Abstract
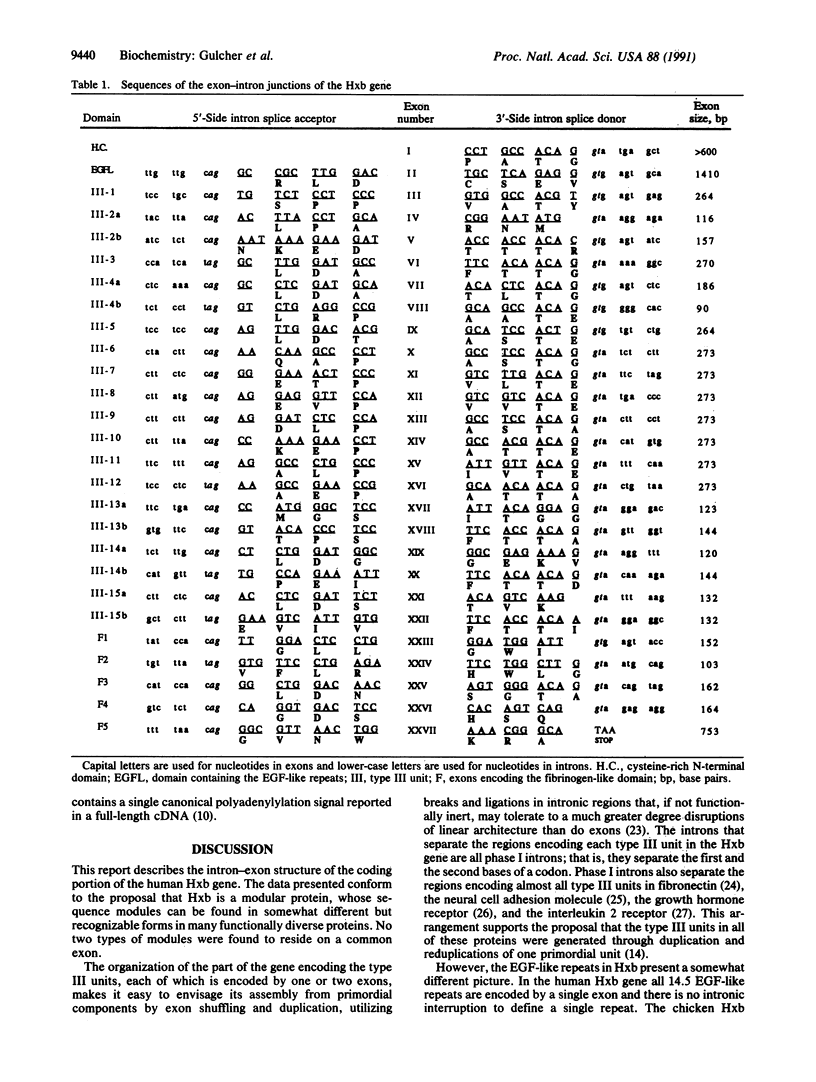
The structure of the gene encoding human hexabrachion (tenascin) has been determined from overlapping clones isolated from a human genomic bacteriophage library. The genomic inserts were characterized by restriction mapping, Southern blot analysis, PCR, and DNA sequencing. The coding region of the hexabrachion gene spans approximately 80 kilobases of DNA and consists of 27 exons separated by 26 introns. The exon-intron structure supports a hypothesis based on the cDNA sequence that the hexabrachion gene is an assembly of DNA modules that are also found elsewhere in the genome. Single exons may encode a module, a portion of a module, or a group of modules. The 15 type III units similar to those found in fibronectin are each encoded either by a single exon or by two exons interrupted by an intron. All type III units known to be spliced out of the smaller forms of the protein are encoded by one exon. The fibrinogen-like domain of 210 amino acids is encoded by five exons. The 14.5 epidermal growth factor-like repeats are all encoded by a single exon.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appella E., Weber I. T., Blasi F. Structure and function of epidermal growth factor-like regions in proteins. FEBS Lett. 1988 Apr 11;231(1):1–4. doi: 10.1016/0014-5793(88)80690-2. [DOI] [PubMed] [Google Scholar]
- Bell G. I., Fong N. M., Stempien M. M., Wormsted M. A., Caput D., Ku L. L., Urdea M. S., Rall L. B., Sanchez-Pescador R. Human epidermal growth factor precursor: cDNA sequence, expression in vitro and gene organization. Nucleic Acids Res. 1986 Nov 11;14(21):8427–8446. doi: 10.1093/nar/14.21.8427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaldo P., Colombatti A. The carboxyl terminus of the chicken alpha 3 chain of collagen VI is a unique mosaic structure with glycoprotein Ib-like, fibronectin type III, and Kunitz modules. J Biol Chem. 1989 Dec 5;264(34):20235–20239. [PubMed] [Google Scholar]
- Boutin J. M., Jolicoeur C., Okamura H., Gagnon J., Edery M., Shirota M., Banville D., Dusanter-Fourt I., Djiane J., Kelly P. A. Cloning and expression of the rat prolactin receptor, a member of the growth hormone/prolactin receptor gene family. Cell. 1988 Apr 8;53(1):69–77. doi: 10.1016/0092-8674(88)90488-6. [DOI] [PubMed] [Google Scholar]
- Brümmendorf T., Wolff J. M., Frank R., Rathjen F. G. Neural cell recognition molecule F11: homology with fibronectin type III and immunoglobulin type C domains. Neuron. 1989 Apr;2(4):1351–1361. doi: 10.1016/0896-6273(89)90073-1. [DOI] [PubMed] [Google Scholar]
- Chiquet-Ehrismann R., Mackie E. J., Pearson C. A., Sakakura T. Tenascin: an extracellular matrix protein involved in tissue interactions during fetal development and oncogenesis. Cell. 1986 Oct 10;47(1):131–139. doi: 10.1016/0092-8674(86)90374-0. [DOI] [PubMed] [Google Scholar]
- Crabtree G. R., Comeau C. M., Fowlkes D. M., Fornace A. J., Jr, Malley J. D., Kant J. A. Evolution and structure of the fibrinogen genes. Random insertion of introns or selective loss? J Mol Biol. 1985 Sep 5;185(1):1–19. doi: 10.1016/0022-2836(85)90179-2. [DOI] [PubMed] [Google Scholar]
