Abstract
A method for transforming Tetrahymena has been established earlier, but its application has been limited because of the lack of selectable markers other than the rRNA-encoding DNA (rDNA). Mutations in the yeast ribosomal protein L29 gene (CYH2) are known that confer cycloheximide resistance. We have cloned and sequenced the homologue of this gene from both a wild-type and a cycloheximide-resistant (ChxA) strain of Tetrahymena. Surprisingly, a comparison shows that the ChxA mutation is not present in the CYH2 homologue. We therefore created the yeast mutations in the Tetrahymena gene by site-directed mutagenesis and used them to transform Tetrahymena either with or without linking to an rDNA vector. All clones transformed by the rDNA vector also became resistant to cycloheximide when the rDNA contained the engineered mutant genes. Without the rDNA vector, the mutant genes transform approximately 1% of injected cells to become resistant to cycloheximide. DNA analysis indicates that transformation occurs by replacement of the host sequence and not by random integration of the injected sequence. The replacement occurs to some but not all copies of this gene in the polyploid macronuclear genome. Thus, transformation in Tetrahymena occurs by specific sequence replacement, and the injected mutant genes can serve as dominant selectable transformation markers in this organism.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austerberry C. F., Yao M. C. Nucleotide sequence structure and consistency of a developmentally regulated DNA deletion in Tetrahymena thermophila. Mol Cell Biol. 1987 Jan;7(1):435–443. doi: 10.1128/mcb.7.1.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belhumeur P., Paterno G. D., Boileau G., Claverie J. M., Skup D. Isolation and characterisation of a murine cDNA clone highly homologous to the yeast L29 ribosomal protein gene. Nucleic Acids Res. 1987 Feb 11;15(3):1019–1029. doi: 10.1093/nar/15.3.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleyman L. K., Bruns P. J. Genetics of cycloheximide resistance in Tetrahymena. Genetics. 1977 Oct;87(2):275–284. doi: 10.1093/genetics/87.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorovsky M. A., Yao M. C., Keevert J. B., Pleger G. L. Isolation of micro- and macronuclei of Tetrahymena pyriformis. Methods Cell Biol. 1975;9(0):311–327. doi: 10.1016/s0091-679x(08)60080-1. [DOI] [PubMed] [Google Scholar]
- Kreader C. A., Heckman J. E. Isolation and characterization of a Neurospora crassa ribosomal protein gene homologous to CYH2 of yeast. Nucleic Acids Res. 1987 Nov 11;15(21):9027–9042. doi: 10.1093/nar/15.21.9027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käufer N. F., Fried H. M., Schwindinger W. F., Jasin M., Warner J. R. Cycloheximide resistance in yeast: the gene and its protein. Nucleic Acids Res. 1983 May 25;11(10):3123–3135. doi: 10.1093/nar/11.10.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martindale D. W. Codon usage in Tetrahymena and other ciliates. J Protozool. 1989 Jan-Feb;36(1):29–34. doi: 10.1111/j.1550-7408.1989.tb02679.x. [DOI] [PubMed] [Google Scholar]
