Abstract
Objective:
To compare refractory convulsive status epilepticus (rSE) management and outcome in children with and without a prior diagnosis of epilepsy and with and without a history of status epilepticus (SE).
Methods:
This was a prospective observational descriptive study performed from June 2011 to May 2016 on pediatric patients (1 month–21 years of age) with rSE.
Results:
We enrolled 189 participants (53% male) with a median (25th–75th percentile) age of 4.2 (1.3–9.6) years. Eighty-nine (47%) patients had a prior diagnosis of epilepsy. Thirty-four (18%) patients had a history of SE. The time to the first benzodiazepine was similar in participants with and without a diagnosis of epilepsy (15 [5–60] vs 16.5 [5–42.75] minutes, p = 0.858). Patients with a diagnosis of epilepsy received their first non-benzodiazepine (BZD) antiepileptic drug (AED) later (93 [46–190] vs 50.5 [28–116] minutes, p = 0.002) and were less likely to receive at least one continuous infusion (35/89 [39.3%] vs 57/100 [57%], p = 0.03). Compared to patients with no history of SE, patients with a history of SE received their first BZD earlier (8 [3.5–22.3] vs 20 [5–60] minutes, p = 0.0073), although they had a similar time to first non-BZD AED (76.5 [45.3–124] vs 65 [32.5–156] minutes, p = 0.749). Differences were mostly driven by the patients with an out-of-hospital rSE onset.
Conclusions:
Our study establishes that children with rSE do not receive more timely treatment if they have a prior diagnosis of epilepsy; however, a history of SE is associated with more timely administration of abortive medication.
Convulsive status epilepticus (SE) is one of the most common pediatric emergencies. It affects approximately 10–25/100,000 children per year,1–3 while posing a substantial economic burden to individuals and society.4,5 Pediatric SE is associated with a short-term mortality of 0%–3%1,6–8 and long-term mortality of approximately 7%.9 Survivors often experience both functional and neurobehavioral sequelae7,8,10 and a reduced quality of life.11 When SE does not respond to initial medications, it is then considered refractory SE (rSE) and its outcome is markedly worse.12
Among the main prognostic factors of SE, time to treatment administration is the most amenable to improvement. Both adults13–17 and children17–20 receive antiepileptic drugs (AEDs) later than recommended by most SE guidelines.21–23 Most episodes of pediatric SE occur in children with no history of seizures.1,19 However, there are no data on whether having a history of seizures or SE results in more timely treatment—including initial abortive medication and escalation of therapies—and better outcomes.
The aim of this study is to address these gaps in knowledge by comparing the management and outcome of rSE in children with and without a prior diagnosis of epilepsy and in children with and without a history of SE.
METHODS
Standard protocol approvals, registrations, and patient consents.
This study was approved by the institutional review board at each participating institution. Written informed consent was obtained from the parents or guardians of each participant.
Study design.
The current study is a prospective observational study. The pediatric Status Epilepticus Research Group (pSERG) is a network of 11 pediatric hospitals in the United States that prospectively collects data on children with SE with the goal of delineating strategies for improving management and prognosis.24
Patients.
We studied patients with rSE in order to asses all of the steps in the management pathway of SE. Inclusion criteria were (1) age from 1 month to 21 years; (2) admission to a pSERG institution between June 1, 2011, and May 1, 2016; and (3) focal or generalized convulsive seizures at onset that continued after administration of at least 2 AEDs, including at least one non-benzodiazepine (BZD) AED or the use of a continuous infusion to treat SE. There was no time threshold to define rSE. Exclusion criteria were (1) nonconvulsive SE detected on EEG lacking convulsive seizure at onset and (2) nonconvulsive SE with motor manifestations limited to infrequent myoclonic jerks. If more than one episode of rSE occurred during the study period, only the first episode was included. The clinical presentation, EEG findings (both ictal and postictal), and follow-up data were not consistent with psychogenic nonepileptic seizures in any of our patients. pSERG centers usually follow published guidelines on SE treatment, but there is no common management protocol: decisions are made based on clinical judgement by the individual treating teams. Detailed data on time to treatment of the first 81 patients in this population have been reported.19
Outcome variables.
The primary descriptive measures for rSE management were the time from seizure onset to the administration of the first BZD, first non-BZD AED, and first continuous infusion. The primary outcome measures were duration of intensive care unit (ICU) stay, duration of convulsive rSE (clinical end of convulsive seizures), proportion of children who returned to pre-rSE baseline at hospital discharge (as evaluated clinically), and in-hospital mortality. The time to AED administration was based on information from families and emergency medical services (EMS) for out-of-hospital seizure onset and from provider documentation and medical records. A prior diagnosis of epilepsy was based on the clinical diagnosis as provided by the individual center. Data were collected with a standardized data acquisition tool and then entered into an electronic database hosted by Cincinnati Children's Hospital Medical Center. Details of the pSERG consortium have been published.24 Unless stated otherwise, continuous variables are provided as median (25th–75th percentile [p25–p75]) and categorical variables are provided as number (percentage).
Statistical analysis.
