Abstract
Numerous studies have attempted to identify successful dietary strategies for weight loss, and many have focused on Low-Fat vs. Low-Carbohydrate comparisons. Despite relatively small between-group differences in weight loss found in most previous studies, researchers have consistently observed relatively large between-subject differences in weight loss within any given diet group (e.g., ~25 kg weight loss to ~5 kg weight gain). The primary objective of this study was to identify predisposing individual factors at baseline that help explain differential weight loss achieved by individuals assigned to the same diet, particularly a pre-determined multi-locus genotype pattern and insulin resistance status. Secondary objectives included discovery strategies for further identifying potential genetic risk scores. Exploratory objectives included investigation of an extensive set of physiological, psychosocial, dietary, and behavioral variables as moderating and/or mediating variables and/or secondary outcomes. The target population was generally healthy, free-living adults with BMI 28-40 kg/m2 (n=600). The intervention consisted of a 12-month protocol of 22 one-hour evening instructional sessions led by registered dietitians, with ~15-20 participants/class. Key objectives of dietary instruction included focusing on maximizing the dietary quality of both Low-Fat and Low-Carbohydrate diets (i.e., Healthy Low-Fat vs. Healthy Low-Carbohydrate), and maximally differentiating the two diets from one another. Rather than seeking to determine if one dietary approach was better than the other for the general population, this study sought to examine whether greater overall weight loss success could be achieved by matching different people to different diets. Here we present the design and methods of the study.
Keywords: Obesity, Low Fat, Low Carbohydrate, Nutrition, Diet, Weight Loss
INTRODUCTION
Obesity has become one of the most significant public health challenges of the 21st century [1, 2]. Numerous studies have attempted to identify successful dietary strategies for weight loss, with a particular emphasis on contrasting Low-Fat to Low-Carbohydrate (Low-Carb) diets. As summarized in multiple meta-analyses, the majority of these diet trials have reported only modest mean weight loss (i.e., < 5% initial body weight) after 12 months or longer, with limited influence of macronutrient differences on average weight loss (i.e., average between-group differences of 2-3 kg) [3, 4]. Notably, despite these relatively small between-group average differences, weight loss variability between subjects within any given diet group in these studies has been substantial, ranging from highly successful to highly unsuccessful (~25 kg weight loss to ~5 kg weight gain) [5-7].
In 2013, our research group received NIH funding (R01 DK 91831) to examine weight loss in overweight/obese adults after randomizing them to Healthy Low-Fat vs. Healthy Low-Carb diets for 12 months. The objective was to identify predisposing and measureable individual differences at baseline that would explain significant amounts of the differential weight loss achieved by individuals assigned to the same weight loss diet. The study was designed to primarily address potential differences in genotype and insulin-glucose dynamics in particular, while also collecting data on an extensive set of potentially relevant physiological, psychosocial, dietary, and behavioral variables. Notably, the study was not designed to identify which of the two study diets was the one best for weight loss, but rather, which diet was best for which individuals for weight loss.- (i.e., the “whiches conundrum”) [8]. Preliminary data suggested there were three distinct multi-locus genotype patterns representing differential weight loss responses to different diets: a Low-Fat Genotype (LFG), a Low-Carbohydrate Genotype (LCG), and a Neither Genotype. The three single nucleotide polymorphisms (SNPs) considered to be components of the multi-locus genotype included FABP2 (rs1799883), PPARG (rs1801282), and ADRB2 (rs1042714). An additional goal was to determine whether other genotypic profiles had a differential impact on the effects of a particular diet on weight loss.
Several additional significant objectives were considered of critical importance to the study design. First, the intervention approach was designed to achieve maximal differentiation in intakes of dietary fat and carbohydrate in the free-living individuals randomized to each of the two diet groups. Both groups were asked to make large initial changes from their baseline habitual diets, such that even after anticipated dietary recidivism over the duration of the protocol, the 12-month differentiation of fat vs. carbohydrate intake would be substantial.
Second, the intervention approach was designed to be comparably challenging for the two groups. There are no standard definitions of “Low-Fat” or “Low-Carb” in terms of grams/day or percent energy intake. Some studies comparing the two have had ambitious goals for one group compared to modest goals for the other, making the comparison unbalanced [9]. This study was designed so that both groups received equally demanding assignments.
Third, both diet approaches emphasized equally high dietary quality in terms of nutrient density (i.e., nutrients/Kcal). In this case, our objective was to avoid employing a study design that favored one diet over the other in terms of overall dietary quality. For example, both diet groups were instructed to incorporate significant variety and quantity of vegetables into their daily diets and to minimize added sugars and refined grains.
In summary, for the three-diet design objectives described above, the protocol was designed to compare two dietary approaches that were maximally differentiated, equally demanding, and equally focused on high quality nutrition.
STUDY DESIGN
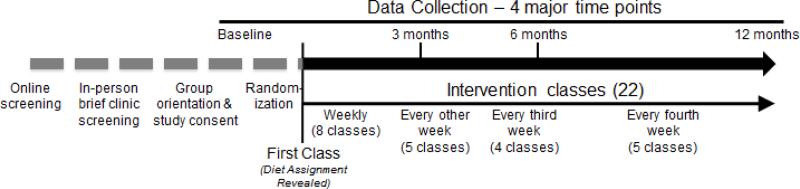
Generally healthy women and men, 18-50 years of age, with a BMI between 28-40 kg/m2, were randomized to a Healthy Low-Fat or Healthy Low-Carb weight loss diet for 12 months. The target sample size was n=600. The intervention involved a series of 22 evening instructional sessions in groups of 12-22 participants per class. Participants attended classes with the same group of individuals over time. Classes were led by health educators who were all registered dietitians (Figure 1). To accommodate the large sample size, enrollment was spread out across five cohorts between the spring of 2013 and the spring of 2015. Target enrollment for the five cohorts was n=80 for Cohort 1, and n=130 for Cohorts 2 through 5. All health educators led a similar number of Low-Fat and Low-Carb classes (i.e., for every cohort, each health educator was assigned one Low-Fat and one Low-Carb class).
Figure 1.
Summary of study protocol flow
SCREENING
Participants were recruited primarily through media advertisements (e.g., radio, online), and e-mail lists. Interested participants were required to complete a 10-minute online survey to determine initial eligibility. Those who remained eligible and interested after the survey were invited to attend a clinical screening that required written informed consent. Potential participants who did not respond to the first invitation received just one additional phone call or email invitation; if there was no response after the second attempt, no additional efforts were made to schedule the clinical screening visit. The 30-minute in-person screening visit included measurements of height, weight, blood pressure, and a fasting fingerstick blood sample to measure glucose, triglycerides, LDL-, HDL-, and total-cholesterol. If ineligible, contact was discontinued after screening results were provided. Table 1 details the inclusion and exclusion criteria used in the screening process.
Table 1.
Inclusion and exclusion criteria (self-reported, unless otherwise indicated)
| Inclusion |
| ■ Age: ≥18 years of age |
| - Women: Pre-menopausal (self-report) and ≤50 years of age |
| - Men: ≤50 years of age |
| ■ Race/Ethnicity: All |
| ■ BMI (body mass index): 28-40 kg/m2 (Measured in clinic) |
| ■ Body weight stable for the last two months, and not actively on a weight loss plan |
| ■ No plans to move from the area over the next 12 months from start of study cohort |
| ■ Available and able to participate in the evaluations and intervention for the study period |
| ■ Willing to accept random assignment |
| ■ To enhance study generalizability, people on medications not noted below as specific exclusions can participate if they have been stable on such medications for at least three months |
| ■ Ability and willingness to give written informed consent |
| ■ No known active psychiatric illness |
| Exclusion |
| ■ Pregnant, lactating, within 6 months post-partum, or planning to become pregnant in the next year |
| ■ Diabetes (type 1 and 2) or history of gestational diabetes or on hypoglycemic medications for any other indication |
| ■ Prevalent diseases: Malabsorption, renal or liver disease, active neoplasms, recent myocardial infarction (<6 months) |
| ■ Currently smoking |
| ■ History of serious arrhythmias, or cerebrovascular disease |
| ■ History of Bariatric Surgery |
| ■ Uncontrolled hyper- or hypothyroidism (TSH not within normal limits, self-report) |
| ■ Medications: Lipid lowering, antihypertensive medications, and those known to affect weight/energy expenditure |
| ■ Excessive alcohol intake (self-reported, ≥ to 2 drinks/day for men or ≥1 drink/day for women) |
| ■ Musculoskeletal disorders precluding regular physical activity |
| ■ Unable to follow either of the two study diets for reasons of food restrictions (e.g., vegan) |
| ■ Currently under psychiatric care, or taking psychiatric medications |
| ■ Inability to communicate effectively with study personnel |
Eligible participants were required to attend an in-person, group-based, 60-75 minute orientation, where study details were explained and full study consent was obtained. Those who attended the orientation and provided written consent were then required to attend a pre-study workshop on the study's dietary assessment methodology using the Nutrition Data System for Research (NDSR). During the hour-long workshop, members of the dietary assessment team reviewed the NDSR program, methods for collecting the 24-hour recalls, and the instructions for using an accompanying Food Amounts Booklet. The workshop also included asking participants to fill out a phone call preference sheet indicating their preferred phone numbers and time of day (i.e., morning, lunch, mid-day, or evening) for their interviewer-administered dietary recalls.
