Abstract
Nicotine dependence is associated with increased risk for emotional, cognitive, and neurological impairments later in life. This study investigated the long-term effects of nicotine exposure during adolescence and adulthood on measures of depression, anxiety, learning, and hippocampal pyramidal cell morphology. Mice (C57BL/6J) received saline or nicotine for 12 days via pumps implanted on postnatal day 32 (adolescent) or 54 (adults). Thirty days after cessation of nicotine/saline, mice were tested for learning using contextual fear conditioning, depression-like behaviors using the forced swim test, or anxiety-like behaviors with the elevated plus maze. Brains from nicotine or saline exposed mice were processed with Golgi stain for whole neuron reconstruction in the CA1 and CA3 regions of the hippocampus. Results demonstrate higher depression-like responses in both adolescent and adult mice when tested during acute nicotine withdrawal. Heightened depression-like behaviors persisted when tested after 30 days of nicotine abstinence in mice exposed as adolescents, but not adults. Adult, but not adolescent, exposure to nicotine resulted in increased open arm time when tested after 30 days of abstinence. Nicotine exposure during adolescence caused deficits in contextual fear learning indicated by lower levels of freezing to the context as compared to controls when tested 30 days later. In addition, reduced dendritic length and complexity in the apical CA1 branches in adult mice exposed to nicotine during adolescence were found. These results demonstrate that nicotine exposure and withdrawal can have long-term effects on emotional and cognitive functioning, particularly when nicotine exposure occurs during the critical period of adolescence.
Keywords: depression, learning, anxiety, memory, withdrawal
Graphical abstract
1. Introduction
Abuse of nicotine by teenagers is a cause for concern as adolescence is a period of high vulnerability to the long-term consequences of nicotine and increased risk for development of nicotine addiction (Breslau and Peterson, 1996; Chassin et al., 1990). This is especially important when considering that 90% of daily smokers began smoking before age 18 years, supporting the idea that adolescence is a unique window for the initiation of smoking and subsequent nicotine dependence (Eaton et al., 2010). Unfortunately, nicotine use by adolescents in the form of e-cigarettes is on the rise with adolescent e-cigarette use nearly tripling between 2012 and 2015 (Center for Disease Control, 2016). This is an alarming trend as the majority of adolescents who report using an e-cigarette have also expressed their willingness to smoke a conventional cigarette if offered (CDC, 2016). Adolescent nicotine use is associated with negative outcomes in cognition and emotional affect. Clinical studies report that use of nicotine by adolescents leads to working memory deficits and these deficits are exacerbated during acute nicotine withdrawal (Jacobsen et al., 2005). Working memory deficits are more pronounced in younger smokers than aged smokers (Falcone et al., 2014) and deficits in working memory following abstinence from nicotine can predict the occurrence of relapse (Patterson et al., 2010). In addition to negative cognitive effects, smokers have a higher risk of developing depression than non-smokers (Breslau et al., 1993). Adolescents who smoke have nearly twice the risk of becoming depressed by their late 20's (Brook et al., 2004; Choi et al., 1997). Therefore, it is important to study the relationship between adolescent exposure to nicotine and working memory and emotional behavior later in life, as well as to investigate potential neurobiological underpinnings of the long-term negative effects of nicotine. The hippocampus plays a crucial role in contextual fear memory (Fanselow, 2000; Kim et al., 1993; Logue et al., 1997). Atrophy of pyramidal cells in the hippocampus is associated with working memory deficits in rodent models (Oliveira-da-Silva et al., 2009; Ribeiro-Carvalho et al., 2011; Spaeth et al., 2010), and reduced hippocampal volume is associated with depression in clinical populations (Bremner et al., 2000). It is unknown, however, how adolescent nicotine affects the morphology of hippocampal pyramidal cells. The aims of this study were to determine if nicotine exposure during mid-adolescence produces long-term deficits in learning and changes in depression- and anxiety-like behaviors as a function of age at drug exposure. The hypothesis that alterations in behavior produced by nicotine may be associated with changes in neuronal morphology and complexity in the hippocampus was tested.
2. Materials and Methods
2.1 Subjects
Male C57BL/6J mice were obtained from Jackson Laboratory (Bar Harbor, ME). Mice in the adolescent groups were delivered at postnatal day (PND) 25, and in the adult groups were delivered at PND47. Mice were housed 4-5 per cage with red igloos for enrichment. The mice were maintained on a 12-hour light-dark cycle (lights on from 7:00-19:00) with unrestricted access to food and water. All experiments took place during the light phase of the light-dark cycle. Separate cohorts of mice were used for each behavioral test and each time point. All procedures were approved by the Temple University Institutional Animal Care and Use Committee and were within the guidelines established by the NIH on the care and use of animals in research.
2.2 Nicotine Administration
Nicotine administration began at PND32 (mid-adolescent) or PND54 (adult) days of age. Nicotine hydrogen tartrate salt (N5260; Sigma Co., St. Louis, MO) was dissolved in saline. Nicotine or saline was delivered by mini-osmotic pumps (Model 1002; Alzet, Cupertino, CA) implanted subcutaneously which delivered drug or vehicle at a rate of 0.25ul/hour to achieve a dose of nicotine base of 12.6 mg/kg/day for 12 days as outlined in previous work (Portugal et al., 2012). Pumps were chosen for continuous delivery of nicotine throughout the 12 days which is a preferred method of chronic nicotine administration because of the rapid metabolism of nicotine in mice (Matta et al., 2007). This dose of nicotine results in similar plasma cotinine levels as seen in adult human smokers and has been shown to produce learning deficits during withdrawal in C57BL6/J mice (Davis et al., 2005; Henningfield and Keenan, 1993). After 12 days, the pumps were removed at PND44 (adolescent) or PND 66 (adult). Testing began either 24 hours or 30 days later.
2.3 Fear Conditioning
Mice that received nicotine or saline as adolescents or adults (n=15/experimental group) were tested in contextual and cued fear conditioning beginning 30 days following drug cessation. Fear conditioning protocols were based on our prior work (Gould and Higgins, 2003; Kutlu and Gould, 2014). On the first day of contextual fear conditioning, mice were trained with a conditioned stimulus (CS, 85dB tone) and an unconditioned stimulus (US, 0.57 mA shock) for 2 CS-US pairings in identical fear conditioning chambers (18.8 × 20 × 18.3 cm). Freezing behavior was assessed once every 10 seconds for 120 seconds before the first (baseline) CS-US pairing; 120 seconds later a second CS-US pairing was administered and mice were left in the fear conditioning chambers for 30 seconds before being placed back in the home cage. Freezing behavior was assessed once every 10 seconds between the first and second CS-US presentation (immediate). Twenty-four hours following training animals were placed back into the same fear conditioning chambers and freezing behaviors were assessed once every ten seconds for a period of 300 seconds (context testing). Approximately one hour later animals were assessed for recall of the auditory cue in a different chamber with an additional olfactory cue (vanilla extract; ACME Markets). Freezing was measured for 180 seconds before the tone was presented (preCS) to assess if there were any differences in generalized freezing and for 180 seconds once the tone was presented (CS) to assess recall of the cued conditioning.
2.4 Forced Swim Test
The forced swim test was used as a measure of depression-like behavior (Castagné et al., 2011; Porsolt et al., 1977; Reith et al., 2012). Separate cohorts of adolescent and adult mice were tested in the forced swim test 24 hours or 30 days following cessation of nicotine or saline administration (n=8-16/experimental group). Mice were placed individually into clear glass cylinders (46 cm tall × 21 cm in diameter) containing 25°C water at a depth of 15 cm for 6 minutes, during which time behavior was video recorded. The latency for the mouse to become immobile and the total time spent immobile during minutes 2 to 6 were measured by an experimenter who was blind to treatment groups.
