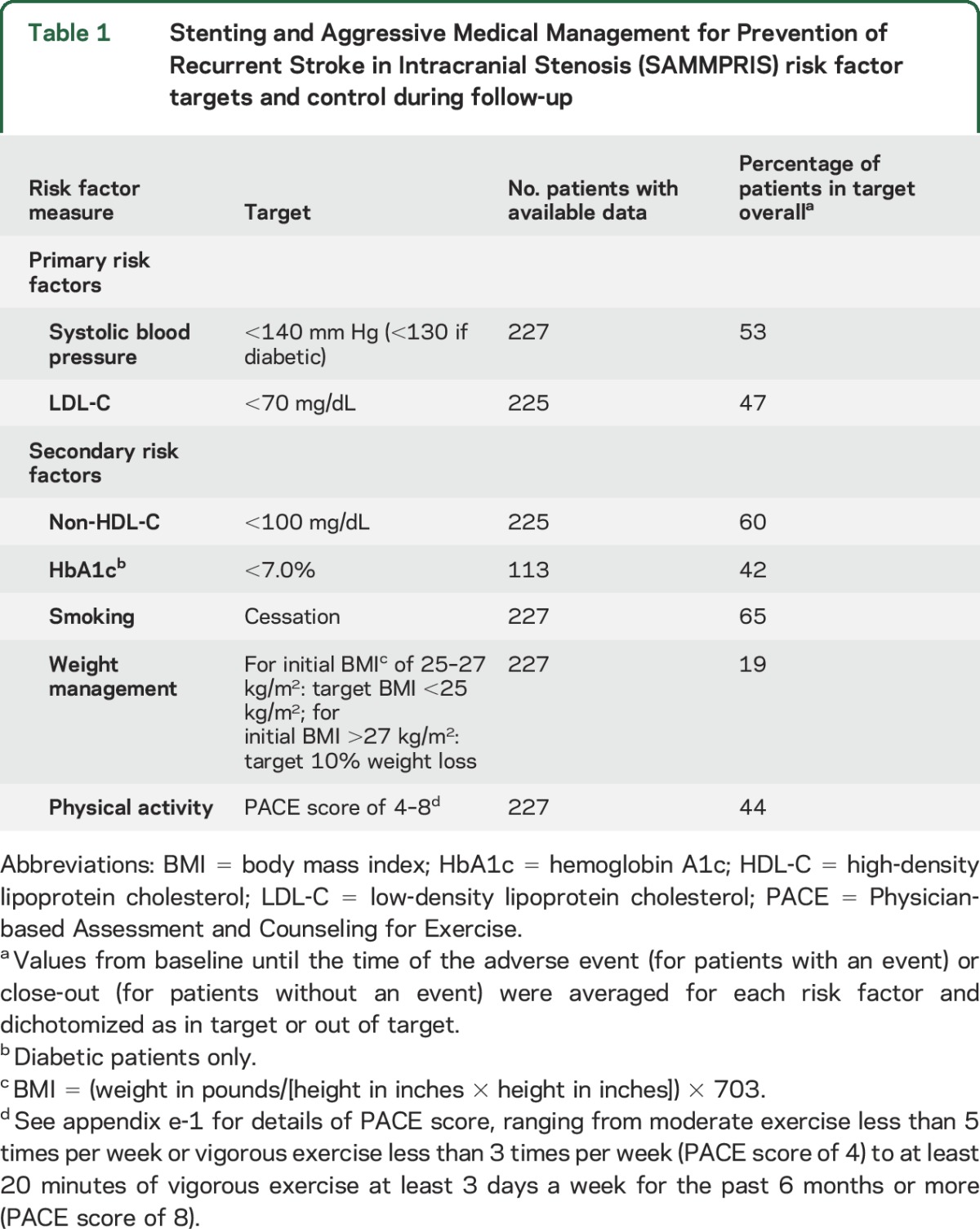
Details of the rationale, design, implementation, baseline risk factor control, and achievement of risk factor control in SAMMPRIS have been described previously.6,7 In brief, aggressive medical therapy consisted of aspirin 325 mg/d during the entire follow-up period (mean follow-up of approximately 32 months), clopidogrel 75 mg/d for 90 days after enrollment, and aggressive risk factor management primarily targeting systolic BP (SBP) <140 mm Hg (<130 if diabetic) and low-density lipoprotein cholesterol (LDL-C) <70 mg/dL. The protocol-defined primary and secondary risk factor targets are listed in table 1. Risk factor management was performed by the study neurologist and coordinator at each site. The following strategies were used to maximize attainment of risk factor targets: providing study medications to patients free of charge, employing medication titration algorithms for primary risk factors, and central oversight of risk factor performance. All participants also received coaching on healthy lifestyle behaviors at regularly scheduled times throughout the study using a commercially available lifestyle modification program (INTERVENT) at no charge.