Demographic and clinical characteristics were summarized with descriptive statistics. We compared nonparametric continuous variables with the Wilcoxon rank-sum test and categorical variables with the Fisher exact test. Time to administration of AEDs was described in a time-to-event analysis and compared with the Gehan-Breslow-Wilcoxon test, which is more sensitive than the log-rank test to differences between groups that occur early and less sensitive to later points in time (likely outliers in this dataset). Given that the location of SE onset—in-hospital vs out-of-hospital—is a potential effect modifier, we supplemented our analysis of the entire study population with a stratified analysis of patients with in-hospital and out-of-hospital SE onset. A conventional 2-sided α level was set at 0.05. We controlled for multiple comparisons with the Benjamini and Hochberg false discovery rate for each subgroup of comparisons (epilepsy vs no epilepsy and SE vs no SE) using a threshold of 0.05.25 The main goal of false discovery rate control is to set significance levels for a collection of tests in such a way that among tests declared significant the proportion of true null hypotheses is lower than a specified threshold: 5% in this case.25 All statistical analyses were performed with R (version 3.2.2) language and environment for statistical computing (R Core Team [2015]; R Foundation for Statistical Computing, Vienna; R-project.org/)26 and the gmodels,27 gdata,28 and survival29 packages.
RESULTS
Study population.
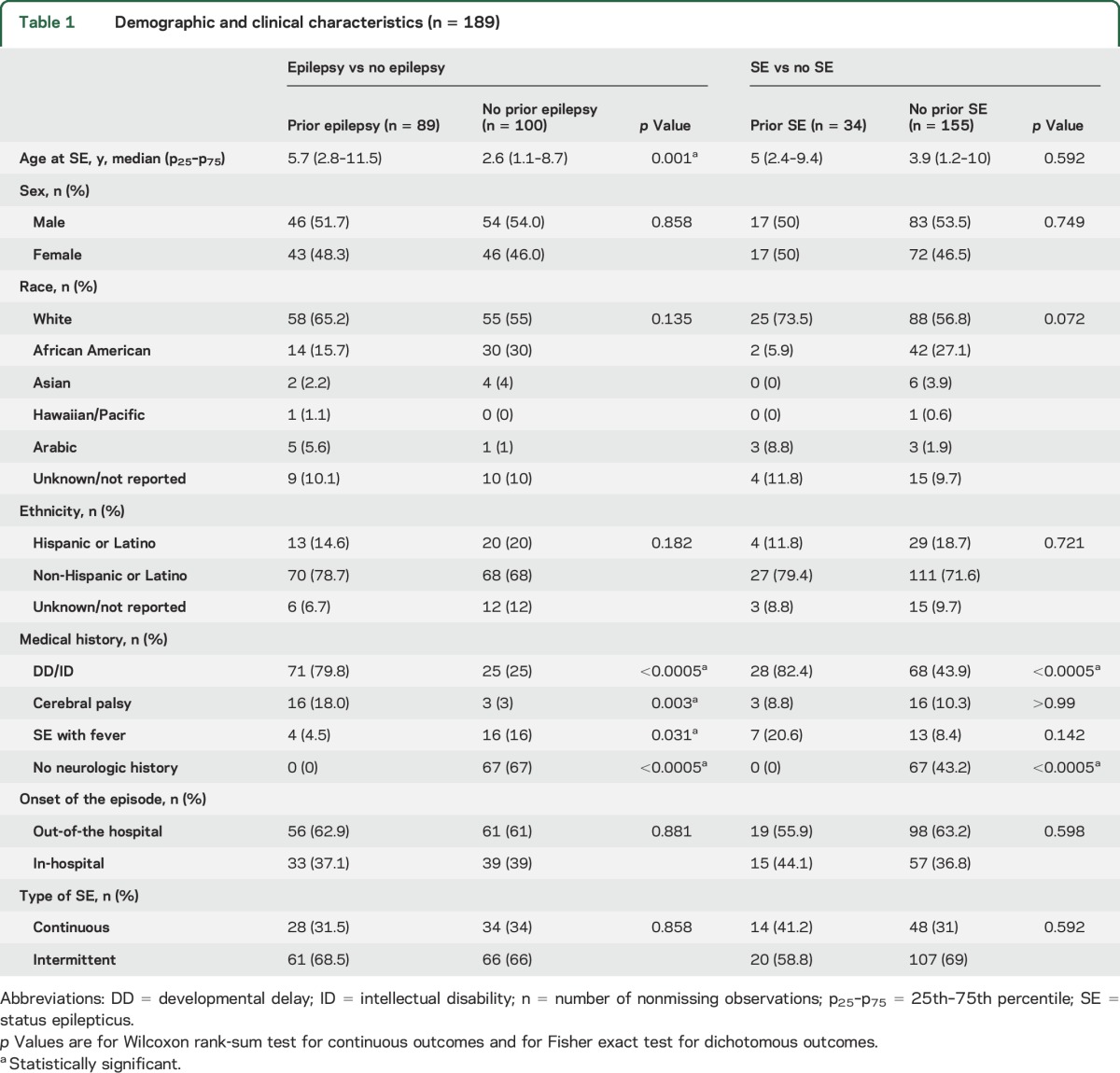
We enrolled 189 participants (53% male) with a median (p25–p75) age of 4.2 (1.3–9.6) years. Eighty-nine (47%) patients had a prior diagnosis of epilepsy. The most common seizure types were tonic-clonic in 89 patients, complex partial in 43 patients, and tonic in 18 patients. Thirty-four (18%) patients had a history of SE (28 had a prior diagnosis of epilepsy and 6 had a history of provoked SE). The main demographic and clinical features of the study cohort are presented in table 1, as well as tables e-1 and e-2 at Neurology.org.
