Abstract
Background
The African rice Oryza glaberrima was domesticated from its wild relative Oryza barthii about 3000 years ago. During the domestication process, panicle complexity changed from a panicle with low complexity in O. barthii, to a highly branched panicle carrying more seeds in O. glaberrima. To understand the basis of this differential panicle development between the two species, we conducted morphological and molecular analyses of early panicle development.
Results
Using X-ray tomography, we analyzed the morphological basis of early developmental stages of panicle development. We uncovered evidence for a wider rachis meristem in O. glaberrima than in O. barthii. At the molecular level, spatial and temporal expression profiles of orthologs of O. sativa genes related to meristem activity and meristem fate control were obtained using in situ hybridization and qRT-PCR. Despite highly conserved spatial expression patterns between O. glaberrima and O. barthii, differences in the expression levels of these early acting genes were detected.
Conclusion
The higher complexity of the O. glaberrima panicle compared to that of its wild relative O. barthii is associated with a wider rachis meristem and a modification of expression of branching-related genes. Our study indicates that the expression of genes in the miR156/miR529/SPL and TAW1 pathways, along with that of their target genes, is altered from the unbranched stage of development. This suggests that differences in panicle complexity between the two African rice species result from early alterations to gene expression during reproductive development.
Electronic supplementary material
The online version of this article (doi:10.1186/s13227-017-0065-y) contains supplementary material, which is available to authorized users.
Keywords: Panicle, Tomography, Branching, Meristem fate, African rice
Background
The African rice Oryza glaberrima and the Asian rice Oryza sativa are the two species of cultivated rice in the world. While O. sativa was domesticated about 10,000 years ago, O. glaberrima has a shorter history as it derived from its wild ancestor Oryza barthii about 3000 years ago along the Niger River in Mali [1–3]. Recently, different studies provided evidence that African rice domestication was linked with a single domestication origin in West Africa, associated with a severe genetic bottleneck [3–7]. However, in contrast to Asian rice domestication that has been the topic of extensive research, African rice domestication has been less studied in terms of molecular genetics. Although African rice maintains a very low genetic diversity compared to Asian rice [3–6], its close relationship to Asian rice species and its simpler domestication history make it an equally good model to study the evolution of morphological traits and associated gene networks in relation to rice domestication.
Several morphological traits were selected during domestication, including tillering, seed color, seed shattering and many other traits collectively referred to as the “domestication syndrome” [8]. In this context, the inflorescence (i.e., the flower bearing structure) architecture is one of the main morphological traits modified during rice domestications [9]. The architecture of the rice inflorescence (panicle) results from the establishment and activity of apical and axillary meristems derived from the vegetative shoot apical meristem (SAM) [10]. In the reproductive phase, the SAM converts into the rachis meristem (RM), which will give primary branch (PB) meristems until its abortion. These PB meristems will contribute to the establishment of the primary branches as well as axillary meristems, which will contribute to the secondary branch (SB), possibly harboring tertiary branch (TB) meristems. Finally, all the axillary and terminal meristems convert to spikelet (Sp) meristems and then florets [11]. In this way, rice panicle architecture is determined overall by two fundamental phases: the process of meristem establishment and branching; and meristem fate transition from branch/axillary to spikelet meristems. A model of inflorescence evolution was proposed on the basis of differences in the time period required for terminal and axillary meristems to acquire floral fate (i.e., heterochrony) [12]. This model is supported by the analysis of various mutants and detailed transcriptomic time course studies in eudicots [13–15]. In tomato and related nightshades (Solanaceae), the diversity of inflorescence structure observed between domesticated and wild relative species is associated with a peak of transcriptome divergence during the reproductive transition, driven by heterochronic shifts [16]. In O. sativa, a large number of genes required for the initiation and development of the panicle have been described [17, 18]. Among these genes, two main categories can be defined: (1) the genes related to the branching phase (i.e., establishment and activity of the indeterminate meristems); and (2) the genes related to the transition from indeterminate meristems to spikelet meristems (i.e., spikelet transition) and subsequently the floret phase [11, 18]. The functional analysis of this class of genes led to the hypothesis that panicle complexity in O. sativa is governed by the fine-tuning of meristem fate change, through the differential regulation of genes involved in the spikelet transition [11]. However, it was shown recently that the miR156/miR529/SPL regulatory pathway, which plays a key role in the transition from the vegetative to the reproductive phase, is also involved in the control of panicle complexity through the regulation of early acting genes such as LAX PANICLE1 (LAX1) and ABERRANT PANICLE ORGANIZATION2 (APO2), as well as the miR172/APETALA2 pathway and the SEPALLATA-like gene PAP2/OsMADS34 involved in spikelet and floret development [19]. Within this framework, it is important to determine to what extent these genes might be associated with panicle structure changes associated with rice domestication.
We previously showed that the differential expression of male-gametogenesis-associated, miR2118-triggered, 21-nucleotide, phased siRNAs could be associated with the differential rate of spikelet development in panicles between O. glaberrima and O. barthii [20]. A more thorough investigation is needed to understand the morphological and molecular basis of the observed differential complexity of panicle architecture in the two African rice species. We thus carried out detailed phenotyping of the early developing panicles using high-resolution X-ray tomography [21]. In order to determine whether differences in panicle complexity between the two African rice species might be associated with differential expression of these landmark genes, spatial and temporal expression profiling was performed using a set of O. sativa genes likely to play important roles in meristem activity and meristem fate control. Our results revealed that the spatial expression patterns of these genes were conserved. However, differences in their expression levels were observed at a very early stage, reflecting the differential inflorescence meristem size of the two African rice species.
Results
African rice panicle structure at mature and early stages
The rice panicle consists of a series of branches of different orders: rachis (main axis) and higher-order axes (PBs, SBs and sometimes TBs) (Fig. 1a). The single-flowered rice spikelets are established on each panicle branch from apical and lateral meristems. To characterize the phenotype of African rice panicles, we used the P-TRAP software on spread panicles [22], to quantify the main morphological traits in different rice accessions: B88 for O. barthii and CG14 for O. glaberrima (Fig. 1). There are structural differences in panicle architecture between the two African species (Fig. 1b). Rachis and primary branch lengths are highly variable between panicles of the same accession for both species (Fig. 1c, Additional file 1). For both parameters, O. glaberrima panicles display higher mean values than panicles of O. barthii (Fig. 1c, Additional file 1). Mature panicles from O. barthii (accession B88) possess only a few PBs (mean of 5 ± 1.59 SD) and SBs (7.06 ± 2.31 SD) bearing relatively few spikelets (47.39 ± 7.41 SD) (Fig. 1c, Additional file 1). O. glaberrima (accession CG14) mature panicles are more highly branched (i.e., they display higher number of PBs and SBs, 12.67 ± 1.33 SD and 35.50 ± 6.95 SD, respectively), carrying larger numbers of spikelets (199.11 ± 23.97 SD) compared to the wild relative (Fig. 1c, Additional file 1).
Fig. 1.
