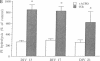Abstract
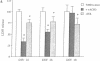
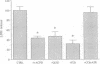
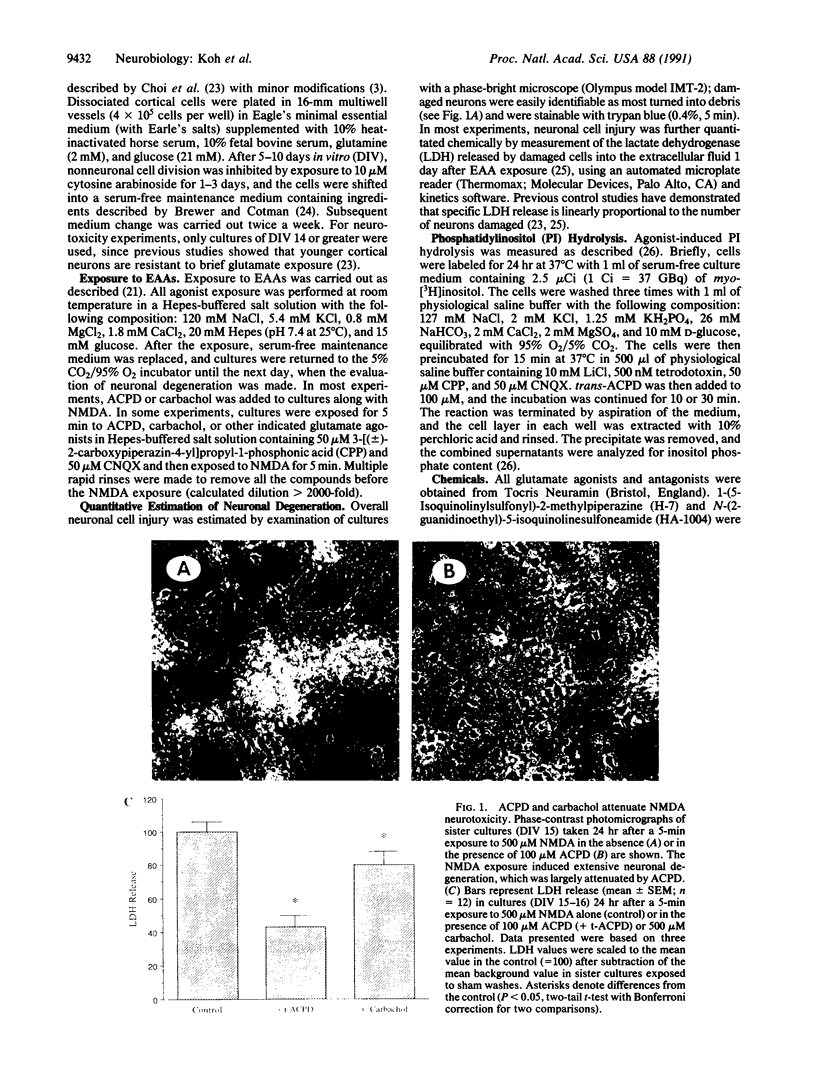
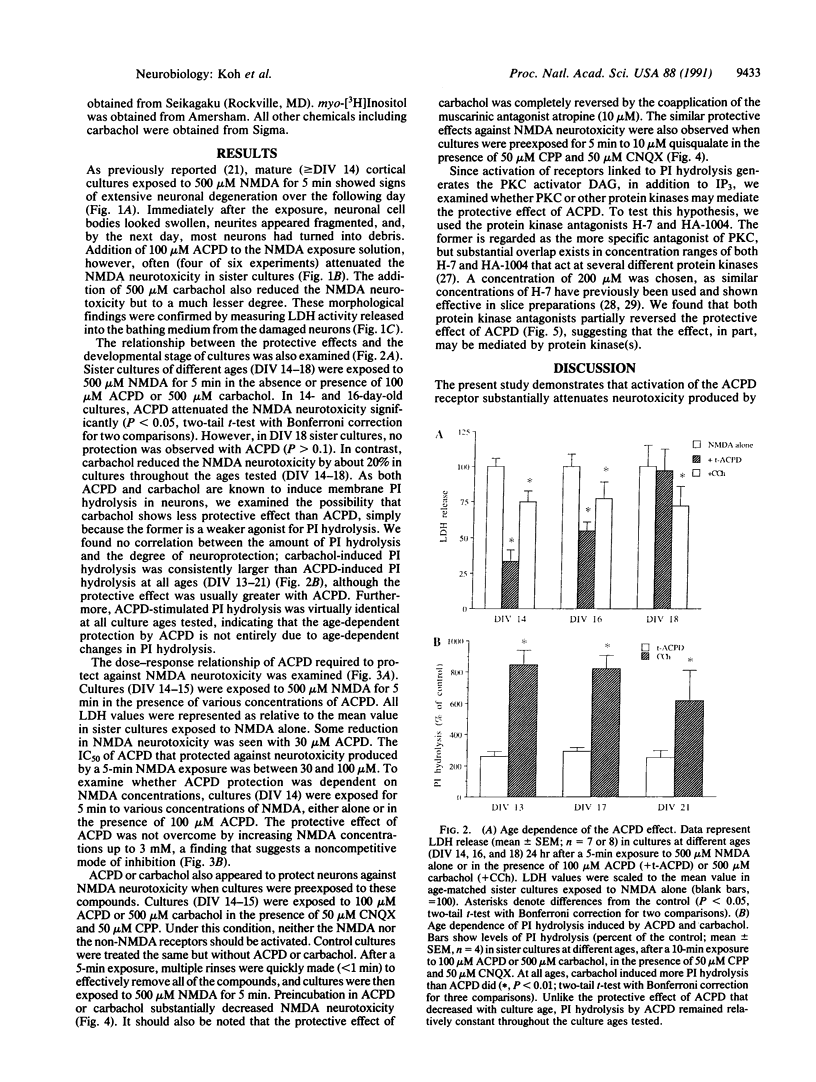
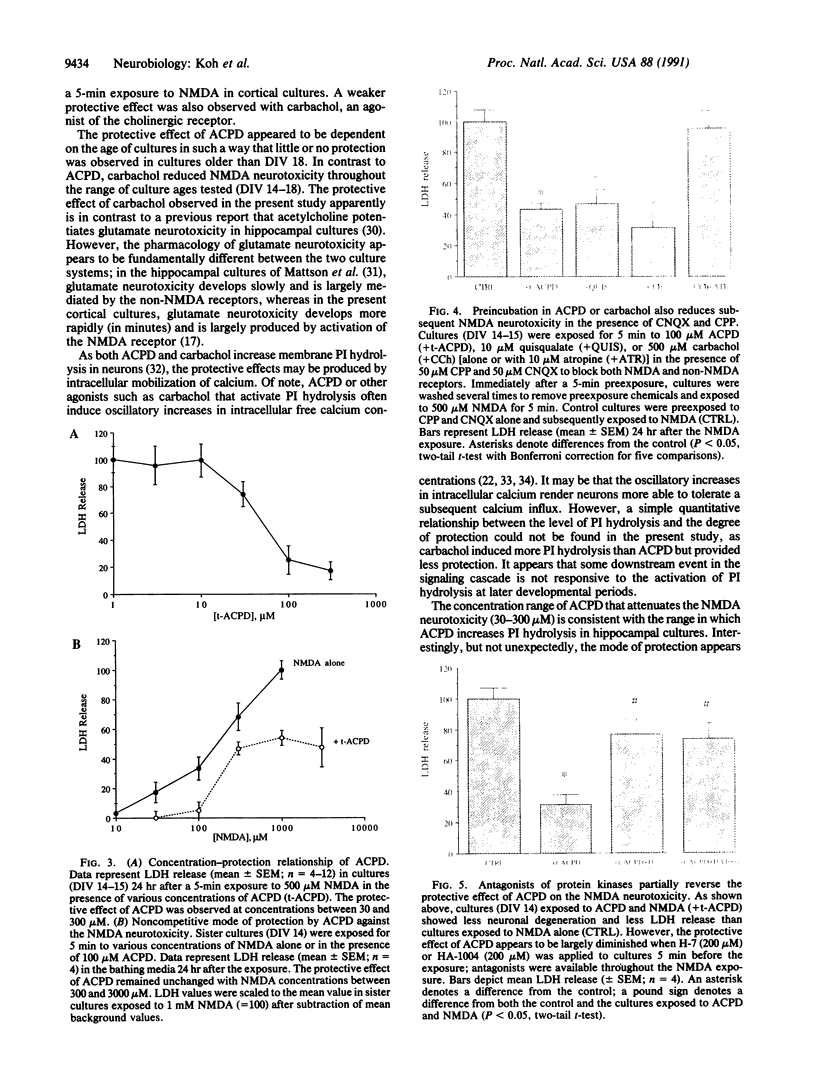
Excitatory amino acid receptor-mediated neurotoxicity (excitotoxicity) has been proposed to contribute to neuronal loss in a wide variety of neurodegenerative conditions. Although considerable evidence has accumulated implicating N-methyl-D-aspartate (NMDA), kainate, and alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors in the processes of excitotoxicity, relatively little research has focused on the ability of other neurotransmitter systems to influence excitotoxic neuronal injury. In the present study, we examined the effects of trans-1-aminocyclopentyl-1,3-dicarboylic acid (ACPD), a selective agonist for the metabotropic glutamate, or ACPD, receptor, and carbachol, an agonist at the acetylcholine receptor, on neuronal degeneration produced by brief exposure to NMDA in murine cortical cultures. Since excitotoxic neuronal injury is probably caused by increases in intracellular Ca2+ concentrations, the two transmitter agonists were of particular interest as both have been shown to mobilize intracellular calcium stores. Contrary to what might be expected, ACPD and, to a lesser degree, carbachol attenuated NMDA neurotoxicity. The neuroprotective effect of ACPD, but not of carbachol, was dependent upon the developmental state of cultures; in older cultures (greater than or equal to 18 days in vitro), the protective effect decreased. The neuroprotection by ACPD may be, in part, mediated by protein kinases, since protection is partially reversed by the protein kinase antagonists H-7 and HA-1004. These data suggest that concomitant activation of the ACPD receptor may serve as a protective mechanism against neurotoxicity that could be produced by brief intense NMDA receptor activation during normal or abnormal brain function.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambrosini A., Meldolesi J. Muscarinic and quisqualate receptor-induced phosphoinositide hydrolysis in primary cultures of striatal and hippocampal neurons. Evidence for differential mechanisms of activation. J Neurochem. 1989 Sep;53(3):825–833. doi: 10.1111/j.1471-4159.1989.tb11779.x. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Galione A. Cytosolic calcium oscillators. FASEB J. 1988 Dec;2(15):3074–3082. doi: 10.1096/fasebj.2.15.2847949. [DOI] [PubMed] [Google Scholar]