Table 1.
Stenting and Aggressive Medical Management for Prevention of Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) risk factor targets and control during follow-up
Collection of risk factor data.
Data from each patient visit throughout the study until the end of year 3 were used to analyze the relationship between risk factor control and vascular events. The accuracy of the SBP measurements was standardized by requiring the use of a highly rated BP monitoring device (Omron HEM-705CP; Omron, Kyoto, Japan) at all sites. The study protocol required the participants to be in the sitting position and the study device to be used with a printout of the BP readings for source documentation. At the baseline visit, 3 BP measurements were averaged and recorded as the initial measurements for each arm. All subsequent BP measurements were done in a selected arm. The right arm was considered the selected arm unless the left arm mean SBP measured >10 mm Hg higher than the right arm at the initial measurement. At each visit, 3 BP measurements were averaged to determine the mean BP for that visit. The frequency of BP data collection depended on the achievement of the SBP target. At any follow-up visit throughout the study, if a patient's mean SBP was above target, an adjustment in the antihypertensive regimen was made, and the patient returned for an extra BP check in 30 days. Once the target BP reading was reached, the patient resumed the normal schedule of follow-up visits every 4 months.
The accuracy of the baseline, 30 days, and 4 months LDL-C and non–high-density lipoprotein cholesterol (HDL-C) measurements was standardized by using a central core lipid laboratory. Patient blood samples were sent to the Emory Lipid Research Laboratory (Atlanta, GA) for measurement of direct LDL-C. Beyond 4 months, LDL-C (direct or calculated) and non-HDL-C were collected annually and were measured at the individual sites. Participants were not required to fast prior to blood sample collection, but fasting status was recorded. Adjustments were made to the lipid-lowering medications based on the LDL-C results.
Physical activity was assessed using the 8-point Physician-based Assessment and Counseling for Exercise (PACE) questionnaire, which the participants completed at each visit.6,9,10 Moderate exercise included activities like brisk walking or slow cycling for at least 10 minutes at a time. Vigorous exercise included activities like jogging or fast cycling for at least 20 minutes at a time. Physical activity out of target for this study was defined as a PACE score of ≤3 (3 = trying to do vigorous or moderate exercise but not exercising regularly, 2 = no vigorous or moderate exercise but thinking of starting in next 6 months, and 1 = no vigorous or moderate exercise and no intention to start in next 6 months). Examples of physical activity in target (PACE ≥4) were as follows: PACE 4 = moderate exercise <5 times per week or vigorous exercise <3 times per week and PACE 6 = at least 30 minutes of moderate exercise a day for at least 5 days a week for the past 6 months or more. See appendix e-1 at Neurology.org for full PACE score.
Smoking and body mass index (BMI) were recorded at all study visits. The protocol recommended testing hemoglobin A1c (HbA1c) in diabetic patients every 6 months in patients with glycemic control in target and quarterly in patients who were out of target. All additional lipid and HbA1c studies performed between scheduled visits, BP measurements performed at extra BP visits, and risk factor results at closeout visits were included in this analysis.
Statistical analysis.
Only data from patients in the medical arm of SAMMPRIS were included in this analysis because the relationship between risk factor control and outcome in the stenting arm was unlikely to be meaningful since most of the strokes in the stenting arm occurred soon after enrollment in the periprocedural period before risk factors had been optimally controlled. For each patient in the medical arm, values from baseline until the time of the vascular event (for patients having these events), the time of close-out, or 3 years of follow-up (if no events) were averaged for each risk factor and dichotomized as in target or out of target. Mean risk factor values were also analyzed as continuous variables.
Binary logistic regressions were performed with the combination of any ischemic stroke, MI, or vascular death as the outcome. Single-predictor (univariate) regression results were used to select risk factors for inclusion in a multivariable model. The analyses were repeated with ischemic stroke alone as the outcome.
