Abstract
Patients with the main clinical phenotypes of multiple sclerosis (MS) manifest varying degrees of brain atrophy beyond that of normal aging. Assessment of atrophy helps to distinguish clinically and cognitively deteriorating patients and predicts those who will have a less-favorable clinical outcome over the long term. Atrophy can be measured from brain MRI scans, and many technological improvements have been made over the last few years. Several software tools, with differing requirements on technical ability and levels of operator intervention, are currently available and have already been applied in research or clinical trial settings. Despite this, the measurement of atrophy in routine clinical practice remains an unmet need. After a short summary of the pathologic substrates of brain atrophy in MS, this review attempts to guide the clinician towards a better understanding of the methods currently used for quantifying brain atrophy in this condition. Important physiologic factors that affect brain volume measures are also considered. Finally, the most recent research on brain atrophy in MS is summarized, including whole brain and various compartments thereof (i.e., white matter, gray matter, selected CNS structures). Current methods provide sufficient precision for cohort studies, but are not adequate for confidently assessing changes in individual patients over the scale of months or a few years.
Clinically relevant irreversible brain tissue loss (i.e., atrophy) occurs in patients with multiple sclerosis (MS) from the early stages of the disease and is useful in differentiating clinical phenotypes and explaining physical disability and cognitive impairment.1–6 Neurodegeneration is among the pathologic hallmarks of MS and halting neurodegeneration and promoting neuroprotection are among the target outcomes of current therapeutic strategies.
Over the last 2 decades, considerable effort has been devoted to elucidating the clinical relevance of brain atrophy in MS, and developing methods for its reliable estimation. Several comprehensive review articles have already been published on this topic.7,8 Since their publication, important technical improvements have been made and several software tools, with differing requirements on technical ability and levels of operator intervention, have been developed. However, brain atrophy quantification is still far from a reliable application in clinical practice. This is in part due to the lack of comparative studies, so that choosing between methods is difficult; the uncertainty in the results when applied to single patient; the difficulty of developing completely automatic methods; of standardizing acquisition; of creating robust postprocessing procedures; and of fitting the lengthy acquisition and analysis procedures into the clinical routine. The absence of a normative database, combined with high intersubject brain volume variability, is another critical issue.
This review provides an update on current understanding of brain atrophy in MS. After a brief summary of the pathologic substrates, we discuss methodologic aspects of quantification, including imaging protocols and clinical applications in cross-sectional and longitudinal settings. Important physiologic factors affecting brain atrophy estimation, which should be controlled for, are also considered. All of this has a profound influence on the feasibility of measuring atrophy in clinical practice. To emphasize the importance of bringing such an assessment closer to clinical use, we then summarize the most recent research into MS brain atrophy (multiple compartments and structures). The effects of currently available disease-modifying treatments on atrophy are not discussed, since they have been the topic of recent reviews.8,9
PATHOLOGIC BASIS
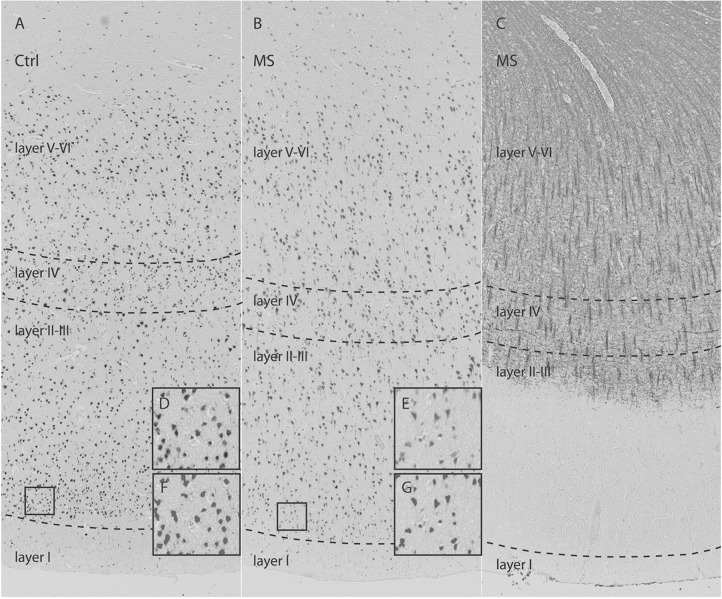
Within white matter (WM) lesions, there is loss of myelin, oligodendrocytes, and axons. Atrophy of nonlesional or normal-appearing WM (NAWM) is likely secondary to myelin loss and axonal damage and loss, the latter partially caused by Wallerian degeneration. Gray matter (GM) pathology is also frequent and widespread. GM demyelination is common in neocortical areas, but is also found in other GM areas, such as the thalamus, hippocampus, and cerebellum. A study relating GM atrophy patterns measured using postmortem MRI to histopathology showed that atrophy is explained predominantly by (neuro)axonal loss and neuronal shrinkage, and is largely independent of demyelination.10 This finding was later confirmed by relating GM lesions and normal-appearing GM more directly (figure 1).11
Figure 1. Neuronal loss in type III cerebral cortical lesions in multiple sclerosis (MS).
Control (Ctrl) (A) and MS (B) sections immunostained for NeuN demonstrate overall neuronal loss in MS cortex. (C) Antimyelin PLP-immunostained section from a patient with MS (B) shows subpial demyelination. High-magnification images from cortical layer II (D, E) with matching pictures (F, G) demonstrate the quality of segmentation scripts. The frontal cortex was selected to investigate possible differences in neuron density and axon density between MS (normal-appearing gray matter and type III lesions) and control cortex. Neuron density (−25.4%; p = 0.001) and axon density (−31.4%; p = 0.001) were significantly reduced in type III lesions compared with control cortex. There was no significant difference in neuron sizes between type III lesions and matched control cortex. From Klaver R, Popescu V, Voorn P, et al. Neuronal and axonal loss in normal-appearing gray matter and subpial lesions in multiple sclerosis. J Neuropathol Exp Neurol 2015;74:453–458,11 by permission of the American Association of Neuropathologists, Inc., Copyright © 2015.
