Abstract
Background
Disasters are mega-scale catastrophic events which cause trauma and mental health sequelae. A review of early pharmacological interventions for the prevention of psychiatric disorders following disasters is sorely needed.
Methods
A literature search of “Psychiatric Sequelae AND Disasters”, “Disaster mental health/Disaster psychiatry”, “Psychotropics AND Disasters”, and “Drug therapy AND Disasters” yielded 213 articles, 38 of which were included in the review.
Results
Common post-disaster psychiatric conditions are: posttraumatic stress disorder (PTSD), depressive and anxiety disorders, substance use disorders and medically-unexplained psychological symptoms. Early psychopharmacological interventions to prevent PTSD provide promising evidence for hydrocortisone in medically ill trauma populations. Less robust benefits were noted for other pharmacological interventions. No reported trials have explored prevention of depression or other common post-disaster psychiatric conditions.
Conclusion
Hydrocortisone shows promise in preventing and reducing the psychiatric sequelae of PTSD following disasters. Further evaluation of hydrocortisone and other potentially beneficial psychopharmacological interventions are needed.
Keywords: disaster, psychopharmacological interventions, prevention, psychiatric conditions, psychiatric sequelae
Introduction
Disasters are mega-scale unanticipated catastrophic events that cause destruction, trauma, various psychological sequelae and death.1,2 Though no standard defines a disaster, mega-scale traumatic events share in common the following negative characteristics: disasters threaten large groups of people with harm or death, disrupt social networks, cause widespread loss of community resources and ultimately place those affected at risk for negative mental and physical health outcomes.1-3
McFarlane and colleagues report that globally an average of at least one disaster occurs every day due to an increasing population density and climate change. Morbidity and mortality from these occurrences are compounded by the effects of trauma exposure on mental health, resulting in increased need for mental health treatment post-disaster.4 According to the International Disaster Database, in 2005, approximately 162 million people were affected globally while in 2010 more than 330 million people were affected.5,6 One large analyses of 60,000 disaster victims showed that samples would struggle psychologically if they endured mass violence (e.g. terrorism, shooting sprees) rather than natural or technological disasters.2,7
Post-disaster psychiatric conditions most commonly described in the literature include: PTSD, MDD, anxiety disorders, substance use disorder (SUD) and other medically unexplained psychological symptoms (MUPS).8 The addition of PTSD to Diagnostic and Statistical Manual of Mental Disorders-third edition in 1980, development of International Society of Traumatic Stress Studies (ISTSS) and the implementation of planning and response guides by World Health Organization (WHO) have made way for the development of evidence-based guidelines for early intervention, treatment and response to mass violence.7 Immediate psychological interventions such as psychological debriefing and psychological first aid have been implemented to prevent or reduce psychological sequelae after disasters with minimal to no evidence of success.9,10 Trauma focused cognitive behavioral therapy within three months of trauma seems to be effective for those patients who seem to reach a clinical threshold for diagnosis of acute stress disorder (ASD) and PTSD.11 As psychological treatments may not be effective or immediately available post-disaster, a working understanding of effective evidence-based pharmacologic treatments for highly traumatized victims at risk for psychiatric sequelae provides an important option for overwhelmed disaster relief workers. This review describes the psychopharmacological interventions that can be recommended either immediately or early following disaster to prevent the development of psychiatric disorders and the psychological sequelae.
Methods
Literature Search
Authors searched PubMed database using MeSh terms such as ‘Disaster’, ‘Psychiatry’, ‘prevention and control’ and ‘Mental Disorders’. Further Boolean terms were employed to narrow the search terms. The following terms were used to conduct a targeted search. “Disasters”[Mesh] AND “Mental Disorders”[Mesh] AND “Therapeutics”[Mesh] retrieved 652 articles. A search conducted using the terms “Disasters”[Mesh] AND “Mental Disorders”[Mesh] AND “prevention and control” [Subheading] retrieved 412 articles.
To be more inclusive, the authors performed a literature search using individual keywords (Non MeSh terms) and combinations of keywords such as: “Psychiatric Sequelae AND Disasters”, “Disaster mental health/Disaster psychiatry”, “Psychotropics AND Disasters”, “Propranolol and PTSD prevention”, “Hydrocortisone and PTSD prevention”, “Morphine and PTSD prevention”, “Disasters AND drug therapy AND mental health”.
Inclusion Criteria: Studies were included if they presented original data between January 1971 and September 2016 that discussed pharmacological interventions initiated immediately and early post disaster. Immediate treatment is defined in the ASD/PTSD literature as pharmacological intervention initiated within hours and early treatment as an intervention initiated within days, weeks or months post-disaster. The same definition was applied to evaluate pharmacological interventions post disaster for other psychiatric disorders described below.
Exclusion criteria: Non-human studies, articles without abstracts and non-English publications were excluded. Articles that did not include psychopharmacological interventions post-disaster were excluded.
Data Extraction: The literature search using the keywords described above yielded 213 articles. Activating the filter “Abstracts,” “Humans,” and “English” decreased it to 191 articles. These titles were screened to evaluate relevance to pharmacological interventions post-disaster, which excluded 118 articles. The remaining 73 abstracts were read in detail. Forty-nine additional articles were not included due to duplication or irrelevance (did not discuss pharmacological interventions). The articles were studied in detail to ensure that no intervention studies were missed. We included the 24 remaining articles consisting of 5 prospective longitudinal studies, 6 cross sectional surveys, 1 randomized placebo-controlled trial and 12 review articles. Of the 29 additional studies discovered in reviewing references from the above literature, 12 reviews and 3 meta-analyses were not counted towards intervention studies, but were referenced in the manuscript. Hence a total of 38 intervention studies were included in this review. See the methods flow chart in Figure 1.
Figure 1.