RUN-IN
The intervention protocol began with a run-in period – between orientation date and the start date of the participants’ first class – of approximately one month (mean ± SD = 32 ± 10 days). During this period potential participants were instructed to maintain their habitual diet, exercise, and body weight so as to maximize the stability of their baseline measures.
BASELINE DATA COLLECTION
Baseline data collection (see Table 2) included the following clinical measures: body weight, height, waist circumference, blood pressure, blood sampling (to later assess such biomarkers as insulin, glucose, lipids, inflammatory markers, genotype) including an oral glucose tolerance test (OGTT), resting energy expenditure (REE), and dual-energy x-ray absorptiometry (DXA). To assess dietary composition, unannounced 24-hour dietary recalls were conducted. Physical activity was assessed by interviewer-administered 7-day recall [10]. Participants also completed a number of psychosocial questionnaires. There were optional sub-studies for which stool samples were collected and fat biopsies were obtained. More detailed information about each of these measures is provided below.
Table 2.
Data Collection Chart
| Assessment a | Screening | Baseline | 3M | 6M | 9M | 12M |
|---|---|---|---|---|---|---|
| Demographics | X | |||||
| Screening Survey | X | |||||
| Weight and Waist circumference | X | X | X | X | ||
| Height | X | X | X | |||
| Blood Pressure | X | X | X | X | ||
| Blood (i.e., OGTT insulin, OGTT glucose, lipids, inflammatory markers) b | X | X | X | X | ||
| Diet Composition – NDSR c | X | X | X | X | ||
| Physical Activity 7-Day Recall | X | X | X | X | ||
| Medication/Supplements taken | X | X | X | X | ||
| Questionnaires | X | X | X | X | ||
| Resting Energy Expenditure (REE) d | X | X | X | |||
| Body Composition (DXA) d | X | X | X | |||
| Fat Biopsy (subset of ~n=100) d | X | X | ||||
| Stool Samples – Microbiome Sub-Study 1 e | X | X | ||||
| Stool Samples – Microbiome Sub-Study 2 f | X | X | X | X | X |
Notes:
Participant In-Class Weight and Attendance were recorded at each of the 22 classes.
OGTT was not measured at 3M.
Diet composition data was collected on two weekdays and one weekend day at each time point.
Collected beginning in Cohort 2.
Stool samples in Sub-Study 1 were only collected in Cohort 2 and were also collected at 10 weeks.
Stool samples in Sub-Study 2 were only collected in Cohort 3, and included whole stool samples for 22 individuals at baseline.
RANDOMIZATION
Participants who completed their baseline clinic visit and data collection were randomized to the Healthy Low-Fat or Healthy Low-Carb diet group using an allocation sequence set by a computerized random number generator as carried out by the study statistician. Once randomized, participants were informed by e-mail of the date and time of their first intervention class. This communication did not inform them of their diet group assignment. They were first informed of their diet group assignment at the start of the first class. The original randomization plan included stratification based on preliminary data on genotype predisposition. However, as will be described below in the section describing the analysis plan, a decision was made, prior to enrollment of the first participant, to simplify this as a straight randomization to one of the two study arms.
SINGLE BLIND
The study was single-blinded. It was not feasible to blind participants to Healthy Low-Fat vs. Healthy Low-Carb dietary assignment. However, for all staff collecting data (e.g., dietary assessment, DXA) and for all laboratory personnel assaying samples (e.g., insulin, glucose), diet group assignments were masked. Only a limited number of staff not involved in data collection or analysis, including the study coordinator and health educators, knew the diet assignments. Subjects were explicitly instructed to not divulge their intervention assignment with assessment staff.
STAGGERED COHORTS
The relatively large sample size of this single-site study required delivery of the intervention to five staggered cohorts so as to maximize efficiency of study staff and space requirements of intervention implementation. The start date of each cohort was staggered to minimize overlap of major data collection time points (baseline and 3, 6, and 12 months; see details in the Assessment Protocol section). Cohorts 1, 2, 3, 4 and 5 started in March 2013, September 2013, April 2014, August 2014 and March 2015, respectively.
INTERVENTION PROTOCOL
CLASS BASED EDUCATION PROGRAM
A team of health educators led the class-based education intervention that was delivered over the 12-month protocol. Altogether there were 36 different class groups across all five cohorts – 18 Healthy Low-Fat class groups and 18 Healthy Low-Carb class groups. Each health educator taught one Healthy Low-Fat and one Healthy Low-Carb group in each cohort. In Cohort 1 there were two health educators and in Cohort 2 an additional two health educators were hired to lead the classes. Before Cohort 3 got started, one of the original health educators left the study, and a new health educator was hired. These four health educators then completed Cohorts 3, 4, and 5. All five health educators were registered dietitians (RDs), four of the five held Masters degrees, and two of the five were certified diabetes educators (CDE). Intervention fidelity across health educators was established through weekly staff meetings, during which time health educators shared information and class experiences and engaged in group problem-solving around any issues that came to light in the course of teaching the classes. Any behavioral issues with participants that arose were discussed with the study's senior behavioral scientist.
All class sessions were held in the evenings, Monday through Thursday. For the first eight weeks of each cohort, the sessions were held weekly. Class sessions then became less frequent – meeting once every two weeks for two months, then once every three weeks until the six-month mark, and finally once every month for the remaining six months. Overall, there were 22 instructional sessions throughout the year for each class group. The size of the class groups ranged from 12 to 22 participants (17 ± 2, mean +/− SD). Once assigned to a particular diet assignment and class group, on a specific night of the week and, at a specific time, participants were not allowed to switch and sit in on any other classes (i.e., not allowed to make-up for a missed class by sitting in on another group's class); the class they attended was always their originally assigned class day and time throughout the 22 sessions. Keeping the classes exclusive in this manner was done with the intention of maximizing overall engagement and retention through the promotion of social cohesion, comfort and privacy, and the minimization of vulnerability (i.e., avoiding newcomers in the class) among class members. For participants who missed a class session, they were provided with the specific information from that session via written materials, as well as through brief email or phone contacts with their health educator.
DIETARY STRATEGY – LIMBO-TITRATE-QUALITY
There were three central components to the dietary strategy that were repeatedly and consistently communicated to the participants regardless of diet assignment. The first was the “go as low as you can go” (Limbo) strategy for the first eight weeks. Participants were instructed to progressively cut back on fat or carbohydrate intake until they had achieved a daily intake of no more than 20 grams of fat or carbohydrate per day, depending on their group assignment. Participants received explicit instructions that the rate of restriction was not critical to the study, and that reaching the 20 grams per day in two vs. four vs. six vs. eight weeks was not considered to carry any advantage or disadvantage. Therefore, their rate could be variable and individually tailored. The instructions also included a clear statement that, even though 20 grams per day was the objective, any individuals who were unable to reach those low levels would not be dropped from the study or considered to be non-compliant; rather, the expectation was more consistent with the concept of the party game Limbo – go as low as you can go. Once participants reached their lowest level of fat or carbohydrate intake, they were encouraged to maintain that level for at least a few weeks. There was no specific set time for maintaining the lowest level. Rather, it was explained to participants that the goal was to provide them with the personal experience of being anchored at the lowest level they could achieve and maintain, at least for a week or two.