- Brewer G. J., Cotman C. W. Survival and growth of hippocampal neurons in defined medium at low density: advantages of a sandwich culture technique or low oxygen. Brain Res. 1989 Aug 7;494(1):65–74. doi: 10.1016/0006-8993(89)90144-3. [DOI] [PubMed] [Google Scholar]
- Choi D. W. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988 Oct;1(8):623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- Choi D. W. Ionic dependence of glutamate neurotoxicity. J Neurosci. 1987 Feb;7(2):369–379. doi: 10.1523/JNEUROSCI.07-02-00369.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D. W., Koh J. Y., Peters S. Pharmacology of glutamate neurotoxicity in cortical cell culture: attenuation by NMDA antagonists. J Neurosci. 1988 Jan;8(1):185–196. doi: 10.1523/JNEUROSCI.08-01-00185.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D. W., Maulucci-Gedde M., Kriegstein A. R. Glutamate neurotoxicity in cortical cell culture. J Neurosci. 1987 Feb;7(2):357–368. doi: 10.1523/JNEUROSCI.07-02-00357.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline H. T., Debski E. A., Constantine-Paton M. N-methyl-D-aspartate receptor antagonist desegregates eye-specific stripes. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4342–4345. doi: 10.1073/pnas.84.12.4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P., Katzman R., Terry R. D. Reduced somatostatin-like immunoreactivity in cerebral cortex from cases of Alzheimer disease and Alzheimer senile dementa. Nature. 1980 Nov 20;288(5788):279–280. doi: 10.1038/288279a0. [DOI] [PubMed] [Google Scholar]
- Davies P., Maloney A. J. Selective loss of central cholinergic neurons in Alzheimer's disease. Lancet. 1976 Dec 25;2(8000):1403–1403. doi: 10.1016/s0140-6736(76)91936-x. [DOI] [PubMed] [Google Scholar]
- Garthwaite G., Garthwaite J. Neurotoxicity of excitatory amino acid receptor agonists in rat cerebellar slices: dependence on calcium concentration. Neurosci Lett. 1986 May 15;66(2):193–198. doi: 10.1016/0304-3940(86)90189-8. [DOI] [PubMed] [Google Scholar]