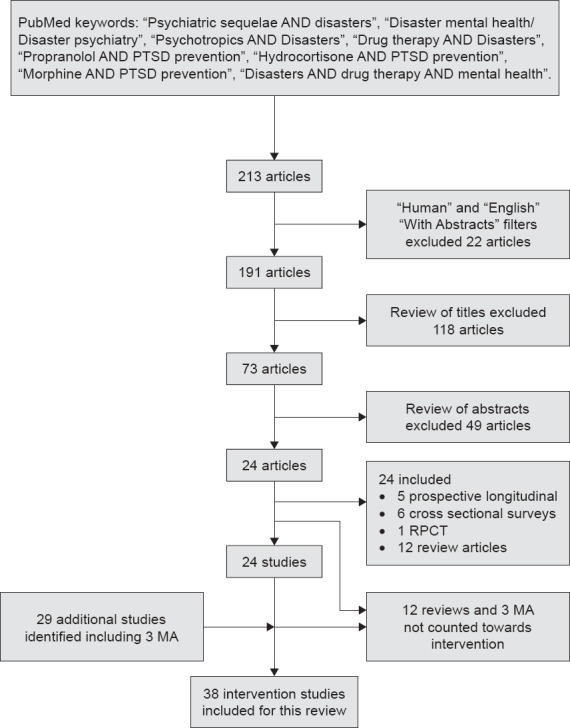
Methodology Flow Chart
Given the unexpected nature and ensuing chaos resulting from most disasters, it is challenging to plan and perform randomized, double-blind, placebo controlled trials in the post-disaster population; to guide treatment choices, this article will delineate all pharmacological interventions trials reported to date. Where feasible we utilize evidence-based medicine guidelines to evaluate psychopharmacological studies on subjects immediately and early post disaster.12
Results
The goals in the immediate aftermath of disaster or any traumatic event are to “preserve life, prevent further harm, and promote recovery”. According to Veteran Affairs-Department of Defense Clinical Practice Guidelines,13 these fundamentals include: taking care of basic needs, ensuring safety, evaluating trauma and stress-related symptoms and imparting required bio-psycho-social interventions that demonstrate evidence for helping survivors in the immediate or early post-trauma period. A significant number of psychiatric surveys have been conducted disaster, which have documented a spectrum of psychiatric disorders. The common psychiatric disorders documented in affected post disaster populations include: acute stress disorder ASD/PTSD, MDD, adjustment disorders, anxiety disorders, SUDs and non-specific somatic symptoms such as dizziness, headaches, body aches and night terrors.8 Amongst the psychiatric disorders, PTSD occurs with the highest prevalence, ranging from 4–60%14 and causes the highest morbidity post-disaster.15 In addition to PTSD, mood disorders including depression with comorbid substance use disorder are often diagnosed. These psychiatric sequelae not only occur with high prevalence, but also can persist over years. A study in one community documented a high prevalence of mental disorders causing significant morbidity even 3–5 years post disaster.16 We focus this review on psychopharmacological interventions tried immediately and early post trauma or post disaster for each of these more common post-disaster psychiatric conditions.
Acute Stress Disorder (ASD)/Posttraumatic Stress Disorder (PTSD)
A systematic review on recent health outcomes among disaster survivors and humanitarian responders showed PTSD as the most fully researched post-trauma condition, with a prevalence ranging from 4–60%.14
As previously reviewed by Birur et al, 2016;17 Noradrenergic, hypothalamic-pituitary-adrenal systems, serotonergic and opioid pathways contribute to the complex neuro-biology of PTSD. Biological findings in PTSD are tachycardia, increased blood pressure, altered galvanic skin responses and abnormal sleep architecture. Based on these biological findings, propranolol, glucocorticoids, opioids, antidepressants, antipsychotics, salbutamol and other pharmacological agents have been investigated in the immediate and early sequel of trauma, to try to prevent PTSD. Below we present the findings of psychopharmacological interventions investigated in the prevention of PTSD after a traumatic event.
Beta Adrenergic Blocker
Studies have found increased urinary norepinephrine in hospitalized patients with PTSD when compared with inpatients with other psychiatric disorders.18 Similar results were replicated in sexually traumatized children compared to healthy controls.19 Pitman postulated that an excess of epinephrine release at the time of trauma makes way to unduly strong emotional memory and fear conditioning that results in the development of PTSD.20 These findings provide a potential pathophysiologic mechanism for a beta-adrenergic blocking agent to prevent PTSD in adults.21 As propranolol non-competitively counteracts beta-1 and beta-2 adrenergic receptors, initiation within hours post trauma could potentially diminish the risk of development of PTSD by modifying levels of stress hormones such as norepinephrine, which typically are enhanced following an emotionally arousing trauma, and act to over consolidate memory in a distinct time dependent process and help in the development of intrusive re-experiencing symptoms in PTSD.22,23
In a randomized placebo controlled trial, efficacy of propranolol in reducing the development of PTSD after acute psychological trauma (MVA) was evaluated in 41 emergency department patients. Within 12 hours of trauma, patients were randomized to propranolol (n = 21; dose up to 240 mg/d) or placebo (n = 20) for 19 days. Researchers conducted psychophysiological and clinical assessments 1–3 months post trauma. At 1 and 3 months, PTSD symptoms were examined using Clinician-Administered PTSD Scale (CAPS)24 and at 5 and 13 weeks and psychophysiological assessments were tested using a script-driven imagery procedure. Results showed that physiological reactivity during script-imagery and PTSD symptom severity and rates of PTSD diagnostic outcomes were not different between the two groups. However subgroup analyses at 5-week post trauma assessment showed lower physiological reactivity in the propranolol group, demonstrating limited support for propranolol in PTSD prevention post-acute trauma.23 Similarly, another randomized double-blind controlled trial of 2 weeks of propranolol (n = 17), anxiolytic antiseizure gabapentin (n = 14) or placebo (n = 17), initiated within 2 days of trauma to patients admitted to a hospital showed that neither drug demonstrated significant superiority over placebo on depressive or posttraumatic stress symptoms when outcome assessments were conducted at 1, 4 and 8 months following injury. Propranolol was started at 20 mg for three doses and up-titrated over 2 days to 40 mg three times a day.25
In another double-blind placebo controlled pilot study, patients were randomized to begin a 10-day course of propranolol (n = 18) at 40 mg QID or placebo (n = 23) within 6 hours of trauma (MVA). At 1 month, mean CAPS score of 11 propranolol completers were 27.6 and of 20 placebo completers were 35.5. At 1 month, PTSD rate was 6/20 (30%) in placebo group and 2/11 (18%) in propranolol group. When evaluated 3 months post-trauma, none of the eight in propranolol group, but six of fourteen in placebo group, were physiologic responders during script driven imagery of trauma. This pilot study results showed propranolol at 160 mg per day may prevent development of subsequent PTSD.21
Another non-randomized study studied the efficacy of propranolol initiated immediately post-trauma (MVA or physical assault). Trauma victims were recruited within 2–20 hours post trauma. Subjective severity of trauma was evaluated using Peritraumatic Distress Inventory and outcome using Treatment Outcome PTSD Scale.26 11 patients who received 40 mg propranolol TID for 1 week followed by a taper over 8–12 days were contrasted with 8 patients who declined propranolol but participated in study. The group who declined propranolol (3/8) showed higher PTSD rates than group who received study medication (1/11). Authors concluded that propranolol might be useful in preventing PTSD.27
A chart review was conducted on children included in a previous randomized-controlled trial that compared groups with and without propranolol treatment on the subsequent occurrence of acute stress disorder (ASD). In the study, pediatric burn patients were randomized to receive propranolol at an average daily dose of 4 mg/kg/d within the first 10 days to sustain treatment benefits and continue through a month after injury. Results showed no difference between the groups and propranolol showed no correlation to risk for subsequent ASD.28 Another retrospective study examined PTSD prevalence in burned soldiers who did (n = 31) and did not receive propranolol (n = 34). Propranolol did not decrease PTSD development. Study limitations included: dosing and timing was not recorded, retrospective study, small sample size and individual patients not matched for total body surface area and injury severity scores.29
A recent meta-analysis summarizing the studies described above, suggests that propranolol does not alter the incidence of PTSD, though psychophysiological responses show improvement. All studies were conducted in emergency room (ER) settings with patients who experienced traumatic events (predominantly motor vehicle accidents). The number of RCT’s conducted in adults to date are limited and characterized by small sample sizes, high attrition rates and frequent lack of assessment for interrater reliability between the assessors. Furthermore time between trauma and exact time of initiation of medication is not clear, hence optimal dosing and duration of treatment to prevent PTSD is unknown.30 It is unclear if findings in the ER setting can be generalized to other settings. These varying results demonstrate a need for trials with propranolol of longer duration and with larger sample size. While the positive evidence is minimal, propranolol may have some utility for reducing physiologic reactivity in post disaster settings, when clinically feasible.