The second component of the dietary strategy (Titrate) involved instructing participants to slowly add fat or carbohydrates back to their diet in increments of 5-15 grams/day, for periods of a week at a time, with no set endpoint goal for a specific level of fat or carbohydrate. For example, for participants who achieved 20 grams/day within the first eight weeks, and then maintained that level for at least a few weeks, they were encouraged to shift their daily goal to 25-35 grams/day for a week or for possibly more than one week. During this process they were instructed to assess how the increased level of fat or carbohydrate affected both their satisfaction with their daily intake (e.g., satiety, palatability, and enjoyment) and their weight loss progress. If satisfaction and weight loss progress were acceptable, they had the option of maintaining that level of fat or carbohydrate intake for another week or adding an incremental 5-15 grams/day. Importantly, while participants were encouraged to slowly add fat or carbohydrate to their diets in this manner, it was also made clear that they should not add back any more than would be necessary to keep them at the lowest possible level over the long term while simultaneously addressing any concerns about long-term satisfaction in areas related to satiety, palatability, and enjoyment. After adding back the designated grams/day, they could also consider reversing that decision and instead reduce their intake based on the factors mentioned above. At this point they could maintain that level for the remainder of the study, or try to add back small amounts of fat or carbohydrate later in the study. Thus, for the purpose of overall guidance, this Titrate component of the dietary strategy was described to participants as having the ultimate objective of having each one of them eventually find their individualized level of fat or carbohydrate that was both:
The lowest they could achieve, and
The lowest they could conceivably maintain for many years to come after the 12-month protocol ended.
Inherent in this approach was the idea that the final level of fat or carbohydrate intake achieved among individuals within both diet arms would vary substantially, with no single set target goal for fat or carbohydrate. It was explained to participants that this was acceptable to the study researchers and was even to be expected due to the central study hypothesis that each of the study diets would be easier for some participants and more difficult for others based on some combination of genetic and/or metabolic predispositions. It was for these reasons that “One Diet Does Not Fit All” was the name of the study used by the research team for communication purposes with participants.
The third component of the overall dietary strategy was promoting high dietary quality (Quality) for both groups for the full 12-month intervention period. Optimizing diet quality was emphasized by giving both diet groups similar instructions to focus on whole, real foods that were mostly prepared at home when possible, and specifically included as many vegetables as possible, every day, however they liked them - grilled, stir-fried, roasted, etc. They were also encouraged to choose lean grass-fed and pasture-raised animal foods as well as sustainable fish. With a focus on mostly consuming whole, real foods, both groups were likewise instructed to eliminate, as much as possible, processed food products, including those with added sugars, refined white flour products, or trans-fats. Participants were encouraged to prepare as much of their own food as possible, and to optimize the inclusion of fresh, seasonal foods. When eating out or traveling, they were encouraged to ask for modifications to standard menu items that would help them adhere to their diet assignment (e.g., ordering salad dressing on the side for the Healthy Low-Fat group or a side of greens instead of mashed potatoes for the Healthy Low-Carb group).
Several of the topics related to Quality were specific to each of the two different diet assignments. Those assigned to Healthy Low-Fat were instructed to choose whole-grain foods (e.g., rather than whole wheat flour products), including steel cut oats, farro, barley, quinoa, brown rice, and wild rice. Healthy Low-Fat participants were also encouraged to explore and consume a wide range of legumes and beans, fresh fruit, low-fat dairy products, and lean meats. Those assigned to Healthy Low-Carb were instructed to choose high quality oils and fats, avocados, hard cheeses, nut butters, and nuts & seeds. During the Titrate phase, and throughout the remainder of the 12-month protocol, as the Healthy Low-Fat group added small amounts of fat back to the diet, and as the Healthy Low-Carb group added small amounts of carbohydrate back to the diet, they were instructed to do so with these same quality foods.
Given that high quality foods can be more expensive than foods that are similar in type but lower in quality, the encouragement to choose quality was framed as a continuum as opposed to an either/or (e.g., for the Healthy Low-Fat participants, organic wheat berries was at the highest level of quality, followed by conventional wheat berries, then whole wheat bread made with a minimal number of ingredients and no additives, then a more conventional whole wheat bread with many ingredients including additives, and, finally, refined white flour bread with many ingredients and additives was considered the lowest end of the quality continuum). In other words, participants were encouraged to choose the highest quality foods that they could reasonably find, realistically afford, and enjoy.
In summary, the diet strategy for both the Healthy Low-Fat and Healthy Low-Carb groups was a Limbo-Titrate-Quality approach, with the goal of having participants achieve an individualized, lowest-possible level of fat or carbohydrate intake of maximal dietary quality, and one that could conceivably be maintained long-term beyond the end of the 12-month protocol. Notably, there were no specific caloric restriction goals for either diet and no single specific percentage of fat or carbohydrate to which they were told to strive as the final goal.
SIMILARITIES IN INTERVENTION STRATEGIES FOR THE TWO DIET GROUPS
Overall, there were four main foci of the instructional sessions: nutrition, behavior, emotions, and physical activity. While all of these were usually touched upon in each class, nutrition was typically the primary focus, and one other component per class was highlighted and explored in more detail. The nutrition focus was strongest in the first two months of the 12-month protocol, after which more and more emphasis was given to the non-dietary components of the sessions, and these were relatively similar in many regards for the two intervention groups (see Appendix for class topics). Specific class topics taught to both diet groups included mindful eating, food and mood, sleep and weight, food addiction, exercise, as well as tips and demonstrations on shopping, preparing, and cooking vegetables. All health educators taught their classes through the lens of helping participants to focus on making sustainable lifestyle changes, not simply following a temporary ‘diet.’ Beyond the classroom instruction, each health educator was available to offer individual contact with their class members via email and phone (e.g., ranging from several times a week to very rarely) to address specific dietary questions and review food logs.
The Quality principle was reinforced similarly for the two diet groups via class food demonstrations highlighting vegetables and the use of simple cooking equipment and techniques to inspire and encourage healthy eating at home. Recipes were provided by health educators via newsletters. Other recipes, selected by the participants and relevant to their dietary assignments, were shared via email and printed booklet. Participants were also encouraged to find and purchase food from local community supported agriculture (CSA) groups or take advantage of home delivery food services that promoted high quality food values (e.g., Good Eggs).
Efforts to maximize similar retention between the two groups included the health educators consistently sending reminder e-mails before class, summary e-mails after class with class materials and pertinent links to other items that came up during class, as well as reaching out via e-mail to individuals directly, as needed. Weekly emails were sent to the groups even during the months when classes were meeting less often. Participants were able to post questions or comments to the whole group as well as privately to their health educator.
PHYSICAL ACTIVITY
The promotion of regular, moderate- or more vigorous-intensity levels of physical activity was identical for the two diet arms and consistent with national guidelines related to weight control. Health educators recommended 60–90 min/day of moderate-intensity physical activity as a target goal [11-14] and encouraged any participants who were not meeting these guidelines at baseline to work up to this level in the first three months of the study. Those who were already exercising at this or a higher level were encouraged to add variety, increase intensity, time, or frequency. Consistent with evidence from recent national reports, health educators discussed the particular importance of physical activity for weight loss maintenance. In Cohorts 1 and 2, participants were provided with pedometers. For subsequent cohorts, while participants were not given pedometers, since health educators realized that they were largely unused due to widespread use among participants of other activity monitors (e.g., Fit Bit, Jawbone), they were encouraged to use these types of wearable devices to regularly track their activity levels. Health educators emphasized a mix of cardiovascular and strength training and proper form to prevent injury. They encouraged participants to start with moderate levels of physical activity (e.g., 10 min/day added to what they were currently doing) and increase activity according to their lifestyle. Lastly, health educators encouraged participants to find ways to be active that were truly enjoyable and practical for the participants, so that their physical activity could be sustainable for the long-term.