- Garthwaite G., Garthwaite J. Quisqualate neurotoxicity: a delayed, CNQX-sensitive process triggered by a CNQX-insensitive mechanism in young rat hippocampal slices. Neurosci Lett. 1989 Apr 24;99(1-2):113–118. doi: 10.1016/0304-3940(89)90274-7. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt A., Bear M. F., Singer W. Blockade of "NMDA" receptors disrupts experience-dependent plasticity of kitten striate cortex. Science. 1987 Oct 16;238(4825):355–358. doi: 10.1126/science.2443978. [DOI] [PubMed] [Google Scholar]
- Koh J. Y., Choi D. W. Quantitative determination of glutamate mediated cortical neuronal injury in cell culture by lactate dehydrogenase efflux assay. J Neurosci Methods. 1987 May;20(1):83–90. doi: 10.1016/0165-0270(87)90041-0. [DOI] [PubMed] [Google Scholar]
- Koh J. Y., Goldberg M. P., Hartley D. M., Choi D. W. Non-NMDA receptor-mediated neurotoxicity in cortical culture. J Neurosci. 1990 Feb;10(2):693–705. doi: 10.1523/JNEUROSCI.10-02-00693.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh J. Y., Yang L. L., Cotman C. W. Beta-amyloid protein increases the vulnerability of cultured cortical neurons to excitotoxic damage. Brain Res. 1990 Nov 19;533(2):315–320. doi: 10.1016/0006-8993(90)91355-k. [DOI] [PubMed] [Google Scholar]
- Malinow R., Madison D. V., Tsien R. W. Persistent protein kinase activity underlying long-term potentiation. Nature. 1988 Oct 27;335(6193):820–824. doi: 10.1038/335820a0. [DOI] [PubMed] [Google Scholar]
- Marcyniuk B., Mann D. M., Yates P. O. The topography of cell loss from locus caeruleus in Alzheimer's disease. J Neurol Sci. 1986 Dec;76(2-3):335–345. doi: 10.1016/0022-510x(86)90179-6. [DOI] [PubMed] [Google Scholar]
- Mattson M. P. Acetylcholine potentiates glutamate-induced neurodegeneration in cultured hippocampal neurons. Brain Res. 1989 Sep 18;497(2):402–406. doi: 10.1016/0006-8993(89)90289-8. [DOI] [PubMed] [Google Scholar]
- Mattson M. P., Guthrie P. B., Hayes B. C., Kater S. B. Roles for mitotic history in the generation and degeneration of hippocampal neuroarchitecture. J Neurosci. 1989 Apr;9(4):1223–1232. doi: 10.1523/JNEUROSCI.09-04-01223.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J. W., Silverstein F. S., Johnston M. V. Neurotoxicity of N-methyl-D-aspartate is markedly enhanced in developing rat central nervous system. Brain Res. 1988 Aug 30;459(1):200–203. doi: 10.1016/0006-8993(88)90306-x. [DOI] [PubMed] [Google Scholar]