Postmortem work compared the results from different GM atrophy MRI analysis tools to cortical thickness measured histopathologically, and showed that there are substantial differences in accuracy between them,12 indicating that future research should focus on improving and validating these different analysis techniques.
METHODOLOGIC ISSUES
Atrophy measures rely on the precise quantification of the volume, or change in volume, of relevant structures. With MRI, the volume of any structure that can be seen with good contrast (that is, a difference in brightness between tissues that exceeds the level of noise) can be quantified. However, raw volumes usually need to be scaled to remove confounding factors that affect volume, giving an index of atrophy that can be compared to normative data. One example is the so-called brain parenchymal fraction (BPF), which is the ratio of the brain volume to the intracranial volume. When follow-up scans of the same patient are acquired, volume changes can be quantified, and indices of change can be made with respect to a baseline scan.
MRI data acquisition.
With careful setup and quality control, an MRI scanner is capable of making precise volume measurements that are not achievable by other means. MRI uses no ionizing radiation and there is no known risk from the low-level heating of the body that occurs during a scan. As a consequence, it can safely be used in serial follow-up studies.
In scientific terminology, accuracy refers to the degree of bias in any measurement, while precision reflects the reproducibility of measurements. The assessment of accuracy and precision is an essential part of the validation process when introducing a new measurement method.13 In the context of MRI assessment of atrophy, precision can be assessed by repeated MRI measurements over a short enough period of time where little real change in volume can have occurred. Accuracy is more difficult to evaluate, unless simulated data or test objects are used, consisting of different structures with known dimensions. Validation should involve measurements made under conditions of different signal-to-noise ratio, contrast, and image geometry, to optimize acquisition and processing parameters, to test method robustness, and to characterize the measurement error. It will only be possible to determine the context in which a method may be applied after rigorous validation involving both healthy controls and patients, studied both cross-sectionally and longitudinally.
The size at which scanned objects appear in MRI is governed by the accurate calibration of the magnetic field gradients used to encode spatial information. The contrast in the images is affected by the radiofrequency (RF) pulse flip angles used to create the signal; this indirectly affects atrophy measures since tissue compartments (CSF, GM, WM) are assigned based on their relative brightness in the image. Thus, stability of the gradient and RF systems are important factors. For longitudinal studies, it is imperative to image any given study participant using the same scanner without upgrades, especially in hardware. This is difficult in clinical practice; however, patients with MS are often referred to the same neurologic center during the course of the disease, and it is likely that they will undergo MRI in the same radiology department. In cases where more than one scanner is available, and it is difficult to guarantee the study of a patient always using the same scanner, the use of quantitative measures may still be possible.14 Attention should also be paid to correction of the effects of gradient distortion.
Brain atrophy is mostly measured from T1-weighted images, where there is good contrast between a bright parenchyma against a dark CSF background and good GM/WM contrast. T2/fluid-attenuated inversion recovery contrast can be utilized for BPF. Most recent studies of atrophy have used high-resolution 3D acquisition, with near-isotropic voxel sizes of around 1 mm3. The high resolution allows the assessment of smaller, thinner structures such as the cortical ribbon, while 3D acquisition and isotropic voxel sizes can minimize the reduction in quality when coregistering serial scans.
Current methods provide sufficient precision for cohort studies, since errors and confounding effects are averaged out, but do not always give enough confidence in the assessment of changes in individual patients with MS. The ability to measure change in a single patient reliably in a particular region depends on the precision of the method used relative to the true change in atrophy over the follow-up, and confounding effects, such as hydration.15 Natural history studies have shown brain atrophy rates in patients with MS in the range 0.7%–1% per year,7 while validation studies report, for example, a median absolute error of 0.15% for longitudinal changes in brain atrophy.16 Thus, it should be possible to measure brain atrophy reliably in a single patient with, say, annual follow-up, although we need to take confounding effects, clinical events, and treatment into account. Regional measures will have poorer precision; for example, cortical thickness has an average variability of around 2.5%–3%.17 The ability to use shorter follow-up periods or to assess substructures must be evaluated case by case.
Methods: Cross-sectional and longitudinal applications.
In radiologic practice, simple linear measures, such as ventricular enlargement, were reproducible at the cost of long training and time spent in manual analysis. Automatic methods now exist that usually involve complex pipelines, combining lesion segmentation, registrations, and tissue/structure segmentation. Table 1 shows a comparison of the current most widely used, freely available software.
Table 1.
Comparison of various widely used, freely available software tools for cross-sectional and longitudinal atrophy measurement

In table 1, the target anatomy of each tool indicates which parts of the brain it examines (e.g., cortex or deep GM), and the measure indicates which aspect of the target anatomy is quantified (e.g., volume or thickness) and whether it is done locally or as a global summary across the brain or structure. Global measures often benefit from averaging over a larger volume and can have greater precision and statistical power. Local measurements more richly describe the anatomical changes and are not diluted by areas where there is little or no change. Prior information is often required when performing segmentation and is usually provided via registration to an atlas. Segmentation of deep GM structures also requires, together with registration, prior anatomical knowledge, though the measures it provides are often more sophisticated (e.g., shape variation or intensity distributions). The set of images used to generate the prior information needs to be appropriate to the studied participants: for example, healthy control priors can cause biases when used with patient images. Some methods (e.g., advanced normalization tools, statistical parametric mapping) mainly use registration to measure local shape differences, having a standard anatomy or the longitudinal acquisition of the same subject as a reference.
WM lesions affect atrophy calculations, since they influence the detection of GM/WM/CSF intensity differences. Lesion filling techniques (table 1) are often employed to alleviate this. This approach is suitable for use with a wide range of atrophy tools (table 1) and is recommended for increased accuracy (figure 2).
Figure 2. Effect of T1-hypointense lesion refilling.