Glucocorticoids
Administration of glucocorticoids such as hydrocortisone immediately after trauma has been shown to prevent PTSD. Glucocorticoids such as cortisol play a vital role in mediating fear response and development of PTSD. Hospitalized veterans with PTSD showed significantly lower urinary free cortisol levels when compared with other hospitalized patients.31 Similar results were demonstrated in holocaust survivors, rape victims and adolescents exposed to natural disasters.32
In a retrospective study at a tertiary care intensive care unit (ICU), 27 patients who received standard treatment for septic shock (controls) were compared with a similar number of patients who received stress doses of hydrocortisone (100 mg bolus, followed by 0.18 mg/kg/hr) in addition to standard therapy. PTSD was diagnosed with the Posttraumatic Stress Syndrome-10 inventory, a self-report scale for PTSD diagnosis. The results showed the hydrocortisone group had a significantly lower incidence of PTSD than the standard treatment group during septic shock, showing promise that stress doses of hydrocortisone could reduce PTSD incidence and improve emotional well-being.33
In a prospective randomized double-blind study comparing stress doses of hydrocortisone (n = 9) vs. placebo (n = 11) during septic shock on PTSD survivors, Shelling demonstrated that only one of nine patients from hydrocortisone group developed PTSD compared with seven of eleven in placebo group when evaluated for PTSD 31 months after discharge from ICU.34 Another prospective randomized study that examined exogenously administered hydrocortisone (stress doses, n = 26) vs. standard treatment (n = 22) during the perioperative cardiac surgery period showed that the hydrocortisone group had fewer symptoms of PTSD and chronic stress at 6 months following cardiac surgery compared to the placebo group.35 Similar results were corroborated in another randomized study examining hydrocortisone (stress doses) vs. placebo in high risk patients following cardiac surgery.36 The authors concluded that glucocorticoids may be useful to attenuate chronic stress symptoms and PTSD after serious medical illness and cardiac surgery.
In another prospective randomized, double-blind, placebo-controlled trial (RDPT), 17 patients with symptoms of acute stress were administered a single intravenous bolus of high-dose hydrocortisone (100–140 mg) (n = 9) or placebo (n = 8) within 6 hours of trauma (traffic accidents). PTSD severity and diagnosis were assessed using CAPS. High dose hydrocortisone reduced the cardinal symptoms of acute stress and of ensuing development of PTSD, thereby altering the trajectory of PTSD following exposure to trauma.37 In another RDPT conducted at a Level 1 trauma unit, 20 mg oral hydrocortisone (n = 31), twice a day for ten days with a six-day taper vs. placebo (n = 33) was administered within 12 hours of injury. The investigators evaluated PTSD symptoms, depression and quality of life at one and three month’s post-trauma. Likewise, the hydrocortisone group experienced fewer symptoms of PTSD and reported a better quality of life.38
Results of the above studies are promising; the administration of hydrocortisone may effectively decrease symptoms of acute stress and reduce the rate of PTSD, when initiated up to 12 hours after trauma. The same findings were confirmed in 2 recent meta-analyses that concluded moderate quality of evidence for the efficacy of hydrocortisone for prevention of PTSD in adults.39,40 While hydrocortisone appears to be beneficial in the prevention of PTSD in selective medically ill populations, clinical recommendations cannot be made regarding its use in post disaster settings. Future studies can shed light on hydrocortisone use post disaster, however when a medical indication for use of hydrocortisone exists following a disaster, a clinician might consider continuing it as a prophylaxis against psychiatric sequelae.
Antidepressants
Selective serotonin reuptake inhibitors (SSRIs) are effective medications for PTSD, major depressive disorders and anxiety disorders such as generalized anxiety and social anxiety disorder.41,42 In a double-blind placebo controlled study, 29 subjects presenting immediately following trauma (vehicle collision, physical or sexual assault within the previous 1 month) who met full or partial criterion for ASD were randomized to 10–20 mg of escitalopram (n = 12) or placebo (n = 17) daily for 24 weeks. Subjects were re-evaluated at 56 weeks and assessed with CAPS. The results showed significant drop in CAPS score in both groups with greater drop in the placebo group, suggesting SSRIs was not efficacious in preventing PTSD.43 However notable limitations included small sample size, baseline differences between groups, and lack of matching of participants for trauma type or ethnicity. Similar findings were replicated in another study by Shalev and colleagues.44
Use of intravenous citalopram (40 mg) in fifteen randomly chosen burn victims (10–50% Total Body Surface Area) for 14 days immediately after admission to ICU demonstrated 100% improvement in post-traumatic stress symptoms when compared with burn victims who did not get citalopram. The citalopram group continued on 20 mg oral citalopram for the duration of 6 months. As a matter of interest, the citalopram group did cosmetically better than control burn group. The benefits of treatment were noted at week 3 after citalopram initiation.45 A prospective, randomized double blind study compared a 7-day trial of imipramine versus chloral hydrate in 25 pediatric burn victim patients suffering from ASD. Interestingly, 10 out of 12 patients on low dose imipramine, compared to 5 of 13 subjects on chloral hydrate responded with attenuation in nightmares, flashbacks, hyperarousal and negative emotions. In a second phase of the study, seven out of eight non-responders to chloral hydrate reported decrease in ASD symptoms when switched to imipramine.46 Review of the literature shows mixed evidence of benefit from antidepressants in the treatment of ASD or PTSD prevention. However for patients presenting with syndromal PTSD in the aftermath of trauma or disaster, it may be reasonable to recommend a trial of the above antidepressants.
Opioid agonists
In addition to beta-blockers and glucocorticoids, opioids such as morphine show promise in the prevention of PTSD. Opiates participate in the stress response by counter-regulating neuroregulatory modulators such as norepinephrine and corticotrophin releasing factor.47 Morphine also attenuates the production of norepinephrine and thereby impairs fear acquisition in the amygdala48 resulting in impaired consolidation of trauma memories.20
Consecutive patients (n = 155) admitted to a hospital following trauma were evaluated for prevailing psychiatric diagnosis, pain and morphine dose seven days post admission and were reevaluated for PTSD and other psychiatric diagnosis 3 months later (n = 120). Seventeen patients (14%) met PTSD diagnosis at 3 months. Interestingly, patients with PTSD diagnosis had received less morphine than patients who did not meet criteria for PTSD. After controlling for age, gender, severity and type of injury, regression analyses demonstrated severity of PTSD at 3 months was predicted by acute pain, mild traumatic brain injury and higher morphine dose 2 days following trauma. The authors concluded that acute morphine administration could prevent PTSD in the aftermath of traumatic injury.49 Similar findings were replicated following morphine use on Iraq veterans following injuries50 and in kids with burns.51,52 In Level 1 trauma patients, a recent study found use of opiates within 2 days after trauma showed reduced PTSD symptoms and diagnosis at 6-weeks and 1-year post-trauma compared to patients who received no opioid medication.53 This review suggests it would be reasonable to initiate opioid agonists early, and perhaps apply generously, at least in traumatized patients with physical trauma.