Table 1.
Demographic and clinical characteristics (n = 189)
Comparison of patients with and without a diagnosis of epilepsy.
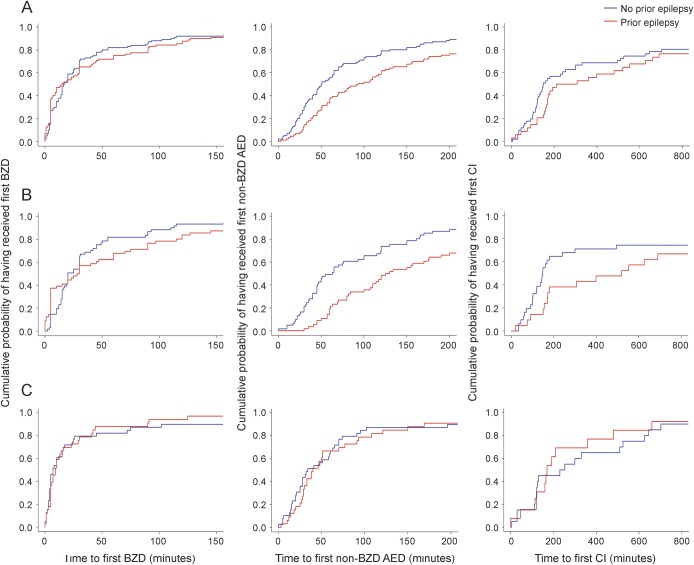
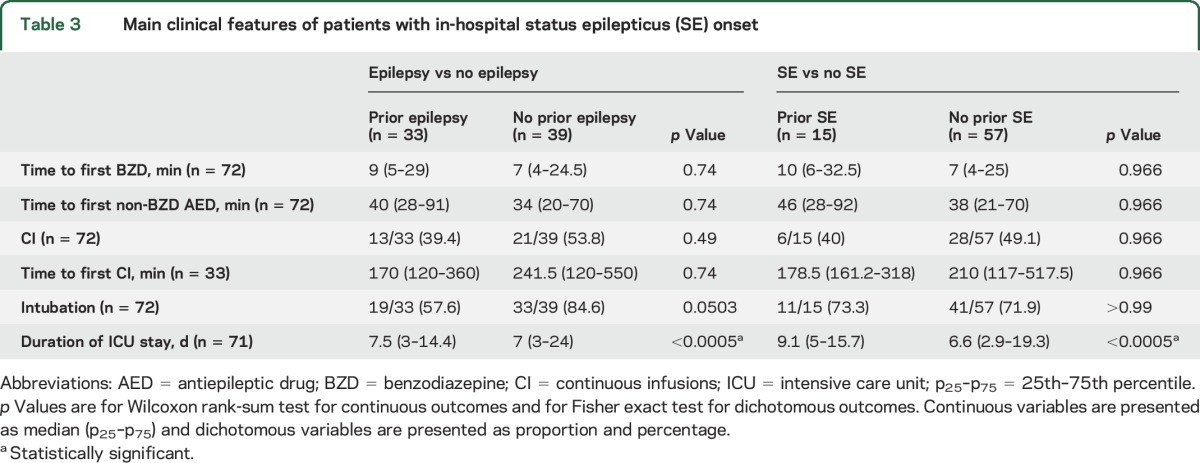
The time to the first BZD was similar in participants with and without a diagnosis of epilepsy (15 [5–60] vs 16.5 [5–42.75] minutes, p = 0.858). Patients with a diagnosis of epilepsy received their first non-BZD AED later (93 [46–190] vs 50.5 [28–116] minutes, p = 0.002) and were less likely to receive at least one continuous infusion (35/89 [39.3%] vs 57/100 [57%], p = 0.03), although the time to the first continuous infusion was similar in participants with and without a diagnosis of epilepsy (258.5 [156.2–682.5] vs 149 [107–575] minutes, p = 0.182) (figure 1A). Patients with a diagnosis of epilepsy were less likely to be intubated (59/89 [66.3%] vs 83/100 [83%], p = 0.026), had a longer duration of SE (174 [88–360] vs 120 [46–245] minutes, p < 0.0005), and had a shorter ICU stay (3.9 [2–9.7] vs 5 [2–17] days, p < 0.0005). At hospital discharge, patients with a diagnosis of epilepsy were more likely to return to baseline (67/89 [75%] vs 65/100 [65%], p = 0.027) and had lower mortality (0/89 [0%] vs 7/100 [7%], p = 0.015). The tendency towards a less timely treatment of SE in patients with a diagnosis of epilepsy was mainly driven by the episodes with an out-of-hospital onset (table 2, figure 1B), while management of SE with in-hospital onset was similar in patients with and without a diagnosis of epilepsy (table 3, figure 1C).
Figure 1. Cumulative probability curves representing time to receive medications for status epilepticus (SE) comparing patients with and without epilepsy.