(A) Three orthogonal views (axial, coronal, and sagittal) of the same patient with multiple sclerosis are shown before (top row) and after (bottom row) T1-hypointense lesion refilling. (B) Results of segmentation without (left) and with (right) T1-hypointense lesion refilling. The gray and white matter tissue classes are shown in 2 different shades of red. Without lesion refilling, the misclassification of lesions as gray matter is evident.
Volumes and thicknesses of brain tissue structures correlate with head size, so it is common practice to produce normalized values. The normalization factors (table 1) are often based on automated measurements of intracranial volume (ICV) or on scaling factors from skull-based or whole-head-based registration to a standard template.
Tools for longitudinal assessment of atrophy are normally designed and optimized to work specifically with pairs of images from each participant. Due to drift in scanner calibration, corrections based on the skull volume (or ICV) are commonly used to isolate true biological changes. Registration of scans of the same subject achieves greater precision than registration to an atlas, because the brain has the same cortical folding pattern.
Pitfalls and limitations.
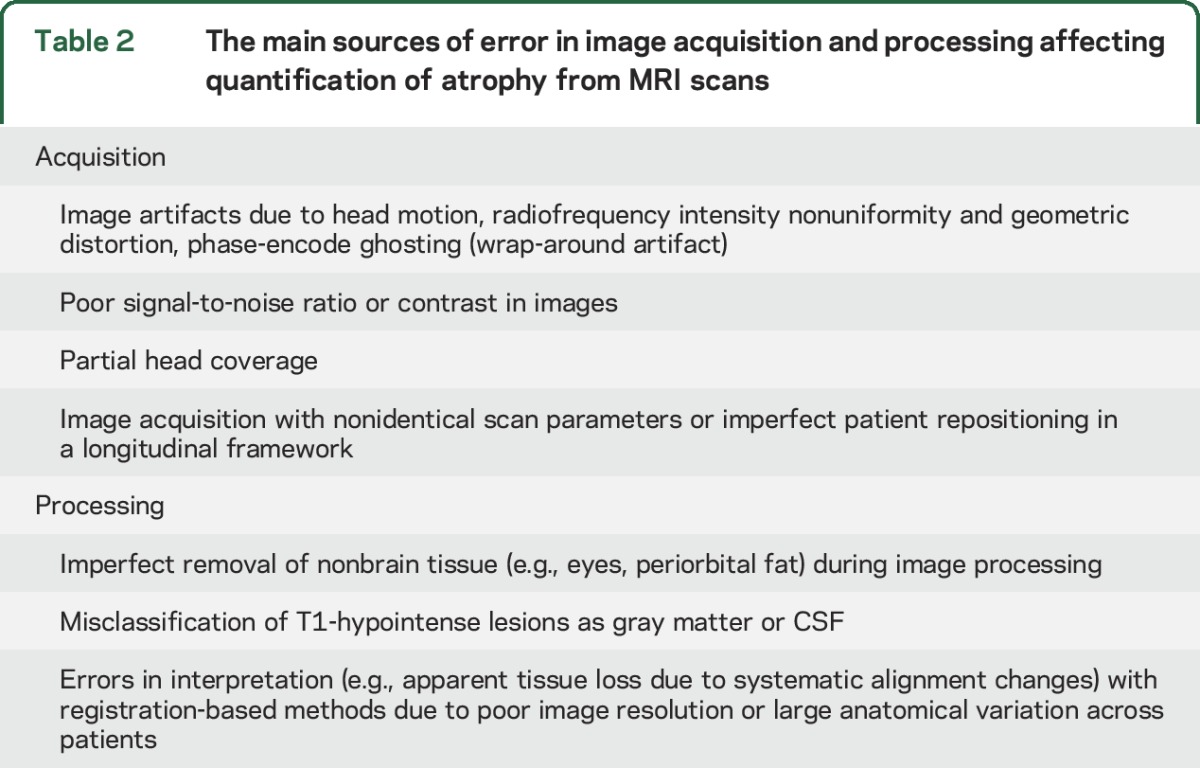
Various sources of error related to image acquisition and processing and variability due to pathophysiologic changes can affect MRI atrophy quantification.8,18 Table 2 lists the main errors due to image acquisition and processing. High-quality and consistent acquisition protocols, together with clear procedures for repositioning, are mandatory for assessing brain atrophy.
Table 2.
The main sources of error in image acquisition and processing affecting quantification of atrophy from MRI scans
The interpretation of apparent brain volume loss needs caution, since numerous factors can have a confounding effect, causing overestimation or underestimation.7–9,18 Factors such as lifestyle (e.g., alcohol, smoking, diet, and dehydration),15 genetics,18 and others (e.g., diabetes, cardiovascular risks) may affect brain volumes. For example, fast (1.5 L in 90 minutes) rehydration after dehydration resulted in a 0.36% increase of brain volume.15 A J-formed association was found between the extent of alcohol ingestion and brain atrophy progression, with heavier drinkers having the highest annual brain atrophy rate (−0.54 ± 0.26%).18 Brain atrophy progresses with age and may be more pronounced when aging is complicated by other risk factors.18 Moreover, brain volumes seem to fluctuate throughout the day, decreasing from morning to evening.19
Because of these difficulties, individualized longitudinal brain volume assessment may not be ready for widespread clinical use. Further studies are needed to establish normative values for brain volume changes (both for healthy individuals and patients with MS).
CLINICAL APPLICATIONS
Global measurements.
Brain atrophy, ventricular system enlargement, and widening of cortical sulci and gyri is evident on visual inspection in the large majority of MS scans. Brain atrophy occurs through all stages of the disease, from clinically isolated syndromes (CIS) to secondary progressive (SP) and primary progressive (PP) disease, being more pronounced in the latter groups.7
In clinically stable and untreated patients, brain volume loss occurs at a rate of about 0.5%–1% per year7 compared to 0.1%–0.3% for healthy controls. Longitudinal studies of different cohorts of patients (including a large group of untreated patients with MS)20 have suggested that the annual rate of brain atrophy loss is not influenced by clinical phenotype.20 A 7.5-year longitudinal study tried to provide specific cutoff values to discriminate physiologic and pathologic brain atrophy rates in patients with MS, which might be of great value in a clinical setting.21 An annualized percentage brain volume change of −0.4% had a specificity of 80% and a sensitivity of 65% for discriminating the presence or absence of pathologic brain atrophy. Further studies, on different cohorts of patients, with different duration of follow-up and using different methods of acquisition and analysis, are needed to confirm the clinical relevance of such a cutoff.