Other pharmacological agents (Benzodiazepines, risperidone, salbutamol, omega-3 fatty acids, oxytocin, docosahexaenoic acid, alcohol)
There is limited data on the use of benzodiazepines in the immediate aftermath of trauma. An uncontrolled pilot study (n = 4) that utilized temazepam for acute stress symptoms (not meeting criteria for ASD) for a period of 7 days (30 mg temazepam for 5 days and 15 mg for 2 days at bed time, initiated within 1–3 weeks of trauma) showed reduction of posttraumatic stress symptoms and improvement in sleep 1 week after cessation of medication. The authors emphasized the importance of sleep regulation before the consolidation of neurobiology of PTSD and thereby prevent the development of chronic PTSD.54
Another study examined benzodiazepine use in the acute period following trauma to prevent PTSD. In a prospective study, 13 trauma survivors received clonazepam (n = 10, dose = 2.7 +/– 0.8 mg/d) or alprazolam (n = 3, dose = 2.5 mg/d) initiated within 6.7 +/– 5.8 days after trauma. Thirteen other trauma survivors matched for gender and symptoms during the seven days post-trauma comprised the control group. Both groups were evaluated for PTSD 1 and 6 months post- trauma utilizing the Horowitz Impact of Event Scale, Mississippi Rating Scale for Combat-Related PTSD-civilian trauma version, CAPS, anxiety, depression and resting heart rate. There were no differences between the benzodiazepine and control groups on PTSD and anxiety scales at 1 and 6 months. Nine benzodiazepine subjects and three in the control group met criteria for PTSD 6 months post-trauma. Hence the authors inferred that early and prolonged use of benzodiazepines after trauma could increase the risk of developing PTSD.55 Although study results are mixed, there is no convincing evidence for the use of benzodiazepines post-disaster and in fact some studies suggest a higher risk for developing PTSD with prolonged use of benzodiazepines.56 Benzodiazepines may be useful immediately post-disaster for hyperarousal, sleep problems and severe anxiety for a short time period.
In a retrospective study, the effectiveness of risperidone (0.5–2 mg at bed time) on acute stress symptoms was examined in 10 adult burn victims. All 10 victims demonstrated improvement in nightmares/flashbacks, sleep disturbances and hyperarousal symptoms 1–2 days after starting risperidone.57 Limitations included retrospective design, small sample and absence of a control group. Based on a chart review, motor vehicle accident survivors (n = 255) who received salbutamol within a day of admission to a hospital, were prospectively evaluated and compared to matched controls at 6 weeks (n = 23) and 12 months post-MVA (n = 20).58 Although statistically insignificant, patients in the salbutamol group did not develop PTSD at follow-up, compared to 8% of the control group who met PTSD diagnosis 12 months after MVA. A small open-label pilot study of omega-3 fatty acids in severely injured patients admitted to an ICU showed reduction of PTSD symptoms.58,59 As a continuation of this study, a randomized double-blind placebo-controlled trial examining docosahexaenoic acid (DHA) plus eicosapentaenoic acid (EPA) vs. placebo for selective prevention of PTSD showed no difference between the two groups.60
Alcohol use (not Alcohol use disorder) has been shown to prevent the development of PTSD in several studies, especially following a disaster.61 A longitudinal study was carried out on 127 patients caught in a ball room fire. Data were analyzed 7–9 months post-trauma by using Composite International Diagnostic Interview (CIDI) and structured interview to assess pre, peri and post-exposure factors. Interestingly alcohol consumption and intoxication preceding trauma decreased the odds of developing PTSD in response to the trauma.62 Drinking alcohol post disaster is not advised and there is no evidence of benefit in the prevention of PTSD. To date anticonvulsants have not been tried in the prevention of PTSD.
Major Depressive Disorder (MDD)
MDD is the most common mental illness in the general population, but is second to PTSD in prevalence in post-disaster victims.2 Studies predictably demonstrate a wide range of MDD prevalence post-disaster, owing to the type of disaster, symptomatology measurement, pre-disaster MDD prevalence and design of the study. An empirical review from 1998–2001 in which 60,000 disaster victims were interviewed and results for 160 samples of disaster victims were coded showed that depression was identified in 58 samples (36%) and was the most prevalent psychiatric problem after PTSD. Additionally, as severity of trauma exposure increased, related symptoms such as suicidality and remorse also increased.2 While scientists have explored the use of antidepressants to prevent PTSD, despite the high prevalence of depression post-disaster, to date there are no systematic studies to explore the role of prophylactic pharmacological agents to prevent depression post disaster.
Anxiety Disorders
In addition to PTSD and MDD, studies have shown an elevated prevalence of anxiety disorders among individuals affected by disaster. As studied by Norris et al, anxiety disorders were identified in 32 (20%) of the 160 samples, which included interviews of 60,000 disaster victims. Among anxiety disorders, generalized anxiety disorder was the most common anxiety disorder found on structured diagnostic instruments.2 Death, anxiety, phobias and panic disorder were also diagnosed in disaster survivors but with less frequency than GAD.62-66 Even though anxiolytics and antidepressants have been trialed to prevent development of PTSD, no psychopharmacological agents have been specifically explored for the purpose of preventing anxiety disorders. However, it has been a common practice to prescribe low dose benzodiazepines post-disaster for brief time periods in developing countries for anxiety symptoms.
Substance Use Disorders (SUDs)
Substance use has been studied less frequently than PTSD or MDD in the post-disaster population. The true prevalence of the development of SUDs post-disaster is unknown. Increases in use of alcohol, drugs and cigarettes have been observed post disaster with evidence suggesting the disaster victims are utilizing alcohol as a coping mechanism.67 For example, 17 out of 182 subjects (9.4%) of Oklahoma City bombing survivors reported using alcohol to cope with their disaster experiences.68 Studies also have shown heightened alcohol, cigarettes and marijuana use post World Trade Center attacks, with 10% of New Yorkers reporting increased cigarette use, 25% reporting increased alcohol use and 3% reporting increased marijuana use.69 A recent review concluded that new-onset of alcohol and substance use disorders is not increased post-disaster, although pre-existing substance use disorders might recur or be exacerbated.70 No psychopharmacological agents have been studied in the prevention of substance use disorders post-disaster.
Medically Unexplained Psychological Symptoms (MUPS) or Somatic Symptom Disorders
In the aftermath of disasters somatic symptoms are often manifested in association with psychological distress. Individuals exposed to disasters frequently report sleep disruption.71,72 Other physical symptoms commonly seen are headache, fatigue, abdominal pain and shortness of breath with prevalence ranging from 3–78%. Even though, somatic symptoms usually subside, some symptoms persist for years post-disaster.67 As studied by Norris et al, in which 60,000-disaster victims were interviewed, self-reported somatic complaints were identified in 36 (23%) of the samples.2 In developing countries medically unexplained symptoms such as dizziness, generalized body aches, vague burning or pulling sensations, backaches and decreased sleep have been reported. To date no psychopharmacological agents have been tried in the prevention of MUPS or somatic symptoms post-disaster.