- McKnight G. L., McConaughy B. L. Selection of functional cDNAs by complementation in yeast. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4412–4416. doi: 10.1073/pnas.80.14.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen H., Andreasen P. H., Dreisig H., Kristiansen K., Engberg J. An intron in a ribosomal protein gene from Tetrahymena. EMBO J. 1986 Oct;5(10):2711–2717. doi: 10.1002/j.1460-2075.1986.tb04555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orias E., Larson D., Hu Y. F., Yu G. L., Karttunen J., Løvlie A., Haller B., Blackburn E. H. Replacement of the macronuclear ribosomal RNA genes of a mutant Tetrahymena using electroporation. Gene. 1988 Oct 30;70(2):295–301. doi: 10.1016/0378-1119(88)90201-6. [DOI] [PubMed] [Google Scholar]
- Orr-Weaver T. L., Szostak J. W., Rothstein R. J. Yeast transformation: a model system for the study of recombination. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6354–6358. doi: 10.1073/pnas.78.10.6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts C. T., Jr, Orias E. A cycloheximide-resistant mutant of Tetrahymena pyriformis. Exp Cell Res. 1973 Oct;81(2):312–316. doi: 10.1016/0014-4827(73)90520-x. [DOI] [PubMed] [Google Scholar]
- Stöcklein W., Piepersberg W., Böck A. Amino acid replacements in ribosomal protein YL24 of Saccharomyces cerevisiae causing resistance to cycloheximide. FEBS Lett. 1981 Dec 28;136(2):265–268. doi: 10.1016/0014-5793(81)80632-1. [DOI] [PubMed] [Google Scholar]
- Sutton C. A., Ares M., Jr, Hallberg R. L. Cycloheximide resistance can be mediated through either ribosomal subunit. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3158–3162. doi: 10.1073/pnas.75.7.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney R., Yao M. C. Identifying functional regions of rRNA by insertion mutagenesis and complete gene replacement in Tetrahymena thermophila. EMBO J. 1989 Mar;8(3):933–938. doi: 10.1002/j.1460-2075.1989.tb03454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tondravi M. M., Yao M. C. Transformation of Tetrahymena thermophila by microinjection of ribosomal RNA genes. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4369–4373. doi: 10.1073/pnas.83.12.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M. C., Kimmel A. R., Gorovsky M. A. A small number of cistrons for ribosomal RNA in the germinal nucleus of a eukaryote, Tetrahymena pyriformis. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3082–3086. doi: 10.1073/pnas.71.8.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
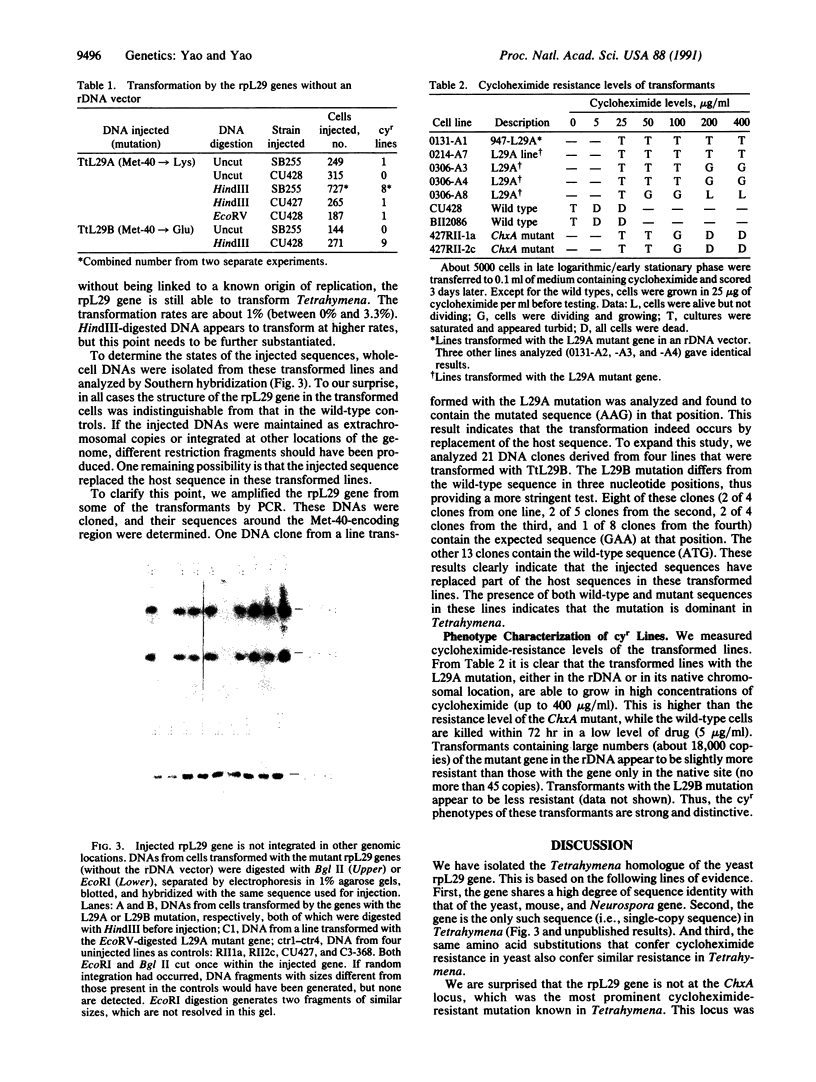
- Yao M. C., Yao C. H. Accurate processing and amplification of cloned germ line copies of ribosomal DNA injected into developing nuclei of Tetrahymena thermophila. Mol Cell Biol. 1989 Mar;9(3):1092–1099. doi: 10.1128/mcb.9.3.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M. C., Yao C. H., Monks B. The controlling sequence for site-specific chromosome breakage in Tetrahymena. Cell. 1990 Nov 16;63(4):763–772. doi: 10.1016/0092-8674(90)90142-2. [DOI] [PubMed] [Google Scholar]
- Yu G. L., Hasson M., Blackburn E. H. Circular ribosomal DNA plasmids transform Tetrahymena thermophila by homologous recombination with endogenous macronuclear ribosomal DNA. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5151–5155. doi: 10.1073/pnas.85.14.5151. [DOI] [PMC free article] [PubMed] [Google Scholar]