Abstract
Objective:
To determine whether the parkinsonian phenotype prevalent in welders is progressive, and whether progression is related to degree of exposure to manganese (Mn)-containing welding fume.
Methods:
This was a trade union–based longitudinal cohort study of 886 American welding-exposed workers with 1,492 examinations by a movement disorders specialist, including 398 workers with 606 follow-up examinations up to 9.9 years after baseline. We performed linear mixed model regression with cumulative Mn exposure as the independent variable and annual change in Unified Parkinson Disease Rating Scale motor subsection part 3 (UPDRS3) as the primary outcome, and subcategories of the UPDRS3 as secondary outcomes. The primary exposure metric was cumulative Mn exposure in mg Mn/m3-year estimated from detailed work histories.
Results:
Progression of parkinsonism increased with cumulative Mn exposure. Specifically, we observed an annual change in UPDRS3 of 0.24 (95% confidence interval 0.10–0.38) for each mg Mn/m3-year of exposure. Exposure was most strongly associated with progression of upper limb bradykinesia, upper and lower limb rigidity, and impairment of speech and facial expression. The association between welding exposure and progression appeared particularly marked in welders who did flux core arc welding in a confined space or workers whose baseline examination was within 5 years of first welding exposure.
Conclusions:
Exposure to Mn-containing welding fume may cause a dose-dependent progression of parkinsonism, especially upper limb bradykinesia, limb rigidity, and impairment of speech and facial expression.
High-level Mn exposure in occupational settings is associated with a severe movement disorder characterized by parkinsonism, dystonia, cognitive dysfunction, and behavioral disturbances.1–3 Exposures causing this phenotype are rarely seen in modern times, although drug addicts using Mn-contaminated ephedrone may have a similar phenotype.4 More recently, we have described a predominantly symmetric parkinsonian syndrome and associated impairments in quality of life in workers with contemporary occupational exposures to Mn-containing welding fume.5 Exposures in these workers are chronic and at levels below occupational exposure standards, and likely far below the exposures seen in IV ephedrone abusers.
Whether these Mn-induced neurologic clinical signs progressively worsen is unclear, since few studies have rigorously assessed patients longitudinally. In original reports of manganism, patients had a rapid progression to severe disability over a short period of time.1,2 Progression of clinical signs of manganism has been highly variable in other small studies.4,6,7 These small case series provide some evidence that patients with manganism have a progressive, likely degenerative, process. It is unclear if the more common parkinsonian phenotype, associated with lower-level chronic Mn exposure in contemporary workplaces, also worsens over time. Determining if these exposures are associated with progressive parkinsonism may provide insight into the pathophysiology of environmental neurotoxins and neurodegenerative diseases. To address this question, we performed longitudinal clinical follow-up in workers exposed to Mn-containing welding fume, to determine if they had dose-dependent progression of their signs of parkinsonism.
METHODS
Standard protocol approvals, registrations, and patient consents.
This study was approved by human subjects committees at Washington University in St. Louis, Missouri, and the University of Washington in Seattle. All participants provided written informed consent. Welders and other workers exposed to welding fume were recruited from 3 US Midwestern worksites, 2 shipyards and 1 heavy machinery fabrication shop, using union membership lists as detailed previously.5 No workers or retirees from these worksites were excluded from participation except as described below.
Clinical assessment.
One of 2 movement disorders–trained neurologists (B.A.R., S.R.C.) performed a neurologic examination that included the Unified Parkinson’s Disease Rating Motor Scale 3 (UPDRS3) on each participant,8 blind to workers' exposure history. Baseline examinations occurred from January 2006 to September 2013. Follow-up examinations occurred from April 2007 to February 2016. Examinations were conducted in local union halls near each worksite. Both examiners rated 10 Parkinson disease (PD) patient videos each year; the intraclass correlation coefficient for these UPDRS3 ratings was >0.90. For validation in the present occupational setting, timed motor task9,10 data were available to validate UPDRS3 scores for 73% of examinations in 70% of participants. As performance on the timed motor task decreased, UPDRS3 scores increased (p < 0.0005). After imputing 1 UPDRS3 subscore for 15 examinations and 2 to 5 subscores for 10 examinations, complete UPDRS3 data were available for all examinations.