In order to assess the effect of age, sex, stroke disability, baseline BMI, and cardiovascular health at baseline on the patients' ability to exercise and thereby potentially confound the outcome, we compared the unadjusted odds ratio (OR) for physical inactivity with the OR adjusted for these covariates: age, sex, NIH Stroke Scale (NIHSS), modified Rankin Scale score (mRS), baseline BMI, and cardiovascular disease at baseline (prior MI, angina, coronary angioplasty or stenting, deep vein thrombosis, pulmonary embolus, cardiac valve abnormality, congestive heart failure) and peripheral vascular disease. The OR for physical activity was also stratified by physical activity status at baseline.
RESULTS
Patients and risk factors.
Data from 227 patients in the aggressive medical management only arm enrolled in the trial were used for these analyses. The number of events (stroke, MI, and vascular death) was 49, including 32 ischemic strokes. The median (and 25th and 75th percentiles) of the number of risk factor values per patient were as follows: SBP: 11 (6, 15), LDL-C: 6 (4, 8), physical activity: 9 (4, 12), smoking: 9 (4, 12), non-HDL-C: 6 (4, 8), HbA1c: 4 (2, 6), BMI: 10 (4, 12). Two patients were missing data from the lipid panel. The percentages of patients with mean risk factor values in target on average during the trial (including baseline and follow-up visits) are shown in table 1. With respect to the primary risk factor targets, 53% of patients had a mean SBP that was in target (<140 mm Hg) during their participation in the study and 47% had a mean LDL-C that was in target (<70 mg/dL).
Univariate analyses.
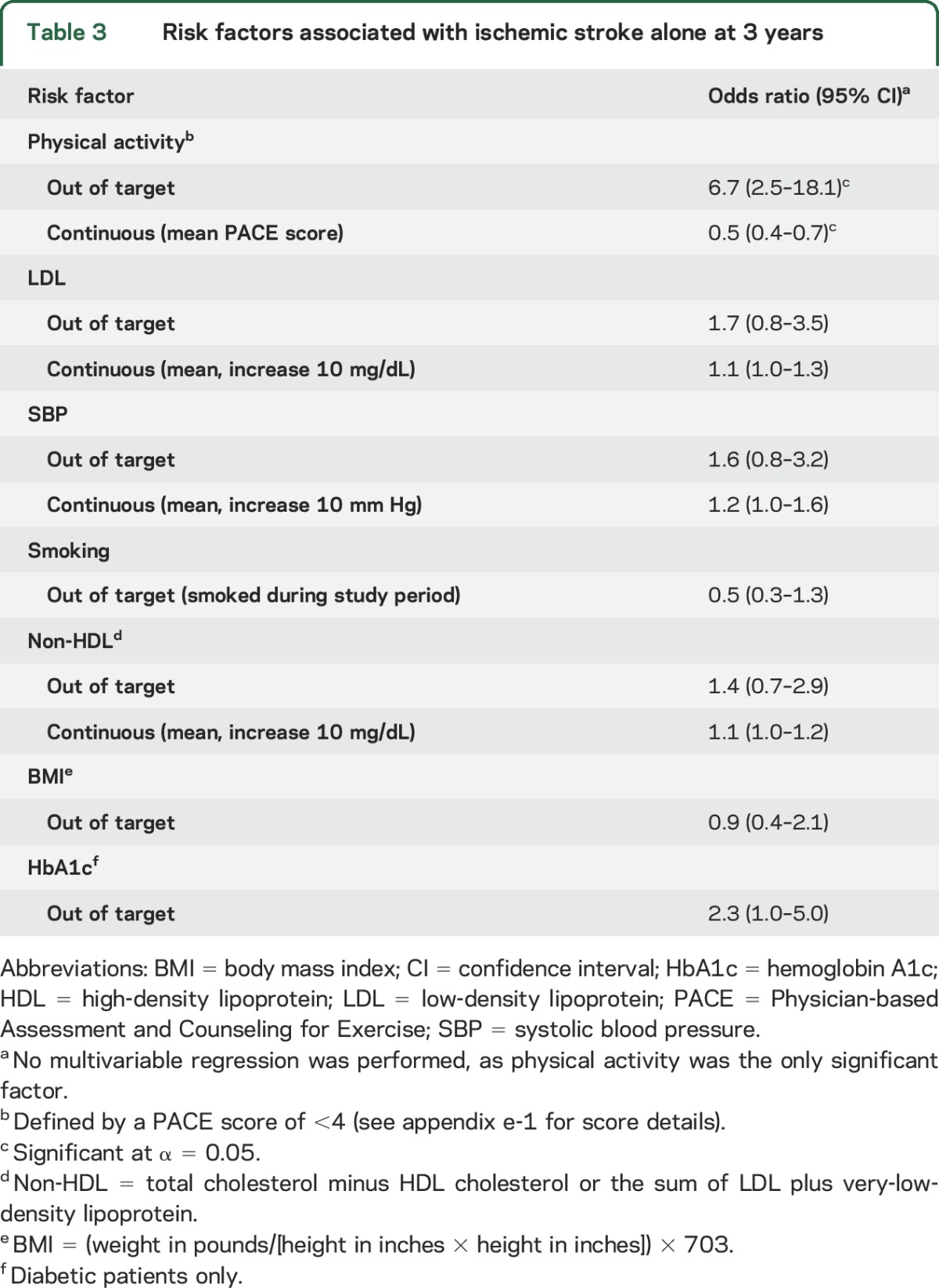
The relationships between control of individual risk factors and various vascular events for patients in the medical arm are shown in tables 2 and 3. For the primary endpoint of ischemic stroke, MI, or vascular death at 3 years (table 2), participants who were in target for SBP and physical activity were significantly less likely to have an event compared to those who did not achieve those targets. Lower LDL and non-HDL were also associated with lower likelihood of vascular events at 3 years when analyzed as continuous variables. For the endpoint of ischemic stroke alone (table 3), physical activity was the only risk factor associated with lower events. Control of other risk factors (HbA1c, smoking, and BMI) did not have a significant effect on any of the vascular outcomes assessed.
Table 2.
Risk factors associated with stroke, myocardial infarction, or vascular death at 3 years
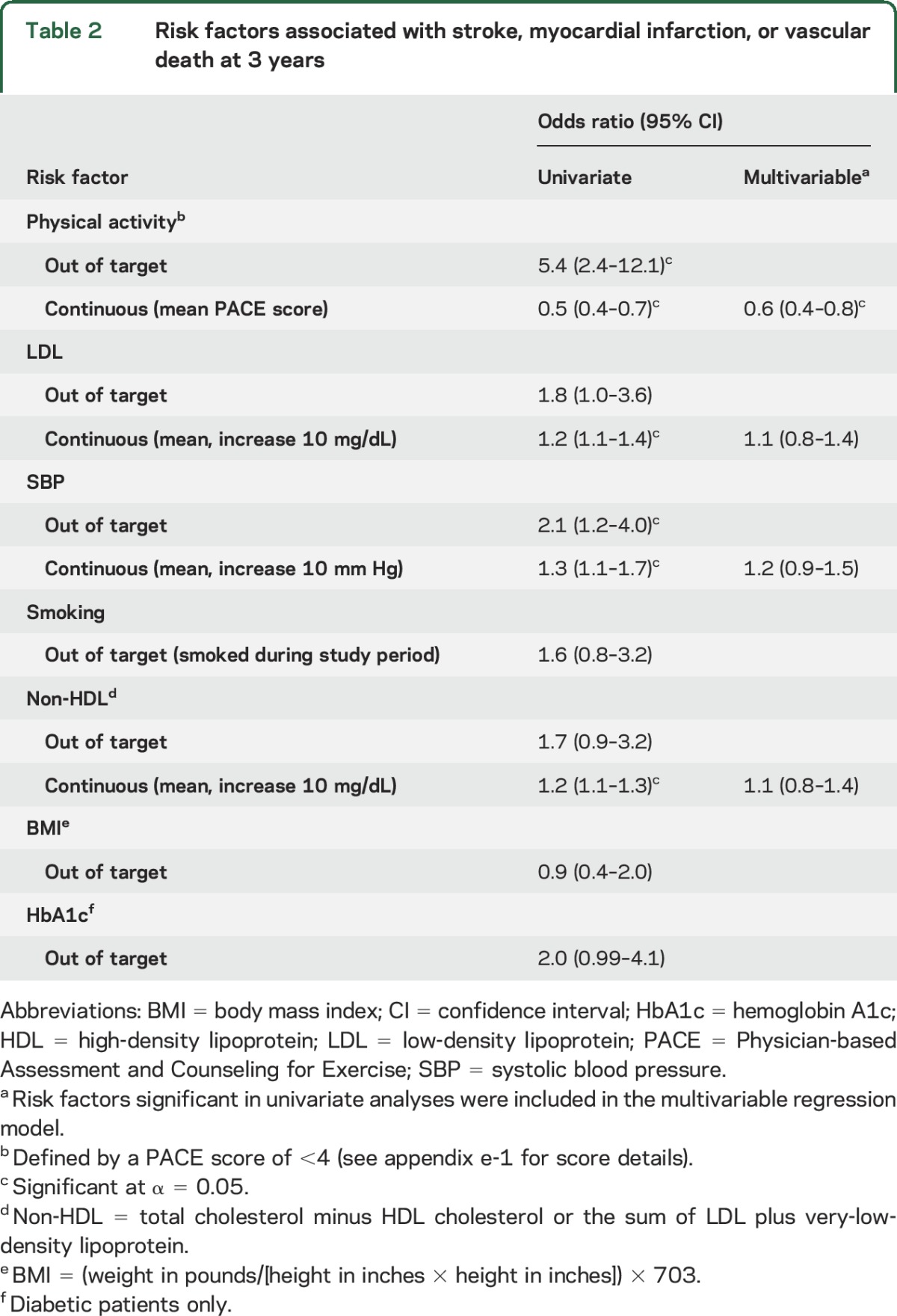
Table 3.