PSYCHOLOGY AND BEHAVIOR MODIFICATION
Health educators emphasized emotional awareness and behavior modification to support the diet and weight loss program. While the initial eight class sessions were focused mostly on nutritional knowledge and understanding what the food changes would be, the majority of the remaining class sessions focused on how to put this knowledge into practice. The behavioral modification strategies were based on a Social Cognitive Model, which views behavior, including health behaviors, as acquired and maintained through a complex set of behavioral, cognitive, and environmental conditions [15, 16]. Social cognitive intervention strategies have been found to be effective in promoting adoption and retention in a number of lifestyle intervention studies with a range of adult populations [17-21]. Health educators exposed participants to empirically-supported principles of self-regulatory behavior change (e.g., goal setting, self-efficacy building, supportive environments, healthful self-reinforcement/rewards, relapse prevention) [18, 20, 22]. Class themes that addressed behavior included ‘the power of habit,’ ‘the practice of mindful eating,’ ‘how emotions drive food decisions,’ ‘the concept of bulk cooking,’ ‘grocery shopping,’ ‘meal planning,’ ‘meal timing,’ ‘dining out,’ and more. The impact of participants’ surrounding environments and contexts (e.g., at work, at home, with friends) and how to deal with the pressures that might derail them were also discussed in detail.
A key concept of behavior change was reinforcing the concept of “One Diet Does Not Fit All” as it applies to behavior change and motivation, as well as Healthy Low-Fat vs. Healthy Low-Carb diets. That is, what works for one individual, may not work for someone else. The 12-month protocol involved helping participants find the right foods to meet individual goals and the personal behaviors and habits needed to allow for sustainable change.
ONGOING INTERVENTION ADAPTATION AND DEVELOPMENT
As the study progressed from Cohort 1 to Cohort 5, the intervention delivery was refined in several minor ways with the intent of maximizing treatment fidelity. Importantly, each of these minor refinements was implemented equally for both the Healthy Low-Fat and the Healthy Low-Carb groups. For example, based on participant feedback in the first and second cohorts, health educators determined that the in-class content could be improved by making it less dense, less didactic, and more interactive. Therefore, the scope of the in-class content was reduced and the interactivity of the material was increased, while retaining the guiding principles. Part of this transition was achieved by creating a set of videos for participants and instructing them to view the videos prior to class (e.g., a “flipped” classroom concept). As another example, in-class cooking activities were made more participatory by including more potlucks and demonstrations of participants’ own recipes.
Additionally, in Cohort 5, an SMS (text messaging) accountability tool was developed for both groups that was introduced after the 6-month data collection time point. Briefly, the tool involved three or four text messages per week with brief queries about adherence to their eating plan, emotions related to their adherence, general well-being, and intentions for any actionable steps to address any lapses in adherence or challenges with well-being. This addition was based on feedback from participants in earlier cohorts that had reported challenges remaining fully engaged in the study in the latter six months of the protocol when the frequency of class meetings was reduced to once per month.
ASSESSMENT PROTOCOL
As presented in Table 2, data were collected across a broad range of domains. The primary data collected for all participants across all five cohorts included demographics, personal and family health history, clinical measures (e.g., weight, waist circumference, and blood work), dietary intake, physical activity, and psychosocial variables. Resting energy expenditure and percent body fat (DXA) were assessed for Cohorts 2-5. Fat biopsies were obtained from a subset of participants who volunteered in Cohorts 2-5, and stool samples were collected from a subset of participants who volunteered from Cohorts 2 and 3. With few exceptions, which are noted below, all data were collected at four major study time points: baseline, 3, 6, and 12 months.
DIETARY ASSESSMENT METHODS
Primary dietary intake was assessed using three unannounced 24-hour dietary recalls within a two-week window at each of the four major data collection time points. Each participant was expected to complete two weekday dietary recalls and one weekend recall at each data collection time point for a total of 12 recalls. Data were collected using NDSR, a computer-based software application developed at the University of Minnesota Nutrition Coordinating Center (NCC). Dietary recalls were collected in a standardized fashion using a multiple-pass interview approach consisting of five steps to ensure completeness and accuracy [23, 24]. First, participants were asked to list all foods and beverages consumed in the previous 24 hours (i.e., midnight to midnight). Second, the interviewer reviewed the list with the participant. Third, the interviewer collected detailed information about each reported food and beverage (e.g. method of preparation and amount consumed). Fourth, the interviewer probed for commonly forgotten foods. Fifth, the information was reviewed for completeness, correctness, and marked as a typical or atypical day. Throughout the recall, the NDSR software searched for foods and brand name products by name and prompted the data collectors with requests for additional detailed information [25]. All data collectors were trained by NDSR certified lead staff and were blinded to the assigned diets.
The NCC Food and Nutrient Database serves as the source of food composition information in NDSR [26]. This database includes over 18,000 foods including 8,000 brand name products. Ingredients and preparation methods allow for more than 160,000 food variants. Values for 165 nutrients, nutrient ratios, and other food groups were generated by the database. The USDA Nutrient Data Laboratory is the primary source for nutrient composition for the database. These values are supplemented by food manufacturers' information and data available in the scientific literature [27]. Standardized, published imputation procedures were applied to minimize missing values [28]. In addition, to the extent possible, the interviewers entered recipes or ingredients for homemade, restaurant, and other items not included in the software. The lead dietary assessment nutritionist conducted a quality check for each cohort after data collection at each study collection point. This involved an in-depth review of both individual and composite reports for completeness and errors.
As mentioned previously, all participants attended a pre-randomization 60-minute, in-person training to learn the procedure for dietary recalls and were provided with a Food Amounts Booklet to be used at the time of each data collection to enhance estimating portion sizes. When an in-person meeting was not possible, this training was done via email and/or telephone (<1% of participants).
During each time point data collection window, participants received unannounced phone calls to complete dietary recalls. If participants could not be reached via phone by the interviewers after approximately five to seven attempted telephone calls, including voicemail messages, then the health educator for that specific participant was notified and assisted in communication by either bringing this to the participant's attention in the next class, or sending an email. If the participant still did not respond, the study coordinator and/or Principal Investigator attempted to contact the participant via email or phone. This process continued until the data were collected, the participant communicated with the study staff his/her wish to discontinue participation, or the data collection window closed.
PARTICIPANT SELF-MONITORING OF DIET
Study participants employed a variety of methods to self-monitor adherence to their diet group assignment throughout the study, particularly in the first eight weeks when the goal was to lower dietary fat or carbohydrate, depending on assignment, to 20 grams/day. The most common dietary monitoring method used was the on-line MyFitnessPal tool [29]. Many of the participants indicated experience with this tool prior to starting the study, and most participants who used it for the first time reported general satisfaction with its ease of use. Participants were able to share access of their MyFitnessPal results with their health educators, who were then able to review entries, as needed, to help guide participants in their diet adherence. Other methods used by those preferring an alternative approach included a paper food log provided by the health educators or one of several web-based tracking tools that are similar to MyFitnessPal, (e.g., MyNetDiary and Lose It!). As the study progressed, and with the increasing popularity of wearable devices, many participants reported tracking via their FitBit and UP wristbands.
PHYSICAL ACTIVITY ASSESSMENT
The Stanford Seven-Day Physical Activity Recall (PAR) was administered by trained study staff at the same time as one of the dietary recalls at each major data collection time point to assess participants’ self-reported level of physical activity [10]. Originally developed at Stanford University in the early 1980's, the PAR is a semi-structured interview that documents the time an individual engages in physical activity, strength, and flexibility activities during the 7 days preceding the interview. An interviewer guides the participant through the recall of daily activities to determine the length and intensity of the physical activities. Physical activity is measured as total energy expenditure and time spent in moderate, hard, and very hard physical activity. Hours per day spent in the various categories of physical activity intensity are then converted to a daily average of metabolic equivalents (METS) and then used to estimate total energy expenditure per day in units of Kcal/kg/day. The questionnaire also captures sleeping time, time spent cooking, and several other lifestyle behaviors.
WEIGHT, HEIGHT AND WAIST CIRCUMFERENCE
Body weight was recorded without shoes to the nearest 0.1 kg using a calibrated Scale-tronix clinical scale. Height was measured to the nearest 0.1 cm using a Seca wall-mounted stadiometer. Waist circumference was measured on the skin at the umbilicus to the nearest 0.1 cm. All measurements were taken by a nurse at the Stanford Clinical & Translational Research Unit (CTRU) at each time point. All clinic visits started between 7:00 and 9:30 am, with participants in a fasted state for at least 10-12 hours.