Exposure assessment.
Workers reported a complete job history in person using a structured, validated questionnaire.11 We used this to estimate weighted welding years, as a measure of cumulative exposure to welding fume,5 which factors in both total duration of welding fume exposure and the intensity of that exposure as estimated from specific job title and classification for all jobs. This metric was designed to provide a relative estimate of cumulative Mn exposure.
To convert weighted welding exposure years to estimated mg Mn/m3-year, we multiplied weighted welding years by 0.14 mg/m3 Mn. We calculated this scalar based upon a model we developed and validated previously11,12 and detailed information on welding type and location, in welders, in the present cohort. The resulting mg Mn/m3-year exposure variable was available for all participants in the cohort. We validated the exposure variable using a gold standard exposure measurement, T1 signal intensity (pallidal index) on MRI,13,14 in a subset of 38 workers in the present cohort who were in a neuroimaging substudy. The pallidal index gold standard significantly increased with each additional mg Mn/m3-year.
We calculated cumulative exposure (mg Mn/m3-year) as of the baseline questionnaire to focus on exposure up to the baseline examination, since our primary hypothesis was that chronic exposure to Mn-containing welding fume would be associated with progression of parkinsonism. We anticipated that progression of parkinsonism after baseline examination could affect ability to continue welding (i.e., interim exposure more biased by the healthy worker survivor effect15). Therefore, our primary analysis excluded all interim exposures, but we considered effects of interim exposures in secondary analyses.
Statistical analysis.
We used a linear mixed model that accounted for dependence of the data to estimate the longitudinal association between cumulative Mn exposure and progression of parkinsonism. Our primary outcome variable was rate of change of UPDRS3 score (difference in adjusted UPDRS3 scores divided by time between examinations). In our primary analyses, we excluded follow-up examinations occurring within 1 year of baseline examination, since their small denominators resulted in highly variable rates of change. As secondary outcomes, we calculated annual rate of change of UPDRS3 subscores. For all models, we modeled exposure linearly, i.e., as a single continuous term. Because UPDRS3 scores were assessed by 2 examiners over 10 years, we adjusted raw scores for examiner and allowed for examiner by time (since January 2006) differences (interaction). Sensitivity analyses included (1) accounting for age, sex, race, tobacco smoking, alcohol consumption, and occupational exposure to pesticides; (2) weighting each subject in the linear mixed regression model by the inverse of the probability of being re-examined (estimated by a logistic regression model containing factors that predicted follow-up [table 1 footnotes]) to give more weight to workers with characteristics associated with loss to follow-up16; and (3) stratified analyses by total follow-up time and by total years of welding exposure prior to baseline examination to investigate the potential influence of the healthy worker survivor effect. For the latter, we tested for interaction on the multiplicative scale.