Psychosis
Research on the incidence of psychosis following disasters and the aftermath of disasters on patients with psychosis is scarce. A study reported increase in rates of admission for schizophrenia during civil wars in 1970’s and 80’s in Lebanon compared to rates during pre-war.73 When compared to the previous year, patients with schizoaffective disorder were found to have longer duration and more severe relapses, 4 months after fall of Berlin Wall.74 In contrast, other uncontrolled studies on hospitalized patients suggests that schizophrenia patients do not have psychotic exacerbations and function at a higher level during periods of disaster.75-77 To date no psychopharmacological interventions have been tried in the prevention of psychotic symptoms post-disaster. However, if clinically recommended, patients with pre-existing psychotic illness should continue taking psychotropics, and have increased professional monitoring post-disaster.
Discussion
The primary focus of psychiatric interventions post disaster is to ensure safety, reduce distress and secondary stressors, enhance coping skills and promote recovery. This literature review shows that despite the potential benefits of pharmacological treatments, there is a paucity of studies examining these interventions immediately post disaster. Controlled trials show promising evidence for hydrocortisone in preventing or attenuating the development of PTSD in ICU populations;39 however whether the same results could be extrapolated to a non-medical trauma population is unexplored. However, further exploration as to whether hydrocortisone can be universally recommended immediately post disaster is warranted in light of previous findings. This review explored several other classes of agents, used predominantly in open trials and noted some positive results for specific types of symptoms mixed with negative results. Controlled studies for some agents are warranted that target specific types of patients. For example, opiates show promise in traumatized patients with physical injuries. Similarly, propranolol showed some promise and requires more study for highly autonomically aroused (perhaps non-injured) patients. Antidepressants in small open trials showed benefit in children and adults who were burn victims and posed a higher risk of developing PTSD in future. Further exploration of risperdal and the use of DHA, EPA and Omega-3 fatty acids as a means of attenuating development of PTSD are required. These interventions could complement evidence-based psychological treatments. Current evidence depicts trauma focused cognitive behavior therapy within 3 months of trauma as the most effective therapy modality for prevention of PTSD after trauma. Psychological interventions such as single session psychological debriefing and psychological first aid are routinely recommended immediately after major traumatic events and disasters despite limited evidence of benefit for these psychological interventions when explored through meta-analyses.
The paucity of evidence may stem in part from the historical belief that the majority of symptoms were self-limiting and use of psychotropics immediately post-trauma was bad clinical practice that would disrupt individuals’ psychological processing of the trauma or lead to pathologizing the normal stress response to trauma.8,78 Preliminary studies discussed in this review indicate that positively modulating neurochemical pathways disrupted by trauma such as the pituitary cortisol axis can attenuate symptomatology independent of sedating or emotionally blunting actions. The small literature on prevention of psychiatric sequelae post disaster focuses on the prevention of PTSD. No psychopharmacological interventions are mentioned for other psychiatric disorders occurring post-disaster despite a high prevalence of these conditions: 36% of disaster victims develop depression, 23% experience ongoing somaticizing symptomatology resembling somatic symptom disorders, 20% anxiety disorder and >9% use excessive amounts of addictive substances. Studies focused on prevention of these conditions post-disaster are needed, in particular for depression prevention.
Review of the post-disaster psychopharmacology literature fails to provide specific guidelines for the general psychopharmacological management of psychiatric symptoms and prevention of psychiatric sequelae following disaster. Even though various pharmacological agents have been tried, these studies are predominantly open label trials with small sample sizes and mixed results. Though a handful of double blind trials have been done utilizing propranolol and hydrocortisone in PTSD prevention, they have been utilized in specific patient populations (physical injuries in ED settings or septic shock patients admitted to ICU). However patients with pre-existing mental illness, or pain would benefit from robust use of appropriate psychotropic medications upon presentation.
Further prospective longitudinal studies, pilot studies and randomized controlled trials are urgently needed to determine what pharmacological agents are indicated immediately post disaster to prevent various psychiatric sequelae. Although previous discussion has focused mainly on ASD and PTSD psychopharmacological prevention strategies, the scope of the literature shows that we need to pay attention to other psychiatric disorders including depression, anxiety disorders, substance use disorders and other medically unexplained symptoms. Even though positive evidence emphasizes the benefits of implementing psychological interventions immediately post disaster, clinicians should not hesitate to use psychotropics when presented with complex psychiatric symptoms such as psychosis, mania, suicidality and severe depression. In addition to immediate pharmacological interventions there is an urgent need for comprehensive care that integrates primary and psychiatric care, and also incorporates individual, family and community needs. One natural limitation of exploring post-disaster victims, as a whole is the lack of uniformity of different types of trauma both in terms of relation to physical injury, intensity of psychological stress endured, degree of ongoing loss or pain and premorbid precipitating factors.
Conclusion
Disasters are mega-scale catastrophic events that cause destruction including physical, mental and psychological sequelae and death. Exposure to disasters is common. There is substantial heterogeneity worldwide in regards to exposure to traumatic events and the various ways the psychiatric sequelae manifest. Post-disaster interventions have been developed to assist victims and survivors in the aftermath of disasters, with the eventual goal of preventing development of medical and psychiatric sequelae. Once the post-disaster period is completed and safety has been ensured, TF-CBT seems to have the most evidence in the prevention and treatment of PTSD when initiated within three months post-trauma, with minimal to no evidence of success for psychological first aid and psychological debriefing. Unfortunately, even though various psychopharmacological interventions have been attempted, predominantly for PTSD, the evidence fails to provide clear guidelines in regards to recommendations for the immediate post disaster population to prevent psychopathology. Though hydrocortisone has shown particular promise in specific patient populations, evidence of its benefit in non-medically ill traumatized populations is needed. Less robust benefits are noted from benzodiazepines, risperidone, salbutamol and omega 3 fatty acids. Opiates for injured patients, and propranolol and antidepressants show mixed evidence requiring further exploration. To date, no reported trials have explored psychopharmacologic interventions immediately post-disaster to prevent the development of depression or other prevalent post disaster psychiatric conditions. There is a pressing need for evidence-based treatments to prevent the development of the other commonly occurring post-disaster psychiatric conditions such as major depressive disorder, anxiety disorders, and substance use disorders.
Footnotes
Funding
There is no grant or funding to disclose in the preparation of this manuscript.
Disclosures of Potential Conflicts of Interest
Drs. Birur, Math and Fargason have no conflict of interest to disclose in the preparation of this manuscript.