BLOOD PRESSURE
After 5 minutes of sitting/resting, CTRU nurses obtained three blood pressure readings on the right arm one minute apart. These were collected automatically using a WelchAllyn, Spot Vital Signs LXi. If a participant's blood pressure was over 160 systolic or 90 diastolic, they rested another five minutes before taking the blood pressure again. If the blood pressure remained high after several readings, the study coordinator and sometimes the study physician were notified. In all cases, the participant was able to continue with the remainder of the clinic visit. For analysis purposes, the first measurement was disregarded, and the second and third measurements were averaged according to NHANES guidelines [30].
BLOOD SAMPLES
Blood was sampled by fingerstick during the screening clinic visit for all potential participants to assess a fasting lipid profile and blood glucose using the Cholestech LDX machine. For all participants determined eligible after screening that consented to participate in the study, subsequent blood samples were taken at baseline, 3, 6 and 12 months via venipuncture at the Stanford CTRU by trained nurses or phlebotomists. Aliquots of plasma and serum were obtained at all time points; buffy coats were collected at baseline, 6 and 12 months.
LIPIDS AND LIPOPROTEINS
Lipids were assessed at all four times points (i.e., baseline, 3, 6, and 12 months) from a fasting blood sample. Blood was collected into purple top EDTA vacutainer tubes. Samples were processed, aliquoted, and frozen directly by the CTRU lab after being drawn. Samples were stored in a −80° freezer until the time of processing for analysis. Plasma triglycerides, total- and HDL-cholesterol were measured by enzymatic endpoint analysis on a clinical chemistry analyzer (Liasys 330) using methodology previously described [31-33]. LDL-cholesterol was calculated using the Friedewald equation. Triglyceride and cholesterol measurements are standardized through the CDC-NHLBI lipid standardization program. Apolipoproteins B and AI were analyzed by immunoturbidimetric assay using the K-assay reagent kits (Kamiya Biomedical). Lipoprotein particle concentrations were measured by ion mobility, a process that allows for direct particle quantification as a function of particle diameter [34]. Ion mobility is based on the principle that particles of a given size behave in a predictable manner when carried in a laminar flow of air and subjected to an electric field. Briefly, a solution of lipoproteins, depleted of other serum proteins, is introduced into a flow of air by electrospray. In the electrospray chamber, the desolvated, highly charged lipoprotein particles are nearly neutralized by ionized air, resulting in a known fraction of singly-charged particles exiting the electrospray chamber. The particles are then carried by airflow to a differential mobility analyzer (DMA), where a variable electric potential, perpendicular to the direction of the airflow, causes the particles to drift toward a collection slit. The velocity of the particles across the airflow is proportional to the particle diameter and to the electrical potential. Only singly charged particles are detectable. Those with different diameters reach the collection slit at different electrical potentials and are then carried to a particle counter where they are detected by light scatter and are counted after transition through the DMA. As a result, lipoprotein particle concentrations are measured directly as a function of their particle diameters. The final numerical ion mobility output is reported in nanomoles per liter for combined bins of particles summed into commonly reported subclasses of lipoproteins. These include: HDL 3, HDL 2b, HDL 2a, LDL 4c, LDL 4b, LDL 4a, LDL 3b, LDL 3a, LDL 2b, LDL 2a, LDL I, IDL 2, IDL 1 and VLDL sm, VLDL med, and VLDL large. The peak LDL particle diameter for each sample is reported as is the LDL phenotype associated with it: pattern A, I, or B. A full description of this method is published elsewhere [34]. All of the lipid assays were performed by the Krauss Lab at the Children's Hospital Oakland Research Institute (CHORI, Oakland, CA) for all cohorts.
GLUCOSE, INSULIN, ORAL GLUCOSE TOLERANCE TEST (OGTT)
Blood was collected to assess post-fasting plasma glucose and insulin via phlebotomy at the Stanford CTRU. Insulin levels were assessed by radioimmunoassay by the Core Laboratory for Clinical Studies Washington University School of Medicine, St. Louis, Missouri [35]. Glucose levels were analyzed using a Beckman Glucose Analyzer II (BGA II) by electrochemical technique [36]. For the OGTT, serial blood sampling was collected under fasting conditions and then at 30, 60 and 120 minutes after consuming 75 g of glucose solution [37, 38]. The Matsuda index was calculated to assess insulin sensitivity according to the methods of Matsuda et al. [39].
TARGETED PROTEOMICS: OLINK PROSEEK® TECHNIQUE
Candidate protein biomarkers were assessed using a high-throughput technique, the OLINK Proseek® Multiplex kits (www.olink.com). Each kit measures 92 disease-related proteins in plasma samples, and three arrays – CVD II, CVD III and Inflammation I – were used to assess samples. The method is based on a proximity extension assay (PEA) in which 92 oligonucleotide-labeled antibody probe pairs can bind to their respective target, present in the sample. The PEA technique has an advantage over conventional multiplex immunoassays, since only correctly matched antibody pairs give rise to a signal, yielding an extremely high specificity. PEA is a homogeneous assay that uses pairs of antibodies equipped with DNA reporter molecules. When binding to their correct targets, they give rise to new DNA amplicons, each ID-barcoding their respective antigens. The amplicons are subsequently quantified using a Fluidigm BioMark™ HD real-time PCR platform. This PCR-based technique offers a high-throughput of simultaneous measurements of 92 protein biomarkers in just one microliter of sample, without any cross-reactivity. Proseek Multiplex provides accurate quantification below picogram per milliliter levels, even in small samples [40].
GENOTYPING
DNA extraction was carried out by the Stanford Cancer Institute (SCI) Biorepository. Briefly, high quality DNA samples were extracted from the buffy coat/red blood cell suspension using laborious phenol/chloroform purification method, a modified procedure of Baas et al. [41], Gustafson et al. [42] and Paul et al. [43]. Processing utilized lysis buffer (144 mM NH4Cl, 14 mM NH4HCO3), pellet buffer (10 mM Tris pH 8.0, 10 mM EDTA, 150 mM NaCl), Proteinase K, 10% SDS, and RNAse, incubated at 50°C for ~16 hours, protein-denaturant buffers, phenol, and chloroform. Processing involved precipitation by NaCl (100-250 mM, 2 volumes of cold Ethanol) and resuspending the dry pellet in TE (10 mM Tris, 0.1 mM EDTA, pH 8.0). A series of QA&QC assays were carried out to determine the DNA purity, concentration, integrity, and digestibility after the DNA was completely dissolved. The working DNA solution was stored at 4°C and the original stock at −80°C.
The Affymetrix Axiom® Genotyping platform was used for analysis of single nucleotide polymorphisms (SNPs) and insertions/deletions (indels). The specific microarray design used was the UK Biobank Axiom® Array [44]. There are 820,967 SNPs and indel markers on the array, which included the three SNPs from the original study design - FABP2 (rs 1799883), PPARG (rs 1801282), and ADRB2 (rs 1042714). The array was designed with imputation-aware algorithms, enabling characterization of millions of additional markers. Simulation results show high concordance between imputed genotypes and genotypes in the Phase I 1000GP release for between 6 and 9 million markers, depending on the population.
DNA extracted as described above was analyzed at the Affymetrix Research Services Laboratory facility using the Axiom® 2.0 Assay Automated Workflow [45]. Total genomic DNA (200 ng) was amplified and randomly fragmented into 25 to 125 base pair (bp) fragments. These fragments are purified, re-suspended, and hybridized to Axiom® Array Plates. Following hybridization, each polymorphic nucleotide was queried via a multi-color ligation event carried out on the array surface. After ligation, the arrays were stained and imaged on the GeneTitan MC Instrument. Data management was performed using Axiom™ Analysis Suite, Affymetrix® Genotyping Console™ Software (GTC) or Affymetrix® Power Tools (APT) and SNPolisher R package to perform quality control analysis (QC), for samples, plates, and SNPs filtering prior to downstream analysis, and advanced genotyping methods [46].
PSYCHOSOCIAL QUESTIONNAIRES
Participants completed a number of psychosocial instruments administered online at baseline, 3, 6 and 12 months (see Appendix B). The questionnaires covered a variety of topics including General Health [47], Sleep Quality [48], Outcome Expectations and Outcome Realizations [49], Diet Self-Efficacy [50], Social Support & Eating Habits [51], Stress [52], Dieting History [53], Eating Inventory [54], Reasons for Dieting [55], Eating Attitudes [56], Food Attitudes [57], Food Choices [58], Body Image [59], Depressive Symptoms [60], Food Addiction [61], Neighborhood Environment Walkability [62], Social Cohesion of Neighborhood [63], Group Cohesion Scale-Revised [64], Self-Control and Self-Management [65], and Emotional Eating [66].