Risk factors associated with ischemic stroke alone at 3 years
Multivariable analyses.
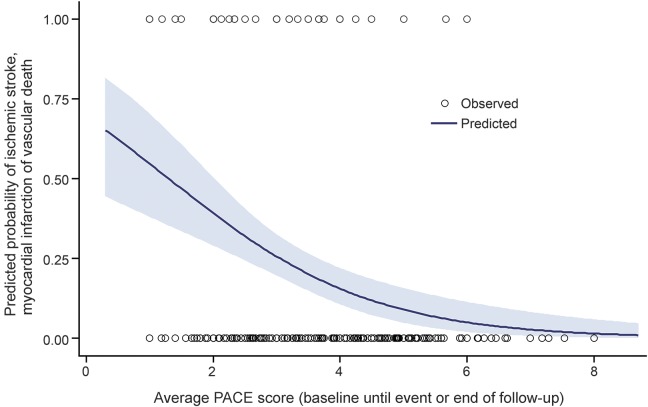
In multivariable analyses that included just those risk factors significant in the univariate analyses, greater physical activity on a continuous scale (higher PACE score) decreased the likelihood of a recurrent stroke, MI, or vascular death (OR 0.6, confidence interval [CI] 0.4–0.8), demonstrating a dose effect of exercise (figure). BP and cholesterol were not significant factors.
Figure. Fitted logistic regression of combined endpoint (stroke, myocardial infarction, or vascular death) predicted by physical activity (Physician-based Assessment and Counseling for Exercise [PACE] score) averaged from baseline until the first event or end of follow-up.
Maximum follow-up was 3 years. The blue shaded band represents 95% confidence intervals for the probability of the event. The markers represent the observed data.
For both the primary endpoint of ischemic stroke, MI, or vascular death and the endpoint of ischemic stroke alone, the unadjusted OR for physical activity (measured on a continuous scale) and the ORs adjusted for age, sex, NIHSS, mRS, baseline BMI, cardiovascular disease, or peripheral vascular disease were 0.5–0.6, suggesting that the association of physical inactivity and events was not confounded by these factors. The ORs for physical activity were similar for participants who were in target (0.6, CI 0.3–1.0) vs out of target (0.5, CI 0.3–0.7) at baseline.
DISCUSSION
This prespecified post hoc analysis of the relationship between risk factor control and outcome in the medical arm of SAMMPRIS demonstrates that physical activity and intensive control of BP and cholesterol are important for reducing vascular events in medically treated patients with severe ICAS. While all 3 of these factors may contribute to the risk of vascular events, the independent effect of physical activity was stronger for the prediction of vascular events than BP and cholesterol.
Remarkably, patients in the medical arm who were physically inactive had up to 5 times the likelihood of having a stroke, MI, or vascular death, as well as 6 times the risk of any ischemic stroke compared to physically active patients. In addition, there appears to be a dose effect of exercise, with higher rates of activity having a more protective effect. Previous meta-analyses have shown that exercise decreases mortality among stroke patients11 and that physical activity decreases the risk of incident stroke among healthy persons,12 but this analysis now demonstrates the benefits of physical activity for prevention of recurrent ischemic stroke.