2.5 Elevated Plus Maze
The elevated plus maze was used to assess anxiety-like behavior (Craige et al., 2015; Pellow et al., 1985; Komada et al., 2008). Separate cohorts of adolescent and adult mice that received nicotine or saline (n=12-16/experimental group) were tested in the elevated plus maze 30 days following drug cessation. The elevated plus maze apparatus was constructed of a wood base with opaque Plexiglas floors and walls. The maze consisted of two opposing arms that were open (30.6cm × 7.6cm; L × W) and two opposing enclosed arms (30.6cmx7.6cmx15.5cm; L × W × H). Light levels were 40 lux when measured from the center of the apparatus. Mice were placed in the center of the plus maze facing a closed arm with their movement recorded by video for 10 minutes, as outlined by Komada et al. (2008). Time spent in open arms was scored by an experimenter blind to the drug conditions. Mice were considered within the open arm when all four paws had crossed into the respective area.
2.6 Golgi-Cox and Neuron Reconstruction
In order to investigate the effects of chronic nicotine administration on neuronal morphology, brains were obtained from the cohort of mice tested on the elevated-plus maze. This cohort was chosen in order to minimize potential effects of learning (fear conditioning cohort) and/or stress (fear conditioning and forced swim procedure) which could impact dendritic length independently of drug administration (Izquierdo et al., 2006; Lai et al., 2012). One week following testing on the elevated plus maze, mice were anesthetized with Avertin® and transcardially perfused with ice-cold phosphate buffered saline. Brains were extracted and placed in a Golgi-Cox solution following the recipe outlined by (Glaser and Van der Loos, 1981) and stored in the dark for a period of 12 days. Golgi-Cox solution was refreshed after the first two days. At the end of the 12-day immersion, brains were placed into a 30% sucrose solution until they were sectioned on a vibratome. Brains were cut into 200 μM coronal sections and slices were collected onto gel coated slides. Sections were processed by the methods previously published (Gibb and Kolb, 1998) where sections are alkalinized in ammonium hydroxide and fixed in Kodak rapid fixer. Afterwards, sections were dehydrated through increasing concentrations of ethanol baths (50%, 70%, 95%, 100% × 2) and cleared in a 1:1:1 solution of xylene, 100% ethanol, and chloroform.
Pyramidal cells in hippocampal subregions CA1 and CA3 were manually reconstructed using the Neurolucida software on a Nikon Optiphot-2 brightfield microscope. Cells were selected based on (1) triangular shape of the soma, (2) thorough impregnation of Golgi-Cox, and (3) dendrites were free of obstructions from other cells or blood vessels and could be traced without interruption. Neurons selected for reconstruction were manually traced under 60x oil immersion magnification. A total of 80 hippocampal pyramidal cells were reconstructed with 40 from the adolescent mice (n=3-4 mice per drug condition) and 40 from the adult mice (n=3-4 mice per drug condition). Each subregion had 20 neurons per age; 10 neurons per nicotine or saline condition. Morphometrics were obtained using the Neurolucida Explorer software (Microrbrightfield Bioscience, Wilson, VT). Parameters obtained were total number of branches (bifurcations), total dendrite length (lengths), and intersections of dendritic branches at each increment. Parameters were quantified using Sholl analysis (10 μM increments) for both basal and apical trees. The number of intersections indicates dendritic complexity whereas bifurcations indicates differences in branching pattern (Ehlinger et al., 2012).
2.7 Statistical Analysis
For fear conditioning data, separate 2 [age: adolescent versus adult] × 2 [drug: saline versus nicotine] ANOVAs were conducted on freezing scores at baseline, context, preCS, and CS. Significant interactions were followed up with simple main effects. For the forced swim test, separate 2 [age: adolescent versus adult] × 2 [drug: saline versus nicotine] ANOVAs were used to analyze latency to immobility and total time spent immobile. Significant interactions were followed up with Bonferroni post-hocs with appropriate corrections for significance. For elevated plus maze, two-way ANOVAs (age: adolescent versus adult × drug: saline versus nicotine) were conducted for open arm time. For morphology data, one way ANOVAs for each age were used to analyze total dendritic length in the apical and basal trees in the CA1 and CA3. Morphometric parameters were analyzed using repeated-measures ANOVA. Significant interactions were followed up with simple main effects. The null hypothesis was rejected with p < 0.05.
3. RESULTS
3.1 Nicotine exposure during adolescence produced deficits in contextual fear learning measured after long-term abstinence
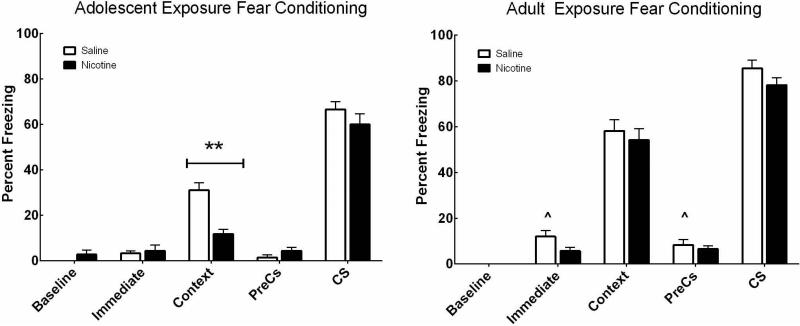
Adolescent and adult mice were treated with nicotine or saline for 12 days. Learning was assessed using contextual fear conditioning 30 days after nicotine cessation, at a time when both groups of mice were adults. As shown in Figure 1A, results demonstrate a significant interaction between age × drug (F(1,58)=3.95, p < .05) when analyzing freezing to the context. Post-hoc tests using simple main effects analysis indicate that there was a significant difference between mice receiving nicotine versus saline as adolescents (F(1,58) = 12.09, p = .001). There was no significant difference between adult mice administered saline or nicotine on freezing to context. Thus, chronic nicotine produced long-term deficits in contextual fear learning only in the mice exposed during adolescence.
Figure 1. Chronic nicotine during adolescence causes deficits in adult contextual fear learning.
Contextual fear conditioning in mice exposed to nicotine or saline as adolescents (A) or adults (B) and tested after 30 days of abstinence. Nicotine exposure during adolescence caused deficits in contextual fear learning indicated by lower percent freezing to the context as compared to saline controls (**p < 0.01). Saline treated adults had higher levels of freezing during the immediate and preCS time points compared to saline treated adolescents (^p < 0.05). There were no differences between nicotine- and saline-exposed mice in cued fear conditioning for either age group. n=15/ experimental group.
Cued recall was also tested. In the preCS condition, freezing before the presentation of the CS was measured. In this condition there was a significant main effect of age (F(1,58)=10.17, p < .01) but no main effect of drug (Figure 1B). Specifically, adult-exposed mice had higher freezing than adolescent-exposed mice (t(60) = 2.68, p = .009). Bonferroni post-hoc analysis indicates saline-treated adult mice had higher scores than saline-treated adolescents, (p < 0.05). However, in the CS condition when freezing was measured during the presentation of the auditory cue, there was no main effect of drug or age.
There were no differences in baseline freezing between groups. A significant difference for the immediate time point was noted, where adult-treated mice had higher levels of freezing than adolescent-treated mice following the first presentation of the CS-US pairing (F(1,58) = 6.29, p < 0.05). Bonferroni post-hoc analysis showed saline-treated adults had higher freezing than saline-treated adolescents (p < 0.05). Taken together these results indicate adolescent nicotine administration selectively impacted contextual learning but not cued learning.