- Erickson H. P., Bourdon M. A. Tenascin: an extracellular matrix protein prominent in specialized embryonic tissues and tumors. Annu Rev Cell Biol. 1989;5:71–92. doi: 10.1146/annurev.cb.05.110189.000443. [DOI] [PubMed] [Google Scholar]
- Erickson H. P., Inglesias J. L. A six-armed oligomer isolated from cell surface fibronectin preparations. Nature. 1984 Sep 20;311(5983):267–269. doi: 10.1038/311267a0. [DOI] [PubMed] [Google Scholar]
- Furley A. J., Morton S. B., Manalo D., Karagogeos D., Dodd J., Jessell T. M. The axonal glycoprotein TAG-1 is an immunoglobulin superfamily member with neurite outgrowth-promoting activity. Cell. 1990 Apr 6;61(1):157–170. doi: 10.1016/0092-8674(90)90223-2. [DOI] [PubMed] [Google Scholar]
- Gilbert W. Why genes in pieces? Nature. 1978 Feb 9;271(5645):501–501. doi: 10.1038/271501a0. [DOI] [PubMed] [Google Scholar]
- Godowski P. J., Leung D. W., Meacham L. R., Galgani J. P., Hellmiss R., Keret R., Rotwein P. S., Parks J. S., Laron Z., Wood W. I. Characterization of the human growth hormone receptor gene and demonstration of a partial gene deletion in two patients with Laron-type dwarfism. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8083–8087. doi: 10.1073/pnas.86.20.8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene J. R., Guarente L. Subcloning. Methods Enzymol. 1987;152:512–522. doi: 10.1016/0076-6879(87)52058-4. [DOI] [PubMed] [Google Scholar]
- Greenwald I. lin-12, a nematode homeotic gene, is homologous to a set of mammalian proteins that includes epidermal growth factor. Cell. 1985 Dec;43(3 Pt 2):583–590. doi: 10.1016/0092-8674(85)90230-2. [DOI] [PubMed] [Google Scholar]
- Gulcher J. R., Alexakos M. J., Le Beau M. M., Lemons R. S., Stefansson K. Chromosomal localization of the human hexabrachion (tenascin) gene and evidence for recent reduplication within the gene. Genomics. 1990 Apr;6(4):616–622. doi: 10.1016/0888-7543(90)90495-g. [DOI] [PubMed] [Google Scholar]
- Gulcher J. R., Nies D. E., Marton L. S., Stefansson K. An alternatively spliced region of the human hexabrachion contains a repeat of potential N-glycosylation sites. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1588–1592. doi: 10.1073/pnas.86.5.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones F. S., Crossin K. L., Cunningham B. A., Edelman G. M. Identification and characterization of the promoter for the cytotactin gene. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6497–6501. doi: 10.1073/pnas.87.17.6497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones F. S., Hoffman S., Cunningham B. A., Edelman G. M. A detailed structural model of cytotactin: protein homologies, alternative RNA splicing, and binding regions. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1905–1909. doi: 10.1073/pnas.86.6.