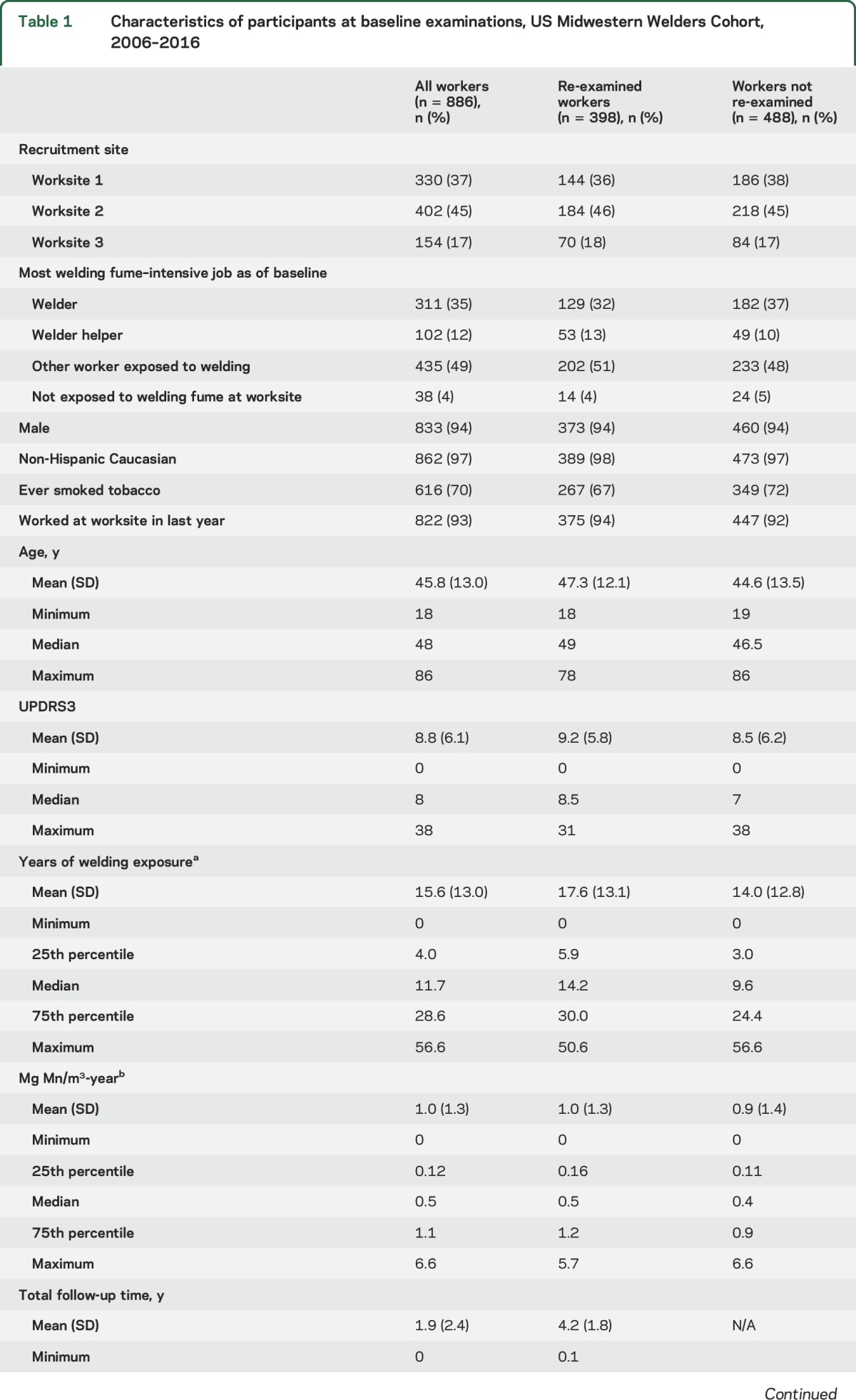
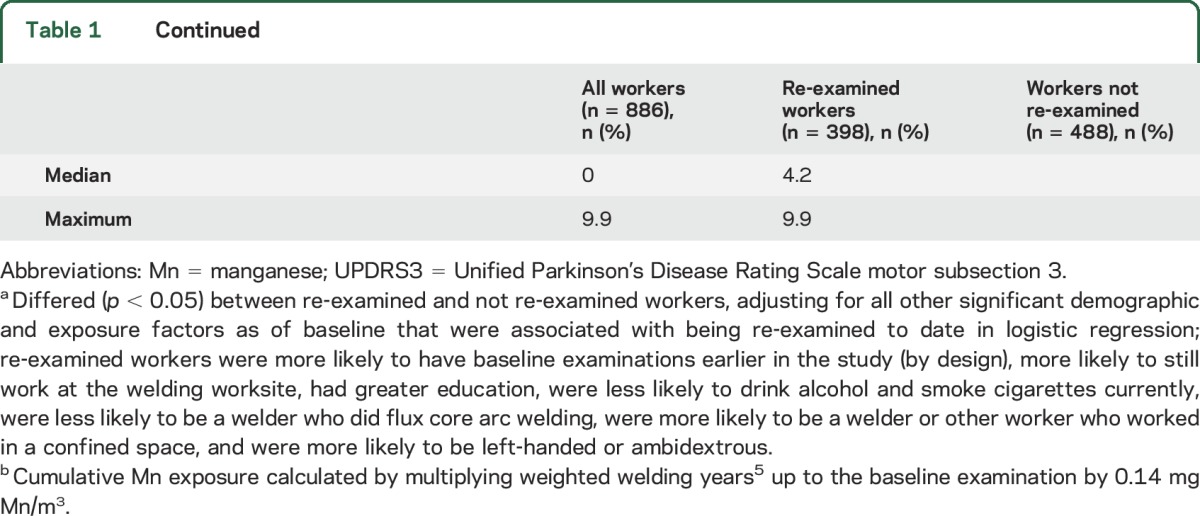
Table 1.