3.2 Nicotine exposure during adolescence increased depression-like behaviors both during early and protracted withdrawal
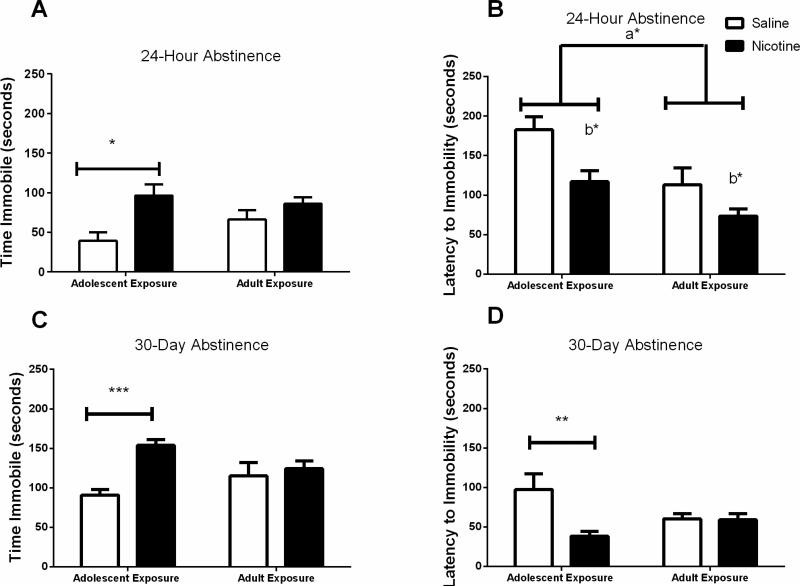
Adolescent and adult mice exposed to nicotine were tested for depression-like behaviors 24 hours or 30 days after cessation of nicotine administration. Two-way ANOVA of time spent immobile (Figure 2A) revealed a significant effect of nicotine treatment (F(1,43)=9.766, p < 0.01) but no significant effect of age at the 24 hour abstinence time point and no interaction between age and drug treatment. Bonferroni posthoc analysis showed that nicotine exposure resulted in significantly greater immobility times for the adolescent mice compared to saline controls (p < 0.05). Two-way ANOVA of latency to become immobile (Figure 2B) showed a significant effect of nicotine treatment (F(1,44)=8.274, p < 0.01) and age (F(1,44)=9.537, p < 0.01), but no significant nicotine versus saline effect within each age group by posthoc analysis.
Figure 2. Depression-like behaviors measured by the forced swim test following adolescent or adult nicotine exposure.
Mice were administered nicotine or saline for 12 days and were tested in the forced swim test 24 hours or 30 days later. A & B) 24-hr abstinence: Adolescent nicotine but not adult nicotine exposure resulted in higher time immobile in the forced swim test than age-matched saline controls (*p<0.05). Nicotine administration significantly shortened the latency to become immobile in both age groups when tested after 24 hours of withdrawal (b* = p<0.01). Adolescent mice had longer latency times to immobility than adult mice regardless of treatment (a*, p < 0.05). C & D) 30-day abstinence. Mice exposed to nicotine during adolescence spent more time immobile in the forced swim test (***p<0.0001) and had shorter latencies to immobility (**p<0.01) when tested after 30 days of withdrawal than saline controls indicating heightened depression-like behaviors after long-term withdrawal from chronic nicotine exposure. Exposure to nicotine during adulthood had no effect on immobility when tested 30 days later. n=8-16/experimental group.
When behavior in the forced swim test was measured after 30 days of abstinence in a separate cohort of mice, two-way ANOVA of time spent immobile (Figure 2C) revealed a significant effect of drug treatment (F(1,43)=13.43, p<0.001) as well as a significant interaction between age of drug exposure and drug treatment (F(1,43)=7.542, p < 0.01). Post-hoc analysis showed that mice treated with nicotine during adolescence had greater immobility times than saline controls (p < 0.01). Two-way ANOVA of latency to immobility (Figure 2D) showed a significant effect of drug administration (F(1,43)=4.157, p < 0.05) at the 30-day withdrawal point, with post-hoc tests showing lower latency to immobility in the mice treated during adolescence (p < 0.0001). No significant differences were seen between adult mice administered nicotine or saline and tested 30 days later. These data demonstrate that exposure to nicotine during adolescence results in a long-lasting depression-like phenotype.
3.3 Effects of chronic nicotine exposure on anxiety-like behaviors
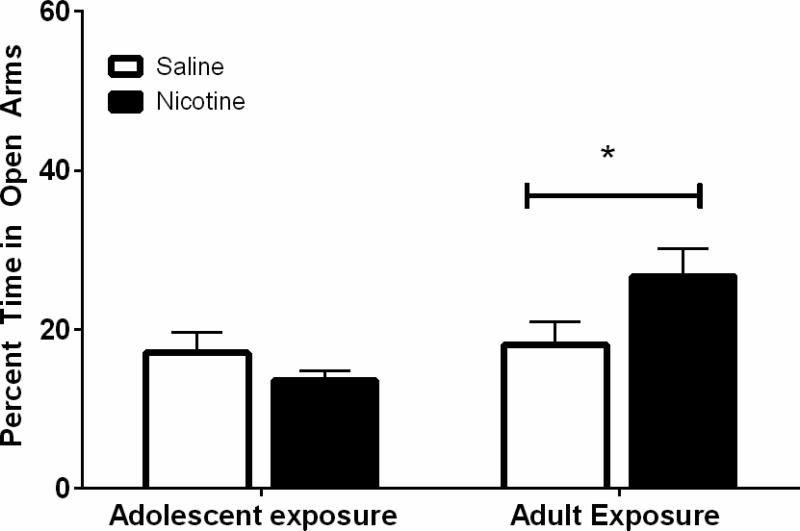
Adolescent and adult mice were exposed to nicotine for 12 days. Thirty days after nicotine cessation, anxiety-like behaviors were measured on the elevated plus maze. A significant interaction between age and drug condition (F(1,53) = 4.50, p < 0.05) was found in the analysis of time spent on open arms (Figure 3). Post-hoc tests using simple main effects revealed that mice administered nicotine in adulthood spent significantly more time in the open arms than their age-matched saline controls when tested 30 days later. There were no significant differences between adolescent nicotine and saline administration on elevated plus maze behaviors measured 30 days later. These data suggest that nicotine reduced anxiety-like behaviors in an age-dependent fashion with the effect being significant when exposure occurred during adulthood.
Figure 3. Anxiety-like behaviors measured on the elevated-plus maze.
Adolescent nicotine exposure and forced abstinence had no effect on time spent in open arms. Nicotine administration in adulthood led to increased time in the open arms compared with saline age-matched controls when tested 30 days after nicotine cessation (*p<0.05). n=12-16/experimental group.