The left column represents the time to receive the first benzodiazepine (BZD), the middle column the time to receive the first non-BZD antiepileptic drug (AED), and the right column the time to receive the first continuous infusion (CI). (A) Whole population. (B) Onset of SE out of the hospital. (C) Onset of SE in the hospital. X-axis arbitrarily truncated at 150 minutes (time to first BZD), at 200 minutes (time to first non-BZD AED), and at 800 minutes (time to first CI) for clarity (outliers not shown in the figure, but evaluated for its calculation).
Table 2.
Main clinical features of patients with an out-of-hospital status epilepticus (SE) onset

Table 3.
Main clinical features of patients with in-hospital status epilepticus (SE) onset
Comparison of patients with and without a history of SE.
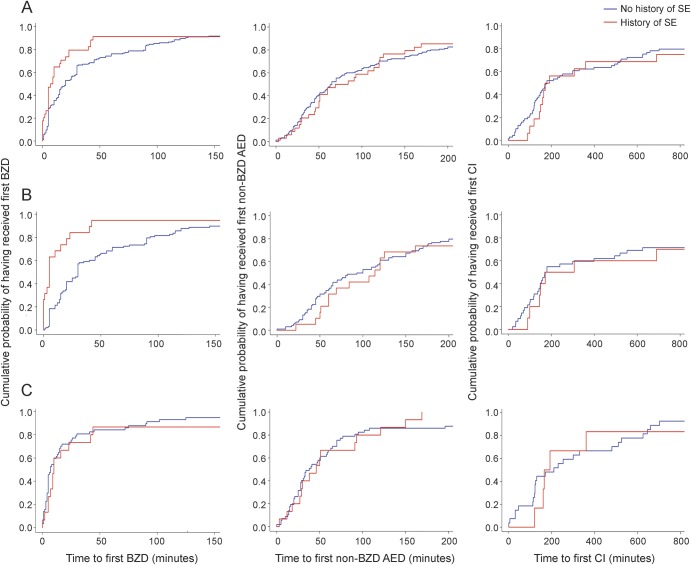
Patients with a history of SE received their first BZD earlier (8 [3.5–22.3] vs 20 [5–60] minutes, p = 0.0073), although the time to first non-BZD AED was similar in patients with and without a history of SE (76.5 [45.3–124] vs 65 [32.5–156] minutes, p = 0.749). Patients with and without a history of SE were as likely to receive continuous infusions (18/34 [52.9%] vs 74/155 [47.7%], p = 0.749), had a similar time to the first continuous infusion (182 [148.8–792] vs 180 [114–626] minutes, p = 0.592) (figure 2A), and were as likely to be intubated (28/34 [82.4%] vs 114/155 [73.5%], p = 0.592). Patients with a history of SE had a longer duration of SE (150 [83.3–285] vs 124.5 [57.5–360] minutes, p < 0.0005) and a shorter ICU stay (5 [3–11.3] vs 4.3 [2–12] days, p < 0.0005). At hospital discharge, return to baseline function was similar between patients with and without a history of SE (26/34 [76.5%] vs 106/155 [68.4%], p = 0.142). All deaths occurred in patients with no history of SE, but the difference was not statistically significant (0/34 [0%] vs 7/155 [4.5%], p = 0.355). The more timely administration of the first BZD in patients with a history of SE was mainly driven by the episodes with an out-of-hospital onset (table 2, figure 2B), while management of SE with in-hospital onset was similar in patients with and without a history of SE (table 3, figure 2C).
Figure 2. Cumulative probability curves representing time to receive medications for status epilepticus (SE) comparing patients with and without a history of SE.
The left column represents the time to receive the first benzodiazepine (BZD), the middle column the time to receive the first non-BZD antiepileptic drug (AED), and the right column the time to receive the first continuous infusion (CI). (A) Whole population. (B) Onset of SE out of the hospital. (C) Onset of SE in the hospital. X-axis arbitrarily truncated at 150 minutes (time to first BZD), at 200 minutes (time to first non-BZD AED), and at 800 minutes (time to first CI) for clarity (outliers not shown in the figure, but evaluated for its calculation).
DISCUSSION
Our data demonstrate that, among children with rSE, those with a diagnosis of epilepsy do not receive more timely treatment than those without. In contrast, those with a history of SE (not only of seizures) receive a more timely administration of abortive medication than children without a history of SE. In our series, differences were mainly driven by rSE episodes with an out-of-hospital onset. Despite less timely management, patients with a diagnosis of epilepsy spend less time in the ICU and are more likely to return to baseline and have lower mortality at hospital discharge.
Current literature recommends rapid administration of AEDs for seizures lasting more than 5 minutes and a rapid escalation between different classes of AEDs if needed.21,23,30,31 Animal models of SE have shown that prolonged seizures cause brain damage independent from the underlying etiology32 and clinical studies have shown that prolonged seizures are associated with worse outcomes.14,33–37 Marked delays to AED administration are well-documented. In a retrospective study of 889 patients (625 adults and 264 children) with SE, approximately 60% of the patients received their first AED after 30 minutes and approximately 25% after 60 minutes.17 In a series of 542 patients with convulsive seizures of at least 10 minutes duration, the median (p25–p75) time from hospital arrival to administration of a non-BZD AED was 24 (15–36) minutes.18 The study with the initial 81 patients in the pSERG consortium showed that the time elapsed since SE onset to administration of the first, second, and third AEDs were 28 (6–67) minutes, 40 (20–85) minutes, and 59 (30–120) minutes, respectively.19 Different endpoints and methodologies complicate direct comparisons, but together, these data suggest that the timeliness of SE treatment needs improvement.