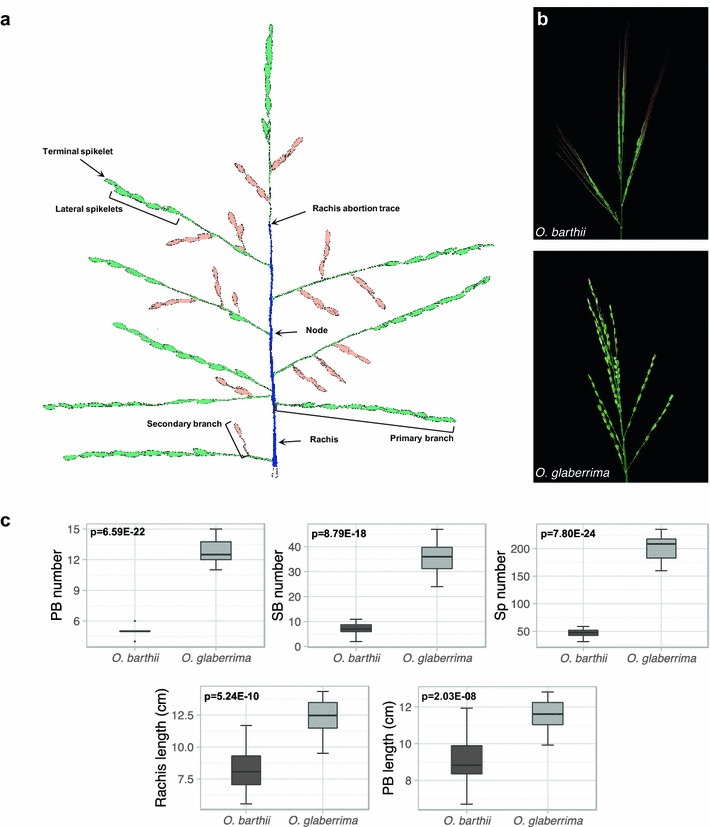
Mature panicle structure in wild and domesticated African species. a Spread panicle from O. glaberrima (CG14), with the rachis in blue, the primary branches in green and the secondary branches in orange. b Mature panicle of O. barthii (Ob) and O. glaberrima (Og). C. Comparison of panicle traits at the mature stage between O. barthii (accession B88) and O. glaberrima (accession CG14). Black lines within box-plots show the median value; box limits indicate the 25th and 75th percentiles. PB_number, primary branches number; SB_number, secondary branches number; Sp_number, spikelet number. Rachis_length, values of rachis length in cm; PB_length, mean values of the length of primary branches per panicle in cm. Morphological traits were quantified using P-TRAP software [21], n = 18 panicles per species. Statistical significance (i.e., t test p values) between the two species for the different panicle traits is indicated
In this study, we focused on the branching process in terms of meristem establishment and activity and the transition phase to spikelets (i.e., meristem fate), because the branching complexity of the rice panicle is determined at this stage. A description of this developmental framework was carried out in the two African species using classical histology (Fig. 2) and X-ray tomography analyses (Additional files 2 and 3). X-ray tomography allows the acquisition of stacks of high-resolution images from whole-mount material. Subsequently, 3D views can be reconstructed and measurements of morphological structures can be taken independently of sample orientation ([21]; see Additional files 2 and 4). For our analysis, we divided early panicle development into four stages (Fig. 2). Stage 1 corresponds to the elongation of the rachis meristem (RM) and the establishment of primary branch meristem (PBm). In stage 2, the PB elongates and higher-order branches are determined (SBs and TBs) from the initiation of axillary meristems (AMs). The transition from axillary meristems to spikelet meristems (SpMs) occurs at stage 3, and the differentiation of floral organs/flower development occurs at stage 4 (Fig. 2). Using X-ray computed tomography and classical histology, we observed that overall panicle morphology is similar between the two species at the early stages of development. However, during the elongation of the PBs (stage 2), more axillary meristems are initiated along the PBs of O. glaberrima compared to those of its wild relative (Fig. 2c–e, j–l). Spikelet differentiation occurs first at the apex of the panicle in both species, after which it progresses toward the base (Fig. 2e, f, l, m; stage 3). At this stage, more SpMs appear to be differentiated in O. barthii than in O. glaberrima. Spikelet and floral organ differentiation then occurs quickly along the panicle, in an acropetal direction, in both species (Fig. 2f, g, m and n; stages 3 and 4).
Fig. 2.
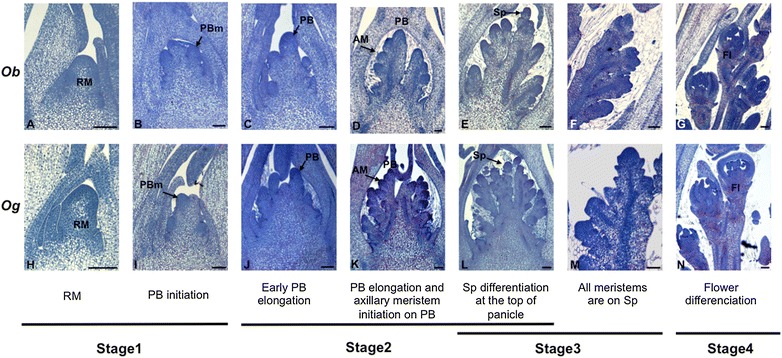
Histological description of early differentiation of African rice panicle and corresponding stages for expression analysis. O. barthii: a–g O. glaberrima: h–n. Developmental stages selected for in situ hybridization and qRT-PCR analyses are indicated in lower panel. Stage 1: unbranched stage with elongation of rachis meristem and formation of primary branch meristems; stage 2: early branching stage with at the end the initiation of spikelet meristem differentiation; stage 3: late branching stage with elongated secondary branch and complete spikelet meristem differentiation; stage 4: floret organ differentiation/development. AM axillary meristem, Fl flower, RM rachis meristem, PBm primary branch meristem, PB primary branch, Sp spikelet. Scale bars 100 µm
Meristem size (i.e., width at 40 µm from top of median meristem, see Fig. 3, Additional files 2 and 4) was measured at four reliable development stages from X-ray tomography images and histological sections: the RM (stage 1); the early PB elongation (stage 2); late PB elongation during the axillary meristem initiation along the PB (stage 2); and at the stage of Sp differentiation at the top of the panicle (stage 3). For each stage, a minimum of nine meristems was analyzed from at least three independent panicles. Sizes of apical and axillary meristems on PBs at the indeterminate branch stage and at the SpM differentiation stage were observed to be similar between the two species. The only difference that could be observed between the two species was in the rachis meristem width, which tends to be wider in O. glaberrima than in O. barthii (Fig. 3).
Fig. 3.
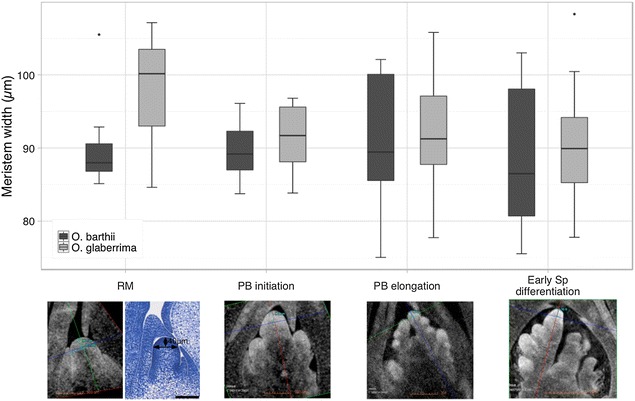
Morphometric traits of panicle meristems in O. barthii (Ob) and O. glaberrima (Og). Measurements were taken at different stages of panicle development via virtual sectioning of 3D micro-computed tomography (micro-CT) images: rachis meristem (RM), early primary branch elongation (PB initiation), primary branch elongation before spikelet differentiation (PB elongation) and just after spikelet differentiation at the top of the panicle (early spikelet differentiation). Upper panel: box-plots of meristem width measured from X-ray or histological images (Additional files 2 and 3). Black lines within box-plots show the median value; box limits indicate the 25th and 75th percentiles. Lower panel: illustration of the considered morphological areas (i.e., width of the meristem at 40 μm from the apical part of the meristem). For the RM stage, meristem widths were quantified from X-ray tomography images (left) and classical histological section (right) of rachis meristem images (n = 9 for each genotype). Black lines represent the area of measurement. For the other developmental stages, meristem width was measured for all apical PB meristems of each panicle (n = 9 meristems at least for each genotypes). Scale bars = 100 μm. T test p values: stage RM, p = 0.06; stage PB initiation, p = 0.22; stage PB elongation, p = 0.35; stage Sp, p = 0.24
Spatial expression pattern of African rice orthologs of panicle-related genes
To obtain further insights into the mechanisms regulating differential panicle development in the two African rice species, the expression patterns of two sets of genes known to be associated with panicle development in O. sativa were analyzed. Firstly, genes controlling the initiation and/or the maintenance of lateral meristems were used as molecular markers of branch meristem activity, such as Oryza sativa homeobox1 (OSH1), a member of the class I KNOX transcription factor family known to be associated with meristematic cell fate control in angiosperms [23]. The transcription factor-encoding genes LAX PANICLE1 (LAX1) and SQUAMOSA promoter binding protein-like14 (SPL14) as well as their microRNA regulators in the panicle miR529 and miR156 were also considered in this set of markers, on account of their involvement in axillary meristem establishment and outgrowth during O. sativa panicle development [24–27]. The second set of genes related to meristem fate control and included the SEPALLATA-like gene LEAFY HULL STERILE1/OsMADS1 (LHS1), which promotes the transition from branch meristems to spikelet meristems [28–31], as well as the LEAFY ortholog ABERRANT PANICLE ORGANIZATION2 (APO2) and TAWAWA1 (TAW1), encoding a member of the ALOG family of transcription regulators, the latter two genes both reported to act as suppressors of the transition from branch meristems to spikelet meristems [32–35].