3.4 Golgi-Cox Staining
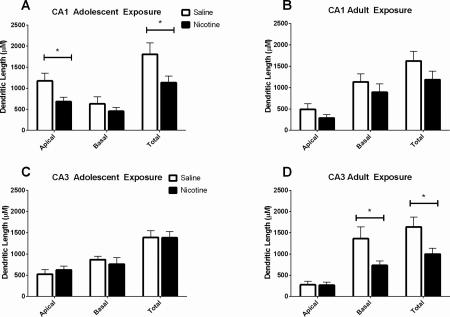
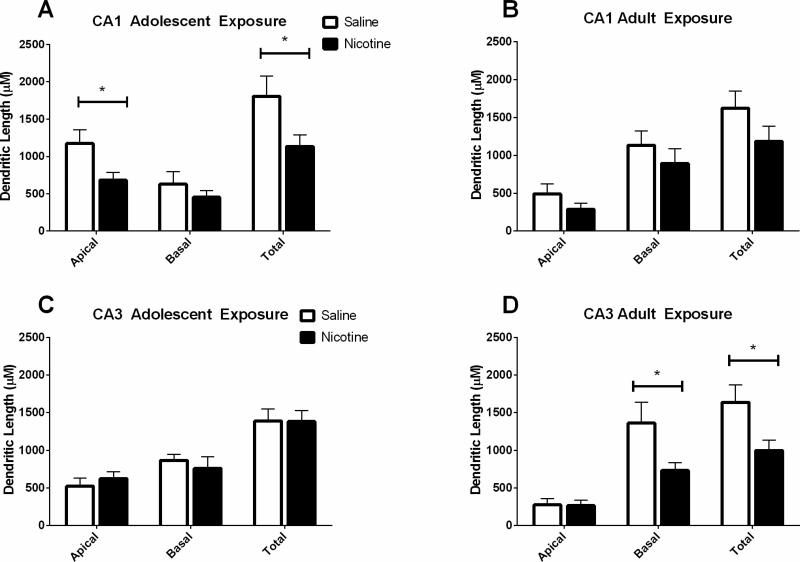
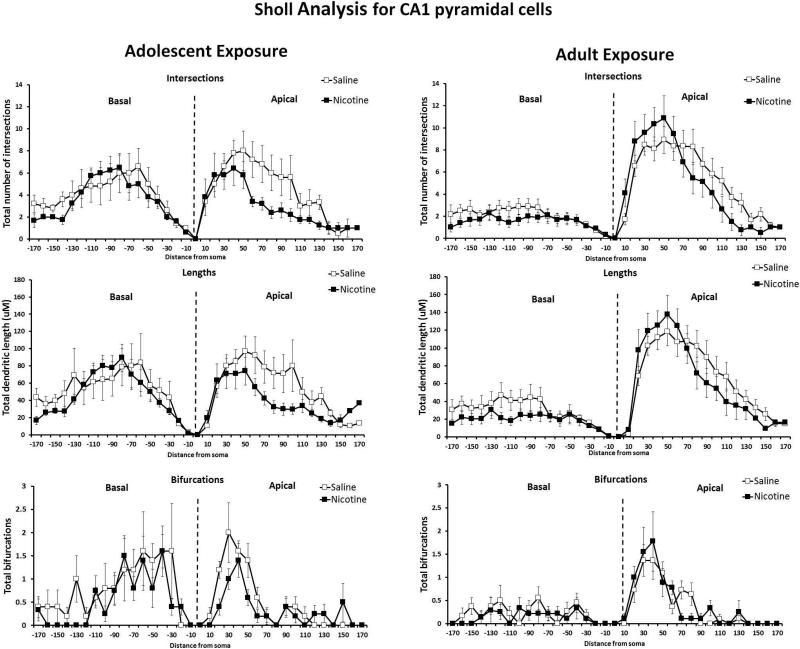
Brains from mice exposed to nicotine or saline followed by 40 days of abstinence were processed for analysis of hippocampal dendritic length and complexity. Anatomical sites of neuronal sampling are shown in Figure 7. Separate one-way ANOVAs were conducted for each age condition and each hippocampal region on basilar and apical dendrites, consistent with other studies using the same statistical tests on reconstructed neurons (Ehlinger et al., 2012). There was a significant effect of drug in the total length of apical dendrites in the CA1 of mice exposed to nicotine during adolescence (F(1,14) = 5.40, p < 0.05), such that shorter total dendritic length was seen in nicotine exposed mice than saline controls (Figure 4A). Additionally, there was a significant difference in number of intersections of dendrites in the apical branch of CA1 pyramidal cells when analyzing Sholl parameters, (F(1,9) = 6.41, p < 0.05) such that mice exposed to nicotine during adolescence had fewer intersections than those administered saline (Figure 5). There were no differences in the CA1 pyramidal cells following abstinence from chronic adolescent nicotine treatment in dendritic lengths and bifurcations by Sholl analysis. Reduced dendritic length and fewer intersections suggest less complexity in the adult CA1 apical tree following chronic nicotine exposure during adolescence.
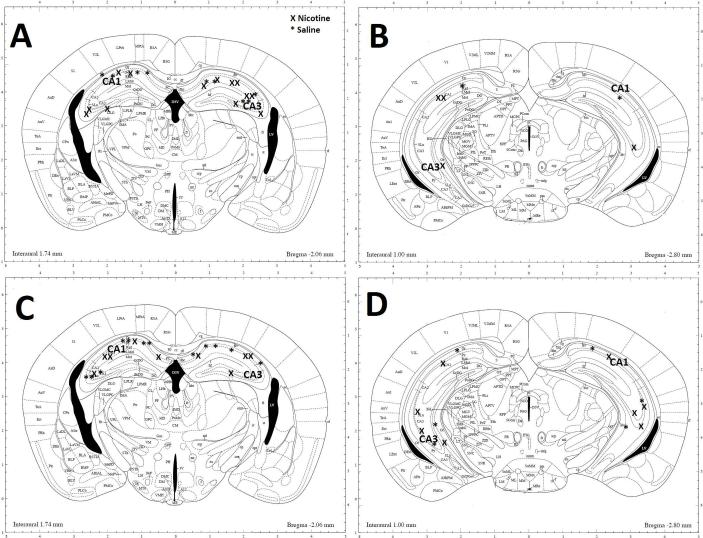
Figure 7. Illustration showing location of neurons in the hippocampus selected for tracing.
Pyramidal neurons were selected bilaterally in the CA1 and CA3 regions of the dorsal and ventral hippocampus following A) & B) adolescent exposure to nicotine (x) or saline (*) or C) & D) adult exposure to nicotine (x) or saline (*). Illustration modified from Mouse Brain Atlas of Paxinos and Franklin (2004).
Figure 4. Total dendritic lengths in CA1 and CA3 apical and basal trees following protracted abstinence from chronic adolescent or adult nicotine or saline administration.
A) Chronic nicotine administration during adolescence resulted in reduced total dendritic length in the apical trees of CA1 pyramidal cells when examined in adulthood following a 30-day drug abstinent period. B) No significant differences in CA1 apical or basal dendritic length were found following chronic nicotine or saline administration to adult mice. C) There were no differences in dendritic length in CA3 apical or basal dendrites between in nicotine or saline exposed adolescent mice. D) Chronic nicotine administration during adulthood reduced the total dendritic length in the basal tree of pyramidal cells in the CA3. * p < 0.05; n = 3-4 mice (10 neurons)/drug/age.
Figure 5. Sholl Analysis: Morphometric parameters of apical and basal trees in the CA1 following protracted abstinence from chronic nicotine or saline administration during adolescence or adulthood.
Data are presented as dendritic characteristic vs distance from soma (μM) for mice exposed to nicotine during adolescence (left) or adulthood (right). Adolescent nicotine exposure resulted in reduced total intersections in the apical trees of the CA1 pyramidal neurons when measured 40 days later (* p < 0.05). No differences in number of intersections between saline and nicotine were found when drug exposure occurred during adulthood. There were no differences between saline and nicotine exposure at either age in total dendritic lengths. No differences in bifurcations in CA1 pyramidal cells were found between saline and nicotine exposure during either adolescent or adulthood. n = 3-4 mice (10 neurons)/age/drug condition.
In mice administered saline or nicotine in adulthood, no significant differences in total dendritic length in the CA1 apical or basal total dendritic length were found between treatment groups (Figure 4B). Sholl analysis (Figure 5) revealed no differences in intersections, lengths, or bifurcations in the CA1 basilar or apical trees between adult saline- and nicotine-treated mice.