Characteristics of participants at baseline examinations, US Midwestern Welders Cohort, 2006–2016
RESULTS
Characteristics of study participants.
The cohort included 886 workers, reflecting the high percentage of 1,265 potentially eligible workers at study initiation who enrolled and a continuing influx of eligible workers to the study worksites over the course of the study. The study neurologists completed 1,537 UPDRS3 examinations on workers recruited at 1 of the 3 welding worksites. After excluding 45 examinations in workers with a history of a stroke, brain tumor, or other medical condition that would compromise the UPDRS3 score, 1,492 (97%) examinations in 886 workers were available. Parkinsonism was the predominant phenotype, with 135 (15.2%) having a UPDRS3 score ≥15. We found mild, asymptomatic limb dystonia in 4 workers and cervical dystonia in 1 worker. No worker had a cock gait, a neurologic sign considered to be pathognomonic of manganism.2,17 Six (0.7%) enrolled workers died during the course of this study, and none was reported to have had a neurodegenerative disorder at death.
Of the 886 workers, 398 (45%) already had at least 1 follow-up UPDRS3 examination to date, with a mean total follow-up time of 4.2 years (SD 1.8 years) and maximum of 9.9 years. Workers who have been re-examined thus far were very similar to the 488 who were not yet examined or lost to follow-up (table 1). Most of the re-examined workers were non-Hispanic white men, with a mean 17.6 (SD 13.1) years of welding exposure as of their baseline examination. Re-examined workers had an average of 1.0 (SD 1.3) mg Mn/m3-year of exposure at baseline.
The number of repeat examinations in these workers was as follows: 1 follow-up examination, 61%; 2 follow-up examinations, 26%; 3 follow-up examinations, 12%; and 4 or 5 follow-up examinations, 1%. Mean time between baseline examination and any follow-up examination was 3.9 years (SD 1.8). Fourteen follow-up examinations occurred less than 1 year after the baseline examination and were excluded in our primary analysis. Six examinations were excluded because stroke or multiple sclerosis occurred between baseline and follow-up examinations, leaving 592 examinations in 389 workers for this analysis.
Progression of parkinsonism.
The average annual change in UPDRS3 score was 0.32 (SD 2.1), across the entire followed cohort, but varied according to job type and cumulative Mn exposure. The average annual change in UPDRS3 score was 0.57 (SD 2.56) among welders, 0.45 (SD 1.88) among welder helpers, and 0.16 (SD 1.78) for workers around welding fume. There did not appear to be any progression among the small number of workers from the welding worksite reportedly not exposed to welding fume (20 follow-up examinations in 14 workers, average change −0.10, SD 1.39). In the mixed linear model, we observed an association between cumulative Mn exposure and annual change in UPDRS3 score, with each additional mg Mn/m3-year of exposure as of baseline conferring an estimated 0.24 UPDRS3 points (95% confidence interval [CI] 0.10–0.38) greater annual change in UPDRS3 score (p < 0.001) (table 2). This association was similar or stronger in sensitivity analyses adjusting for age at baseline, sex, race, tobacco smoking, alcohol consumption, and occupational pesticide exposure; weighting each subject by the reciprocal of the probability of being followed; or including follow-up examinations conducted <1 year after the baseline examination. In these sensitivity analyses, the point estimate for the annual change in UPDRS3 per mg Mn/m3-year ranged from 0.23 to 0.33. In contrast, when we extended the exposure estimate to include interim exposures that could exacerbate the healthy worker survivor effect, the association was slightly attenuated (0.20, 95% CI 0.07–0.33). In additional exploratory analyses, we observed that the strong, consistent association between cumulative Mn exposure and parkinsonism progression was influenced by workers with short follow-up time (e.g., ≤3 years) or welders and welder helpers who ever performed flux core arc welding (FCAW), especially in a confined space (table 2). The association between Mn exposure and parkinsonism progression did not as clearly differ by location alone (confined space vs not confined space; data not shown in tables). We did not consider differences according to other welding types, as FCAW generates markedly higher particulates than other welding processes.12 The association between Mn exposure and parkinsonism progression appeared to be exceptionally strong among workers first examined relatively soon after Mn exposure first began (e.g., ≤5 years) (table 2).