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblihtt A. R., Umezawa K., Vibe-Pedersen K., Baralle F. E. Primary structure of human fibronectin: differential splicing may generate at least 10 polypeptides from a single gene. EMBO J. 1985 Jul;4(7):1755–1759. doi: 10.1002/j.1460-2075.1985.tb03847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubomura S., Obara M., Karasaki Y., Taniguchi H., Gotoh S., Tsuda T., Higashi K., Ohsato K., Hirano H. Genetic analysis of the cell binding domain region of the chicken fibronectin gene. Biochim Biophys Acta. 1987 Nov 20;910(2):171–181. doi: 10.1016/0167-4781(87)90070-4. [DOI] [PubMed] [Google Scholar]
- Larsen A., Davis T., Curtis B. M., Gimpel S., Sims J. E., Cosman D., Park L., Sorensen E., March C. J., Smith C. A. Expression cloning of a human granulocyte colony-stimulating factor receptor: a structural mosaic of hematopoietin receptor, immunoglobulin, and fibronectin domains. J Exp Med. 1990 Dec 1;172(6):1559–1570. doi: 10.1084/jem.172.6.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightner V. A., Gumkowski F., Bigner D. D., Erickson H. P. Tenascin/hexabrachion in human skin: biochemical identification and localization by light and electron microscopy. J Cell Biol. 1989 Jun;108(6):2483–2493. doi: 10.1083/jcb.108.6.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos M., Tacke R., Scherer H., Teplow D., Früh K., Schachner M. Neural adhesion molecule L1 as a member of the immunoglobulin superfamily with binding domains similar to fibronectin. Nature. 1988 Aug 25;334(6184):701–703. doi: 10.1038/334701a0. [DOI] [PubMed] [Google Scholar]
- Nies D. E., Hemesath T. J., Kim J. H., Gulcher J. R., Stefansson K. The complete cDNA sequence of human hexabrachion (Tenascin). A multidomain protein containing unique epidermal growth factor repeats. J Biol Chem. 1991 Feb 15;266(5):2818–2823. [PubMed] [Google Scholar]
- Odermatt E., Tamkun J. W., Hynes R. O. Repeating modular structure of the fibronectin gene: relationship to protein structure and subunit variation. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6571–6575. doi: 10.1073/pnas.82.19.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens G. C., Edelman G. M., Cunningham B. A. Organization of the neural cell adhesion molecule (N-CAM) gene: alternative exon usage as the basis for different membrane-associated domains. Proc Natl Acad Sci U S A. 1987 Jan;84(1):294–298. doi: 10.1073/pnas.84.1.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patthy L. Homology of a domain of the growth hormone/prolactin receptor family with type III modules of fibronectin. Cell. 1990 Apr 6;61(1):13–14. doi: 10.1016/0092-8674(90)90208-v. [DOI] [PubMed] [Google Scholar]