While treatment delays in SE are well documented, there are no data on what causes these delays. The present study aimed to understand whether a diagnosis of epilepsy or a history of SE modifies treatment timeliness. One might surmise that the families and the health care environment of patients with a diagnosis of epilepsy would be familiar with SE management and, therefore, would give more timely treatment. Contrary to our expectations, we found that patients with a diagnosis of epilepsy were assisted by EMS later, arrived to the hospital later, and received the first non-BZD AED later than patients with no diagnosis of epilepsy. Specifically, it took approximately twice as much time to receive that management in patients with a diagnosis of epilepsy. These results may reflect that families and health care providers of patients with epilepsy are less disturbed by seizures, have difficulty recognizing SE, and only activate the EMS or present to the hospital when seizures are far more prolonged than usual. In contrast, when a child without a diagnosis of epilepsy has a seizure, the families and health care providers might provide more timely treatment. Patients with a history of SE received their first BZD almost 3 times as quickly (8 vs 20 minutes) than patients without a history of SE. This difference was largely driven by patients with out-of-hospital SE onset, who received their first BZD 6 times faster (5 vs 30 minutes) when they had a history of SE. These results suggest that it is the experience of a prior episode of SE and not the history of epilepsy that drives more timely treatment of such patients. To further support this point, patients with a diagnosis of epilepsy were less likely to receive at least one continuous infusion (39% vs 57%), were less likely to be intubated (66% vs 83%), and had a longer duration of SE (174 vs 120 minutes). These findings suggest that delays in treatment are not attributable to lack of access to treatment but to an insufficiently timely treatment of a prolonged seizure in the already epileptic patient. Education about SE as a life-threatening emergency might help improve times to treatment. In particular, our results suggest that a more detailed discussion with patients on when to intervene with rescue medication and individualized seizure action plans may improve times to treatment.
Although we cannot necessarily infer causality, the literature supports that longer delays to treatment are associated with longer SE duration. In a series of 45 episodes of generalized convulsive SE in children, the duration of SE was shorter (32 vs 60 minutes) in the 19 episodes treated with out-of-hospital diazepam than in the 26 episodes without prehospital treatment.30 A study of 182 children with convulsive SE evaluated the effect of delays: for each minute from seizure onset to arrival at the emergency room, there was a 5% cumulative increase in the risk of SE lasting more than 60 minutes.34 In a previous pSERG study, the longer the delays to the first, second, and third AED, the longer the duration of SE.19
Despite having more prolonged SE episodes, patients with a diagnosis of epilepsy were more likely to return to baseline function at hospital discharge and to have lower in-hospital mortality. The reasons for better short-term outcomes in patients with a prior diagnosis of epilepsy are unknown. Some had argued that, as there is less risk for poor outcome in patients with prior epilepsy, prompt treatment is not as crucial as in patients with no prior epilepsy.38 A more reasonable interpretation is that among patients who present with rSE with no prior epilepsy, there are a higher proportion of acute etiologies with a worse prognosis. Also, the baseline functional condition of patients with a diagnosis of epilepsy is likely worse (and easier to return to) than for patients with a normal functional baseline, as reflected in the higher rate of intellectual disability and cerebral palsy in patients with a diagnosis of epilepsy. Our results are concordant with prior literature that suggests that mortality in SE depends largely on the underlying etiology and is higher in children without prior epilepsy, who are more likely to have an acute symptomatic etiology.8,39,40
Our study describes management and outcome in 189 cases of pediatric rSE, which to our knowledge places this series among the largest in the current literature.1,34 Our population is not necessarily representative of all children with epilepsy or with SE, as our cohort received care for rSE in tertiary care reference centers. In order to gather a large enough population of children with rSE, our study enrolled patients from reference hospitals with greater volume. Episodes of SE that occur in the community and never reach large tertiary care hospitals could not be studied with this approach. However, to evaluate all stages of SE management and compare large populations, a multicenter study in reference centers is necessary. Further, this study focuses on convulsive rSE and did not consider nonconvulsive SE. Times were assessed based on family and EMS information for out-of-hospital onset and from provider information and hospital records once in the hospital. We cross-referenced information on times with families, EMS, nurses, and medication administration records when available to reduce potential information and recall bias.
We accounted for multiple comparisons using false discovery rate adjustment. Our preplanned data analysis showed a consistent tendency, frequently reaching statistical significance, towards less aggressive treatment of SE in patients with a diagnosis of epilepsy. Where the SE started—in or out of the hospital—is a potentially important effect modifier if unevenly distributed between patients with and without epilepsy. Our large sample size allowed a stratification of results by location of SE onset.
Children who experience rSE and have a diagnosis of epilepsy are treated less timely than children without a diagnosis of epilepsy. Our data show that children with a history of SE are treated more aggressively than children with no history of SE. We do not have data on what causes less timely treatment in patients with a diagnosis of epilepsy. We can only hypothesize that in patients with no prior diagnosis of epilepsy, the protocol for SE is implemented as soon as possible. In contrast, in patients with a diagnosis of epilepsy the treatment is likely based on prior therapies or the neurologist's opinion. Despite this lack of knowledge, our results clearly identify specific steps in SE management (e.g., arrival to the hospital, time to initiation of non-BZD AEDs) and specific populations (e.g., patients with a diagnosis of epilepsy) in which to focus education and policies for timely treatment of prolonged seizures.