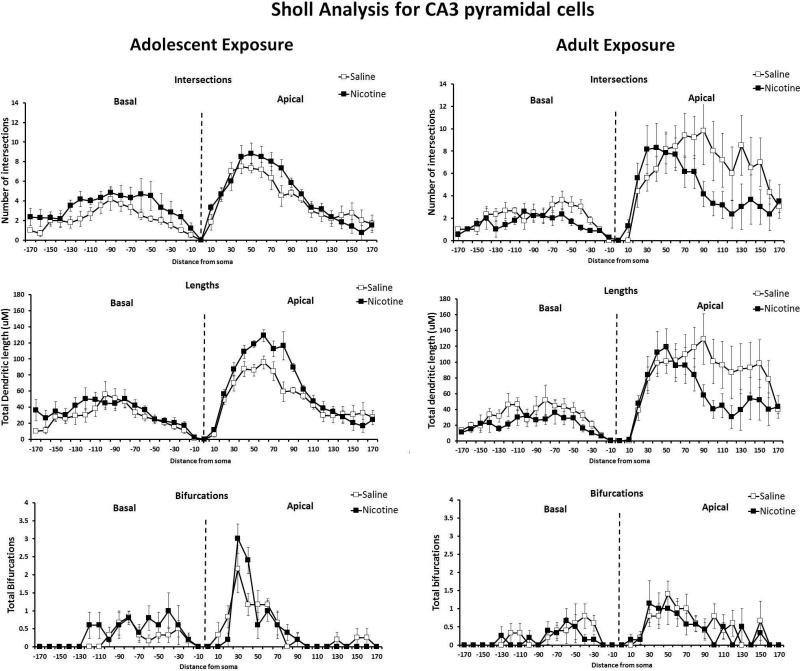
In the CA3 region of the hippocampus, there were no differences in the total length of apical or basilar dendrites in the CA3 region following adolescent administration of nicotine or saline (Figure 4B). There were also no significant between treatment differences in the CA3 apical or basal region in intersections, lengths, or bifurcations when analyzing Sholl parameters (Figure 6).
Figure 6. Sholl Analysis: Morphometric parameters of apical and basal trees in the CA3 following protracted abstinence from chronic nicotine or saline administration during adulthood or adolescence.
Data are presented as dendritic characteristic vs distance from soma (μM) for mice exposed to nicotine during adolescence (left) or adulthood (right). No differences in intersections in CA3 pyramidal cells of adult mice administered saline or nicotine during adolescence or adulthood were found. There were no differences in dendritic length in the CA3 pyramidal cells between saline or nicotine exposed mice of either age. No differences were found between nicotine and saline exposure at either age in bifurcations in CA3 pyramidal cells. n = 3-4 mice (10 neurons)/age/drug condition.
Mice treated in adulthood with nicotine had significant reductions in total length of basilar dendrites in the CA3 compared to saline age-matched controls (F(1,14) = 5.03, p < .05) (Figure 4D). Sholl analysis (Figure 6) revealed no significant differences between saline and nicotine groups in the number of intersections, dendritic lengths, or bifurcations in the CA3 basilar dendrites. In the apical dendrites of the CA3 region, there were no significant differences in dendritic intersections, lengths, or bifurcations between mice exposed to saline or nicotine as adults.
4. DISCUSSION
Adolescence is a vulnerable period for the initiation of substance abuse, and tobacco is one of the most frequently used drugs (NIDA, 2014). Clinical research has shown associations between adolescent nicotine use and negative consequences on cognition and affect later in life (e.g., Choi et al., 1997; Falcone et al., 2014; Jacobsen et al., 2005). The negative effects of nicotine on the adolescent were mirrored in this pre-clinical mouse study which showed that chronic nicotine exposure during mid-adolescence created long-term impairments in contextual fear learning, increased depressive-like behaviors, and reduced the dendritic length of neurons in the CA1 region of the hippocampus. When exposure occurred during adulthood, nicotine did not produce the same long-term impairments in learning and increases in depression-like behaviors, but uniquely altered behavior on the elevated plus maze. Prior research, including our own, has shown that chronic nicotine administration during adolescence leads to long-term changes in learning and memory in rodent models (Counotte et al., 2011; Iñiguez et al., 2009; Portugal et al., 2012; Slawecki et al., 2003; Spaeth et al., 2010). The present study using mid-adolescent mice (PND32) extends previous work with early and late adolescent mice (Portugal et al., 2012) by demonstrating additional effects on depression-like behaviors following withdrawal from nicotine.
Results presented herein indicate that adult mice that received nicotine during mid-adolescence had long-term deficits in contextual fear learning, as indicated by lower freezing to a context previously associated with a shock when tested 30 days later. This was an age-specific response as nicotine administration to adult mice did not produce similar deficits. Contextual fear learning is a hippocampus-dependent task (Kim et a., 1993; Fanselow, 2000; Logue et al., 1997). The age-dependent long-term behavioral deficits noted here may be due to structural changes in the dendritic arborization of pyramidal cells in CA1 subregion of the hippocampus. These cells are crucial to the consolidation and recall of contextual memories and thus alterations of this neuronal population could lead to learning deficits (Daumas et al., 2005; Ji and Maren, 2008; Lee and Kesner, 2004). Neurons from mice exposed to nicotine during adolescence showed atrophy in the apical dendrites of pyramidal cells in the CA1 region. Since these neurons were Golgi-impregnated 40 days following nicotine cessation, the results demonstrate that there are long-term changes in pyramidal cell structure which may underlie the observed learning deficits produced by adolescent nicotine exposure. Other studies report age-specific effects of nicotine on synaptic transmission which could lead to the observed structural changes. For example, nicotine applied to immature hippocampal neurons impairs LTP in the Schaffer-CA1 pathway but enhances LTP in mature hippocampal neurons (Maggi et al., 2004).
Much evidence supports the importance of CA1 neuronal function in cognition. Evidence from research on Alzheimer's disease suggests that dendritic atrophy in the CA1 region precedes cognitive impairments (Kerchner et al., 2012). In primates, lesions of the CA1 region of the hippocampus causes learning deficits in both acquisition and retrieval of novel object recognition (Ridley et al., 1995). It is possible that the atrophy in the CA1 region of the hippocampus of mice exposed to nicotine during adolescence results in altered signaling to the subiculum. The subiculum receives input from the CA1 region of the hippocampus and acts as a relay between the hippocampus and other parts of the cortex (O'Mara et al., 2000). Lesions to this region result in impaired acquisition and recall of contextual fear (Maren, 1999), as well as disruption of latent inhibition which is context dependent and mediated through the hippocampus (Quintero et al., 2011). Thus, if reduced dendritic length in the CA1 leads to suboptimal output to the subiculum, this could be a potential mechanism for the observed deficits in contextual fear learning identified in this study. In contrast to adolescent nicotine exposure, neurons from mice exposed to nicotine in adulthood showed atrophy in the basilar CA3 dendrites. Interestingly, a number of studies indicate that dendritic atrophy in the CA3 region occurs independently of changes in behavior (Conrad et al., 1999; Lopes et al., 2013; Magariños et al., 1996). Our results are consonant with these previous studies as mice that received nicotine in adulthood did not display contextual fear deficits, although dendritic atrophy in the CA3 region was apparent. Thus, the dissociation between the atrophy in the CA1 and CA3 regions that occurs in an age-dependent manner following chronic nicotine exposure and withdrawal may explain why only mice that received nicotine as adolescents demonstrated persistent impairments in a hippocampus-dependent task. It should be noted, however, that our experimental design did not allow for correlation analysis between dendritic morphology and performance in the fear conditioning task as the two measures were performed in separate groups of mice. Further studies are needed to firmly establish a potential causative relationship between dendritic atrophy and cognitive impairments produced by adolescent nicotine exposure.