Physical inactivity is highly prevalent after stroke due to factors such as fatigue, poststroke disability, and depression.13 In SAMMPRIS, patients were enrolled after having a TIA or stroke within the previous 30 days, so it is not surprising that the percentage of patients who were in target for physical activity at study entry was low (32%). However, at the 4-month follow-up visit, this number had increased to 56%.14,15 This increase was largely due to compliance with the lifestyle modification program provided in the trial, which also contributed to the high percentage of patients achieving other risk factor targets.16 Indeed, education and motivation are critical to enlisting stroke patients in an exercise program, and physicians typically lack the time to provide the level of support needed. Comprehensive lifestyle risk-reduction programs have been highlighted as an effective way to bridge the gap between recommendations for physical activity in guidelines and implementation of physical activity in practice,13 and our results support the effectiveness of such an approach.
The association of poor BP and cholesterol control with recurrent vascular events in medically treated patients in SAMMPRIS confirms the same findings in the WASID trial,4,5 which together provide strong evidence that control of these 2 risk factors is very important for lowering the risk of vascular events in patients with ICAS. This analysis also demonstrates that intensive lowering of BP early after a recent stroke or TIA appears to be safe in high-risk patients with ICAS. In WASID, patients were randomized within 90 days of a stroke or TIA (median time from qualifying event to enrollment was 17 days) and had their first follow-up visit at 4 months. Limited conclusions could be drawn about the safety of early lowering of BP in WASID4,5 because treatment of BP was not intensive and, in many cases, was delayed because of the extended time between qualifying event and enrollment. In contrast, SAMMPRIS patients were enrolled within 30 days of the qualifying event (median time to enrollment of 7 days), were started on intensive BP control at enrollment, and had their first follow-up visit in 30 days. Given the earlier time from qualifying event to enrollment in SAMMPRIS, one could hypothesize that SAMMPRIS patients were less hemodynamically stable than WASID patients and, therefore, more susceptible to ischemia distal to the stenotic artery from intensive lowering of BP after enrollment. However, this was not found to be the case in SAMMPRIS as the rate of vascular events was lower among patients with controlled SBP. Furthermore, this lower event rate among patients who are in target for SBP is not likely driven solely by patients who were already in target at baseline, given that within the first 30 days the percentage of patients achieving SBP target increased from 33.8% at baseline to 47.6% at 30 days.15
We were unable to demonstrate that control of some of the secondary risk factors (e.g., diabetes, weight, and smoking) significantly reduced recurrent vascular events in these analyses. This finding is similar to analyses from WASID that failed to show a relationship between control of diabetes and smoking with outcome.5 However, in both WASID and SAMMPRIS, patients with better diabetes control had lower rates of events than patients with poorer control, but the differences were not statistically significant. The inability of these analyses to demonstrate a benefit of control of the secondary risk factors targets may be due to a true lack of benefit, but could also be due to a lack of power to detect an effect given the small number of patients in some groups (i.e., type II error).
Our study has some limitations. The risk factor values were averaged over the duration of follow-up, which may have diluted the effect of particularly high or low levels that could have been temporally associated with an event (e.g., a particularly high BP that immediately preceded a stroke). However, the goal of this analysis was to assess the effect of overall risk factor control on outcome, not the effect of individual fluctuations in risk factors. While any methodologic approach to determining the relationship between risk factors and vascular events has limitations, we chose a measure that takes into account the long-term pathophysiology of atherosclerosis. In addition, since prior analyses of the relationship between risk factor control and outcome in patients with stroke due to large artery disease averaged risk factors over follow-up,4,17 this method was used for comparison purposes. Other possible risk factor interventions, such as dietary modification, were not analyzed, so their effect cannot be addressed in this study. Another limitation is that this was a post hoc analysis (albeit prespecified) and not a randomized trial of different risk factor treatment targets. Therefore, we cannot rule out the effect of unknown confounders on our results. However, given that the effect of BP and cholesterol control in SAMMPRIS is consistent with findings from WASID, the findings appear to be reliable.
Well-controlled BP, cholesterol, and physical activity during follow-up were associated with fewer vascular events in SAMMPRIS. These findings support treatment of elevated BP, cholesterol, and physical inactivity in patients with intracranial atherosclerosis to prevent future vascular events.
Supplementary Material
ACKNOWLEDGMENT
The PACE self-assessment forms for smoking cessation and physical activity were provided by the San Diego Center for Health Interventions, LLC.