- Petersen T. E., Thøgersen H. C., Skorstengaard K., Vibe-Pedersen K., Sahl P., Sottrup-Jensen L., Magnusson S. Partial primary structure of bovine plasma fibronectin: three types of internal homology. Proc Natl Acad Sci U S A. 1983 Jan;80(1):137–141. doi: 10.1073/pnas.80.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranscht B. Sequence of contactin, a 130-kD glycoprotein concentrated in areas of interneuronal contact, defines a new member of the immunoglobulin supergene family in the nervous system. J Cell Biol. 1988 Oct;107(4):1561–1573. doi: 10.1083/jcb.107.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M., Kato S., Kohno K., Martin G. R., Yamada Y. Sequence of the cDNA encoding the laminin B1 chain reveals a multidomain protein containing cysteine-rich repeats. Proc Natl Acad Sci U S A. 1987 Feb;84(4):935–939. doi: 10.1073/pnas.84.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf S. J., Horn G. T., Erlich H. A. Direct cloning and sequence analysis of enzymatically amplified genomic sequences. Science. 1986 Sep 5;233(4768):1076–1078. doi: 10.1126/science.3461561. [DOI] [PubMed] [Google Scholar]
- Schwarzbauer J. E., Patel R. S., Fonda D., Hynes R. O. Multiple sites of alternative splicing of the rat fibronectin gene transcript. EMBO J. 1987 Sep;6(9):2573–2580. doi: 10.1002/j.1460-2075.1987.tb02547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya H., Yoneyama M., Nakamura Y., Harada H., Hatakeyama M., Minamoto S., Kono T., Doi T., White R., Taniguchi T. The human interleukin-2 receptor beta-chain gene: genomic organization, promoter analysis and chromosomal assignment. Nucleic Acids Res. 1990 Jul 11;18(13):3697–3703. doi: 10.1093/nar/18.13.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siri A., Carnemolla B., Saginati M., Leprini A., Casari G., Baralle F., Zardi L. Human tenascin: primary structure, pre-mRNA splicing patterns and localization of the epitopes recognized by two monoclonal antibodies. Nucleic Acids Res. 1991 Feb 11;19(3):525–531. doi: 10.1093/nar/19.3.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring J., Beck K., Chiquet-Ehrismann R. Two contrary functions of tenascin: dissection of the active sites by recombinant tenascin fragments. Cell. 1989 Oct 20;59(2):325–334. doi: 10.1016/0092-8674(89)90294-8. [DOI] [PubMed] [Google Scholar]
- Südhof T. C., Russell D. W., Goldstein J. L., Brown M. S., Sanchez-Pescador R., Bell G. I. Cassette of eight exons shared by genes for LDL receptor and EGF precursor. Science. 1985 May 17;228(4701):893–895. doi: 10.1126/science.3873704. [DOI] [PubMed] [Google Scholar]
- Vibe-Pedersen K., Kornblihtt A. R., Baralle F. E. Expression of a human alpha-globin/fibronectin gene hybrid generates two mRNAs by alternative splicing. EMBO J. 1984 Nov;3(11):2511–2516. doi: 10.1002/j.1460-2075.1984.tb02165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Suzuki K., Oyanagi W., Ohnishi K., Tanaka H. Gene cloning of chitinase A1 from Bacillus circulans WL-12 revealed its evolutionary relationship to Serratia chitinase and to the type III homology units of fibronectin. J Biol Chem. 1990 Sep 15;265(26):15659–15665. [PubMed] [Google Scholar]
- Weller A., Beck S., Ekblom P. Amino acid sequence of mouse tenascin and differential expression of two tenascin isoforms during embryogenesis. J Cell Biol. 1991 Jan;112(2):355–362. doi: 10.1083/jcb.112.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]