Spatial expression profiling of these genes in the two rice species was carried out using in situ hybridization analysis (Fig. 4, Additional file 5). A strong accumulation of OSH1 transcripts was observed at all developmental stages in the vascular bundles and also in the entire branch meristems (but not in the epidermis) until the stage of initiation of spikelet/floret meristems (Fig. 4, Additional file 5), as previously reported in O. sativa [36]. In contrast, LAX1 transcripts were observed to be restricted specifically to the adaxial boundary region of new BMs and to persist in the young SpMs (Fig. 4, Additional file 5), as reported by [27] in O. sativa. Similarly, SPL14 transcripts were detected in the boundary region of new BMs in both species (Fig. 4, Additional file 5) but not in the spikelets, as reported by [37] in O. sativa. In contrast, miR156 and miR529, the SPL14 microRNA regulators, were detected in the entire new BMs (including epidermis) and persisted in the florets with differentiating organs (Fig. 4, Additional file 5). This finding would suggest that the microRNA-mediated regulation of SPL14 transcripts acts not as a dampening system but rather as an exclusion/restriction-type mechanism.
Fig. 4.
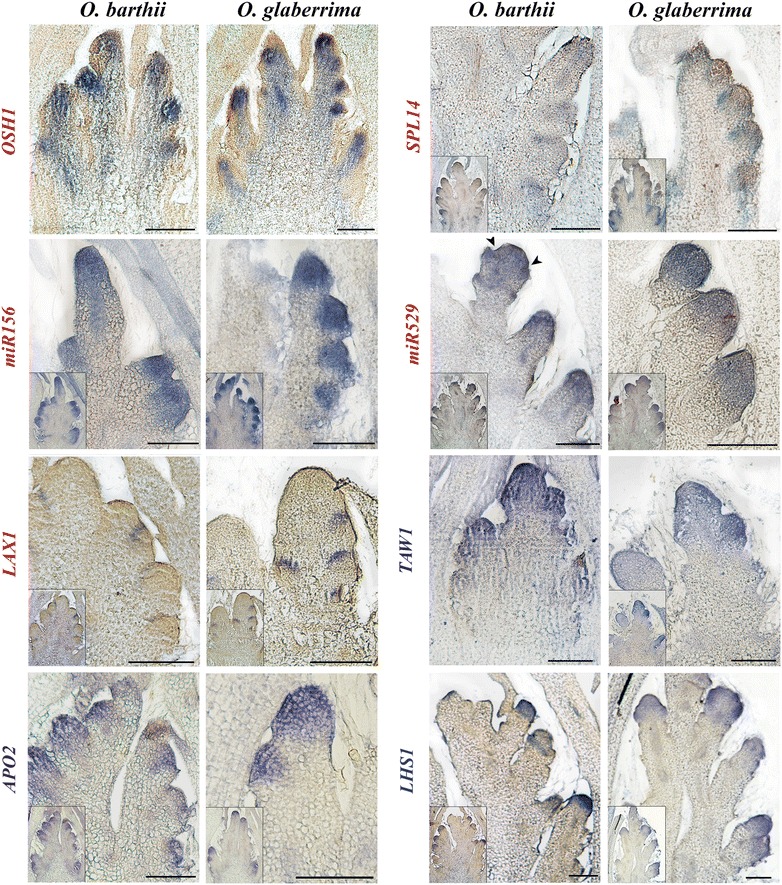
In situ expression patterns of African rice orthologs of panicle-related genes. In situ hybridization analysis of OSH1, SPL14, miR529, miR159, LAX1, APO2, TAW1 and LHS1 transcripts in branch meristems (stages 2–3) in O. barthii and O. glaberrima. Genes reported as being involved in branch meristem establishment and outgrowth are indicated in red. Those reported as being involved in branch to spikelet fate transition (both activator and inhibitor) are indicated in blue. The inset photographs show the complete panicle section corresponding to the close-up views of the branch. Scale bars 100 µm
APO2 and TAW1 ortholog transcripts were detected with a similar patterning: in all branch meristems from early stage 1 of panicle development (Fig. 4, Additional file 5), and also in the SpMs and developing florets. The APO2 ortholog expression pattern observed in our study is similar to that described by [32] in O. sativa, which differs from the pattern described by [34]. In the latter case, a transient down-regulation was observed in late branch meristems and spikelet meristems, which was not observed in our study. The TAW1 ortholog expression pattern observed here differs from that reported for O. sativa [35], in which transcripts were detected only in branch meristems and not in spikelet meristems. In agreement with the report of [38] for O. sativa, a strong signal corresponding to LHS1 transcripts was detected in the spikelet meristem and weakly in the palea and lemma of FMs but not in the inner organs of the differentiating floret nor in branch meristems (Fig. 4, Additional file 5).
Overall, a number of distinct expression patterns were observed for these genes, with profiles that marked branch and/or spikelet meristems or the boundary regions between the axillary meristem and rachis. However, spatial patterning was strictly conserved between the two African rice species and similar to the patterns reported for O. sativa.
Relative expression of panicle-related genes during African rice panicle development
In parallel with morphological analysis, relative accumulation of panicle-related gene transcripts in O. barthii and O. glaberrima was monitored by qRT-PCR at developmental stage 1 (i.e., RM elongation, establishment and initial elongation of PBs; see Fig. 5). Overall, this profiling revealed a higher relative accumulation of transcripts in O. glaberrima compared to O. barthii for the branching-related genes with one exception, the LAX1 gene. However, the latter gene displayed a peak of mRNA accumulation at stage 2 in O. glaberrima rather than at stage 1 as in O. barthii (see Additional file 6). In a same way, a lower accumulation of OSH1 was observed at stage 1 in O. glaberrima but higher accumulation was observed later in development, compared to O. barthii (Additional file 6). A lower accumulation of miR529 in O. glaberrima mirrored the higher accumulation of SPL14 in this species (Fig. 5), in agreement with a miR529-dependent regulation of SPL14 transcript accumulation levels rather than miR156-dependency at this stage.
Fig. 5.
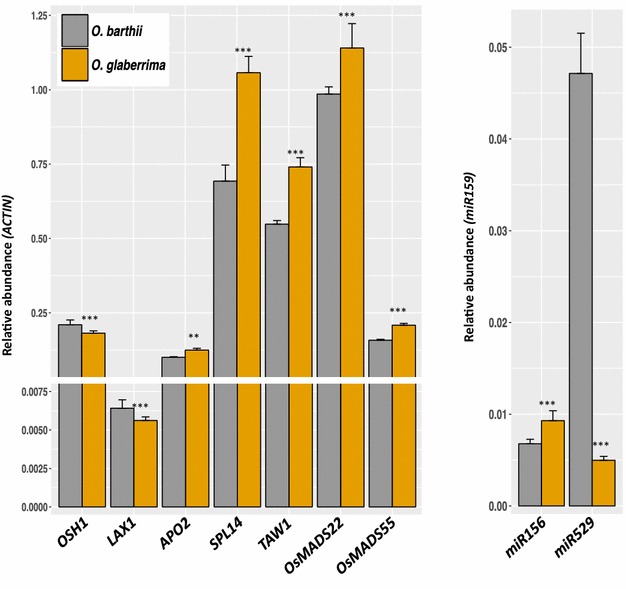
Expression profiling of branching-related genes at unbranched stage in O. barthii and O. glaberrima. qRT-PCR analysis of OSH1, LAX1, SPL14, APO2, TAW1, OsMADS22, OsMADS55, miR529 and miR156 accumulation levels at stage 1 (unbranched stage with elongation of rachis meristem and formation of primary branch meristems) in O. barthii (gray lines) and in O. glaberrima (orange lines). Target mRNA and small RNA accumulation levels were normalized using rice Actin gene (LOC_Os03g50885) transcript and mature miR159 microRNA accumulation levels, respectively. For mRNAs, the graph is divided into two sections due to scale range. Statistical significances (i.e., t test) between the two species for the relative expression levels of each gene are as follows: *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001
Overall, differences in expression levels were observed for orthologous genes between the two species, with higher expression levels for the branching-related genes SPL14, LAX1, APO2, TAW1 (and its targets OsMADS22 and OsMADS55) in O. glaberrima, leading to the hypothesis that both the miR156/miR529/SPL and TAW1 regulatory pathways are differentially active between the two African rice species from the unbranched stage of panicle development.