Table 2.
Annual change in Unified Parkinson’s Disease Rating Motor Scale 3 (UPDRS3) score (95% confidence interval) per mg Mn/m3-year,a overall and by selected worker characteristics, US Midwestern Welders Cohort, 2006–2016 (n = 981 examinations in 389 workers)b

When we examined which UPDRS3 subscores were associated with cumulative Mn exposure, we observed that progression of subscores for upper limb bradykinesia, upper and lower limb rigidity, speech, and facial masking increased with exposure (table 3). When we explored which subscores contributed to the remarkably large association between cumulative Mn and parkinsonism progression among workers first examined within 5 years of their initial Mn exposure, we observed that the annual increase in upper limb bradykinesia was particularly marked and statistically significant (data not shown).
Table 3.
Annual change in Unified Parkinson’s Disease Rating Scale motor subsection 3 subscoresa (95% confidence intervals) per mg Mn/m3-year,b US Midwestern Welders Cohort, 2006–2016 (n = 981 examinations in 389 workers)c

DISCUSSION
In this large cohort of workers from welding worksites, progression of parkinsonism increased as cumulative exposure to Mn-containing welding fume increased. In particular, progression of parkinsonism increased with exposure as of baseline examination, suggesting that some of these workers may possibly have a neurodegenerative pathophysiology. This is consistent with prior, small case series of patients with manganism, which observed that workers with parkinsonism had progression of their parkinsonian signs, despite removal from exposure.4,7 Our study is unique in that we followed a cohort of largely healthy welding-exposed workers longitudinally, over a relatively short period of time, and still were able to demonstrate progression of the parkinsonian phenotype.5 In addition, we used a clinically relevant scale for parkinsonism, and evaluations were conducted by movement disorders specialists. Moreover, the rates of progression were clinically meaningful, with average annual increases in UPDRS3 of more than 0.3 points in relation to welding exposure. Although some groups of workers influenced the magnitude of the association between progression and cumulative Mn exposure, a comprehensive set of rigorous sensitivity analyses consistently confirmed the overall association.
Workers who did some FCAW in confined spaces appeared to have particularly marked worsening of UPDRS3. This is notable because Mn concentration from welding is greatest in confined spaces, and FCAW is the welding process that generates the greatest levels of particulate matter.12 In addition, workers followed for less than 3 years had particularly marked progression; that is, parkinsonism progression appeared to weaken in relation to total follow-up time, as would be expected if we were able to follow the slowest progressing workers the longest. This is consistent with the presence of a healthy worker survivor effect, whereby workers with higher levels of exposure develop parkinsonism and then, as a result of their parkinsonism, drop out of the workforce.15 In the presence of the healthy worker survivor effect, we may be underestimating the magnitude of the association. We explored the effect of weighting by the inverse probability of being followed, to attempt to address bias from the healthy worker survivor effect (and other potential biases related to loss to follow-up). However, this weighting could not address the fact that the majority of the workers had already survived substantial exposure as of the baseline examination. This is important given the particularly strong association observed among the small group of workers who had baseline examinations relatively soon after first becoming exposed. Since our results were resilient to numerous sensitivity analyses and we observed some evidence of muting due to the healthy worker survivor effect, this study provides compelling evidence that Mn-containing welding fume can result in progressive parkinsonism.