- Michaels R. L., Rothman S. M. Glutamate neurotoxicity in vitro: antagonist pharmacology and intracellular calcium concentrations. J Neurosci. 1990 Jan;10(1):283–292. doi: 10.1523/JNEUROSCI.10-01-00283.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan D. T., Bridges R. J., Cotman C. W. The excitatory amino acid receptors: their classes, pharmacology, and distinct properties in the function of the central nervous system. Annu Rev Pharmacol Toxicol. 1989;29:365–402. doi: 10.1146/annurev.pa.29.040189.002053. [DOI] [PubMed] [Google Scholar]
- Muller D., Buchs P. A., Dunant Y., Lynch G. Protein kinase C activity is not responsible for the expression of long-term potentiation in hippocampus. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4073–4077. doi: 10.1073/pnas.87.11.4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase K., Randic M., Shirasaki T., Nakagawa T., Akaike N. Serotonin suppresses N-methyl-D-aspartate responses in acutely isolated spinal dorsal horn neurons of the rat. Brain Res. 1990 Aug 13;525(1):84–91. doi: 10.1016/0006-8993(90)91323-9. [DOI] [PubMed] [Google Scholar]
- Murphy S. N., Miller R. J. Two distinct quisqualate receptors regulate Ca2+ homeostasis in hippocampal neurons in vitro. Mol Pharmacol. 1989 May;35(5):671–680. [PubMed] [Google Scholar]
- Nedergaard S., Engberg I., Flatman J. A. Serotonin facilitates NMDA responses of cat neocortical neurones. Acta Physiol Scand. 1986 Oct;128(2):323–325. doi: 10.1111/j.1748-1716.1986.tb07983.x. [DOI] [PubMed] [Google Scholar]
- Nicoletti F., Iadarola M. J., Wroblewski J. T., Costa E. Excitatory amino acid recognition sites coupled with inositol phospholipid metabolism: developmental changes and interaction with alpha 1-adrenoceptors. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1931–1935. doi: 10.1073/pnas.83.6.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Palmer E., Monaghan D. T., Cotman C. W. Glutamate receptors and phosphoinositide metabolism: stimulation via quisqualate receptors is inhibited by N-methyl-D-aspartate receptor activation. Brain Res. 1988 Sep;464(2):161–165. doi: 10.1016/0169-328x(88)90008-3. [DOI] [PubMed] [Google Scholar]
- Palmer E., Monaghan D. T., Cotman C. W. Trans-ACPD, a selective agonist of the phosphoinositide-coupled excitatory amino acid receptor. Eur J Pharmacol. 1989 Aug 3;166(3):585–587. doi: 10.1016/0014-2999(89)90383-x. [DOI] [PubMed] [Google Scholar]