Divergence of promoter structure between O. glaberrima and O. barthii
We next investigated whether the differences in expression observed between the two African species might be explained by the genes in question being subject to differential cis-regulation. Taking advantage of the recent release of the O. glaberrima CG14 genome and O. barthii genomic data [7] and local genomic resources, genomic sequences of the landmark genes in CG14 and B88 accessions were analyzed to determine whether any observed polymorphisms fell within gene and promoter sequences. A high conservation of coding sequences was observed between the two African rice species. The recognition sites of the miR529 and miR156 microRNAs in the SPL14 gene of the two African species and O. sativa are strictly conserved, indicating that differential expression levels of SPL14 between the two African rice species were not due to an alteration of these sequences (Additional file 7). In contrast, polymorphic sites between O. barthii and O. glaberrima were detected in the promoter region of LAX1 (5 SNPs), SPL14 (24 SNPs), APO2 (32 SNPs), TAW1 (23 SNPs) and LHS1 (11 SNPs) (Additional file 8). Several GTAC motifs, the binding site of SPL proteins [19], were found in the promoters of SPL14, APO2, LAX1, TAW1 and LHS1. However, none of them were affected by polymorphism between the two African rice species. Based on the observed polymorphism in these promoter regions, alteration of expression related to the modification of cis-regulatory elements could therefore not be ruled out.
Discussion
Spatial expression patterns of panicle-related genes are conserved between the two African rice species
This study was based on the assumption that orthologous genes have a conserved function in African rice species compared to O. sativa. This assumption was supported by the fact that there are no non-synonymous changes in orthologous genes between the two species. Moreover, we observed that the spatial expression patterns of the panicle-related genes tested in this study were similar in O. glaberrima and O. barthii.
The O. sativa SPL14 and LAX1 genes and their orthologs in the two African rice species (our study) and in maize (TASSELSHEATH4 and BARREN STALK1, respectively) are expressed at the adaxial boundary adjacent to all branch meristems [25, 37, 39–42]. The O. sativa miR156/miR529/SPL14 regulatory pathway was shown to be involved in the control of panicle branching, notably through the regulation of LAX1 gene expression, which is also reported to be involved in axillary meristem initiation [19, 24, 25, 39–41]. In situ hybridization analysis of SPL14 gene and miRNA expression patterns in the two African rice species revealed that their expression patterns either did not overlap or only overlapped partially: miR529 and miR156 were detected in the center but not in the flank of branch meristems where SPL14 mRNAs were accumulated (Fig. 5). These spatially separated expression domains suggest a regulatory mechanism based on spatial restriction or mutual exclusion rather than on dampening regulation [43]. In other species, separated patterns were observed for miR156 and SPL14-like genes in Arabidopsis thaliana (SPL9 gene) and in O. sativa during the vegetative phase albeit with slight differences, with SPL14-like gene transcripts observed along with miR156 accumulation in both the shoot apical meristem and in leaf primordia [44–46]. This suggests a similar microRNA-dependent regulatory mechanism of SPL gene expression, irrespective of the type of microRNA and of the developmental context. However, the spatial expression of SPL9 was not affected in A. thaliana se1 and ago1-27 mutants, which have reduced miR156 accumulation, suggesting that miR156 is not the main regulator of SPL9 spatial accumulation in leaf primordia [44].
The O. sativa APO2/RFL gene, orthologous to the eudicot floral promoting gene LEAFY (LFY) [14], and the TAW1 gene belonging to the small ALOG (Arabidopsis LSH1 and Oryza G1) gene family have been described as negative regulators of the transition to spikelet meristem fate [32, 34, 35]. However, based on their expression patterns and mutant phenotypes, it would be more accurate to consider these two genes as promoting factors of indeterminate meristematic activity in grass inflorescences. The delay of spikelet meristem specification in the loss of function mutant background may be considered as a consequence of an alteration of branch meristem functioning. In the present study, transcripts of the African rice species orthologs of O. sativa APO2/RFL and TAW1 were detected in both branch and spikelet meristems. The APO2 ortholog expression pattern observed in our study is similar to the one described by [32] in O. sativa. Similarly, a recent analysis of gene expression profiling of reproductive meristem types in early rice inflorescences by laser microdissection has shown that the APO2/RFL gene is expressed in spikelet meristems [47]. The TAW1 ortholog expression pattern observed in the two African rice species differed from the pattern reported in O. sativa [35, 47], in which transcripts were detected only in branch meristems and not in spikelet meristems. However, the expression level of the O. barthii and O. glaberrima TAW1 orthologs is still lower in spikelet than in branch meristems (Additional file 6). This suggests that partial divergence of function might have occurred between TAW1 orthologs genes in Asian and African rice species.
In contrast to the aforementioned genes, the O. sativa spikelet-promoting LHS1/OsMADS1 gene, along with its African rice orthologs, is only expressed in the spikelet meristems ([20, 38]; this study). The maize LHS1 orthologs ZMM8 and ZMM14 are expressed only in the upper floret, and within floral organs of certain sampled taxa, indicating that these genes are involved in the determinacy of the spikelet meristem and in the distinction of upper florets and lower florets in maize inflorescences [48]. In addition, the wheat LHS1 ortholog (WLHS1) is also expressed slightly differently from rice LHS1, the transcript of WLHS1 accumulating at high levels in floret organs (i.e., lemma, palea, pistil, glume) [49]. The LHS1-like SEPALLATA (SEP) genes have been linked with the origin and diversification of the grass spikelet [50].
Overall, we observed a strict conservation of the spatial expression domains of these genes between O. glaberrima and O. barthii, indicating that the spatial regulation of these genes was not affected during African rice domestication. In addition, an extension of the expression domain of TAW1 orthologs to spikelets and floret meristems was observed compared to O. sativa, suggesting an extension of the functional domain of TAW1 orthologs in African rice.
Differential levels of branching-related gene expression at the unbranched developmental stage of African rice panicles
A previous study, based on deep sequencing of panicle-derived small RNA transcriptomes in O. glaberrima and O. barthii, suggested that the spikelet/floret fate acquisition rate differs between the two species: for a similar morphological complexity at the early branching stage, all meristems are converted into spikelets in O. barthii, whereas only those of the apical region of the panicle branches are converted in O. glaberrima, as suggested by the expression pattern of the miR2118-triggered 21-nt phasiRNA pathway (i.e., miR2118, MEL1, lncRNAs and phasiRNAs) and the spikelet-associated MADS-box gene LHS1/OsMADS1 [20].
The present study revealed that in addition to the spikelet/floret marker genes, genes implicated in panicle branching activity were also differentially expressed, in quantitative terms, between the two species, despite their conserved spatial expression patterns, and this from the unbranched stage (i.e., in the rachis meristem before primary branch establishment). All these genes were more highly expressed in the crop species O. glaberrima, with the exception of miR529, as might be expected from its known function. These modifications of expression suggest a higher branching activity in O. glaberrima compared to its wild relative for the initial reproductive stage in the rachis meristem.
This differential expression during panicle development between the two species may be a consequence of genomic evolution affecting cis- and/or trans-regulatory mechanisms. This is often the case for traits associated with dynamic processes which are more readily modified through their regulation (i.e., cis- and/or trans-elements) rather than through coding mutations [51]. Despite the low sequence divergence between the two African rice species [5–7], several SNPs were evident in the promoter regions of the O. glaberrima LHS1, APO2, TAW1, SPL14 and LAX1 genes compared to O. barthii. Consequently, the possibility of alteration of expression through the modification of cis-regulatory elements could not be ruled out.
The observed global alteration of expression of these genes in O. glaberrima with respect to its wild relative would suggest that the expression or activity of one or more very early acting factors in panicle development might be affected. Some parallels might be drawn with the key role of the miR156/miR529/SPL regulatory pathway in the control of panicle development through the branch meristem-promoting genes LAX1 and APO2, and also the miR172/AP2 and PAP2/RCN1 regulatory pathways involved in spikelet transition [19]. However, the relationship between the APO2 and miR156/miR529/SPL pathways still needs to be clarified with regard to the early stages of the transition to the plant reproductive phase. Nevertheless, gene expression profiling of reproductive meristem types in early rice inflorescences by laser microdissection has shown that the APO2 gene reaches a peak of expression in elongating primary branch/axillary meristems and decreases in spikelet meristems, after the SPL14 expression peak in initial primary branch meristems [47]. In the same study, the TAW1 gene as well as its identified target genes (SVP-like MADS-box genes OsMADS22 and OsMADS55) displayed a peak of expression in the rachis meristem and decreased in expression between primary branch and spikelet meristems [47]. The genetic and functional relationship between the miR156/miR529/SPL pathway and TAW1/SVP pathway is not known. However, the present study would suggest a relationship between the two regulatory pathways, as both are more active in O. glaberrima than in O. barthii.