The impact of nicotine on emotional behavior was also investigated herein. Results from the forced swim test demonstrate that exposure to nicotine during adolescence resulted in increased depression-like behavior, and this phenotype persisted into adulthood when tested 30 days after nicotine cessation. Adolescent mice that were exposed to nicotine spent more time immobile than their saline age-matched controls after both a 24-hour and 30-day abstinent period. Likewise, adolescent mice had a shorter latency to become immobile compared to the saline controls after long-term abstinence from nicotine, indicating an elevation in depression-like behaviors when nicotine exposure occurred during adolescence. The finding that chronic nicotine exposure during adolescence induces a depression-like phenotype in adulthood is consistent with other literature demonstrating 15 days of nicotine administered as twice daily injections to rats during adolescence, but not during adulthood, increased immobility time when tested 1 month later (Iñiguez et al., 2009).These observations are also consonant with clinical reports documenting that adolescents who smoke cigarettes have a higher prevalence of depression throughout their life-times than do non-smoking teenagers (Breslau and Peterson, 1996; Brook et al., 2004; Choi et al., 1997; Park et al., 2013; Steuber and Danner, 2006). The long-term pro-depressant effect of nicotine withdrawal was specific for adolescent nicotine exposure in the present study, since mice administered nicotine during adulthood showed no differences from saline age-matched controls when tested after 30 days of nicotine abstinence. An additional finding from the forced swim test was that control adolescent mice had longer latencies to immobility and shorter immobility times than control adult mice at the 24-hour time point (at P45 and P67 respectively). Despite the apparent resilience of the adolescent mice to the stressor in this test, they were none-the-less more susceptible to the pro-depressive effects of nicotine withdrawal than the adult mice.
Anxiety-like behaviors after withdrawal from chronic nicotine exposure during adolescence and adulthood were also investigated. The results demonstrate that mice exposed to nicotine during adolescence showed no differences in anxiety-like behaviors compared to age-matched controls when tested 30 days later in adulthood. In contrast to adolescent nicotine exposure, adult nicotine exposure and withdrawal led to a reduction in anxiety-like behaviors compared to saline age-matched controls, as shown by an increase in time spent in open arms on the elevated plus maze. The finding that adolescent nicotine exposure and withdrawal did not increase anxiety is in agreement with another study that showed that chronic nicotine exposure during adolescence does not increase anxiety-like behaviors on the elevated plus maze after either short or long-term withdrawal (Abreu-Villaça et al., 2007). It isinconsistent, however, with a study by Bolanos-Guzman and colleagues (Iniguez et al., 2009) who demonstrate reduced time in open arms of the elevated plus maze in adult rats that were injected with nicotine twice daily for 15 days as adolescents and assessed for anxiety-like behaviors 30 days later. Likewise, Slawecki and colleagues (Slawecki et al., 2003) report reduced center time in an open field test in late adolescent rats treated with nicotine during peri-adolescence. The current study also examined changes in anxiety behaviors following protracted abstinence from adult nicotine exposure. Our results demonstrate reduced anxiety-like behaviors when tested 30 days after chronic nicotine administration during adulthood. This is in contrast to Iniguez et al (2009) who report no changes in elevated plus maze performance by adult rats injected with chronically with nicotine. It is possible that dose, length of nicotine exposure, age of exposure, or species plays a role in the disparate findings. Other studies have investigated short-term nicotine withdrawal in adult rodents and found an anxiogenic response. For example, short-term withdrawal (ie, 24 hrs) from repeated nicotine injections in adult rats increases anxiety-like behaviors on the elevated plus maze (Irvine et al., 2001), as does mecamylamine-precipitated withdrawal, an effect not seen in adolescent rats (Wilmouth and Spear, 2006). The transition from acute anxiogenic to protracted anxiolytic responses following withdrawal from adult nicotine exposure deserves further investigation. It is possible, however, that the increase in open arm time measured following adult nicotine exposure is related to an increase in impulsivity rather than a change in anxiety, and future work should investigate this possibility.
The results from the current study extend the body of knowledge showing adolescents and adults differ in long-term effects of nicotine. The strong propensity for adolescent initiation of nicotine use, leading to increased risk for nicotine dependence in adulthood, may be attributed to several molecular events occurring within the drug-exposed adolescent brain. Age-related differences in reward circuitry and nicotinic cholinergic receptor regulation likely play a role in the development of nicotine dependence and associated behavioral sequela in the adolescent population (eg., Christensen et al., 2014; Doura et al., 2008; Trauth et al., 2000). The adolescent brain undergoes vast developmental changes that may be related to the heightened sensitivity of this age-group to the stimulating and addictive effects of nicotine. Further, the long-term morphological changes in hippocampal neurons induced by nicotine exposure during adolescence demonstrated in the present study may contribute to persistent learning deficits and increased risk of continued nicotine use in adulthood. Clinical studies report greater cognitive deficits in young smokers compared to non-smokers and impairments in cognition are associated with relapse during periods of abstinence (Jacobsen et al., 2005; Falcone et al., 2014; Patterson et al., 2010). Our results support this as chronic nicotine exposure during the adolescent period produced long-lasting deficits in adult contextual fear learning, increased adult depressive-like behaviors, and decreased dendritic length in the apical dendrites of the hippocampal CA1 region. Thus, the results from the current study extend the growing body of literature demonstrating that nicotine exposure during adolescence produces long-lasting changes in emotional behaviors and associative learning, which may be related to its impact on hippocampal pyramidal cell morphology.
HIGHLIGHTS.
Chronic nicotine administration starting during mid-adolescence resulted in deficits in contextual fear learning when tested after 30 days of drug abstinence during adulthood.
Chronic nicotine administration during adolescence increased depressive-like behaviors which persisted in adulthood.
Chronic nicotine administration during adolescence decreased the length and complexity of hippocampal CA1 apical dendrites when measured during adulthood.
Acknowledgements
This work was supported by a grant from Temple University and the PA Department of Health. Additional support came from NIDA/NIH grants, DA017949 (TJG), P30 DA13429 (EMU) and T32 DA007237 (EMU/CO & KLC). The authors would also like to thank Dr. Jeffrey Barr for his contributions to this project.
Footnotes
The authors have no conflicts of interest to report.
References
- Abreu-Villaça Y, Nunes F, Queiroz-Gomes F. do E., Manhães AC, Filgueiras CC. Combined Exposure to Nicotine and Ethanol in Adolescent Mice Differentially Affects Anxiety Levels during Exposure, Short-Term, and Long-Term Withdrawal. Neuropsychopharmacology. 2007;33:599–610. doi: 10.1038/sj.npp.1301429. doi:10.1038/sj.npp.1301429. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS. Hippocampal Volume Reduction in Major Depression. Am. J. Psychiatry. 2000;157:115–118. doi: 10.1176/ajp.157.1.115. doi:10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- Breslau N, Kilbey MM, Andreski P. Nicotine dependence and major depression. New evidence from a prospective investigation. Arch Gen Psychiatry. 1993;50:31–5. doi: 10.1001/archpsyc.1993.01820130033006. [DOI] [PubMed] [Google Scholar]
- Breslau N, Peterson EL. Smoking cessation in young adults: age at initiation of cigarette smoking and other suspected influences. Am. J. Public Health. 1996;86:214–220. doi: 10.2105/ajph.86.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook JS, Schuster E, Zhang C. Cigarette smoking and depressive symptoms: a longitudinal study of adolescents and young adults. Psychol Rep. 2004;95:159–66. doi: 10.2466/pr0.95.1.159-166. doi:10.2466/pr0.95.1.159-166. [DOI] [PubMed] [Google Scholar]
- Castagné V, Moser P, Roux S, Porsolt RD. Rodent models of depression: forced swim and tail suspension behavioral despair tests in rats and mice. Curr. Protoc. Neurosci. Editor. Board Jacqueline N Crawley Al. 2011 doi: 10.1002/0471142301.ns0810as55. Chapter 8, Unit 8.10A. doi:10.1002/0471142301.ns0810as55. [DOI] [PubMed] [Google Scholar]
- Center for Disease Control E-cigarette use by youth is dangerous [WWW Document]. [2.23.16];Cent. Dis. Control Prev. 2016 URL http://www.cdc.gov/vitalsigns/ecigarette-ads/index.html.