References
- 1.Neria Y, Nandi A, Galea S. Post-traumatic stress disorder following disasters: a systematic review. Psychological Medicine. 2008;38(4):467–480. doi: 10.1017/S0033291707001353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Norris FH, Friedman MJ, Watson PJ, Byrne CM, Diaz E, Kaniasty K. 60,000 disaster victims speak: Part I An empirical review of the empirical literature, 1981-2001. Psychiatry. 2002;65(3):207–239. doi: 10.1521/psyc.65.3.207.20173. [DOI] [PubMed] [Google Scholar]
- 3.McFarlane AC, Van Hooff M, Goodhew F. Anxiety Disorders and PTSD. New York; Cambridge Univ. Press: 2009. [Google Scholar]
- 4.McFarlane AC, Williams R. Mental health services required after disasters: learning from the lasting effects of disasters. Depression Research and Treatment. 2012;2012:970194. doi: 10.1155/2012/970194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Off. US Foreign Diast. Assit. (OFDA)/Cent. Res. Epidemiol. Diast. (CRED) 2006 http://www.cred.be/emdat. [Google Scholar]
- 6.Off. US Foreign Diast. Assist. (OFDA)/Cent. Res. Epidemiol. Diast. (CRED) 2010 http://www.cred.be/emdat. [Google Scholar]
- 7.Raphael B, Maguire PA. Disaster Mental Health Research: Past, Present and Future. Cambridge Univ. Press; 2009. [Google Scholar]
- 8.Math SB, Nirmala MC, Moirangthem S, Kumar NC. Disaster management: mental health perspective. Indian Journal of Psychological Medicine. 2015;37(3):261–271. doi: 10.4103/0253-7176.162915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dieltjens T, Moonens I, Van Praet K, De Buck E, Vandekerckhove P. A systematic literature search on psychological first aid: lack of evidence to develop guidelines. PLoS One. 2014;9(12):e114714. doi: 10.1371/journal.pone.0114714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rose S, Bisson J, Churchill R, Wessely S. Psychological debriefing for preventing post traumatic stress disorder (PTSD) The Cochrane Database of Systematic Reviewse. 2002;(2):Cd000560. doi: 10.1002/14651858.CD000560. [DOI] [PubMed] [Google Scholar]
- 11.Kornor H, Winje D, Ekeberg O et al. Early trauma-focused cognitive-behavioural therapy to prevent chronic post-traumatic stress disorder and related symptoms: a systematic review and meta-analysis. BMC Psychiatry. 2008;8:81. doi: 10.1186/1471-244X-8-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shekelle PG, Woolf SH, Eccles M, Grimshaw J. Clinical guidelines: developing guidelines. BMJ (Clinical Research ed.) 1999;318(7183):593–596. doi: 10.1136/bmj.318.7183.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nash WP, Watson PJ. Review of VA/DOD Clinical Practice Guideline on management of acute stress and interventions to prevent posttraumatic stress disorder. Journal of Rehabilitation Research and Development. 2012;49(5):637–648. doi: 10.1682/jrrd.2011.10.0194. [DOI] [PubMed] [Google Scholar]
- 14.Young BH, Ford JD, Ruzek JI, Friedman MJ, Gusman FD. Disaster mental health services: A Guide for Clinicians and Administrators. Palo Alto, California: 2008. [Google Scholar]
- 15.Ursano RJ, Norwood AE, North CS. Trauma and Disaster, Responses and Management, Review of Psychiatry Series. Vol. 22. Virginia: American Psychiatric Publishing, Inc; 2003. [Google Scholar]
- 16.Liu A, Tan H, Zhou J et al. An epidemiologic study of posttraumatic stress disorder in flood victims in Hunan China. Canadian Journal of Psychiatry Revue Canadienne de Psychiatrie. 2006;51(6):350–354. doi: 10.1177/070674370605100603. [DOI] [PubMed] [Google Scholar]
- 17.Birur B, Moore NC, Davis LL. An evidence-based review of early intervention and prevention of posttraumatic stress disorder. Community Mental Health Journal. 2016 doi: 10.1007/s10597-016-0047-x. [DOI] [PubMed] [Google Scholar]
- 18.Kosten TR, Mason JW, Giller EL, Ostroff RB, Harkness L. Sustained urinary norepinephrine and epinephrine elevation in post-traumatic stress disorder. Psychoneuroendocrinology. 1987;12(1):13–20. doi: 10.1016/0306-4530(87)90017-5. [DOI] [PubMed] [Google Scholar]
- 19.De Bellis MD, Lefter L, Trickett PK, Putnam FW., Jr Urinary catecholamine excretion in sexually abused girls. Journal of the American Academy of Child and Adolescent Psychiatry. 1994;33(3):320–327. doi: 10.1097/00004583-199403000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Pitman RK. Post-traumatic stress disorder, hormones, and memory. Biol Psychiatry. 1989;26(3):221–223. doi: 10.1016/0006-3223(89)90033-4. [DOI] [PubMed] [Google Scholar]
- 21.Pitman RK, Sanders KM, Zusman RM et al. Pilot study of secondary prevention of posttraumatic stress disorder with propranolol. Biol Psychiatry. 2002;51(2):189–192. doi: 10.1016/s0006-3223(01)01279-3. [DOI] [PubMed] [Google Scholar]
- 22.Debiec J, Bush DE, LeDoux JE. Noradrenergic enhancement of reconsolidation in the amygdala impairs extinction of conditioned fear in rats—a possible mechanism for the persistence of traumatic memories in PTSD. Depression and Anxiety. 2011;28(3):186–193. doi: 10.1002/da.20803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoge EA, Worthington JJ, Nagurney JT et al. Effect of acute posttrauma propranolol on PTSD outcome and physiological responses during script-driven imagery. CNS Neuroscience & Therapeutics. 2012;18(1):21–27. doi: 10.1111/j.1755-5949.2010.00227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blake DD, Weathers FW, Nagy LM et al. The development of a Clinician-Administered PTSD Scale. Journal of Traumatic Stress. 1995;8(1):75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 25.Stein MB, Kerridge C, Dimsdale JE, Hoyt DB. Pharmacotherapy to prevent PTSD: results from a randomized controlled proof-of-concept trial in physically injured patients. Journal of Traumatic Stress. 2007;20(6):923–932. doi: 10.1002/jts.20270. [DOI] [PubMed] [Google Scholar]
- 26.Davidson JR, Book SW, Colket JT et al. Assessment of a new self-rating scale for post-traumatic stress disorder. Psychological Medicine. 1997;27(1):153–160. doi: 10.1017/s0033291796004229. [DOI] [PubMed] [Google Scholar]
- 27.Vaiva G, Ducrocq F, Jezequel K et al. Immediate treatment with propranolol decreases posttraumatic stress disorder two months after trauma. Biol Psychiatry. 2003;54(9):947–949. doi: 10.1016/s0006-3223(03)00412-8. [DOI] [PubMed] [Google Scholar]
- 28.Sharp S, Thomas C, Rosenberg L, Rosenberg M, Meyer W., 3rd Propranolol does not reduce risk for acute stress disorder in pediatric burn trauma. The Journal of Trauma. 2010;68(1):193–197. doi: 10.1097/TA.0b013e3181a8b326. [DOI] [PubMed] [Google Scholar]
- 29.McGhee LL, Maani CV, Garza TH, Desocio PA, Gaylord KM, Black IH. The effect of propranolol on posttraumatic stress disorder in burned service members. Journal of Burn Care & Research: Official Publication of the American Burn Association. 2009;30(1):92–97. doi: 10.1097/BCR.0b013e3181921f51. [DOI] [PubMed] [Google Scholar]