Impact of inflorescence meristem size on panicle architecture
Variations in inflorescence architecture can be explained in part by the rate of initiation of branch meristems and by the rate of transition to floral meristems [12, 15]. However, shoot and inflorescence architectures can be initially influenced by meristem size and maintenance [52]. For example, mutations in CLV and ERECTA pathway genes cause meristems to enlarge, leading to increased shoot and inflorescence branching, more flowers and extra organs in flowers and fruits [53–55]. A comparison of several varieties of O. sativa showed that the number of primary branches was related to the initial size of the reproductive apex and that the number of spikelets per primary branch was positively associated with cell division activity during apex growth [56]. Similarly, it was reported that the alteration of O. sativa panicle architecture in apo1 and apo2 mutants compared to wild-type plants might be associated with a change in inflorescence meristem size in relation to cell proliferation rate [34, 57]. The small panicle with little primary and secondary branching observed in the aforementioned loss of function mutants is associated with a smaller inflorescence meristem displaying reduced cell proliferation activity. Conversely, APO1-over-expressing plants, which are characterized by small, packed but highly branched panicles, have a larger inflorescence meristem [57]. Similarly, plants over-expressing the SPL14 regulator miR156, which are characterized by smaller panicles with low branching complexity, also have a smaller inflorescence meristem [19]. It would be interesting to analyze the SPL and TAW1 mis-expressing plants in this context. Overall, these observations would suggest that the modulation of expression of these early acting genes involved in the miR156/miR529/SPL pathway might influence the cell proliferation activity of the inflorescence/rachis meristem, leading to a modulation of branching activity in O. sativa.
The rachis meristem in O. glaberrima tends to be larger than in its wild relative O. barthii. Given the higher expression levels we observed for the early acting SPL14, APO2 and LAX1 gene orthologs in O. glaberrima compared to O. barthii, it appears that the difference in panicle architecture between the two species could be related to very early differences in the regulation of inflorescence meristem functioning. However, none of the genes studied here co-localized with genomic regions under a selective sweep in O. glaberrima in comparison with O. barthii [7], suggesting that these genes were not directly affected by human selection during African rice domestication. This would suggest that one or more factors upstream of this pathway might have been the target of selection.
Conclusion
The higher complexity of the O. glaberrima panicle compared to its wild relative O. barthii is associated with a wider inflorescence meristem and a modification of expression of early acting genes affecting branching activity. Our study suggests that the miR156/miR529/SPL and TAW1 pathways, in association with early targeted genes (APO2, LAX1, OsMADS22 and OsMADS55), might be affected at the molecular regulatory level. Even though spatial patterns were observed to be conserved, the expression patterns of these genes were found to be modified quantitatively in the rachis meristem. This alteration of gene expression suggests a higher branching activity in O. glaberrima and, as a consequence, delayed spikelet meristem fate acquisition. It will be of great interest to dissect the regulatory mechanisms governing the activity of these genes in rice species in order to understand the initial steps of panicle architecture control and its evolution. Moreover, it will be important to highlight to what extent early morphological events and gene expression were affected in similar ways by the two independent domestication events in Asia and Africa, resulting in the phenotypic convergence of panicle complexity that can now be seen.
Methods
Plant materials
Plants of Oryza glaberrima cv. CG14 (IRRI IRGC acc. #96717, ADN_ID code 142; [6]) and Oryza barthii var. B88 (IRRI IRGC acc. #104141, ADN_ID code 589 W; [6]) were grown in a growth chamber for 7 weeks in long-day conditions (14- to 10-h day/night cycle at 32/28 °C and humidity at 60%) at IRD, Montpellier (France). Plants were then transferred and maintained in short-day conditions (10- to 14-h day/night cycle at 32/28 °C and humidity at 60%) for flowering induction. Panicles were collected at four different stages for histology, RNA isolation and in situ hybridization (Fig. 2): stage 1, rachis and primary branch meristem; stage 2, elongated primary branch and secondary meristem, early spikelet differentiation; stage 3, spikelet differentiation; stage 4, young flowers with differentiated organs.
Histological analyses
All samples were fixed in 4% (w/v) PFA (paraformaldehyde) − 1 × PBS (phosphate-buffered saline) solutions under vacuum for 15 min and then incubated for 16 h at 4 °C. Samples were then treated in serial solutions of 1 × PBS and then dehydrated through a graded ethanol series [50, 70, 80, 90 and 100% (v/v)] for 2 h and stored at 4 °C. They were then transferred into absolute butanol for 2 days and then embedded in resin (Technovit resin, Heraeus Kulzer, Wehrheim, Germany) according to the manufacturer’s instructions. Histological sections (4–5 μm) were made using a rotary microtome (Microm HM 355 S). Sections were double-stained with periodic acid-Schiff’s reagent (Sigma-Aldrich, Lyon, France) and naphthol blue black (NBB, Sigma-Aldrich). Slides were then observed with a Leica DMRB microscope in conjunction with an Evolution MP5.0 color Media Cybernetics camera. Images were processed using Photoshop CS6.
X-ray micro-computed tomography (micro-CT) and morphometric analysis
For X-ray micro-CT, panicle of the main tiller of three independent plants was collected from 6 to 20 days after floral transition. All samples were treated with a solution of 1% (w/v) of phosphotungstic acid in FAA following the protocols given in [21]. All micro-CT data acquisition was performed on a MicroXCT-200 system (Zeiss Microscopy). Treated samples and scanning conditions are summarized in Additional file 2. 3D data reconstruction was performed via XMReconstructor 8.1.6599 (Zeiss Microscopy). The TMX3D Viewer software (Zeiss Microscopy) was used to perform the morphological and morphometric analyses. In order to carry out exact measurements of meristem size, 2D pictures were generated in TMX3D Viewer software. For each stage, a minimum of nine meristems was analyzed from at least three independent panicles.
qRT-PCR expression analysis
Total RNAs (mRNAs and small RNAs) from different stages (stage 1 to stage 4) of rice panicle development were extracted using an RNeasy Plant Mini Kit with RLT and RWT buffers (Qiagen, France). DNAse treatments were performed using the RNAeasy-free DNase set (Qiagen). Reverse transcript (RT) samples preparation and qRT-PCR analysis on mRNAs and miRNAs were performed according to [20], using LightCycler 480 thermocycler (Roche, France). Each set of experiments was repeated twice (with three technical replicates each) using independent RTs from the same biological samples. The efficiency of each primer pair used was measured using a dilution series of a mix of RTs from the two accessions and the four stages. The relative quantification method with an efficiency-corrected calculation model [58] was used to evaluate quantitative variation. In miRNA qRT-PCR, accumulation levels were normalized with respect to the mature miR159 expression level. In mRNA qRT-PCR, mRNAs were normalized with respect to the rice Actin gene (LOC_Os03g50885.1). The primers used are listed in Additional file 9.
In situ hybridization
PCR amplifications were performed with gene-specific antisense primers tailed with a T7 RNA polymerase binding site (see Additional file 9 for primer sequences). The resulting DNA fragments were used directly as templates for synthesizing antisense ribo-probes incorporating UTP–digoxigenin (Roche) as the label with the aid of a T7 Maxi Script kit (Ambion). For miR156 and miR529 detection, 0.02 M of a 5′ digoxigenin-labeled LNA probe complementary to the target (see Additional file 9 for primer sequences) was used. In situ hybridization experiments were carried out as described by [59]. Detection was performed using the Vector Blue Alkaline Phosphatase Substrate Kit III (Vector Laboratories). Slides were observed and photographed using an Evolution MP5.0 color Media Cybernetics camera in conjunction with a Leica DMRB microscope, and images were processed using Photoshop CS6. Each experiment was repeated at least twice, using at least two sample series (i.e., two paraffin blocks) each time, and the reported spatial patterns were observed in at least two repeats.
Gene sequencing and data processing
SNP polymorphisms of the genomic sequences of the landmark genes from O. glaberrima and O. barthii (i.e., promoters, UTRs, CDS and introns) were obtained by using O. sativa MSU7.0 reference sequence (http://rice.plantbiology.msu.edu/) for each locus in conjunction with genomic sequences from O. glaberrima CG14 [60] and from O. barthii B88 accession (kindly provided by F. Sabot, http://irigin.org). The identified polymorphic sites were validated against a previous version of CG14 genomic sequence [7] using the Gramene database (http://blast.gramene.org/Multi/blastview) in conjunction with the BLASTn program [61]. A 2.5-Kb-long region upstream from the ATG codon was used for promoter sequence analysis. Identification of putative transcription factor binding sites (TFBSs) in promoter regions was performed using the Genomatix software package (http://www.genomatix.de/) in conjunction with the O. glaberrima CG14 genomic sequence from Gramene database.