The progression of parkinsonism in this welder cohort was largely due to upper limb bradykinesia and limb rigidity. Progression of parkinsonian speech and facial expression abnormalities also contributed. In a previous study, we found that welders with parkinsonism had worse quality of life than welders without parkinsonism, with the strongest associations with activities of daily living (ADLs) and speech.18 The effect of Mn exposure on ADLs would be consistent with our finding of a strong relationship between Mn exposure and progression of upper limb bradykinesia and rigidity. The speech finding is intriguing given that early reports of manganism described profound speech impairment.2
We previously found that the phenotype of parkinsonian welders overlapped with that of patients with early PD,5 as did the quality of life disturbance.18 We have also demonstrated that welders with only mild parkinsonian signs have reduced uptake of the radioligand 6-[18F]fluoro-l-DOPA in the caudate nucleus, using PET, indicating presynaptic nigrostriatal dopamine dysfunction. Taken together, this current study and previous studies suggest that Mn-exposed welders have a dose-dependent, progressive parkinsonian syndrome, as well as presynaptic dopaminergic dysfunction. While this does not necessarily indicate that these workers have PD, these combined studies suggest that Mn-exposed workers may provide a useful model for environmental basal ganglia neurodegeneration and could provide some insight into common pathogenic mechanisms.
Occupational Mn exposure limits have been gradually reduced based upon data linking low-level occupational Mn exposures to adverse health outcomes. We observed particularly marked progression among welders who reported ever having a job that entailed FCAW in a confined space. Previous studies of welders indicate that FCAW produced markedly greater particulate and greater Mn concentration than other welding types.12,19 The American Conference of Governmental Industrial Hygienists (ACGIH) threshold limit value (TLV) for Mn is 0.2 mg Mn/m3 and was based upon neurologic health effects.20–22 While we do not know the actual peak air Mn concentration experienced by workers who performed FCAW in our study, the ACGIH TLV is higher than the mean time-weighted Mn concentration (0.14 mg Mn/m3) to which we estimate the welders and welder helpers in this cohort were exposed. We cannot exclude an effect of other metals in FCAW fume, particulate in general, or other coexposures, notably paint and solvents used as degreasing agents. However, these are mainly handled by workers who are around welding rather than by the welders themselves.
As with any study, there are some limitations. We were unable to measure workers' cumulative Mn exposure directly, since there are no reliable biomarkers of cumulative Mn exposure that can be applied to such a large cohort. In addition, even though there are regulatory limits for US workplace Mn exposures, employers are not required to monitor workers, so monitoring data from these worksites were limited, thus we relied on exposure modeling of job history information. While the UPDRS3 was designed to monitor progression of PD in clinical trials, this scale does not measure other clinical features commonly cited as indicative of the clinical Mn neurotoxicity spectrum, such as dystonia and cognitive dysfunction. However, all workers were examined by a movement disorders expert; dystonia was rare, and no participant had a cock gait. Finally, our estimate of a 0.24 annual increase in UPDRS3 score for each mg Mn/m3-year should be used with caution when attempting to predict progression for individuals, given that results were markedly influenced by some worker groups and the association may not be linear. We observed a remarkably large progression of parkinsonism among workers whose baseline UPDRS3 examination was within 5 years of first exposure to Mn. A major contributor to this association was upper limb bradykinesia, which we hypothesize would have the greatest effect on worker dexterity and work performance. This may reflect the healthy worker survivor effect. Alternatively, the association between cumulative Mn exposure and progression of parkinsonism, which we modeled linearly, may be nonlinear, with more marked worsening of UPDRS3 in the initial years, rather than later years of exposure. Even if parkinsonism progression is linear, the rate of change in UPDRS3 we estimated should not be extrapolated beyond 10 years of follow-up time, since we did not follow any workers beyond that period. However, even after only 10 years of follow-up, the overall increase in UPDRS3 score due to welding fume exposure, particularly among welders themselves, is predicted to be clinically meaningful (figure).
Figure. Increase in Unified Parkinson’s Disease Rating Motor Scale 3 (UPDRS3) score due to welding fume exposure by years as a welder.