- 30.Argolo FC, Cavalcanti-Ribeiro P, Netto LR, Quarantini LC. Prevention of posttraumatic stress disorder with propranolol: A meta-analytic review. Journal of Psychosomatic Research. 2015;79(2):89–93. doi: 10.1016/j.jpsychores.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 31.Mason JW, Giller EL, Kosten TR, Ostroff RB, Podd L. Urinary free-cortisol levels in posttraumatic stress disorder patients. The Journal of Nervous and Mental Disease. 1986;174(3):145–149. doi: 10.1097/00005053-198603000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Vedantham K, Brunet A, Neylan TC, Weiss DS, Mannar CR. Neurobiological findings in posttraumatic stress disorder: a review. Dialogues in Clinical Neuroscience. 2000;2(1):23–29. doi: 10.31887/DCNS.2000.2.1/kvedantham. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schelling G, Stoll C, Kapfhammer HP et al. The effect of stress doses of hydrocortisone during septic shock on posttraumatic stress disorder and health-related quality of life in survivors. Critical Care Medicine. 1999;27(12):2678–2683. doi: 10.1097/00003246-199912000-00012. [DOI] [PubMed] [Google Scholar]
- 34.Schelling G, Briegel J, Roozendaal B, Stoll C, Rothenhausler HB, Kapfhammer HP. The effect of stress doses of hydrocortisone during septic shock on posttraumatic stress disorder in survivors. Biol Psychiatry. 2001;50(12):978–985. doi: 10.1016/s0006-3223(01)01270-7. [DOI] [PubMed] [Google Scholar]
- 35.Schelling G, Roozendaal B, De Quervain DJ. Can posttraumatic stress disorder be prevented with glucocorticoids? Annals of the New York Academy of Sciences. 2004;1032:158–166. doi: 10.1196/annals.1314.013. [DOI] [PubMed] [Google Scholar]
- 36.Weis F, Kilger E, Roozendaal B et al. Stress doses of hydrocortisone reduce chronic stress symptoms and improve health-related quality of life in high-risk patients after cardiac surgery: a randomized study. The Journal of Thoracic and Cardiovascular Surgery. 2006;131(2):277–282. doi: 10.1016/j.jtcvs.2005.07.063. [DOI] [PubMed] [Google Scholar]
- 37.Zohar J, Yahalom H, Kozlovsky N et al. High dose hydrocortisone immediately after trauma may alter the trajectory of PTSD: interplay between clinical and animal studies. European Neuropsychopharmacology: the Journal of the European College of Neuropsychopharmacology. 2011;21(11):796–809. doi: 10.1016/j.euroneuro.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 38.Delahanty DL, Gabert-Quillen C, Ostrowski SA et al. The efficacy of initial hydrocortisone administration at preventing posttraumatic distress in adult trauma patients: a randomized trial. CNS Spectrums. 2013;18(2):103–111. doi: 10.1017/S1092852913000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sijbrandij M, Kleiboer A, Bisson JI, Barbui C, Cuijpers P. Pharmacological prevention of post-traumatic stress disorder and acute stress disorder: a systematic review and meta-analysis. The Lancet Psychiatry. 2015;2(5):413–421. doi: 10.1016/S2215-0366(14)00121-7. [DOI] [PubMed] [Google Scholar]
- 40.Amos T, Stein DJ, Ipser JC. Pharmacological interventions for preventing post-traumatic stress disorder (PTSD) The Cochrane Database of Systematic Reviews. 2014;7:Cd006239. doi: 10.1002/14651858.CD006239.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jakubovski E, Varigonda AL, Freemantle N, Taylor MJ, Bloch MH. Systematic review and meta-analysis: dose-response relationship of selective serotonin reuptake inhibitors in major depressive disorder. The American Journal of Psychiatry. 2016;173(2):174–183. doi: 10.1176/appi.ajp.2015.15030331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ipser JC, Wilson D, Akindipe TO, Sager C, Stein DJ. Pharmacotherapy for anxiety and comorbid alcohol use disorders. The Cochrane Database of Systematic Reviews. 2015;1(Cd007505) doi: 10.1002/14651858.CD007505.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suliman S, Seedat S, Pingo J, Sutherland T, Zohar J, Stein DJ. Escitalopram in the prevention of posttraumatic stress disorder: a pilot randomized controlled trial. BMC Psychiatry. 2015;15:24. doi: 10.1186/s12888-015-0391-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shalev AY, Ankri Y, Israeli-Shalev Y, Peleg T, Adessky R, Freedman S. Prevention of posttraumatic stress disorder by early treatment: results from the Jerusalem Trauma Outreach And Prevention study. Archives of General Psychiatry. 2012;69(2):166–176. doi: 10.1001/archgenpsychiatry.2011.127. [DOI] [PubMed] [Google Scholar]
- 45.Blaha J, Svobodova K, Kapounkova Z. Therapeutical aspects of using citalopram in burns. Acta Chirurgiae Plasticae. 1999;41(1):25–32. [PubMed] [Google Scholar]
- 46.Robert R, Blakeney PE, Villarreal C, Rosenberg L, Meyer WJ, 3rd. Imipramine treatment in pediatric burn patients with symptoms of acute stress disorder: a pilot study. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38(7):873–882. doi: 10.1097/00004583-199907000-00018. [DOI] [PubMed] [Google Scholar]
- 47.Morgan CA, 3rd, Krystal JH, Southwick SM. Toward early pharmacological posttraumatic stress intervention. Biol Psychiatry. 2003;53(9):834–843. doi: 10.1016/s0006-3223(03)00116-1. [DOI] [PubMed] [Google Scholar]
- 48.Tanaka M, Kohno Y, Tsuda A et al. Differential effects of morphine on noradrenaline release in brain regions of stressed and non-stressed rats. Brain Research. 1983;275(1):105–115. doi: 10.1016/0006-8993(83)90422-5. [DOI] [PubMed] [Google Scholar]
- 49.Bryant RA, Creamer M, O’Donnell M, Silove D, McFarlane AC. A study of the protective function of acute morphine administration on subsequent posttraumatic stress disorder. Biol Psychiatry. 2009;65(5):438–440. doi: 10.1016/j.biopsych.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 50.Holbrook TL, Galarneau MR, Dye JL, Quinn K, Dougherty AL. Morphine use after combat injury in Iraq and post-traumatic stress disorder. The New England Journal of Medicine. 2010;362(2):110–117. doi: 10.1056/NEJMoa0903326. [DOI] [PubMed] [Google Scholar]
- 51.Saxe G, Stoddard F, Courtney D et al. Relationship between acute morphine and the course of PTSD in children with burns. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40(8):915–921. doi: 10.1097/00004583-200108000-00013. [DOI] [PubMed] [Google Scholar]
- 52.Stoddard FJ, Jr, Sorrentino EA, Ceranoglu TA et al. Preliminary evidence for the effects of morphine on posttraumatic stress disorder symptoms in one- to four-year-olds with burns. Journal of Burn Care & Research: Official Publication of the American Burn Association. 2009;30(5):836–843. doi: 10.1097/BCR.0b013e3181b48102. [DOI] [PubMed] [Google Scholar]