Authors’ contributions
KNT performed molecular analyses (PCR, RT-PCR, in situ hybridization); HA performed the sample preparation and the analysis of histological and X-ray tomography data. YS conducted the X-ray tomography analysis with the support of JS. TH and JT contributed to the analysis of morphological and molecular data. SJ and HA initiated and led the project with the support of PG, NVD and AG. KNT, HA, YS JT, TH, PG and SJ wrote manuscript together. All authors read and approved the final manuscript.
Acknowledgements
We would like to thank F. Morcillo and S. Dussert for access to Genomatix software, M. Collin for technical advice for histological work as well as S. Chéron and H. Chrestin for plant care in the greenhouse. F. Sabot, C. Tranchant-Dubreuil and H. Pidon are also thanked for their bioinformatic support.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article and its additional files.
Funding
This project was jointly supported by Agropolis Foundation [through the “Investissements d’avenir” programme (ANR-10-LABX-0001-01)] and Fondazione Cariplo under the reference ID EVOREPRICE 1201-004. This work was also supported by the Institut de Recherche pour le Développement (IRD) institutional funding, Vietnamese government (program 322 to TKN) and the Global Rice Science Partnership (GRiSP) scholarship program (TKN).
Abbreviations
- AM
axillary meristem
- Fl
flower
- micro-CT
X-ray micro-computed tomography
- PB
primary branch
- PBm
primary branch meristem
- qRT-PCR
quantitative real-time reverse transcription polymerase chain reaction
- RM
rachis meristem
- SAM
shoot apical meristem
- SB
secondary branch
- SD
standard deviation
- Sp
spikelet
- SpM
spikelet meristem
- TB
tertiary branch
- TFBS
transcription factor binding site
Additional files
Additional file 1. Mean, median and standard deviation (SD) values of panicle-related traits in O. barthii (B88) and O. glaberrima (CG14). PB primary branch, Sb secondary branch, Sp spikelet. For each species, n = 18.
Additional file 2. Illustration of X-ray tomography reconstructed images of the sample scanning and 3D model. a Reconstructed transverse section (y–x view) of the sample, b reconstructed longitudinal (x–z view) section of the sample, c reconstructed longitudinal (y–z view) section of the sample, d 3D model of the sample. Meristem width was measured on a reconstructed transverse section at 40 μm from the top of the meristem. Scale bars = 200 μm.
Additional file 3. X-ray tomography scanning parameters of O. glaberrima and O. barthii samples.
Additional file 4. longitudinal sections and 3D models of O. glaberrima and O. barthii panicles at very early developmental stages using X-Ray tomography.
Additional file 5. Spatial expression patterns of OSH1, LAX1, SPL14, miR156, miR529, LHS1, TAW1 and APO2 genes during panicle development of O. glaberrima and O. barthii. This figure illustrates the in situ hybridization data during panicle development not presented in Fig. 4 of the main text.
Additional file 6. Expression profiling of panicle-related genes during panicle development in O. barthii and O. glaberrima. qRT-PCR analysis of OSH1, LAX1, SPL14, APO2, TAW1, LHS1, OsMADS22, OsMADS55, miR529 and miR156 accumulation levels during panicle development (from stage 1 to stage 3) in O. barthii (black lines) and in O. glaberrima (gray lines). Target mRNA and small RNA accumulation levels were normalized using the rice Actin gene (LOC_Os03g50885) transcript and mature miR159 microRNA accumulation levels, respectively. Expression levels are relative to O. barthii B88 stage 1 (y-axis).
Additional file 7. miR156 and miR529 recognition sites in SPL14 mRNA target in O. sativa (OsSPL14), O. glaberrima (OgSPL14) and O. barthii (ObSPL14). The single nucleotide change in O. sativa from C to A at the Osa-miR156 targeted site in OsSPL14 as reported in the japonica cultivars Aikawa1 and Shaoniejing (SNJ) by [24, 25], respectively, is highlighted in red. Numbers above the sequence indicate the location of the nucleotide in the OsSPL14 coding sequence. Dots indicate identical nucleotide sequences in the region corresponding to the recognition sites of Osa-miR156 and Osa-miR529.
Additional file 8. Promoter sequence comparisons of LHS1, APO2 and SPL14 orthologous genes in O. glaberrima and O. barthii. Vertical bars represent SNPs in O. barthii vs. O. glaberrima. Putative transcription factor binding sites related to SNPs are indicated by arrowheads (indicating site orientation). These binding sites are described as being involved in hormone response (ABA and jasmonate, JAS) [62, 63, 65] and in RNA polymerase II transcription activity [64]. The sequence alignments of these sites between O. barthii (Ob) and O. glaberrima (Og) are indicated on the right, and the corresponding polymorphic site is highlighted.
Additional file 9. List of primers used in this study. The underlined sequences correspond to the T7 promoter used for RNA probe synthesis. The sequences in italic correspond to the stem-loop region used for stem-loop qRT-PCRs. Bases shown in square brackets were LNA-modified for in situ hybridization.
Footnotes
K. N. Ta and H. Adam contributed equally to this work
Contributor Information
K. N. Ta, Email: nhungtakim161@gmail.com
H. Adam, Email: helene.adam@ird.fr
Y. M. Staedler, Email: yannick.staedler@univie.ac.at
J. Schönenberger, Email: juerg.schoenenberger@univie.ac.at
T. Harrop, Email: thomas.harrop@ird.fr
J. Tregear, Email: james.tregear@ird.fr
N. V. Do, Email: nangvinhdo@gmail.com
P. Gantet, Email: pascal.gantet@univ-montp2.fr
A. Ghesquière, Email: alain.ghesquiere@ird.fr
S. Jouannic, Phone: +33 (0)4 67 41 62 41, Email: stephane.jouannic@ird.fr
References
- 1.Vaughan DA, Lu BR, Tomooka N. The evolving story of rice evolution. Plant Sci. 2008;174:394–408. doi: 10.1016/j.plantsci.2008.01.016. [DOI] [Google Scholar]
- 2.Huang X, Kurata N, Wei X, Wang ZX, Wang A, Zhao Q, Zhao Y, Liu K, Lu H, Li W, Guo Y, Lu Y, Zhou C, Huang T, Zhang L, Wang Y, Feng L, Furuumi H, Kubo T, Miyabayashi T, Yuan X, Xu A, Dong G, Zhan Q, Li C, Fujiyam A, Toyada A, Lu A, Feng Q, Aian Q, Li J, Han B. A map of rice genome variation reveals the origin of cultivated rice. Nature. 2012;490:497–501. doi: 10.1038/nature11532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Q-J, Zhu T, Xia E-H, Shi C, Liu Y-L, Zhang Y, Liu Y, Jiang W-K, Zhao Y-J, Mao S-Y, Zhang L-P, Huang H, Jiao J-Y, Xu P-Z, Yao Q-Y, Zeng F-C, Yang L-L, Gao J, Tao D-Y, Wang Y-J, Bennetzen JL, Gao L-Z. Rapid diversification of five Oryza AA genomes associated with rice adaptation. Proc Natl Acad Sci USA. 2014;111:E4954–E4962. doi: 10.1073/pnas.1418307111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li ZM, Zheng XM, Ge S. Genetic diversity and domestication history of African rice (Oryza glaberrima) as inferred from multiple gene sequences. Theor Appl Genet. 2011;123:21–31. doi: 10.1007/s00122-011-1563-2. [DOI] [PubMed] [Google Scholar]
- 5.Nabholz B, Sarah G, Sabot F, Ruiz M, Adam H, Nidelet S, Ghesquière A, Santoni S, David J, Glémin S. Transcriptome population genomics reveals severe bottleneck and domestication cost in the African Rice (Oryza glaberrima) Mol Ecol. 2014;23:2210–2227. doi: 10.1111/mec.12738. [DOI] [PubMed] [Google Scholar]
- 6.Orjuela J, Sabot F, Chéron S, Vigouroux Y, Adam H, Chrestin H, Sanni K, Lorieux M, Ghesquière A. An extensive analysis of the African rice genetic diversity through a global genotyping. Theor Appl Genet. 2014;127:2211–2223. doi: 10.1007/s00122-014-2374-z. [DOI] [PubMed] [Google Scholar]