Our study establishes that children with rSE do not receive more timely treatment if they have a diagnosis of epilepsy and it is the history of SE, not seizures, that is associated with a more timely administration of abortive medication. These differences are mainly driven by SE episodes with an out-of-hospital onset.
Supplementary Material
GLOSSARY
- AED
antiepileptic drug
- BZD
benzodiazepine
- EMS
emergency medical services
- ICU
intensive care unit
- p25–p75
25th–75th percentile
- pSERG
pediatric Status Epilepticus Research Group
- rSE
refractory status epilepticus
- SE
status epilepticus
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
I.S.F. participated in drafting and revising the manuscript for content, including medical writing for content, study concept and design, analysis and interpretation of data, acquisition of data, statistical analysis, and study supervision or coordination. M.C.J. participated in drafting and revising the manuscript for content, including medical writing for content, study concept and design, analysis and interpretation of data, acquisition of data, and study supervision or coordination. N.S.A. participated in revising the manuscript for content, including medical writing for content, analysis and interpretation of data, acquisition of data, and study supervision or coordination. R.A. participated in revising the manuscript for content, including medical writing for content, analysis and interpretation of data, acquisition of data, and study supervision or coordination. J.N.B. participated in revising the manuscript for content, including medical writing for content, analysis and interpretation of data, acquisition of data, and study supervision or coordination. J.L.C. participated in revising the manuscript for content, including medical writing for content, analysis and interpretation of data, acquisition of data, and study supervision or coordination. K.E.C. participated in revising the manuscript for content, including medical writing for content, analysis and interpretation of data, acquisition of data, and study supervision or coordination. W.D.G. participated in revising the manuscript for content, including medical writing for content, analysis and interpretation of data, acquisition of data, and study supervision or coordination. M.G.L. participated in revising the manuscript for content, including medical writing for content, analysis and interpretation of data, acquisition of data, and study supervision or coordination. T.A.G. participated in revising the manuscript for content, including medical writing for content, analysis and interpretation of data, acquisition of data, and study supervision or coordination. J.L.G. participated in revising the manuscript for content, including medical writing for content, analysis and interpretation of data, acquisition of data, and study supervision or coordination. H.P.G. participated in revising the manuscript for content, including medical writing for content, analysis and interpretation of data, acquisition of data, and study supervision or coordination. A.H. participated in revising the manuscript for content, including medical writing for content, analysis and interpretation of data, acquisition of data, and study supervision or coordination. K.K. participated in revising the manuscript for content, including medical writing for content, analysis and interpretation of data, acquisition of data, and study supervision or coordination. T.M. participated in revising the manuscript for content, including medical writing for content, analysis and interpretation of data, acquisition of data, and study supervision or coordination. M.A.M. participated in revising the manuscript for content, including medical writing for content, analysis and interpretation of data, acquisition of data, and study supervision or coordination. K.P. participated in revising the manuscript for content, including medical writing for content, analysis and interpretation of data, acquisition of data, and study supervision or coordination. J.R. participated in revising the manuscript for content, including medical writing for content, analysis and interpretation of data, acquisition of data, and study supervision or coordination. R.C.T. participated in revising the manuscript for content, including medical writing for content, analysis and interpretation of data, acquisition of data, and study supervision or coordination. A.A.T. participated in revising the manuscript for content, including medical writing for content, analysis and interpretation of data, acquisition of data, and study supervision or coordination. M.S.W. participated in revising the manuscript for content, including medical writing for content, analysis and interpretation of data, acquisition of data, and study supervision or coordination. A.W. participated in revising the manuscript for content, including medical writing for content, analysis and interpretation of data, acquisition of data, and study supervision or coordination. K.W. participated in revising the manuscript for content, including medical writing for content, analysis and interpretation of data, acquisition of data, and study supervision or coordination. T.L. participated in drafting and revising the manuscript for content, including medical writing for content, study concept and design, analysis and interpretation of data, acquisition of data, statistical analysis, and study supervision or coordination.
STUDY FUNDING
This study and consortium was funded by the Epilepsy Foundation of America (EF-213583, Targeted Initiative for Health Outcomes), by the American Epilepsy Society/Epilepsy Foundation of America Infrastructure Award, and by the Pediatric Epilepsy Research Foundation.