SUB-STUDIES
In addition to the above-mentioned measurements, participants had an opportunity to participate in several sub-studies. Some were only offered to certain cohorts but were required of all in that cohort, while others were optional.
DUAL ENERGY X-RAY ABSORPTIOMETRY (DXA)
Dual Energy X-ray Absorptiometry (DXA) scans were performed to examine whole body adiposity, lean body mass, and bone density at baseline, 6 and 12 months. Scans provided body composition measurements for six specific body areas (i.e., left arm, right arm, left leg, right leg, trunk, and head) as well as whole body composition. Each individual underwent DXA scans using a Hologic QDR-4500W fan-beam scanner (Bedford, MA) based on the manufacturer's guidelines. Quality control procedures were carried out regularly based on the manufacturer's recommendations and the instrument was calibrated weekly using appropriate phantoms supplied by the manufacturer. DXA data were collected for Cohorts 2 through 5 (i.e., resources were not available at the onset of the study for cohort 1). One technician completed all scans for all participants at all time points to minimize potential variability.
RESTING ENERGY EXPENDITURE (REE)
Respiratory gas exchange was measured at the Stanford CTRU. The assessment was performed at the same time (i.e., 7:00 am – 9:30 am) and in the same room at each visit to minimize deviations in environmental conditions. Participants were measured in a fasting state after lying supine for 5 minutes at their baseline, 6 and 12 month clinic visit. Specifically, measurements were taken after vitals but before the first blood draw for the OGTT protocol. This was done using the PravoMedics TrueOne 2400 metabolic cart [67, 68]. Similar to the DXA data, REE data were collected for Cohorts 2 through 5 (i.e., resources were not available at the onset of the study for Cohort 1).
Due to the high volume of our study population, two separate metabolic carts at the CTRU were purchased and used to measure REE [69]. The equipment was allowed to warm up for 30 minutes prior to calibration and testing. Flow and gas calibration was performed every morning the metabolic carts were to be used. Resting measurements were taken for a minimum of 20 minutes for each participant, and the first 5 minutes were discarded from analysis. Data were collected every 60 seconds. Output files included the following variables: VO2, VO2/kg, METS, VCO2, VE, RQ, RR, Vt, FEO2, FECO2, and REE. Our analyses focused on the average values for those variables from minute 6 to minute 20.
FAT BIOPSIES
Beginning in Cohort 2, fat biopsies were collected to assess fat cell biology from approximately 20% of participants. The procedure was offered on a rolling basis to the first 20-30 volunteers per cohort. Participants were instructed to remain weight stable and to refrain from using any over the counter anti-inflammatories for a week before the procedure. Lower abdominal fat biopsies were taken at baseline. For all biopsies, participants were asked to fast from food and all non-water liquids for 12 hours prior to the procedure. Follow-up fat biopsies were taken at 6 months from those meeting the following two eligibility criteria: (1) being in the Healthy Low-Carb group and consuming less than 55 g of carbohydrates per day on average, or being in the Healthy Low-Fat group and consuming less than 40 g of fat per day on average, as determined during the 3-month NDSR diet data collection, and (2) losing ≥10 lbs in the six months since baseline.
A subset of those who were eligible for the six month follow-up and met the additional eligibility criteria of having a TG:HDL-C ratio (at the time of their screening visit) of equal to or greater than 1.5 for women and 2.5 for men, were asked to participate in a more elaborate 6 month follow-up consisting of a meal tolerance test with fat biopsy. For those completing the meal tolerance test, similar to the OGTT, eligible participants fasted for 12 hours prior to the appointment. Researchers took an initial fat biopsy at the beginning of the appointment, then provided participants with a Healthy Low-Fat or Healthy Low-Carb meal, depending on group assignment. A second fat biopsy was taken two hours after the meal. Blood was collected from each participant every hour for four hours, after the initial fasting blood draw was taking.
STOOL COLLECTION FOR MICROBIOME ANALYSES
Stool samples were collected from a subset of volunteers from Cohorts 2 and 3 to examine the microbiome. In Cohort 2, two samples of stool taken one week apart were collected from participants at baseline, and then subsequent samples were collected at 10 weeks and 6 months. Participants were instructed to, “use the spoon attached to the cap to put several scoopfuls of stool into the collection tube until each tube has a portion of the stool specimen equivalent to large marble or a walnut.”
Stool sample collection in Cohort 3 involved five time points: baseline, 3, 6, 9 and 12 months. For the majority of the sampling, two tubes of a walnut size sample of stool were taken from a single bowel movement. The baseline collection differed from the others in two ways. First, for baseline, two samples were collected one week apart (i.e., each with two tubes). Second, for approximately one quarter of the participants one of the two samples was a complete stool collection rather than just a walnut sized portion.
Participants from both cohorts were given ice packs and supplies to freeze the samples as soon as possible after collection and to keep the samples on ice until they were delivered to the research unit. Once samples were delivered to the research unit they were put into storage at –80°C.
ANALYSIS PLAN, INCLUDING EARLY MODIFICATIONS TO THE STUDY DESIGN
An overview of the original analysis plan as described in the grant application to the NIH is provided below. However, modifications have been made to this original plan due to four important developments.
First, after NIH funding was obtained in 2012, additional funding was acquired to augment the original study which enabled increasing the sample size from n=400 to n=600, as well as adding some of the metabolic and physiological assessments that are described above.
Second, and related, the racial/ethnic composition of the study sample was modified. The original study population was intended to be restricted to Caucasians because of the primary focus on a genetic interaction involving SNPs, for which allele frequencies have been established for Caucasians but not other racial/ethnic groups. The three SNPs of original interest were considered to be components of a multi-locus genotype that included FABP2 (rs 1799883), PPARG (rs 1801282), and ADRB2 (rs 1042714). Preliminary data[70] suggested that there were three distinct multi-locus genotype patterns with differential weight loss response to different diets: a Low-Fat Genotype (LFG), a Low-Carbohydrate Genotype (LCG), and a Neither Genotype, with rough distributions among Caucasians of 40:40:20. To maintain the intent of the originally funded NIH study, it was decided to complete the enrollment of n=400 Caucasians. In order to broaden the generalizability of the study, it was decided to recruit the additional n=200 from participants of all non-Caucasian race/ethnicities (which included Hispanics) that met all other study inclusion/exclusion criteria.
Third, the original plan to stratify randomization to the two diet groups by multi-locus genotype pattern was altered. Prior to initiating the study we determined that the evidence supporting the proposed genotype patterns remained unclear, and we wanted to explore other possible genotype interactions and associations. We still plan to test the original multi-locus genotype patterns, but we will also evaluate other SNPs secondarily in an exploratory manner, as described in the original aims of the grant application.
Fourth, the original plan for primary analysis was to employ ANOVA to evaluate change in weight at 12 months as a function of study arm and genotype. This analysis – known as a complete-case analysis – includes only those subjects who contribute body weight data at both baseline and 12 months. To address this issue of missing data, we initially proposed secondary analyses that incorporated multiple imputation-based methods. This would allow insight into the effect of assumptions regarding the missing data on our primary findings. We currently propose using mixed effects regression methods to evaluate change in body weight at 12 months as the primary analysis, keeping the secondary analyses as proposed and adding the complete-case analysis as another secondary analysis. This allows us to better adhere to intent-to-treat principles as all subjects randomized will be included in the analysis, even if they do not contribute weight data at 12 months. We will use maximum likelihood techniques for estimation, allowing subjects with any data points to contribute to the analysis, and borrowing strength from data contributed across subjects.
HYPOTHESES
The first primary hypothesis of the study is that there will be a significant diet-genotype interaction for weight loss success. We predict that diet and genotype main effects will be non-significant and that only by taking into account an interaction between the two factors can we predict weight loss success.