Measuring atrophy progression provides clinically relevant information. More pronounced ventricular enlargement occurs in patients with CIS who evolve to MS compared to those who do not.1 Greater brain atrophy develops in patients with worsening disability than in those who are clinically stable.2 Whole brain atrophy correlates with cognitive dysfunction22 and mood disturbances.23 Finally, quantification of brain volume on early scans provides prognostic measures of clinical status not only for medium and long-term follow-up,24 but also for short-term (over 6 months) decline.25
Cross-sectional and longitudinal studies have investigated the mechanisms driving brain atrophy development. T2-hyperintense lesion volume correlates with brain atrophy at a given timepoint and over time.26 A 14-year longitudinal study suggested that this relationship might be dynamic, since early rather than later T2 focal lesion accumulation was related to the subsequent long-term atrophy.27 Correlative studies with MRI-detected disease activity (new T2 or gadolinium-enhancing lesions) have shown that inflammation can result in a transient increase in brain volume, which can dramatically resolve (pseudoatrophy) following steroid treatment.28 While some authors have suggested faster brain volume reduction in APOE ε4 MS carriers,29 others did not confirm these findings.30
There is recent evidence of more severe brain atrophy in patients with MS harboring one or more cardiovascular risk factors.31 Accumulation of damage in sites directly connected to the brain, such as the optic nerves and spinal cord, may also influence brain atrophy through secondary degeneration. For instance, a 4-year study showed an association between retinal layer abnormalities and brain atrophy progression.32 Other studies33 found a correlation between brain atrophy and imaging measures of cervical cord damage.
GM and WM atrophy.
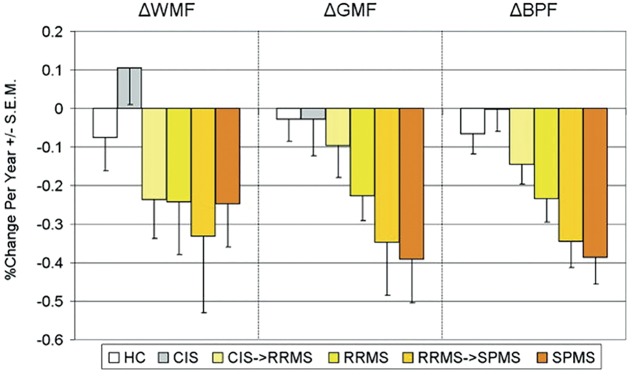
The ability to analyze brain WM and GM involvement separately has been one of the main advances in atrophy quantification. Although atrophy involves both these compartments, GM atrophy assessment provides more clinically relevant information than does WM atrophy. Significant GM fraction reduction was found in patients with CIS who developed MS over the subsequent 3 years.34 Confirming several smaller studies, in a large (n = 927) cross-sectional cohort, GM atrophy helped to distinguish the different disease forms (being more severe in SPMS) and was correlated with disability and cognitive impairment.3 Even though these results need to be confirmed on a larger dataset, expressed as an increase relative to controls, GM atrophy over a 4-year period accumulated with disease stage, being 8.1 times greater in patients with relapsing-remitting MS (RRMS), 12.4 times greater in patients converting from RRMS to SPMS, and 14 times greater in patients with SPMS (figure 3).4 In contrast, WM atrophy rates were constant across all MS disease stages.4 Reduced GM volume explains deteriorating cognitive function35 and impairment in selected cognitive domains. A 13-year study in relapse-onset patients with MS demonstrated that baseline GM atrophy was the only imaging predictor of disability worsening.6
Figure 3. Longitudinal changes in gray matter (GM) and white matter (WM) atrophy in multiple sclerosis (MS) phenotypes.
Plot of the mean annualized rates of change for WM fraction (WMF), GM fraction (GMF), and brain parenchymal fraction (BPF) in patients with MS with different disease clinical phenotypes, including healthy controls (HCs; white bars; n = 17), patients with clinically isolated syndromes (CIS; gray bars) throughout the 4‐year study (n = 7), patients who started the study with a diagnosis of CIS and converted to relapsing‐remitting MS (CIS→RRMS; light yellow bars; n = 8), patients with RRMS throughout the study (RRMS; dark yellow bars; n = 28), patients who started the study with a diagnosis of RRMS and progressed to secondary progressive MS (RRMS→SPMS; light orange bars; n = 7), and patients with SPMS (orange bars) throughout the study (n = 19). Error bars indicate SEM. From Fisher E, Lee JC, Nakamura K, Rudick RA. Gray matter atrophy in multiple sclerosis: a longitudinal study. Ann Neurol 2008;64:255–265,4 by permission of Wiley-Liss, Inc., © 2008 American Neurological Association.