UPDRS3 increases with each additional year of work as a welder (mg [Mn]/m3-year exposure) as of the baseline examination. Among workers whose welding fume exposure was consistently as a welder, mg Mn/m3-year exposure is proportional to the number of years worked as a welder. A worker who had been a welder for 20 years prior to the baseline examination, for example, would have had an estimated 2.8 mg Mn/m3-year exposure and would be predicted to have nearly a 7-point increase in UPDRS3 score related to that welding fume exposure. This exposure estimate assumes a Mn concentration of 0.14 mg Mn/m3. CI = confidence interval.
Cumulative Mn exposure due to occupational exposure to welding fume at estimated Mn concentrations near some regulatory thresholds appeared to increase progression of parkinsonism in a dose-dependent manner. More stringent workplace monitoring of Mn exposures, greater use of personal protective equipment and ventilation, and systematic worker assessment may be indicated to reduce morbidity.
Supplementary Material
GLOSSARY
- ACGIH
American Conference of Governmental Industrial Hygienists
- ADL
activities of daily living
- CI
confidence interval
- FCAW
flux core arc welding
- Mn
manganese
- PD
Parkinson disease
- TLV
threshold limit value
- UPDRS3
Unified Parkinson's Disease Rating Motor Scale 3
Footnotes
Editorial, page 338
AUTHOR CONTRIBUTIONS
Brad A. Racette supervised data collection, oversaw data analysis, and wrote the first draft of the manuscript. Susan Searles Nielsen performed data analysis and edited the manuscript. Susan R. Criswell assisted with data collection and edited the manuscript. Lianne Sheppard oversaw data analysis and edited the manuscript. Noah Seixas provided occupational hygiene expertise for the exposure reconstruction and edited the manuscript. Mark N. Warden performed data analysis. Harvey Checkoway contributed to the study design and edited the manuscript. Statistical analysis was conducted by Susan Searles Nielsen, Mark N. Warden, and Lianne Sheppard. Dr. Racette takes full responsibility for the data, the analyses and interpretation, and the conduct of the research; he has full access to all of the data; and he has the right to publish any and all data separate and apart from any sponsor.
STUDY FUNDING
Study funded by the National Institute for Environmental Health Sciences (R01ES021488, K24ES017765, P42ES004696, R01ES021488, K23ES021444), the Michael J. Fox Foundation, National Institute of Neurologic Disorders and Stroke National Center for Research Resources, and NIH Roadmap for Medical Research grant UL1 RR024992.
DISCLOSURE
B. Racette: research support from Teva (PI), Adamas Pharmaceuticals (PI), Auspex Pharmaceuticals (PI), Eisai (PI), Allergan (PI), Merz Pharmaceuticals GmbH (PI), Pfizer (PI), Civitas Therapeutics (PI), Kyowa Hakko Kinn Pharma (PI), and AbbVie (PI); government research support from NIH (K24ES017765 [PI], R21ES17504 [PI], R01ES021488 [PI], R01ES021488-02S1 [PI], P42ES004696 [co-I]); and research support from the Michael J. Fox Foundation. S. Searles Nielsen: government research support from NIH (P42ES004696). S. Criswell: research support from the American Parkinson Disease Association (APDA), Merck (PI), Chiltern (Sub-I), TEVA (Sub-I); research support from TEVA (PI), Medivation (PI), Biotie (PI), MERZ (PI), Pfizer (PI), ADAMAS (PI), Accorda Therapeutics (PI), Allergan (PI), Solstice Neurosciences (Sub-I), NIH (KL2 RR024994, R01 ES013743-01A2, K23ES021444-01); and consultant for Analysis Group. L. Sheppard: government research support from NIH (P42ES004696). N. Seixas: government research support from NIH (P42ES004696). M. Warden reports no disclosures relevant to the manuscript. H. Checkoway: government research support from NIH (P42ES004696). Go to Neurology.org for full disclosures.