- Palmer E., Nangel-Taylor K., Krause J. D., Roxas A., Cotman C. W. Changes in excitatory amino acid modulation of phosphoinositide metabolism during development. Brain Res Dev Brain Res. 1990 Jan 1;51(1):132–134. doi: 10.1016/0165-3806(90)90266-2. [DOI] [PubMed] [Google Scholar]
- Perry E. K., Tomlinson B. E., Blessed G., Bergmann K., Gibson P. H., Perry R. H. Correlation of cholinergic abnormalities with senile plaques and mental test scores in senile dementia. Br Med J. 1978 Nov 25;2(6150):1457–1459. doi: 10.1136/bmj.2.6150.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossor M. N., Emson P. C., Mountjoy C. Q., Roth M., Iversen L. L. Reduced amounts of immunoreactive somatostatin in the temporal cortex in senile dementia of Alzheimer type. Neurosci Lett. 1980 Dec;20(3):373–377. doi: 10.1016/0304-3940(80)90177-9. [DOI] [PubMed] [Google Scholar]
- Rothman S. M., Olney J. W. Glutamate and the pathophysiology of hypoxic--ischemic brain damage. Ann Neurol. 1986 Feb;19(2):105–111. doi: 10.1002/ana.410190202. [DOI] [PubMed] [Google Scholar]
- Sladeczek F., Pin J. P., Récasens M., Bockaert J., Weiss S. Glutamate stimulates inositol phosphate formation in striatal neurones. Nature. 1985 Oct 24;317(6039):717–719. doi: 10.1038/317717a0. [DOI] [PubMed] [Google Scholar]
- Sugiyama H., Ito I., Hirono C. A new type of glutamate receptor linked to inositol phospholipid metabolism. Nature. 1987 Feb 5;325(6104):531–533. doi: 10.1038/325531a0. [DOI] [PubMed] [Google Scholar]
- Tremblay E., Roisin M. P., Represa A., Charriaut-Marlangue C., Ben-Ari Y. Transient increased density of NMDA binding sites in the developing rat hippocampus. Brain Res. 1988 Oct 4;461(2):393–396. doi: 10.1016/0006-8993(88)90275-2. [DOI] [PubMed] [Google Scholar]
- Weiss J. H., Hartley D. M., Koh J., Choi D. W. The calcium channel blocker nifedipine attenuates slow excitatory amino acid neurotoxicity. Science. 1990 Mar 23;247(4949 Pt 1):1474–1477. doi: 10.1126/science.247.4949.1474. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Hirano A. Nucleus raphe dorsalis in Alzheimer's disease: neurofibrillary tangles and loss of large neurons. Ann Neurol. 1985 Jun;17(6):573–577. doi: 10.1002/ana.410170608. [DOI] [PubMed] [Google Scholar]