GM atrophy is unevenly distributed across the brain. At the beginning of the disease (i.e., CIS and pediatric36 MS), GM involvement might start from deep GM (DGM) nuclei and then progressively extend to cortical GM regions.37 In PPMS, early involvement of the cingulate cortex occurred, which continued at a steady rate over a 5-year period, contributing to clinical deterioration.38
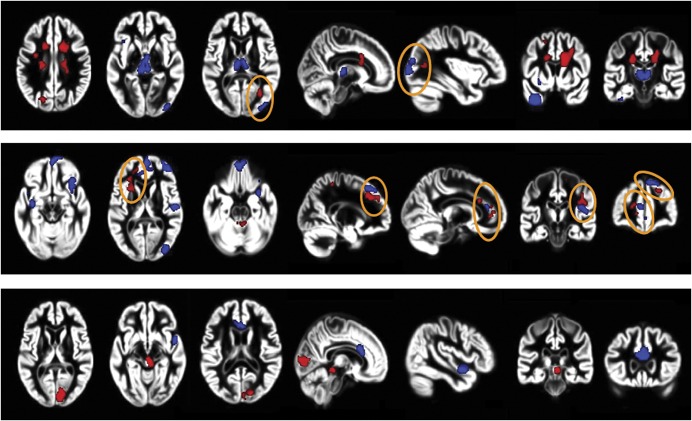
Atrophy of selected GM regions helps to explain specific clinical deficits or variability between clinical phenotypes. A well-characterized pattern of regional GM atrophy involving several frontal, parietal, and temporal regions distinguishes cognitively impaired patients with MS (figure 4)39; patients with MS with longstanding disease or severe disability show focal thinning of the primary sensorimotor cortex40; atrophy of the hippocampus and its subfield correlates with cognitive deficits.41 Applying radial mapping analysis (measured as the distance from hippocampal medial core to the surface), an expansion of the dentate gyrus of the hippocampus has been described, mostly in patients with RRMS, which may represent an inflammation-induced neurogenic process.42 In patients with CIS, inflammation may cause an early, paradoxical increase of GM volume.43
Figure 4. T2 lesions, gray matter (GM) atrophy, and cognitive impairment in multiple sclerosis (MS).
Distribution of regions of significant GM atrophy (blue) and T2‐visible lesions (red) in cognitively impaired vs cognitively preserved patients with MS according to the clinical phenotype. Top row: relapsing-remitting MS; middle row: secondary progressive MS; bottom row: primary progressive MS. Orange circles identify regions with a correspondence between presence of T2-visible lesions and GM atrophy. Images are oriented with neurologic convention. From Riccitelli G, Rocca MA, Pagani E, et al. Cognitive impairment in multiple sclerosis is associated to different patterns of gray matter atrophy according to clinical phenotype. Hum Brain Mapp 2011;32:1535–1543,39 by permission of Wiley-Liss, Inc., © 2010.
Several mechanisms drive GM atrophy. WM lesion accumulation influences GM atrophy, as suggested by cross-sectional44 and longitudinal studies that found a correlation between atrophy of selected GM regions over time and WM lesion progression.45 A 2-year study found that halting new T2 lesion formation may result in less GM atrophy, with no effect on cognitive deterioration.46 Another study described an association between increased periventricular T2 lesion volume and decreased cortical thickness in RRMS, but not in CIS,47 suggesting that common CSF-mediated factors might play a role in GM and WM damage accumulation. Studies that have assessed the anatomical correspondence between regional GM and WM damage found only a partial correspondence between damaging processes occurring in these 2 brain compartments.48 In early PPMS, cortical damage over a 2-year period was mostly a sequela of NAWM pathology, which, in turn, was predicted by abnormalities within WM lesions.49
Cortical atrophy can also occur as a result of focal GM lesions. However, the use of voxel-wise measures has shown that regional GM atrophy and cortical lesions are not spatially coupled.50
A 2-year longitudinal study demonstrated that fluctuations in inflammatory WM lesion activity can profoundly influence WM volume changes (pseudoatrophy), whereas it does not affect GM volume changes.51
Deep GM nuclei.
In early work, 3rd ventricle width (TVW) correlated with performance on many cognitive tests.5 A postmortem/MRI correlative study showed increased TVW (105%) and decreased thalamic volume (17%) in 10 patients, with a 22% reduction of neuronal density.52 In 86 patients with MS,53 volume was reduced in caudate, putamen, pallidum, nucleus accumbens, and thalamus. The effect on the thalamus was nearly twice that of any other DGM structure. In 120 patients,54 MRI/clinical correlations were again between thalamus and measures of cognitive processing speed and executive function, and between caudate and tests emphasizing working memory and verbal memory.
The thalamus is an assembly of multiple interconnected nuclei, with unique efferents of the cerebral cortex. The next research goal is to identify tracts emanating from specific thalamic nuclei or subregions and measure the correlation between microstructural tract injury and associated seed nuclei. Recent multicenter work55 performed a diffusion tractography–based parcellation of the thalamus and its WM connections and found that cognitive impairment in RRMS was associated with increased fractional anisotropy in most thalamic regions, atrophy of the frontal subregion that includes the anterior nucleus, and abnormal fractional anisotropy of all corticothalamic tracts.
In a longitudinal study,56 the rate of atrophy over 3 years was modest in patients with MS when compared to healthy controls, and was more pronounced in patients with low cognitive reserve. In another investigation, decreased cognitive processing speed over 3 years was related to localized atrophy of the anterior and superior surface of the left thalamus.57
Measuring DGM nuclei atrophy may provide important prognostic information, as suggested by patients with CIS, in whom decreased thalamic volume over a 2-year period was associated with development of MS,58 or in relapse-onset patients with MS, in whom baseline thalamic atrophy was associated with the accumulation of disability after an 8-year follow-up.59
The mechanisms influencing DGM tissue loss are those previously described for the whole GM, although a significant regional effect of T2 lesions has been described early in the disease course.60
DISCUSSION
Many technical improvements have occurred that have enhanced the quantification of brain atrophy, and there is an increased understanding of the clinical relevance of atrophy and its underlying substrates in patients with MS. Multiple physiologic and disease-related factors are now recognized as potential modifiers of brain atrophy estimates, which suggests that caution is needed when moving atrophy measures into clinical practice. The choice of the analysis method and target tissue area should be guided by clinical questions (i.e., monitoring of disease course, obtaining prognostic information, explaining specific symptoms). At present, it seems that a global measure of brain volume or a simple measure of GM volume may be sufficient for clarifying many of the processes at work in MS. To move atrophy quantification definitively into clinical practice, effort should be devoted to creating a worldwide standardized protocol for image acquisition not only for research studies, but also for individual patient management. As is the case for many other paraclinical diagnostic tools, specialized analysis by expert laboratories may be the future.