DISCLOSURE
I. Sánchez Fernández is funded by Fundación Alfonso Martín Escudero and the HHV6 Foundation. M. Jackson, N. Abend, R. Arya, N. Brenton, J. Carpenter, K. Chapman, W. Gaillard, and M. Gaínza Lein report no disclosures relevant to the manuscript. T. Glauser is funded by NIH grants 2U01-NS045911, U10-NS077311, R01-NS065840, and R01-HD073115. He has received consulting fees from Supernus. He had served as an expert consultant for the US Department of Justice and has received compensation for work as an expert on medico-legal cases. He receives royalties from a patent license from AssureX Health. J. Goldstein reports no disclosures relevant to the manuscript. H. Goodkin is a member of the clinical standardization team for Sage Therapeutics. A. Reynolds, K. Kapur, T. McDonough, M. Mikati, K. Peariso, J. Riviello, R. Tasker, A. Topjian, and M. Wainwright report no disclosures relevant to the manuscript. A. Wilfong reports research grant funding for epilepsy from GW Pharm, Novartis, Acorda, Upsher-Smith, Lundbeck, and Eisai, and royalties from publication of Up To Date chapters on epilepsy. K. Williams reports no disclosures relevant to the manuscript. T. Loddenkemper serves on the Laboratory Accreditation Board for Long-Term (Epilepsy and Intensive Care Unit) Monitoring, on the Council (and as Treasurer) of the American Clinical Neurophysiology Society, on the American Board of Clinical Neurophysiology, as an Associate Editor for Seizure, as Contributing Editor for Epilepsy Currents, and as an Associate Editor for Wyllie's Treatment of Epilepsy 6th edition. He is part of pending patent applications to detect and predict seizures and to diagnose epilepsy. He receives research support from the Epilepsy Research Fund, the American Epilepsy Society, the Epilepsy Foundation of America, the Epilepsy Therapy Project, PCORI, the Pediatric Epilepsy Research Foundation, CURE, and HHV-6 Foundation, and received research grants from Lundbeck, Eisai, Upsher-Smith, Acorda, and Pfizer. He serves as a consultant for Zogenix, Upsher-Smith, and Lundbeck. He performs video EEG long-term and ICU monitoring, EEG, and other electrophysiologic studies at Boston Children's Hospital and affiliated hospitals and bills for these procedures and he evaluates pediatric neurology patients and bills for clinical care. He has received speaker honoraria from national societies including the American Academy of Neurology, American Epilepsy Society, and American Clinical Neurophysiology Society, and for grand rounds at various academic centers. His wife, Dr. Karen Stannard, is a pediatric neurologist and she performs video EEG long-term and ICU monitoring, EEG, and other electrophysiologic studies and bills for these procedures and she evaluates pediatric neurology patients and bills for clinical care. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Chin RF, Neville BG, Peckham C, et al. Incidence, cause, and short-term outcome of convulsive status epilepticus in childhood: prospective population-based study. Lancet 2006;368:222–229. [DOI] [PubMed] [Google Scholar]
- 2.Dham BS, Hunter K, Rincon F. The epidemiology of status epilepticus in the United States. Neurocrit Care 2014;20:476–483. [DOI] [PubMed] [Google Scholar]
- 3.Hesdorffer DC, Logroscino G, Cascino G, Annegers JF, Hauser WA. Incidence of status epilepticus in Rochester, Minnesota, 1965-1984. Neurology 1998;50:735–741. [DOI] [PubMed] [Google Scholar]
- 4.Penberthy LT, Towne A, Garnett LK, Perlin JB, DeLorenzo RJ. Estimating the economic burden of status epilepticus to the health care system. Seizure 2005;14:46–51. [DOI] [PubMed] [Google Scholar]
- 5.Strzelczyk A, Knake S, Oertel WH, Rosenow F, Hamer HM. Inpatient treatment costs of status epilepticus in adults in Germany. Seizure 2013;22:882–885. [DOI] [PubMed] [Google Scholar]
- 6.Loddenkemper T, Syed TU, Ramgopal S, et al. Risk factors associated with death in in-hospital pediatric convulsive status epilepticus. PLoS One 2012;7:e47474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maegaki Y, Kurozawa Y, Tamasaki A, et al. Early predictors of status epilepticus-associated mortality and morbidity in children. Brain Dev 2015;37:478–486. [DOI] [PubMed] [Google Scholar]
- 8.Maytal J, Shinnar S, Moshe SL, Alvarez LA. Low morbidity and mortality of status epilepticus in children. Pediatrics 1989;83:323–331. [PubMed] [Google Scholar]
- 9.Chin RF, Neville BG, Scott RC. A systematic review of the epidemiology of status epilepticus. Eur J Neurol 2004;11:800–810. [DOI] [PubMed] [Google Scholar]
- 10.Raspall-Chaure M, Chin RF, Neville BG, Scott RC. Outcome of paediatric convulsive status epilepticus: a systematic review. Lancet Neurol 2006;5:769–779. [DOI] [PubMed] [Google Scholar]
- 11.Ferro MA, Chin RF, Camfield CS, Wiebe S, Levin SD, Speechley KN. Convulsive status epilepticus and health-related quality of life in children with epilepsy. Neurology 2014;83:752–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kravljanac R, Djuric M, Jankovic B, Pekmezovic T. Etiology, clinical course and response to the treatment of status epilepticus in children: a 16-year single-center experience based on 602 episodes of status epilepticus. Eur J Paediatr Neurol 2015;19:584–590. [DOI] [PubMed] [Google Scholar]