- Chassin L, Presson CC, Sherman SJ, Edwards DA. The natural history of cigarette smoking: predicting young-adult smoking outcomes from adolescent smoking patterns. Health Psychol. 1990;9:701. doi: 10.1037//0278-6133.9.6.701. [DOI] [PubMed] [Google Scholar]
- Choi WS, Patten CA, Gillin JC, Kaplan RM, Pierce JP. Cigarette smoking predicts development of depressive symptoms among U.S. adolescents. Ann Behav Med. 1997;19:42–50. doi: 10.1007/BF02883426. [DOI] [PubMed] [Google Scholar]
- Christensen MH, Ishibashi M, Nielsen ML, Leonard CS, Kohlmeier KA. Age-related changes in nicotine response of cholinergic and non-cholinergic laterodorsal tegmental neurons: implications for the heightened adolescent susceptibility to nicotine addiction. Neuropharmacology. 2014;85:263–283. doi: 10.1016/j.neuropharm.2014.05.010. doi:10.1016/j.neuropharm.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, LeDoux JE, Magariños AM, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav. Neurosci. 1999;113:902–913. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- Counotte DS, Smit AB, Pattij T, Spijker S. Development of the motivational system during adolescence, and its sensitivity to disruption by nicotine. Accid. Anal. Prev. 2011;1:430–443. doi: 10.1016/j.dcn.2011.05.010. doi:10.1016/j.dcn.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craige CP, Lewandowski S, Kirby LG, Unterwald EM. Dorsal raphe 5-HT(2C) receptor and GABA networks regulate anxiety produced by cocaine withdrawal. Neuropharmacology. 2015;93:41–51. doi: 10.1016/j.neuropharm.2015.01.021. doi:10.1016/j.neuropharm.2015.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daumas S, Halley H, Francés B, Lassalle J-M. Encoding, consolidation, and retrieval of contextual memory: differential involvement of dorsal CA3 and CA1 hippocampal subregions. Learn. Mem. Cold Spring Harb. N. 2005;12:375–382. doi: 10.1101/lm.81905. doi:10.1101/lm.81905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, James JR, Siegel SJ, Gould TJ. Withdrawal from chronic nicotine administration impairs contextual fear conditioning in C57BL/6 mice. J. Neurosci. 2005;25:8708–13. doi: 10.1523/JNEUROSCI.2853-05.2005. doi:10.1523/JNEUROSCI.2853-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doura MB, Gold AB, Keller AB, Perry DC. Adult and periadolescent rats differ in expression of nicotinic cholinergic receptor subtypes and in the response of these subtypes to chronic nicotine exposure. Brain Res. 2008;1215:40–52. doi: 10.1016/j.brainres.2008.03.056. doi:10.1016/j.brainres.2008.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton DK, Kann L, Kinchen S, Shanklin S, Ross J, Hawkins J, Harris WA, Lowry R, McManus T, Chyen D, Lim C, Whittle L, Brener ND, Wechsler H, Control, C. for D., (CDC), P. Youth risk behavior surveillance - United States, 2009. MMWR Surveill Summ. 2010;59:1–142. [PubMed] [Google Scholar]
- Ehlinger DG, Bergstrom HC, McDonald CG, Smith RF. Nicotine-induced dendritic remodeling in the insular cortex. Neurosci. Lett. 2012;516:89–93. doi: 10.1016/j.neulet.2012.03.064. doi:10.1016/j.neulet.2012.03.064. [DOI] [PubMed] [Google Scholar]
- Falcone M, Wileyto EP, Ruparel K, Gerraty RT, LaPrate L, Detre JA, Gur R, Loughead J, Lerman C. Age-related differences in working memory deficits during nicotine withdrawal. Addict. Biol. 2014;19:907–917. doi: 10.1111/adb.12051. doi:10.1111/adb.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS. Contextual fear, gestalt memories, and the hippocampus. Behav. Brain Res. 2000;110:73–81. doi: 10.1016/s0166-4328(99)00186-2. [DOI] [PubMed] [Google Scholar]
- Gibb R, Kolb B. A method for vibratome sectioning of Golgi-Cox stained whole rat brain. J. Neurosci. Methods. 1998;79:1–4. doi: 10.1016/s0165-0270(97)00163-5. [DOI] [PubMed] [Google Scholar]
- Glaser EM, Van der Loos H. Analysis of thick brain sections by obverse-reverse computer microscopy: application of a new, high clarity Golgi-Nissl stain. J. Neurosci. Methods. 1981;4:117–125. doi: 10.1016/0165-0270(81)90045-5. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Higgins JS. Nicotine enhances contextual fear conditioning in C57BL/6J mice at 1 and 7 days post-training. Neurobiol. Learn. Mem. 2003;80:147–57. doi: 10.1016/s1074-7427(03)00057-1. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Keenan RM. Nicotine delivery kinetics and abuse liability. J. Consult. Clin. Psychol. 1993;61:743–750. doi: 10.1037//0022-006x.61.5.743. [DOI] [PubMed] [Google Scholar]
- Iñiguez SD, Warren BL, Parise EM, Alcantara LF, Schuh B, Maffeo ML, Manojlovic Z, Bolaños- Guzmán CA. Nicotine exposure during adolescence induces a depression-like state in adulthood. Neuropsychopharmacology. 2009;34:1609–24. doi: 10.1038/npp.2008.220. doi:10.1038/npp.2008.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine EE, Cheeta S, File SE. Tolerance to nicotine's effects in the elevated plus-maze and increased anxiety during withdrawal. Pharmacol. Biochem. Behav. 2001;68:319–325. doi: 10.1016/s0091-3057(00)00449-4. doi:10.1016/S0091-3057(00)00449-4. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Wellman CL, Holmes A. Brief Uncontrollable Stress Causes Dendritic Retraction in Infralimbic Cortex and Resistance to Fear Extinction in Mice. J. Neurosci. 2006;26:5733–5738. doi: 10.1523/JNEUROSCI.0474-06.2006. doi:10.1523/JNEUROSCI.0474-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen LK, Krystal JH, Mencl WE, Westerveld M, Frost SJ, Pugh KR. Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biol Psychiatry. 2005;57:56–66. doi: 10.1016/j.biopsych.2004.10.022. doi:10.1016/j.biopsych.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Ji J, Maren S. Differential roles for hippocampal areas CA1 and CA3 in the contextual encoding and retrieval of extinguished fear. Learn. Mem. Cold Spring Harb. N. 2008;15:244–251. doi: 10.1101/lm.794808. doi:10.1101/lm.794808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerchner GA, Deutsch GK, Zeineh M, Dougherty RF, Saranathan M, Rutt BK. Hippocampal CA1 apical neuropil atrophy and memory performance in Alzheimer's disease. NeuroImage. 2012;63:194–202. doi: 10.1016/j.neuroimage.2012.06.048. doi:10.1016/j.neuroimage.2012.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Rison RA, Fanselow MS. Effects of amygdala, hippocampus, and periaqueductal gray lesions on short- and long-term contextual fear. Behav. Neurosci. 1993;107:1093–1098. doi: 10.1037//0735-7044.107.6.1093. [DOI] [PubMed] [Google Scholar]
- Komada M, Takao K, Miyakawa T. Elevated Plus Maze for Mice. J. Vis. Exp. JoVE. 2008 doi: 10.3791/1088. doi:10.3791/1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutlu MG, Gould TJ. Acute nicotine delays extinction of contextual fear in mice. Behav. Brain Res. 2014;263:133–137. doi: 10.1016/j.bbr.2014.01.031. doi:10.1016/j.bbr.2014.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CSW, Franke TF, Gan W-B. Opposite effects of fear conditioning and extinction on dendritic spine remodelling. Nature. 2012;483:87–91. doi: 10.1038/nature10792. doi:10.1038/nature10792. [DOI] [PubMed] [Google Scholar]