GLOSSARY
- BMI
body mass index
- BP
blood pressure
- CI
confidence interval
- HbA1c
hemoglobin A1c
- HDL-C
high-density lipoprotein cholesterol
- ICAS
intracranial atherosclerotic stenosis
- LDL-C
low-density lipoprotein cholesterol
- MI
myocardial infarction
- mRS
modified Rankin Scale score
- NIHSS
NIH Stroke Scale
- OR
odds ratio
- PACE
Physician-based Assessment and Counseling for Exercise
- SAMMPRIS
Stenting and Aggressive Medical Management for Prevention of Recurrent Stroke in Intracranial Stenosis
- SBP
systolic blood pressure
- WASID
Warfarin Aspirin Symptomatic Intracranial Disease
Footnotes
Supplemental data at Neurology.org
Editorial, page 342
AUTHOR CONTRIBUTIONS
T. Turan: study concept and design, drafting the manuscript, study supervision A. Nizam: analysis and interpretation of the data. M. Lynn: study concept and design, analysis and interpretation of the data, study supervision. B. Egan: acquisition of data. N.A. Le: acquisition of data. M. Lopes-Virella: acquisition of data. K. Hermayer: acquisition of data. J. Harrell: acquisition of data. C. Derdeyn: study concept and design, study supervision. D. Fiorella: study concept and design, study supervision. L.S. Janis: study supervision. B. Lane: study concept and design, study supervision. J. Montgomery: acquisition of data. M. Chimowitz: study concept and design, study supervision.
STUDY FUNDING
This study was funded by a research grant (U01 NS058728) from the US Public Health Service National Institute of Neurologic Disorders and Stroke (NINDS). In addition, the following Clinical and Translational Science Awards, funded by the NIH, provided local support for the evaluation of patients in the trial: Medical University of South Carolina (UL1RR029882), University of Florida (UL1RR029889), University of Cincinnati (UL1RR029890), and University of California, San Francisco (UL1RR024131). Corporate Support: Stryker Neurovascular (formerly Boston Scientific Neurovascular) provided study devices and supplemental funding for third-party device distribution, site monitoring, and study auditing. This research was also supported by the Investigator-Sponsored Study Program of AstraZeneca, which donated rosuvastatin (Crestor) to study patients. Vendors: INTERVENT provided the lifestyle modification program to the study at a discounted rate. The Regulatory and Clinical Research Institute (RCRI) (Minneapolis, MN) provided assistance in designing the site monitoring processes and performing the site monitoring visits. The VA Cooperative Studies Program Clinical Research Pharmacy Coordinating Center (Albuquerque, NM) handled the procurement, labeling, distribution, and inventory management of the study devices and rosuvastatin. Walgreens pharmacies provided study medications except rosuvastatin to patients at a discounted price (paid for by the study).
DISCLOSURE
T. Turan reports grants from NIH/NINDS related to this study and personal fees from Gore and Boehringer Ingelheim for participating as a stroke adjudicator in clinical trials unrelated to this work. A. Nizam reports grants from NINDS during the conduct of the study. M. Lynn reports grants from NINDS during the conduct of the study. B. Egan received research support from Medtronic, Takeda, and Novartis, and has been a consultant for Medtronic and Blue Cross. N.A. Le reports grants from NINDS during the conduct of the study. M. Lopez-Virella reports no disclosures relevant to the manuscript. K. Hermayer received research support from the American Diabetes Association (ADA), Sanofi-Aventis, Eli Lilly, Novo Nordisk, and the NIH (DCCT/EDIC trial). Dr. Hermayer has received speakers' bureau appointment payments from Sanofi-Aventis, Eli Lilly, Amylin, and Boehringer Ingelheim. J. Harrell reports no disclosures relevant to the manuscript. C. Derdeyn reports grants from NINDS during the conduct of the study and has relationships with companies that manufacture medical devices for the treatment of cerebrovascular disease in general, although none was directly involved in this study (W.L. Gore and Associates [Scientific Advisory Board and Consultant]; Microvention, Inc. [Angiographic Core Lab for clinical trial]; Penumbra, Inc. [DSMB member for clinical trial]; and Pulse Therapeutics [Chair, Scientific Advisory Board]). D. Fiorella reports Siemens Microintervention institutional payment for research/salary support; Covidien Ev3, Cordis, NFocus Medical, and Micrus Endovascular consulting fees; Codman & Shurtleff (REVIVE) royalties; and Vascular Simulators LLC, TDC Technologies, and CVSL ownership and stock interests. L. Janis, B. Lane, and J. Montgomery report no disclosures relevant to the manuscript. M. Chimowitz reports grants from NIH/NINDS and other support from AstraZeneca and Stryker Neurovascular (formerly Boston Scientific Neurovascular) related to this study; other grants from NIH/NINDS and personal fees from Gore Associates, Merck/Parexel, and Medtronic for participating as a stroke adjudicator or data safety monitoring board member on clinical trials unrelated to the submitted work; and personal fees as an expert witness in medical legal cases related to stroke. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Gorelick PB, Wong KS, Bae HJ, Pandey DK. Large artery intracranial occlusive disease, a large worldwide burden but a relatively neglected frontier. Stroke 2008;39:2396–2399. [DOI] [PubMed] [Google Scholar]
- 2.Chimowitz MI, Lynn MJ, Howlett-Smith H, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med 2005;352:1305–1316. [DOI] [PubMed] [Google Scholar]
- 3.Mazighi M, Tanasescu R, Ducrocq X, et al. Prospective study of symptomatic atherothrombotic intracranial stenoses: the GESICA study. Neurology 2006;66:1187–1191. [DOI] [PubMed] [Google Scholar]
- 4.Turan TN, Cotsonis G, Lynn MJ, Chaturvedi S, Chimowitz M; Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) Trial Investigators. Relationship between blood pressure and stroke recurrence in patients with intracranial arterial stenosis. Circulation 2007;115:2969–2975. [DOI] [PubMed] [Google Scholar]
- 5.Chaturvedi S, Turan TN, Lynn MJ, et al. Risk factor status and vascular events in patients with symptomatic intracranial stenosis. Neurology 2007;69:2063–2068. [DOI] [PubMed] [Google Scholar]
- 6.Turan TN, Lynn MJ, Nizam A, et al. Rationale, design, and implementation of aggressive risk factor management in the Stenting and Aggressive Medical Management for Prevention of Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) trial. Circ Cardiovasc Qual Outcomes 2012;5:e51–e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Derdeyn CP, Chimowitz MI, Lynn MJ, et al. ; Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis Trial Investigators. Aggressive medical treatment with or without stenting in high-risk patients with intracranial artery stenosis (SAMMPRIS): the final results of a randomised trial. Lancet 2014;383:333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chimowitz MI, Lynn MJ, Turan TN, et al. Design of the stenting and aggressive medical management for preventing recurrent stroke in intracranial stenosis trial. J Stroke Cerebrovasc Dis 2011;20:357–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calfas KJ, Long BJ, Sallis JF, Wooten WJ, Pratt M, Patrick K. A controlled trial of physician counseling to promote the adoption of physical activity. Prev Med 1996;25:225–233. [DOI] [PubMed] [Google Scholar]
- 10.Ainsworth BE, Youmans CP. Tools for physical activity counseling in medical practice. Obes Res 2002;10(1 suppl):69S–75S. [DOI] [PubMed] [Google Scholar]
- 11.Naci H, Ioannidis JP. Comparative effectiveness of exercise and drug interventions on mortality outcomes: metaepidemiological study. BMJ 2013;347:f5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J, Siegrist J. Physical activity and risk of cardiovascular disease: a meta-analysis of prospective cohort studies. Int J Environ Res Public Health 2012;9:391–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Billinger SA, Arena R, Bernhardt J, et al. ; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Lifestyle and Cardiometabolic Health; Council on Epidemiology and Prevention; Council on Clinical Cardiology. Physical activity and exercise recommendations for stroke survivors: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45:2532–2553. [DOI] [PubMed] [Google Scholar]
- 14.Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med 2011;365:993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turan TN, Nizram A, Lynn MJ, et al. ; SAMMPRIS Investigators. Impact of an aggressive medical management protocol on early risk factor measures in the Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) trial. Stroke 2012;43:A141. Abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turan TN, Nizam A, Lynn MJ, et al. Relationship between compliance with the lifestyle modification program and risk factor control in the Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) trial. Stroke 2014;45(suppl 1):ATMP105. Abstract. [DOI] [PubMed] [Google Scholar]
- 17.Powers WJ, Clarke WR, Grubb RL Jr, et al. Lower stroke risk with lower blood pressure in hemodynamic cerebral ischemia. Neurology 2014;82:1027–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.