- 7.Wang M, Yu Y, Haberer G, Marri PR, Fan C, Goicoechea JL, Zuccolo A, Song X, Kudrna D, Ammiraju JSS, Cossu RM, Maldonado C, Chen J, Lee S, Sisneros N, de Baynast K, Golser W, Wissotski M, Kim W, Sanchez P, Ndjiondjop MN, Sanni K, Long M, Carney J, Panaud O, Wicker T, Machado CA, Chen M, Mayer KFX, Rounsley S, Wing RA. The genome sequence of African rice (Oryza glaberrima) and evidence for independent domestication. Nat Genet. 2014;46:982–988. doi: 10.1038/ng.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doebley JF, Brandon SG, Bruce DS. The molecular genetics of crop domestication. Cell. 2006;127:1309–1321. doi: 10.1016/j.cell.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Sweeney M, McCouch S. The complex history of the domestication of rice. Ann Bot. 2007;100:951–957. doi: 10.1093/aob/mcm128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ikeda K, Sunohara H, Nagato Y. Developmental course of inflorescence and spikelet in rice. Breed Sci. 2004;54:147–156. doi: 10.1270/jsbbs.54.147. [DOI] [Google Scholar]
- 11.Kyozuka J, Tokunaga H, Yoshida A. Control of grass inflorescence form by the fine-tuning of meristem phase change. Curr Opin Plant Biol. 2014;17:110–115. doi: 10.1016/j.pbi.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 12.Prusinkiewicz P, Erasmus Y, Lane B, Harder LD, Coen E. Evolution and development of inflorescence architectures. Science. 2007;316:1452–1456. doi: 10.1126/science.1140429. [DOI] [PubMed] [Google Scholar]
- 13.Koes R. Evolution and development of virtual inflorescences. Trends Plant Sci. 2008;13:1–3. doi: 10.1016/j.tplants.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Moyroud E, Kusters E, Monniaux M, Koes R, Parcy F. LEAFY blossoms. Trends Plant Sci. 2010;15:346–352. doi: 10.1016/j.tplants.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Park SJ, Eshed Y, Lippman ZB. Meristem maturation and inflorescence architecture: lessons from the Solanaceae. Curr Opin Plant Biol. 2014;17:70–77. doi: 10.1016/j.pbi.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Lemmon ZH, Park SJ, Jiang K, Van Eck J, Schatz MC, Lippman ZB. The evolution of inflorescence diversity in the nightshades and heterochrony during meristem maturation. Genome Res. 2016;26:1676–1686. doi: 10.1101/gr.207837.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xing Y, Zhang Q. Genetic and molecular bases of rice yield. Ann Rev Plant Biol. 2010;61:421–442. doi: 10.1146/annurev-arplant-042809-112209. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Li J. Branching in rice. Curr Opin Plant Biol. 2011;14:94–99. doi: 10.1016/j.pbi.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Wang L, Sun S, Jin J, Fu D, Yang X, Weng X, Xu C, Li X, Xiao J, Zhang Q. Coordinated regulation of vegetative and reproductive branching in rice. Proc Natl Acad Sci USA. 2015;112:15504–15509. doi: 10.1073/pnas.1521949112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ta KN, Sabot F, Adam H, Vigouroux Y, De Mita S, Ghesquière A, Do NV, Gantet P, Jouannic S. miR2118-triggered phased siRNAs are differentially expressed during the panicle development of wild and domesticated African rice species. Rice (NY) 2016;9:10. doi: 10.1186/s12284-016-0082-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Staedler YM, David M, Jürg S. Plant tissues in 3D via X-ray tomography: simple contrasting methods allow high resolution imaging. PloS ONE. 2013;8:e75295. doi: 10.1371/journal.pone.0075295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.AL-Tam F, Adam H, Anjos AD, Lorieux M, Larmande P, Ghesquière A, Jouannic S, Shahbazkia HR. P-TRAP: a Panicle TRAit Phenotyping tool. BMC Plant Biol. 2013;13:122. doi: 10.1186/1471-2229-13-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsuda K, Hake S. Diverse functions of KNOX transcription factors in the diploid body plan of plants. Curr Opin Plant Biol. 2015;27:91–96. doi: 10.1016/j.pbi.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 24.Jiao Y, Wang Y, Xue D, Wang J, Yan M, Liu G, Dong G, Zeng D, Lu Z, Zhu X, Qian Q, Li J. Regulation of OsSPL14 by OsmiR156 defines ideal plant architecture in rice. Nat Genet. 2010;42:541–544. doi: 10.1038/ng.591. [DOI] [PubMed] [Google Scholar]
- 25.Miura K, Ikeda M, Matsubara A, Song XJ, Ito M, Asano K, Matsuoka M, Kitano H, Ashikari M. OsSPL14 promotes panicle branching and higher grain productivity in rice. Nat Genet. 2010;42:545–549. doi: 10.1038/ng.592. [DOI] [PubMed] [Google Scholar]
- 26.Jeong DH, Park S, Zhai J, Gurazada SGR, De Paoli E, Meyers BC, Green PJ. Massive analysis of rice small RNAs: mechanistic implications of regulated microRNAs and variants for differential target RNA cleavage. Plant Cell. 2011;23:4185–4207. doi: 10.1105/tpc.111.089045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Komatsu K, Maekawa M, Ujiie S, Satake Y, Furutani I, Okamoto H, Shimamoto K, Kyozuka J. LAX and SPA: major regulators of shoot branching in rice. Proc Natl Acad Sci USA. 2003;100:11765–11770. doi: 10.1073/pnas.1932414100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agrawal GK, Abe K, Yamazaki M, Miyao A, Hirochika H. Conservation of the E-function for floral organ identity in rice revealed by the analysis of tissue culture-induced loss-of-function mutants of the OsMADS1 gene. Plant Mol Biol. 2005;59:125–135. doi: 10.1007/s11103-005-2161-y. [DOI] [PubMed] [Google Scholar]
- 29.Jeon JS, Jang S, Lee S, Nam J, Kim C, Lee SH, Chung YY, Kim SR, Lee YH, Cho YG, et al. leafy hull sterile1 is a homeotic mutation in a rice MADS box gene affecting rice flower development. Plant Cell. 2000;12:871–884. doi: 10.1105/tpc.12.6.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen ZX, Wu JG, Ding WN, Chen HM, Wu P, Shi CH. Morphogenesis and molecular basis on naked seed rice, a novel homeotic mutation of OsMADS1 regulating transcript level of AP3 homologue in rice. Planta. 2006;5:882–890. doi: 10.1007/s00425-005-0141-8. [DOI] [PubMed] [Google Scholar]
- 31.Khanday I, Yadav SR, Vijayraghavan U. Rice LHS1/OsMADS1 controls floret meristem specification by coordinated regulation of transcription factors and hormone signaling pathways. Plant Physiol. 2013;161:1970–1983. doi: 10.1104/pp.112.212423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rao NN, Prasad K, Kumar PR, Vijayraghavan U. Distinct regulatory role for RFL, the rice LFY homolog, in determining flowering time and plant architecture. Proc Natl Acad Sci USA. 2008;105:3646–3651. doi: 10.1073/pnas.0709059105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ikeda K, Ito M, Nagasawa N, Kyozuka J, Nagato Y. Rice ABERRANT PANICLE ORGANIZATION1, encoding an F-box protein, regulates meristem fate. Plant J. 2007;51:1030–1040. doi: 10.1111/j.1365-313X.2007.03200.x. [DOI] [PubMed] [Google Scholar]
- 34.Ikeda-Kawakatsu K, Maekawa M, Takeshi I, Tioh JI, Nagato Y. ABERRANT PANICLE ORGANIZATION 2/RFL, the rice ortholog of Arabidopsis LEAFY, suppresses the transition from inflorescence meristem to floral meristem through interaction with APO1. Plant J. 2012;69:168–180. doi: 10.1111/j.1365-313X.2011.04781.x. [DOI] [PubMed] [Google Scholar]