We have planned a number of follow-up exploratory analyses as well. One hypothesis is that other obesity-relevant SNPs will predict weight loss success. This process includes evaluating the contribution of the newly identified SNPs in predicting weight change. These SNPs will each be evaluated in turn and also expressed as a weighted linear combination or score. The additional SNPs we will consider are those that have been previously and robustly documented to have genome-wide significant associations with weight, waist circumference, and/or metabolic phenotypes (e.g., lipid levels, type 2 diabetes, and insulin resistance) in previous studies We will systematically review these studies for these associated phenotypes using the database of the Catalog of Genome-Wide Association studies, which is continuously updated by the European Molecular Biology Laboratory-European Bioinformatics Institute [71] and will include all independent genetic variants with corresponding p-values smaller than 5×10-8 for any of these phenotypes. These exploratory analyses would serve to identify additional potential gene loci that may regulate response to specific diets. We expect these exploratory associations to be studied in other larger studies in the future. There are currently almost 200 such independent genetic variants that have been discovered, and we anticipate that approximately 250 or more may be available in the next year or so.
A second primary hypothesis is that there will be a significant diet X insulin sensitivity interaction for weight loss success. Based on previous findings [5, 72-75], we predict that weight loss success will be greater for those on the Healthy Low-Carb diet who are more insulin resistant at baseline and greater for those on the Healthy Low-Fat diet who are more insulin sensitive.
Exploratory analyses will test whether various factors mediate the relationship between matched assignment and weight loss. We will use contemporary mediation analyses techniques [76, 77]. Selected mediators for these analyses include insulin sensitivity, energy intake, perceived appetite, satiety and hunger, resting energy expenditure, and physical activity.
STATISTICAL ANALYSES
To include subjects with missing data, the primary hypothesis of diet-genotype interaction will be assessed using a linear mixed model with weight as the outcome. The intention-to-treat (ITT) principle will be followed. All participants who were randomized will be included in the analysis and analyzed according to their assigned treatment, irrespective of compliance. Weight change over time (i.e., baseline, 3, 6 and 12 months) between the intervention groups will be assessed using a linear mixed effects model with main effect terms for time and whether or not a subject was a “match” (i.e., assigned to the diet implied by their genotype). The model will include interaction terms for time and match and subject-specific intercepts to account for within-subject correlation over time. Since participants were randomly assigned to diet groups, no baseline characteristics will be adjusted in the analysis. For the original genotypic classification of interest, statistical significance will be assessed at the 0.05 level. For all other SNPs, we will assess significance after controlling the false discovery rate to be no more than 0.05.
MISSING DATA, DROP-OUTS, AND INTENT-TO-TREAT
Analyses that do not account for missing data can lead to biased and inefficient estimates. To address such issues, our original plan was to perform both a complete-case analysis that excludes individuals missing at least one variable in the model as well as a multiple imputation-based model. The latter allows adherence to intention-to-treat principles in that all subjects randomized are included in the analysis regardless of drop out. Importantly, multiple imputation provides statistically valid results when the data are missing at random (i.e., the reason for missingness is related to observed variables only)[78]. Our current plan is to utilize mixed effects regression techniques that provide statistically valid results under the same condition (i.e., missing at random) as those upon which multiple imputation relies. In addition, these methods allow us to adhere to intention-to-treat principles as all subjects randomized will be included in the analysis, regardless of attrition.
CONCLUSION
At the core of the current study is a weight loss diet intervention comparing a Healthy Low-Fat vs. a Healthy Low-Carb diet among non-diabetic and generally healthy adults ages 18-50 years with a BMI in the range of 28-40 kg/m2. However, the study was not designed to simply test whether Healthy Low-Fat or Healthy Low-Carb is better overall for weight loss success. The study was designed with the recognition from more than a dozen previous Low-Fat vs. Low-Carb studies that the variability in weight loss within diet groups typically ranges from highly successful to very disappointing, while the difference in average weight loss between diet groups is typically negligible. Thus, this study was designed to examine interactions between diet group assignment and genotype and metabolism (e.g., insulin resistance). Beyond the primary hypotheses about interactions with genotype and metabolism, the current study will generate a rich data set to examine a wide range of physiological and psychosocial factors that likely contribute to the heterogeneity of response to weight loss diets. The study is intended to test these hypotheses and then generate many more. It has been designed to reframe a central question about diet and weight loss: Rather than searching for the one best diet to recommend to all, this study seeks to determine if overall success will be greater when different diets are matched to different people based on predisposing individual differences.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to acknowledge the many study team members who contributed to various aspects of the original design and/or implementation the study, and for either contributing sections to the manuscript or contributing helpful review. These team members include our health educators Jae Berman, Dalia Perelman, and Mandy Carroll, our diet assessment team of Molly Shimer-John, Valeria Alaimo and Diane Demis, Katherine Dotter who oversaw innovations and technology, Kyla Kent who provided expertise in DXA assessment, Alexandra Rossi who managed blood samples, and Dr. Kenji Nagao who provided helpful review of the manuscript. We would like to acknowledge the work early in the study of Antonella Dewell as the initial project coordinator and Josephine Lin as a research assistant. And, finally we would like to acknowledge the many participants who made this study possible.
Sources of Support: National Institute of Diabetes and Digestive and Kidney Diseases NIH 1R01DK091831, Nutrition Science Initiative, National Heart, Lung, and Blood Institute NIH T32HL007034, NIH 1 K12 GM088033, Stanford Clinical and Translational Science Award (CTSA) to Spectrum NIH UL1 TR001085, and the War-Related Injury and Illness Study Center and VA Palo Alto Health Care System. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or other funders.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Swinburn BA, Sacks G, Hall KD, McPherson K, Finegood DT, Moodie ML, Gortmaker SL. The global obesity pandemic: shaped by global drivers and local environments. Lancet. 2011;378(9793):804–14. doi: 10.1016/S0140-6736(11)60813-1. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311(8):806–14. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnston BC, Kanters S, Bandayrel K, Wu P, Naji F, Siemieniuk RA, Ball GD, Busse JW, Thorlund K, Guyatt G, Jansen JP, Mills EJ. Comparison of weight loss among named diet programs in overweight and obese adults: a meta-analysis. JAMA. 2014;312(9):923–33. doi: 10.1001/jama.2014.10397. [DOI] [PubMed] [Google Scholar]
- 4.Nordmann AJ, Nordmann A, Briel M, Keller U, Yancy WS, Jr., Brehm BJ, Bucher HC. Effects of low-carbohydrate vs low-fat diets on weight loss and cardiovascular risk factors: a meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166(3):285–93. doi: 10.1001/archinte.166.3.285. [DOI] [PubMed] [Google Scholar]
- 5.Gardner CD, Kiazand A, Alhassan S, Kim S, Stafford RS, Balise RR, Kraemer HC, King AC. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: a randomized trial. JAMA. 2007;297(9):969–77. doi: 10.1001/jama.297.9.969. [DOI] [PubMed] [Google Scholar]
- 6.Greenberg I, Stampfer MJ, Schwarzfuchs D, Shai I, Group D. Adherence and success in long-term weight loss diets: the dietary intervention randomized controlled trial (DIRECT) J Am Coll Nutr. 2009;28(2):159–68. doi: 10.1080/07315724.2009.10719767. [DOI] [PubMed] [Google Scholar]
- 7.Yancy WS, Jr., Westman EC, McDuffie JR, Grambow SC, Jeffreys AS, Bolton J, Chalecki A, Oddone EZ. A randomized trial of a low-carbohydrate diet vs orlistat plus a low-fat diet for weight loss. Arch Intern Med. 2010;170(2):136–45. doi: 10.1001/archinternmed.2009.492. [DOI] [PubMed] [Google Scholar]
- 8.King AC. Behavioral medicine in the 21st century: transforming “the Road Less Traveled” into the “American Way of Life”. Ann Behav Med. 2014;47(1):71–8. doi: 10.1007/s12160-013-9530-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bazzano LA, Hu T, Reynolds K, Yao L, Bunol C, Liu Y, Chen CS, Klag MJ, Whelton PK, He J. Effects of low-carbohydrate and low-fat diets: a randomized trial. Ann Intern Med. 2014;161(5):309–18. doi: 10.7326/M14-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sallis JF, Haskell WL, Wood PD, Fortmann SP, Rogers T, Blair SN, Paffenbarger RS., Jr. Physical activity assessment methodology in the Five-City Project. Am J Epidemiol. 1985;121(1):91–106. doi: 10.1093/oxfordjournals.aje.a113987. [DOI] [PubMed] [Google Scholar]
- 11.Physical Activity Guidelines Advisory Committee . Physical Activity Guidelines Advisory Committee Report. U.S. Department of Health and Human Services; Washington, DC: 2008. [DOI] [PubMed] [Google Scholar]
- 12.Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, Franklin B, Kris-Etherton P, Harris WS, Howard B, Karanja N, Lefevre M, Rudel L, Sacks F, Van Horn L, Winston M, Wylie-Rosett J. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114(1):82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 13.Saris WHM, Blair SN, van Baak MA, Eaton SB, Davies PSW, DiPierto L, Fogelholm M, Rissanen A, Schoeller D, Swinburn B, Temblay A, Westerterp KR, Wyatt H. How much physical activity is enough to prevent unhealthy weight gain? Outcome of the IASO 1st Stock Conference and consensus statement. Obes Rev. 2003;4:101–104. doi: 10.1046/j.1467-789x.2003.00101.x. [DOI] [PubMed] [Google Scholar]
- 14.Department of Health and Human Services Dietary Guidelines. 2005 < http://www.healthierus.gov/dietaryguidelines>.