REFERENCES
- 1.Couper J. On the effects of black oxide of manganese when inhaled into the lungs. Br Ann Med Pharmacol 1837;1:41–42. [Google Scholar]
- 2.Rodier J. Manganese poisoning in Moroccan miners. Br J Ind Med 1955;12:21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang JD, Huang CC, Hwang YH, Chiang JR, Lin JM, Chen JS. Manganese induced parkinsonism: an outbreak due to an unrepaired ventilation control system in a ferromanganese smelter. Br J Ind Med 1989;46:856–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Selikhova M, Fedoryshyn L, Matviyenko Y, et al. Parkinsonism and dystonia caused by the illicit use of ephedrone: a longitudinal study. Mov Disord 2008;23:2224–2231. [DOI] [PubMed] [Google Scholar]
- 5.Racette BA, Criswell SR, Lundin JI, et al. Increased risk of parkinsonism associated with welding exposure. Neurotoxicology 2012;33:1356–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang CC, Lu CS, Chu NS, et al. Progression after chronic manganese exposure. Neurology 1993;43:1479–1483. [DOI] [PubMed] [Google Scholar]
- 7.Huang CC, Chu NS, Lu CS, Chen RS, Calne DB. Long-term progression in chronic manganism: ten years of follow-up. Neurology 1998;50:698–700. [DOI] [PubMed] [Google Scholar]
- 8.Fahn S, Elton RL; Members of the UPDRS Development Committee. Unified Parkinson's Disease Rating Scale. In: Fahn S, Marsden CD, Goldstein M, Calne DB, eds. Recent Developments in Parkinson's Disease. New York: Macmillan; 1987:153–163. [Google Scholar]
- 9.Parkinson's Study Group. DATATOP: a multicenter clinical trial in early Parkinson's disease. Arch Neurol 1989;46:1052–1060. [DOI] [PubMed] [Google Scholar]
- 10.Criswell S, Sterling C, Swisher L, Evanoff B, Racette BA. Sensitivity and specificity of the finger tapping task for the detection of psychogenic movement disorders. Parkinsonism Relat Disord 2010;16:197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hobson AJ, Sterling DA, Emo B, et al. Validity and reliability of an occupational exposure questionnaire for parkinsonism in welders. J Occup Environ Hyg 2009;6:324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hobson A, Seixas N, Sterling D, Racette BA. Estimation of particulate mass and manganese exposure levels among welders. Ann Occup Hyg 2011;55:113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Criswell SR, Perlmutter JS, Huang JL, et al. Basal ganglia intensity indices and diffusion weighted imaging in manganese-exposed welders. Occup Environ Med 2012;69:437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dietz MC, Ihrig A, Wrazidlo W, Bader M, Jansen O, Triebig G. Results of magnetic resonance imaging in long-term manganese dioxide-exposed workers. Environ Res 2001;85:37–40. [DOI] [PubMed] [Google Scholar]
- 15.Pearce N, Checkoway H, Kriebel D. Bias in occupational epidemiology studies. Occup Environ Med 2007;64:562–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seaman SR, White IR. Review of inverse probability weighting for dealing with missing data. Stat Methods Med Res 2013;22:278–295. [DOI] [PubMed] [Google Scholar]
- 17.Huang CC, Chu NS, Lu CS, Calne DB. Cock gait in manganese intoxication. Mov Disord 1997;12:807–808. [DOI] [PubMed] [Google Scholar]
- 18.Harris RC, Lundin JI, Criswell SR, et al. Effects of parkinsonism on health status in welding exposed workers. Parkinsonism Relat Disord 2011;17:672–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baker MG, Simpson CD, Stover B, et al. Blood as an exposure biomarker: state of the evidence. J Occup Environ Hyg 2014;11:210–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Conference of Governmental Industrial Health. Documentation of TLVs. Cincinnati: ACGIH; 1992. [Google Scholar]
- 21.Korczynski RE. Occupational health concerns in the welding industry. Appl Occup Environ Hyg 2000;15:936–945. [DOI] [PubMed] [Google Scholar]
- 22.Susi P, Goldberg M, Barnes P, Stafford E. The use of a task-based exposure assessment model (T-BEAM) for assessment of metal fume exposures during welding and thermal cutting. Appl Occup Environ Hyg 2000;15:26–38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