SEARCH STRATEGY AND SELECTION CRITERIA
References for this review were identified through searches of PubMed with the search terms atrophy, multiple sclerosis, tissue loss, white matter, lesions, gray matter, brain, phenotype/s, disability, prognosis, and cognitive impairment from 1979 until September 2016. Articles were also identified through searches of the authors' own files. Only articles published in English were reviewed. The final reference list was generated on the basis of originality and relevance to the broad scope of this review.
Supplementary Material
GLOSSARY
- BPF
brain parenchymal fraction
- CIS
clinically isolated syndrome
- DGM
deep gray matter
- GM
gray matter
- ICV
intracranial volume
- MS
multiple sclerosis
- NAWM
normal-appearing white matter
- PP
primary progressive
- RF
radiofrequency
- RR
relapsing-remitting
- SP
secondary progressive
- TVW
3rd ventricle width
- WM
white matter
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
M.F. drafted the introduction and conclusions as well as the global measurement section. M.A.R. drafted the gray matter and white matter atrophy section. J.J.G.G. drafted the pathologic basis section. M.A.H., R.G.H., M.J., and E.P drafted the methodologic issues section. N.D.S. and M.B. drafted the pitfalls and limitations section. R.H.B.B. drafted the deep gray matter nuclei section. The complete article was commented on, revised, and approved by all authors.
STUDY FUNDING
This article reports the conclusions of the Eighteenth Advanced Course on Magnetic Resonance Techniques in Multiple Sclerosis, which was held in Milan on March 19–20, 2015, and subsequent discussion among meeting participants. The course was supported by an unrestricted educational grant from Biogen Idec. The funding source had no role in the preparation of the manuscript.
DISCLOSURE
M.A. Rocca received speakers' honoraria from Biogen Idec, Novartis, Genzyme, Sanofi-Aventis, Teva Pharmaceutical Industries, and Excemed and receives research support from the Italian Ministry of Health and Fondazione Italiana Sclerosi Multipla. M. Battaglini reports no disclosures relevant to the manuscript. R. Benedict receives research support from Accorda, Novartis, Genzyme, Biogen, and Mallinckrodt Pharmaceuticals, is on the speakers' bureau for EMD Serono (designing CME courses), consults for Biogen, Genentech, Genzyme, and Novartis, and receives royalties from Psychological Assessment Resources. N. De Stefano has received honoraria from Schering, Biogen-Idec, Teva, Novartis, Genzyme, and Merck Serono S.A. for consulting services, and speaking and travel support. He serves on advisory boards for Biogen-Idec Merck Serono S.A. and Novartis. J. Geurts serves on the editorial board of Multiple Sclerosis Journal and Neurology®; received speaker honoraria from Novartis, Biogen, Genzyme, Merck Serono, and Teva; and served on the board of the Dutch MS Research Foundation and the progressive MS Alliance. R. Henry has received grants from Stem Cells Inc, Hoffmann La Roche, and Genentech. Consultancies have been compensated for Novartis, Abbvie, and Mallinckrodt. M. Horsfield is an employee and stockholder of Xinapse Systems Ltd. M. Jenkinson receives royalties from ISIS (University of Oxford's commercialization company) for licensing of FSL to commercial enterprises and received speaker honoraria from Novartis. E. Pagani reports no disclosures relevant to the manuscript. M. Filippi is Editor-in-Chief of the Journal of Neurology; serves on the scientific advisory board for Teva Pharmaceutical Industries; has received compensation for consulting services and/or speaking activities from Biogen Idec, Excemed, Novartis, and Teva Pharmaceutical Industries; and receives research support from Biogen Idec, Teva Pharmaceutical Industries, Novartis, Italian Ministry of Health, Fondazione Italiana Sclerosi Multipla, Cure PSP, Alzheimer's Drug Discovery Foundation (ADDF), the Jacques and Gloria Gossweiler Foundation (Switzerland), and ARiSLA (Fondazione Italiana di Ricerca per la SLA). Go to Neurology.org for full disclosures.
REFERENCES
- 1.Dalton CM, Brex PA, Jenkins R, et al. Progressive ventricular enlargement in patients with clinically isolated syndromes is associated with the early development of multiple sclerosis. J Neurol Neurosurg Psychiatry 2002;73:141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ingle GT, Stevenson VL, Miller DH, Thompson AJ. Primary progressive multiple sclerosis: a 5-year clinical and MR study. Brain 2003;126:2528–2536. [DOI] [PubMed] [Google Scholar]
- 3.Roosendaal SD, Bendfeldt K, Vrenken H, et al. Grey matter volume in a large cohort of MS patients: relation to MRI parameters and disability. Mult Scler 2011;17:1098–1106. [DOI] [PubMed] [Google Scholar]
- 4.Fisher E, Lee JC, Nakamura K, Rudick RA. Gray matter atrophy in multiple sclerosis: a longitudinal study. Ann Neurol 2008;64:255–265. [DOI] [PubMed] [Google Scholar]
- 5.Benedict RHB, Weinstock-Guttman B, Fishman I, Sharma J, Tjoa CW, Bakshi R. Prediction of neuropsychological impairment in multiple sclerosis: comparison of conventional magnetic resonance imaging measures of atrophy and lesion burden. Arch Neurol 2004;61:226–230. [DOI] [PubMed] [Google Scholar]
- 6.Filippi M, Preziosa P, Copetti M, et al. Gray matter damage predicts the accumulation of disability 13 years later. Neurology 2013;81:1759–1767. [DOI] [PubMed] [Google Scholar]
- 7.Bermel RA, Bakshi R. The measurement and clinical relevance of brain atrophy in multiple sclerosis. Lancet Neurol 2006;5:158–170. [DOI] [PubMed] [Google Scholar]