- 35.Yoshida A, Sasao M, Yasuno N, Takagi K, Daimon Y, Chen R, Yamazaki R, Tokunaga H, Kitaguchi Y, Sato Y, Nagamura Y, Ushijima T, Kumamaru T, Iida S, Maekawa M, Kyozuka J. TAWAWA1, a regulator of rice inflorescence architecture, functions through the suppression of meristem phase transition. Proc Natl Acad Sci USA. 2013;110:767–772. doi: 10.1073/pnas.1216151110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sentoku N, Sato Y, Kurata N, Ito Y, Kitano H, Matsuoka M. Regional expression of the rice KN1-type homeobox gene family during embryo, shoot, and flower development. Plant Cell. 1999;11:1651–1664. doi: 10.1105/tpc.11.9.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luo L, Li W, Miura K, Ashikari M, Kyozuka J. Control of tiller growth of rice by OsSPL14 and strigolactones, which work in two independent pathways. Plant Cell Physiol. 2012;53:1793–1801. doi: 10.1093/pcp/pcs122. [DOI] [PubMed] [Google Scholar]
- 38.Kobayashi K, Maekawa M, Miyao A, Hirochika H. Kyozuka J PANICLE PHYTOMER2 (PAP2), encoding a SEPALLATA subfamily MADS-Box protein, positively controls spikelet meristem identity in rice. Plant Cell Physiol. 2010;51:47–57. doi: 10.1093/pcp/pcp166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Komatsu M, Maekawa M, Shimamoto K, Kyozuka J. The LAX1 and FRIZZY PANICLE 2 genes determine the inflorescence architecture of rice by controlling rachis-branch and spikelet development. Dev Biol. 2001;231:364–373. doi: 10.1006/dbio.2000.9988. [DOI] [PubMed] [Google Scholar]
- 40.Gallavotti A, Zhao Q, Kyozuka J, Meeley RB, Ritter MK, Doebley JF, Pè ME, Schmidt RJ. The role of barren stalk1 in the architecture of maize. Nature. 2004;432:630–635. doi: 10.1038/nature03148. [DOI] [PubMed] [Google Scholar]
- 41.Oikawa T, Kyozuka J. Two-step regulation of LAX PANICLE1 protein accumulation in axillary meristem formation in rice. Plant Cell. 2009;21:1095–1108. doi: 10.1105/tpc.108.065425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chuck G, Whipple C, Jackson D, Hake S. The maize SBP-Box transcription factor encoded by tasselsheath4 regulates bract development and the establishment of meristem boundaries. Development. 2010;137:1243–1250. doi: 10.1242/dev.048348. [DOI] [PubMed] [Google Scholar]
- 43.Voinnet O. Origin, biogenesis, and activity of plant microRNAs. Cell. 2009;136:669–687. doi: 10.1016/j.cell.2009.01.046. [DOI] [PubMed] [Google Scholar]
- 44.Wang J-W, Schwab R, Czech B, Mica E, Weigel D. Dual effects of miR156-targeted SPL genes and CYP78A5/KLUH on plastochron length and organ size in Arabidopsis thaliana. Plant Cell. 2008;20:1231–1243. doi: 10.1105/tpc.108.058180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang J-W, Czech B, Weigel D. miR156-regulated SPL transcription factors define an endogenous flowering pathway in Arabidopsis thaliana. Cell. 2009;138:738–749. doi: 10.1016/j.cell.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 46.Xie K, Shen J, Hou X, Yao J, Li X, Xiao J, Xiong L. Gradual increase of miR156 regulates temporal expression changes of numerous genes during leaf development in rice. Plant Physiol. 2012;158:1382–1394. doi: 10.1104/pp.111.190488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harrop TWR, Ud Din I, Gregis V, Osnato M, Jouannic S, Adam H, Kater MM. Gene expression profiling of reproductive meristem types in early rice inflorescences by laser microdissection. Plant J. 2016;86:75–88. doi: 10.1111/tpj.13147. [DOI] [PubMed] [Google Scholar]
- 48.Cacharron NJ, Saedler H, Theissen G. Expression of MADS box genes ZMM8 and ZMM14 during inflorescence development of Zea Mays discriminates between the upper and the lower floret of each spikelet. Dev Genes Evol. 1999;209:411–420. doi: 10.1007/s004270050271. [DOI] [PubMed] [Google Scholar]
- 49.Shitsukawa N, Tahira C, Kassai KI, Hirabayashi C, Shimizu T, Takumi S, Mochida K, Kawaura K, Ogihara Y, Murai K. Genetic and epigenetic alteration among three homoeologous genes of a class E MADS box gene in hexaploid wheat. Plant Cell. 2007;19:1723–1737. doi: 10.1105/tpc.107.051813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Malcomber ST, Preston JC, Reinheimer R, Kossuth J, Kellogg EA. Developmental gene evolution and the origin of grass inflorescence diversity. Adv Bot Res. 2006;44:425–481. doi: 10.1016/S0065-2296(06)44011-8. [DOI] [Google Scholar]
- 51.Wray GA. The evolutionary significance of cis-regulatory mutations. Nat Rev Genet. 2007;8:206–216. doi: 10.1038/nrg2063. [DOI] [PubMed] [Google Scholar]
- 52.Laufs P, Olivier G, Claudia J, Kiên K, Traas J. Cellular parameters of the shoot apical meristem in Arabidopsis. Plant Cell. 1998;10:1375–1389. doi: 10.1105/tpc.10.8.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bommert P, Nagasawa NS, Jackson D. Quantitative variation in maize kernel row number is controlled by the FASCIATED EAR2 locus. Nat Genet. 2013;45:334–3347. doi: 10.1038/ng.2534. [DOI] [PubMed] [Google Scholar]
- 54.Mandel T, Fanny M, Yaarit K, Jennifer CF, Cristel C, Leor EW. The ERECTA receptor kinase regulates Arabidopsis shoot apical meristem size, phyllotaxy and floral meristem identity. Development. 2014;141:830–841. doi: 10.1242/dev.104687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu C, Liberatore KL, MacAlister CA, Huang Z, Chu YH, Jiang K, Brooks C, Ogawa-Ohnishi M, Xiong G, Pauly M, Van Eck J, Matsubayashi Y, van der Knaap E, Lippman ZB. A cascade of arabinosyltransferases controls shoot meristem size in tomato. Nat Genet. 2015;47:784–792. doi: 10.1038/ng.3309. [DOI] [PubMed] [Google Scholar]
- 56.Mu C, Nemoto K, You Z, Yamagishi J. Size and activity of shoot apical meristems as determinants of floret number in rice panicles. Plant Prod Sci. 2015;8:51–59. doi: 10.1626/pps.8.51. [DOI] [Google Scholar]
- 57.Ikeda-Kawakatsu K, Yasuno N, Oikawa T, Iida S, Nagato Y, Maekawa M, Kyozuka J. Expression level of ABERRANT PANICLE ORGANIZATION1 determines rice inflorescence form through control of cell proliferation in the meristem. Plant Physiol. 2009;150:736–747. doi: 10.1104/pp.109.136739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pfaffl MW. Relative quantification. In: Dorak T, editor. Real-time PCR. San Diego: International University Line; 2006.
- 59.Adam H, Marguerettaz M, Qadri R, Adroher B, Richaud F, Collin M, Thuillet AC, Vigouroux Y, Laufs P, Tregear JW, Jouannic S. Divergent expression patterns of miR164 and CUP-SHAPED COTYLEDON genes in palms and other monocots: implication for the evolution of meristem function in angiosperms. Mol Biol Evol. 2011;28:1439–1454. doi: 10.1093/molbev/msq328. [DOI] [PubMed] [Google Scholar]
- 60.Monat C, Pera B, Ndjiondjop MN, Sow M, Tranchant-Dubreuil C, Bastianelli L, Ghesquière A, Sabot F. De novo assemblies of three Oryza glaberrima accessions provide first insights about pan-genome of African rices. Genome Biol Evol. 2016 doi: 10.1093/gbe/evw253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 62.Busk K, Pagès M. Protein binding to the abscisic acid-responsive element is independent of VIVIPAROUS1 in vivo. Plant Cell. 1997;9:2261–2270. doi: 10.1105/tpc.9.12.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Figueroa P, Browse J. The Arabidopsis JAZ2 promoter contains a G-Box and thymidine-rich module that are necessary and sufficient for jasmonate-dependent activation by MYC transcription factors and repression by JAZ proteins. Plant Cell Physiol. 2012;53:330–343. doi: 10.1093/pcp/pcr178. [DOI] [PubMed] [Google Scholar]
- 64.Ohler U, Wassarman DA. Promoting developmental transcription. Development. 2010;137:15–26. doi: 10.1242/dev.035493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Simpson SD, Nakashima K, Narusaka Y, Seki M, Shinozaki K, Shinozaki KY. Two different novel cis-acting elements of ERD1, a CLPa homologous Arabidopsis gene function in induction by dehydration stress and dark-induced senescence. Plant J. 2003;33:259–270. doi: 10.1046/j.1365-313X.2003.01624.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article and its additional files.