- 53.Mouthaan J, Sijbrandij M, Reitsma JB et al. The role of early pharmacotherapy in the development of posttraumatic stress disorder symptoms after traumatic injury: an observational cohort study in consecutive patients. General Hospital Psychiatry. 2015;37(3):230–235. doi: 10.1016/j.genhosppsych.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 54.Mellman TA, Byers PM, Augenstein JS. Pilot evaluation of hypnotic medication during acute traumatic stress response. Journal of Traumatic Stress. 1998;11(3):563–569. doi: 10.1023/A:1024460814230. [DOI] [PubMed] [Google Scholar]
- 55.Gelpin E, Bonne O, Peri T, Brandes D, Shalev AY. Treatment of recent trauma survivors with benzodiazepines: a prospective study. The Journal of Clinical Psychiatry. 1996;57(9):390–394. [PubMed] [Google Scholar]
- 56.Simon A, Gorman J. Psychopharmacological possibilities in the acute disaster setting. The Psychiatric Clinics of North America. 2004;27(3):425–458. doi: 10.1016/j.psc.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 57.Stanovic JK, James KA, Vandevere CA. The effectiveness of risperidone on acute stress symptoms in adult burn patients: a preliminary retrospective pilot study. The Journal of Burn Care & Rehabilitation. 2001;22(3):210–213. doi: 10.1097/00004630-200105000-00005. [DOI] [PubMed] [Google Scholar]
- 58.Kobayashi I, Sledjeski E, Fallon W, Jr, Spoonster E, Riccio D, Delahanty D. Effects of early albuterol (salbutamol) administration on the development of posttraumatic stress symptoms. Psychiatry Research. 2011;185(1-2):296–298. doi: 10.1016/j.psychres.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matsuoka Y, Nishi D, Yonemoto N, Hamazaki K, Hashimoto K, Hamazaki T. Omega-3 fatty acids for secondary prevention of posttraumatic stress disorder after accidental injury: an open-label pilot study. Journal of Clinical Psychopharmacology. 2010;30(2):217–219. doi: 10.1097/JCP.0b013e3181d48830. [DOI] [PubMed] [Google Scholar]
- 60.Matsuoka Y, Nishi D, Hamazaki K et al. Docosahexaenoic acid for selective prevention of posttraumatic stress disorder among severely injured patients: a randomized, placebo-controlled trial. The Journal of Clinical Psychiatry. 2015;76(8):e1015–1022. doi: 10.4088/JCP.14m09260. [DOI] [PubMed] [Google Scholar]
- 61.McFarlane AC. Epidemiological evidence about the relationship between PTSD and alcohol abuse: the nature of the association. Addictive Behaviors. 1998;23(6):813–825. doi: 10.1016/s0306-4603(98)00098-7. [DOI] [PubMed] [Google Scholar]
- 62.Maes M, Delmeire L, Mylle J, Altamura C. Risk and preventive factors of post-traumatic stress disorder (PTSD): alcohol consumption and intoxication prior to a traumatic event diminishes the relative risk to develop PTSD in response to that trauma. Journal of Affective Disorders. 2001;63(1-3):113–121. doi: 10.1016/s0165-0327(00)00173-7. [DOI] [PubMed] [Google Scholar]
- 63.Armenian HK, Morikawa M, Melkonian AK et al. Loss as a determinant of PTSD in a cohort of adult survivors of the 1988 earthquake in Armenia: implications for policy. Acta Psychiatrica Scandinavica. 2000;102(1):58–64. doi: 10.1034/j.1600-0447.2000.102001058.x. [DOI] [PubMed] [Google Scholar]
- 64.Bolton D, O’Ryan D, Udwin O, Boyle S, Yule W. The long-term psychological effects of a disaster experienced in adolescence: II: General psychopathology. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2000;41(4):513–523. [PubMed] [Google Scholar]
- 65.Chung MC, Chung C, Easthope Y. Traumatic stress and death anxiety among community residents exposed to an aircraft crash. Death Studies. 2000;24(8):689–704. doi: 10.1080/074811800750036578. [DOI] [PubMed] [Google Scholar]
- 66.David D, Mellman TA, Mendoza LM, Kulick-Bell R, Ironson G, Schneiderman N. Psychiatric morbidity following Hurricane Andrew. Journal of Traumatic Stress. 1996;9(3):607–612. doi: 10.1007/BF02103669. [DOI] [PubMed] [Google Scholar]
- 67.Goldmann E, Galea S. Mental health consequences of disasters. Annual Review of Public Health. 2014;35:169–183. doi: 10.1146/annurev-publhealth-032013-182435. [DOI] [PubMed] [Google Scholar]
- 68.North CS, Nixon SJ, Shariat S et al. Psychiatric disorders among survivors of the Oklahoma City bombing. Jama. 1999;282(8):755–762. doi: 10.1001/jama.282.8.755. [DOI] [PubMed] [Google Scholar]
- 69.Vlahov D, Galea S, Ahern J et al. Alcohol drinking problems among New York City residents after the September 11 terrorist attacks. Substance use & Misuse. 2006;41(9):1295–1311. doi: 10.1080/10826080600754900. [DOI] [PubMed] [Google Scholar]
- 70.North CS, Pfefferbaum B. Mental health response to community disasters: a systematic review. Jama. 2013;310(5):507–518. doi: 10.1001/jama.2013.107799. [DOI] [PubMed] [Google Scholar]
- 71.Mellman TA, David D, Kulick-Bell R, Hebding J, Nolan B. Sleep disturbance and its relationship to psychiatric morbidity after Hurricane Andrew. The American Journal of Psychiatry. 1995;152(11):1659–1663. doi: 10.1176/ajp.152.11.1659. [DOI] [PubMed] [Google Scholar]
- 72.Ursano RJ, Zhang L, Li H et al. PTSD and traumatic stress from gene to community and bench to bedside. Brain Research. 2009;1293:2–12. doi: 10.1016/j.brainres.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 73.Yaktin US, Labban S. Traumatic war Stress & schizophrenia. Journal of Psychosocial Nursing and Mental Health Services. 1992;30(6):29–33. doi: 10.3928/0279-3695-19920601-09. [DOI] [PubMed] [Google Scholar]
- 74.Bohlken J, Priebe S. Political change and course of affective psychoses: Berlin 1989-90. Psychiatry Research. 1991;37(1):1–4. doi: 10.1016/0165-1781(91)90100-4. [DOI] [PubMed] [Google Scholar]
- 75.Godleski LS, Luke KN, DiPreta JE, Kline AE, Carlton BS. Responses of state hospital patients to Hurricane Iniki. Hospital & Community Psychiatry. 1994;45(9):931–933. doi: 10.1176/ps.45.9.931. [DOI] [PubMed] [Google Scholar]
- 76.Chubb HL, Bisson JI. Early psychological reactions in a group of individuals with pre-existing and enduring mental health difficulties following a major coach accident. The British Journal of Psychiatry: the Journal of Mental Science. 1996;169(4):430–433. doi: 10.1192/bjp.169.4.430. [DOI] [PubMed] [Google Scholar]
- 77.McMurray L, Steiner W. Natural disasters and service delivery to individuals with severe mental illness—ice storm 1998. Canadian Journal of Psychiatry Revue Canadienne de Psychiatrie. 2000;45(4):383–385. doi: 10.1177/070674370004500408. [DOI] [PubMed] [Google Scholar]
- 78.Lundin T. The treatment of acute trauma Post-traumatic stress disorder prevention. The Psychiatric Clinics of North America. 1994;17(2):385–391. [PubMed] [Google Scholar]