- 8.De Stefano N, Airas L, Grigoriadis N, et al. Clinical relevance of brain volume measures in multiple sclerosis. CNS Drugs 2014;28:147–156. [DOI] [PubMed] [Google Scholar]
- 9.Zivadinov R, Reder AT, Filippi M, et al. Mechanisms of action of disease-modifying agents and brain volume changes in multiple sclerosis. Neurology 2008;71:136–144. [DOI] [PubMed] [Google Scholar]
- 10.Popescu V, Klaver R, Voorn P, et al. What drives MRI-measured cortical atrophy in multiple sclerosis? Mult Scler 2015;21:1280–1290. [DOI] [PubMed] [Google Scholar]
- 11.Klaver R, Popescu V, Voorn P, et al. Neuronal and axonal loss in normal-appearing gray matter and subpial lesions in multiple sclerosis. J Neuropathol Exp Neurol 2015;74:453–458. [DOI] [PubMed] [Google Scholar]
- 12.Popescu V, Klaver R, Versteeg A, et al. Postmortem validation of MRI cortical volume measurements in MS. Hum Brain Mapp 2016;37:2223–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sullivan DC, Obuchowski NA, Kessler LG, et al. Metrology standards for quantitative imaging biomarkers. Radiology 2015;277:813–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deoni SC. Quantitative relaxometry of the brain. Top Magn Reson Imaging 2010;21:101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamura K, Brown RA, Araujo D, Narayanan S, Arnold DL. Correlation between brain volume change and T2 relaxation time induced by dehydration and rehydration: implications for monitoring atrophy in clinical studies. Neuroimage Clin 2014;6:166–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith SM, Zhang Y, Jenkinson M, et al. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage 2002;17:479–489. [DOI] [PubMed] [Google Scholar]
- 17.Tustison NJ, Cook PA, Klein A, et al. Large-scale evaluation of ANTs and FreeSurfer cortical thickness measurements. Neuroimage 2014;99:166–179. [DOI] [PubMed] [Google Scholar]
- 18.Enzinger C, Fazekas F, Matthews PM, et al. Risk factors for progression of brain atrophy in aging: six-year follow-up of normal subjects. Neurology 2005;64:1704–1711. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura K, Brown RA, Narayanan S, Collins DL, Arnold DL; Alzheimer's Disease Neuroimaging Initiative. Diurnal fluctuations in brain volume: statistical analyses of MRI from large populations. Neuroimage 2015;118:126–132. [DOI] [PubMed] [Google Scholar]
- 20.De Stefano N, Giorgio A, Battaglini M, et al. Assessing brain atrophy rates in a large population of untreated multiple sclerosis subtypes. Neurology 2010;74:1868–1876. [DOI] [PubMed] [Google Scholar]
- 21.De Stefano N, Stromillo ML, Giorgio A, et al. Establishing pathological cut-offs of brain atrophy rates in multiple sclerosis. J Neurol Neurosurg Psychiatry 2016;87:93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rao SM, Leo GJ, Haughton VM, St Aubin-Faubert P, Bernardin L. Correlation of magnetic resonance imaging with neuropsychological testing in multiple sclerosis. Neurology 1989;39:161–166. [DOI] [PubMed] [Google Scholar]
- 23.Bakshi R, Czarnecki D, Shaikh ZA, et al. Brain MRI lesions and atrophy are related to depression in multiple sclerosis. Neuroreport 2000;11:1153–1158. [DOI] [PubMed] [Google Scholar]
- 24.Fisher E, Rudick RA, Simon JH, et al. Eight-year follow-up study of brain atrophy in patients with MS. Neurology 2002;59:1412–1420. [DOI] [PubMed] [Google Scholar]
- 25.Gauthier SA, Mandel M, Guttmann CR, et al. Predicting short-term disability in multiple sclerosis. Neurology 2007;68:2059–2065. [DOI] [PubMed] [Google Scholar]
- 26.Radue EW, Barkhof F, Kappos L, et al. Correlation between brain volume loss and clinical and MRI outcomes in multiple sclerosis. Neurology 2015;84:784–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chard DT, Brex PA, Ciccarelli O, et al. The longitudinal relation between brain lesion load and atrophy in multiple sclerosis: a 14 year follow up study. J Neurol Neurosurg Psychiatry 2003;74:1551–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheriyan J, Kim S, Wolansky LJ, Cook SD, Cadavid D. Impact of inflammation on brain volume in multiple sclerosis. Arch Neurol 2012;69:82–88. [DOI] [PubMed] [Google Scholar]
- 29.Enzinger C, Ropele S, Smith S, et al. Accelerated evolution of brain atrophy and “black holes” in MS patients with APOE-epsilon 4. Ann Neurol 2004;55:563–569. [DOI] [PubMed] [Google Scholar]
- 30.van der Walt A, Stankovich J, Bahlo M, et al. Apolipoprotein genotype does not influence MS severity, cognition, or brain atrophy. Neurology 2009;73:1018–1025. [DOI] [PubMed] [Google Scholar]
- 31.Kappus N, Weinstock-Guttman B, Hagemeier J, et al. Cardiovascular risk factors are associated with increased lesion burden and brain atrophy in multiple sclerosis. J Neurol Neurosurg Psychiatry 2016;87:181–187. [DOI] [PubMed] [Google Scholar]
- 32.Saidha S, Al-Louzi O, Ratchford JN, et al. Optical coherence tomography reflects brain atrophy in multiple sclerosis: a four-year study. Ann Neurol 2015;78:801–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lukas C, Sombekke MH, Bellenberg B, et al. Relevance of spinal cord abnormalities to clinical disability in multiple sclerosis: MR imaging findings in a large cohort of patients. Radiology 2013;269:542–552. [DOI] [PubMed] [Google Scholar]
- 34.Dalton CM, Chard DT, Davies GR, et al. Early development of multiple sclerosis is associated with progressive grey matter atrophy in patients presenting with clinically isolated syndromes. Brain 2004;127:1101–1107. [DOI] [PubMed] [Google Scholar]