- Lee I, Kesner RP. Differential contributions of dorsal hippocampal subregions to memory acquisition and retrieval in contextual fear-conditioning. Hippocampus. 2004;14:301–310. doi: 10.1002/hipo.10177. doi:10.1002/hipo.10177. [DOI] [PubMed] [Google Scholar]
- Logue SF, Paylor R, Wehner JM. Hippocampal lesions cause learning deficits in inbred mice in the Morris water maze and conditioned-fear task. Behav Neurosci. 1997;111:104–13. doi: 10.1037//0735-7044.111.1.104. [DOI] [PubMed] [Google Scholar]
- Lopes A, Torres DB, Rodrigues AJ, Cerqueira JJ, Pêgo JM, Sousa N, Gontijo J. a. R., Boer PA. Gestational protein restriction induces CA3 dendritic atrophy in dorsal hippocampal neurons but does not alter learning and memory performance in adult offspring. Int. J. Dev. Neurosci. Off. J. Int. Soc. Dev. Neurosci. 2013;31:151–156. doi: 10.1016/j.ijdevneu.2012.12.003. doi:10.1016/j.ijdevneu.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Magariños AM, McEwen BS, Flügge G, Fuchs E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J Neurosci. 1996;16:3534–40. doi: 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi L, Sola E, Minneci F, Le Magueresse C, Changeux JP, Cherubini E. Persistent decrease in synaptic efficacy induced by nicotine at Schaffer collateral-CA1 synapses in the immature rat hippocampus. J. Physiol. 2004;559:863–874. doi: 10.1113/jphysiol.2004.067041. doi:10.1113/jphysiol.2004.067041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Neurotoxic or electrolytic lesions of the ventral subiculum produce deficits in the acquisition and expression of Pavlovian fear conditioning in rats. Behav. Neurosci. 1999;113:283–290. doi: 10.1037//0735-7044.113.2.283. doi:10.1037/0735-7044.113.2.283. [DOI] [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, Gardiner PS, Grady SR, Heberlein U, Leonard SS, Levin ED, Lukas RJ, Markou A, Marks MJ, McCallum SE, Parameswaran N, Perkins KA, Picciotto MR, Quik M, Rose JE, Rothenfluh A, Schafer WR, Stolerman IP, Tyndale RF, Wehner JM, Zirger JM. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl.) 2007;190:269–319. doi: 10.1007/s00213-006-0441-0. doi:10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- NIDA [10.1.15];What drugs are most frequently used by adolescents? [WWW Document] 2014 URL http://www.drugabuse.gov/publications/principles-adolescent-substance-use-disorder-treatment-research-based-guide/frequently-asked-questions/what-drugs-are-most-frequently-used-by-adolescents.
- Oliveira-da-Silva A, Vieira FB, Cristina-Rodrigues F, Filgueiras CC, Manhães AC, Abreu-Villaça Y. Increased apoptosis and reduced neuronal and glial densities in the hippocampus due to nicotine and ethanol exposure in adolescent mice. Int. J. Dev. Neurosci. 2009;27:539–548. doi: 10.1016/j.ijdevneu.2009.06.009. doi:10.1016/j.ijdevneu.2009.06.009. [DOI] [PubMed] [Google Scholar]
- O'Mara SM, Commins S, Anderson M. Synaptic plasticity in the hippocampal area CA1- subiculum projection: implications for theories of memory. Hippocampus. 2000;10:447–456. doi: 10.1002/1098-1063(2000)10:4<447::AID-HIPO11>3.0.CO;2-2. doi:10.1002/1098-1063(2000)10:4<447::AID-HIPO11>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Park S, Romer D, Lim S. Does smoking initiation in adolescence increase risk for depression across the lifespan? Evidence from the South korean national health and nutrition examination survey. J. Addict. Nurs. 2013;24:142–148. doi: 10.1097/JAN.0b013e3182a4cad3. doi:10.1097/JAN.0b013e3182a4cad3. [DOI] [PubMed] [Google Scholar]
- Patterson F, Jepson C, Loughead J, Perkins K, Strasser AA, Siegel S, Frey J, Gur R, Lerman C. Working memory deficits predict short-term smoking resumption following brief abstinence. Drug Alcohol Depend. 2010;106:61–4. doi: 10.1016/j.drugalcdep.2009.07.020. doi:10.1016/j.drugalcdep.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. Gulf Professional Publishing; 2004. [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Portugal GS, Wilkinson DS, Turner JR, Blendy JA, Gould TJ. Developmental effects of acute, chronic, and withdrawal from chronic nicotine on fear conditioning. Neurobiol. Learn. Mem. 2012;97:482–494. doi: 10.1016/j.nlm.2012.04.003. doi:10.1016/j.nlm.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero E, Díaz E, Vargas JP, Schmajuk N, López JC, De la Casa LG. Effects of context novelty vs. familiarity on latent inhibition with a conditioned taste aversion procedure. Behav. Processes. 2011;86:242–249. doi: 10.1016/j.beproc.2010.12.011. doi:10.1016/j.beproc.2010.12.011. [DOI] [PubMed] [Google Scholar]
- Reith MEA, Ali S, Hashim A, Sheikh IS, Theddu N, Gaddiraju NV, Mehrotra S, Schmitt KC, Murray TF, Sershen H, Unterwald EM, Davis FA. Novel C-1 Substituted Cocaine Analogs Unlike Cocaine or Benztropine. J. Pharmacol. Exp. Ther. 2012;343:413–425. doi: 10.1124/jpet.112.193771. doi:10.1124/jpet.112.193771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro-Carvalho A, Lima CS, Nunes-Freitas AL, Filgueiras CC, Manhães AC, Abreu-Villaça Y. Exposure to nicotine and ethanol in adolescent mice: Effects on depressive-like behavior during exposure and withdrawal. Behav. Brain Res. 2011;221:282–289. doi: 10.1016/j.bbr.2011.03.014. doi:10.1016/j.bbr.2011.03.014. [DOI] [PubMed] [Google Scholar]
- Ridley RM, Timothy CJ, Maclean CJ, Baker HF. Conditional learning and memory impairments following neurotoxic lesion of the CA1 field of the hippocampus. Neuroscience. 1995;67:263–275. doi: 10.1016/0306-4522(95)00063-o. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Gilder A, Roth J, Ehlers CL. Increased anxiety-like behavior in adult rats exposed to nicotine as adolescents. Pharmacol. Biochem. Behav. 2003;75:355–361. doi: 10.1016/s0091-3057(03)00093-5. [DOI] [PubMed] [Google Scholar]
- Spaeth AM, Barnet RC, Hunt PS, Burk JA. Adolescent nicotine exposure disrupts context conditioning in adulthood in rats. Pharmacol Biochem Behav. 2010;96:501–506. doi: 10.1016/j.pbb.2010.07.011. doi:10.1016/j.pbb.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steuber TL, Danner F. Adolescent smoking and depression: which comes first? Addict. Behav. 2006;31:133–136. doi: 10.1016/j.addbeh.2005.04.010. doi:10.1016/j.addbeh.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Trauth JA, Seidler FJ, Slotkin TA. An animal model of adolescent nicotine exposure: effects on gene expression and macromolecular constituents in rat brain regions. Brain Res. 2000;867:29–39. doi: 10.1016/s0006-8993(00)02208-3. [DOI] [PubMed] [Google Scholar]
- Wilmouth CE, Spear LP. Withdrawal from chronic nicotine in adolescent and adult rats. Pharmacol Biochem Behav. 2006;85:648–57. doi: 10.1016/j.pbb.2006.10.021. doi:10.1016/j.pbb.2006.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]