- 13.Alldredge BK, Gelb AM, Isaacs SM, et al. A comparison of lorazepam, diazepam, and placebo for the treatment of out-of-hospital status epilepticus. N Engl J Med 2001;345:631–637. [DOI] [PubMed] [Google Scholar]
- 14.Aranda A, Foucart G, Ducasse JL, Grolleau S, McGonigal A, Valton L. Generalized convulsive status epilepticus management in adults: a cohort study with evaluation of professional practice. Epilepsia 2010;51:2159–2167. [DOI] [PubMed] [Google Scholar]
- 15.Jordan KG. Status epilepticus: a perspective from the neuroscience intensive care unit. Neurosurg Clin N Am 1994;5:671–686. [PubMed] [Google Scholar]
- 16.Kämppi L, Mustonen H, Soinila S. Analysis of the delay components in the treatment of status epilepticus. Neurocrit Care 2013;19:10–18. [DOI] [PubMed] [Google Scholar]
- 17.Pellock JM, Marmarou A, DeLorenzo R. Time to treatment in prolonged seizure episodes. Epilepsy Behav 2004;5:192–196. [DOI] [PubMed] [Google Scholar]
- 18.Lewena S, Pennington V, Acworth J, et al. Emergency management of pediatric convulsive status epilepticus: a multicenter study of 542 patients. Pediatr Emerg Care 2009;25:83–87. [DOI] [PubMed] [Google Scholar]
- 19.Sánchez Fernández I, Abend NS, Agadi S, et al. Time from convulsive status epilepticus onset to anticonvulsant administration in children. Neurology 2015;84:2304–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seinfeld S, Shinnar S, Sun S, et al. Emergency management of febrile status epilepticus: results of the FEBSTAT study. Epilepsia 2014;55:388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brophy GM, Bell R, Claassen J, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care 2012;17:3–23. [DOI] [PubMed] [Google Scholar]
- 22.Loddenkemper T, Goodkin HP. Treatment of pediatric status epilepticus. Curr Treat Options Neurol 2011;13:560–573. [DOI] [PubMed] [Google Scholar]
- 23.Wilkes R, Tasker RC. Pediatric intensive care treatment of uncontrolled status epilepticus. Crit Care Clin 2013;29:239–257. [DOI] [PubMed] [Google Scholar]
- 24.Sánchez Fernández I, Abend NS, Agadi S, et al. Gaps and opportunities in refractory status epilepticus research in children: a multi-center approach by the Pediatric Status Epilepticus Research Group (pSERG). Seizure 2014;23:87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glickman ME, Rao SR, Schultz MR. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. J Clin Epidemiol 2014;67:850–857. [DOI] [PubMed] [Google Scholar]
- 26.R Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Available at: R-project.org/. Accessed 2015. [Google Scholar]
- 27.Warnes GR, Bolker B, Lumley T, Johnson RC. Gmodels: Various R Programming Tools for Model Fitting. R package version 2.16.2. Available at: CRAN.R-project.org/package=gmodels. Accessed 2015. [Google Scholar]
- 28.Warnes G, Bolker BG, Grothendieck G, et al. gdata: Various R Programming Tools for Data Manipulation. R Package version 2.17.0. Available at: CRAN.R-project.org/package=gdata. Accessed 2015. [Google Scholar]
- 29.Therneau T. A Package for Survival Analysis in S. version 2.38. Available at: CRAN.R-project.org/package=survival. Accessed 2015. [Google Scholar]
- 30.Abend NS, Loddenkemper T. Pediatric status epilepticus management. Curr Opin Pediatr 2014;26:668–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riviello JJ Jr., Claassen J, LaRoche SM, et al. Treatment of status epilepticus: an international survey of experts. Neurocrit Care 2013;18:193–200. [DOI] [PubMed] [Google Scholar]
- 32.Lothman E. The biochemical basis and pathophysiology of status epilepticus. Neurology 1990;40:13–23. [PubMed] [Google Scholar]
- 33.Alldredge BK, Wall DB, Ferriero DM. Effect of prehospital treatment on the outcome of status epilepticus in children. Pediatr Neurol 1995;12:213–216. [DOI] [PubMed] [Google Scholar]
- 34.Chin RF, Neville BG, Peckham C, Wade A, Bedford H, Scott RC. Treatment of community-onset, childhood convulsive status epilepticus: a prospective, population-based study. Lancet Neurol 2008;7:696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eriksson K, Metsaranta P, Huhtala H, Auvinen A, Kuusela AL, Koivikko M. Treatment delay and the risk of prolonged status epilepticus. Neurology 2005;65:1316–1318. [DOI] [PubMed] [Google Scholar]
- 36.Sagduyu A, Tarlaci S, Sirin H. Generalized tonic-clonic status epilepticus: causes, treatment, complications and predictors of case fatality. J Neurol 1998;245:640–646. [DOI] [PubMed] [Google Scholar]
- 37.Towne AR, Pellock JM, Ko D, DeLorenzo RJ. Determinants of mortality in status epilepticus. Epilepsia 1994;35:27–34. [DOI] [PubMed] [Google Scholar]
- 38.Freeman JM. Status epilepticus: it's not what we've thought or taught. Pediatrics 1989;83:444–445. [PubMed] [Google Scholar]
- 39.Logroscino G, Hesdorffer DC, Cascino G, Annegers JF, Hauser WA. Short-term mortality after a first episode of status epilepticus. Epilepsia 1997;38:1344–1349. [DOI] [PubMed] [Google Scholar]
- 40.Maytal J, Shinnar S. Febrile status epilepticus. Pediatrics 1990;86:611–616. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.