- 35.Amato MP, Portaccio E, Stromillo ML, et al. Cognitive assessment and quantitative magnetic resonance metrics can help to identify benign multiple sclerosis. Neurology 2008;71:632–638. [DOI] [PubMed] [Google Scholar]
- 36.Mesaros S, Rovaris M, Pagani E, et al. A magnetic resonance imaging voxel-based morphometry study of regional gray matter atrophy in patients with benign multiple sclerosis. Arch Neurol 2008;65:1223–1230. [DOI] [PubMed] [Google Scholar]
- 37.Ceccarelli A, Rocca MA, Pagani E, et al. A voxel-based morphometry study of grey matter loss in MS patients with different clinical phenotypes. Neuroimage 2008;42:315–322. [DOI] [PubMed] [Google Scholar]
- 38.Eshaghi A, Bodini B, Ridgway GR, et al. Temporal and spatial evolution of grey matter atrophy in primary progressive multiple sclerosis. Neuroimage 2014;86:257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Riccitelli G, Rocca MA, Pagani E, et al. Cognitive impairment in multiple sclerosis is associated to different patterns of gray matter atrophy according to clinical phenotype. Hum Brain Mapp 2011;32:1535–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sailer M, Fischl B, Salat D, et al. Focal thinning of the cerebral cortex in multiple sclerosis. Brain 2003;126:1734–1744. [DOI] [PubMed] [Google Scholar]
- 41.Longoni G, Rocca MA, Pagani E, et al. Deficits in memory and visuospatial learning correlate with regional hippocampal atrophy in MS. Brain Struct Funct 2015;220:435–444. [DOI] [PubMed] [Google Scholar]
- 42.Rocca MA, Longoni G, Pagani E, et al. In vivo evidence of hippocampal dentate gyrus expansion in multiple sclerosis. Hum Brain Mapp 2015;36:4702–4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rocca MA, Preziosa P, Mesaros S, et al. Clinically isolated syndrome suggestive of multiple sclerosis: dynamic patterns of gray and white matter changes: a 2-year MR imaging study. Radiology 2016;278:841–853. [DOI] [PubMed] [Google Scholar]
- 44.Charil A, Dagher A, Lerch JP, Zijdenbos AP, Worsley KJ, Evans AC. Focal cortical atrophy in multiple sclerosis: relation to lesion load and disability. Neuroimage 2007;34:509–517. [DOI] [PubMed] [Google Scholar]
- 45.Battaglini M, Giorgio A, Stromillo ML, et al. Voxel-wise assessment of progression of regional brain atrophy in relapsing-remitting multiple sclerosis. J Neurol Sci 2009;282:55–60. [DOI] [PubMed] [Google Scholar]
- 46.Damasceno A, Damasceno BP, Cendes F. No evidence of disease activity in multiple sclerosis: implications on cognition and brain atrophy. Mult Scler 2016;22:64–72. [DOI] [PubMed] [Google Scholar]
- 47.Jehna M, Pirpamer L, Khalil M, et al. Periventricular lesions correlate with cortical thinning in multiple sclerosis. Ann Neurol 2015;78:530–539. [DOI] [PubMed] [Google Scholar]
- 48.Riccitelli G, Rocca MA, Forn C, Colombo B, Comi G, Filippi M. Voxelwise assessment of the regional distribution of damage in the brains of patients with multiple sclerosis and fatigue. AJNR Am J Neuroradiol 2011;32:874–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bodini B, Chard D, Altmann DR, et al. White and gray matter damage in primary progressive MS: the chicken or the egg? Neurology 2016;86:170–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van de Pavert SH, Muhlert N, Sethi V, et al. DIR-visible grey matter lesions and atrophy in multiple sclerosis: partners in crime? J Neurol Neurosurg Psychiatry 2016;87:461–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tiberio M, Chard DT, Altmann DR, et al. Gray and white matter volume changes in early RRMS: a 2-year longitudinal study. Neurology 2005;64:1001–1007. [DOI] [PubMed] [Google Scholar]
- 52.Cifelli A, Arridge M, Jezzard P, Esiri MM, Palace J, Matthews PM. Thalamic neurodegeneration in multiple sclerosis. Ann Neurol 2002;52:650–653. [DOI] [PubMed] [Google Scholar]
- 53.Batista S, Zivadinov R, Hoogs M, et al. Basal ganglia, thalamus and neocortical atrophy predicting slowed cognitive processing in multiple sclerosis. J Neurol 2012;259:139–146. [DOI] [PubMed] [Google Scholar]
- 54.Schoonheim MM, Popescu V, Rueda Lopes FC, et al. Subcortical atrophy and cognition: sex effects in multiple sclerosis. Neurology 2012;79:1754–1761. [DOI] [PubMed] [Google Scholar]
- 55.Bisecco A, Rocca MA, Pagani E, et al. Connectivity-based parcellation of the thalamus in multiple sclerosis and its implications for cognitive impairment: a multicenter study. Hum Brain Mapp 2015;36:2809–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Modica CM, Bergsland N, Dwyer MG, et al. Cognitive reserve moderates the impact of subcortical gray matter atrophy on neuropsychological status in multiple sclerosis. Mult Scler 2016;22:36–42. [DOI] [PubMed] [Google Scholar]
- 57.Bergsland N, Zivadinov R, Dwyer MG, Weinstock-Guttman B, Benedict RH. Localized atrophy of the thalamus and slowed cognitive processing speed in MS patients. Mult Scler 2016;22:1327–1336. [DOI] [PubMed] [Google Scholar]
- 58.Zivadinov R, Havrdova E, Bergsland N, et al. Thalamic atrophy is associated with development of clinically definite multiple sclerosis. Radiology 2013;268:831–841. [DOI] [PubMed] [Google Scholar]
- 59.Rocca MA, Mesaros S, Pagani E, Sormani MP, Comi G, Filippi M. Thalamic damage and long-term progression of disability in multiple sclerosis. Radiology 2010;257:463–469. [DOI] [PubMed] [Google Scholar]
- 60.Muhlau M, Buck D, Forschler A, et al. White-matter lesions drive deep gray-matter atrophy in early multiple sclerosis: support from structural MRI. Mult Scler 2013;19:1485–1492. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.