- 15.Bandura A. Social foundations of thought and action: a social cognitive theory. Prentice Hall; Englewood Cliffs, New Jersey: 1986. [Google Scholar]
- 16.Bandura A. Self-efficacy: The exercise of control. W. H. Freeman and Company; New York, New York: 1997. [Google Scholar]
- 17.Brownell KD, Cohen LR. Adherence to dietary regimens. 2: Components of effective interventions. Behav Med. 1995;20(4):155–64. doi: 10.1080/08964289.1995.9933732. [DOI] [PubMed] [Google Scholar]
- 18.Foreyt JP, Goodrick GK. Impact on behavior therapy on weight loss. Am J Health Promot. 1994;8(6):466–8. doi: 10.4278/0890-1171-8.6.466. [DOI] [PubMed] [Google Scholar]
- 19.Jeffery RW, Epstein LH, Wilson G, Drewnowski A, Stunkard AJ, Wing RR. Long-term maintenance of weight loss: Current status. Health Psychology. 2000;19(Suppl 1):5–16. doi: 10.1037/0278-6133.19.suppl1.5. [DOI] [PubMed] [Google Scholar]
- 20.King AC, Frey-Hewitt B, Dreon DM, Wood PD. Diet vs exercise in weight maintenance. The effects of minimal intervention strategies on long-term outcomes in men. Arch Intern Med. 1989;149(12):2741–6. doi: 10.1001/archinte.149.12.2741. [DOI] [PubMed] [Google Scholar]
- 21.Wing RR, Tate DF, Gorin AA, Raynor HA, Fava JL. A self-regulation program for maintenance of weight loss. N Engl J Med. 2006;355(15):1563–71. doi: 10.1056/NEJMoa061883. [DOI] [PubMed] [Google Scholar]
- 22.Brownell KD. The LEARN Manual for weight management. 10th ed. American Health Publishing Company; Dallas, TX: 2004. [Google Scholar]
- 23.Feskanich D, Sielaff BH, Chong K, Buzzard IM. Computerized collection and analysis of dietary intake information. Comput Methods Programs Biomed. 1989;30(1):47–57. doi: 10.1016/0169-2607(89)90122-3. [DOI] [PubMed] [Google Scholar]
- 24.Johnson RK, Driscoll P, Goran MI. Comparison of multiple-pass 24-hour recall estimates of energy intake with total energy expenditure determined by the doubly labeled water method in young children. J Am Diet Assoc. 1996;96(11):1140–4. doi: 10.1016/S0002-8223(96)00293-3. [DOI] [PubMed] [Google Scholar]
- 25.Harnack L, Stevens M, Van Heel N, Schakel S, Dwyer JT, Himes J. A computer-based approach for assessing dietary supplement use in conjunction with dietary recalls. J Food Compost Anal. 2008;21:S78–S82. doi: 10.1016/j.jfca.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sievert YA, Schakel SF, Buzzard IM. Maintenance of a nutrient database for clinical trials. Control Clin Trials. 1989;10(4):416–25. doi: 10.1016/0197-2456(89)90006-8. [DOI] [PubMed] [Google Scholar]
- 27.Schakel S, Sievert YA, Buzzard IM. Sources of data for developing and maintaining a nutrient database. J Am Diet Assoc. 1988;88(10):1268–71. [PubMed] [Google Scholar]
- 28.Schakel S, Buzzard I, Gebhardt S. Procedures for estimating nutrient values for food composition databases. J Food Comp Anal. 1997;10:102–114. [Google Scholar]
- 29.Lee A, Lee M. [December 1.2015];MyFitnessPal, LLC. 2015 < www.myfitnesspal.com>. [Google Scholar]
- 30.National Center for Health Statistics, Centers for Disease Control and Prevention, National Health and Nutrition Examination Survey 1999-2000 . In: Data Documentation, Codebook, and Frequencies: Blood Pressure (BPX) C.f.D.C.a.P. US Department of Health and Human Services, editor. Hyattsville, MD: 2002. [Google Scholar]
- 31.Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20(4):470–5. [PubMed] [Google Scholar]
- 32.Nagele U, Hagele EO, Sauer G, Wiedemann E, Lehmann P, Wahlefeld AW, Gruber W. Reagent for the enzymatic determination of serum total triglycerides with improved lipolytic efficiency. J Clin Chem Clin Biochem. 1984;22(2):165–74. doi: 10.1515/cclm.1984.22.2.165. [DOI] [PubMed] [Google Scholar]
- 33.Warnick GR, Nguyen T, Albers AA. Comparison of improved precipitation methods for quantification of high-density lipoprotein cholesterol. Clin Chem. 1985;31(2):217–22. [PubMed] [Google Scholar]
- 34.Caulfield MP, Li S, Lee G, Blanche PJ, Salameh WA, Benner WH, Reitz RE, Krauss RM. Direct determination of lipoprotein particle sizes and concentrations by ion mobility analysis. Clin Chem. 2008;54(8):1307–16. doi: 10.1373/clinchem.2007.100586. [DOI] [PubMed] [Google Scholar]
- 35.Morgan CR, Lazarow A. Immunoassay of insulin: two antibody system: plasma insulin levels in normal, subdiabetic and diabetic rats. Diabetes. 1963;12:115–126. [Google Scholar]
- 36.Kadish AH, Hall DA. A new method for the continuous monitoring of blood glucose by measurement of dissolved oxygen. Clin Chem. 1965;11(9):869–75. [PubMed] [Google Scholar]
- 37.Mooy JM, Grootenhuis PA, de Vries H, Kostense PJ, Popp-Snijders C, Bouter LM, Heine RJ. Intra-individual variation of glucose, specific insulin and proinsulin concentrations measured by two oral glucose tolerance tests in a general Caucasian population: the Hoorn Study. Diabetologia. 1996;39(3):298–305. doi: 10.1007/BF00418345. [DOI] [PubMed] [Google Scholar]
- 38.Olijhoek JK, Banga JD, Doevendans PA, Visseren FL. Oral glucose tolerance test or metabolic syndrome criteria to predict risk in patients with coronary heart disease. Eur Heart J. 2005;26(6):623. doi: 10.1093/eurheartj/ehi133. author reply 623-4. [DOI] [PubMed] [Google Scholar]
- 39.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes care. 1999;22(9):1462–70. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 40.Olink Proteomics Proseek Multiplex Product Site. 2016 < http://www.olink.com/products/proseek-multiplex/>.
- 41.Baas F, Bikker H, van Ommen GJ, de Vijlder JJ. Unusual scarcity of restriction site polymorphism in the human thyroglobulin gene. A linkage study suggesting autosomal dominance of a defective thyroglobulin allele. Hum Genet. 1984;67(3):301–5. doi: 10.1007/BF00291357. [DOI] [PubMed] [Google Scholar]
- 42.Gustafson S, Proper JA, Bowie EJ, Sommer SS. Parameters affecting the yield of DNA from human blood. Anal Biochem. 1987;165(2):294–9. doi: 10.1016/0003-2697(87)90272-7. [DOI] [PubMed] [Google Scholar]
- 43.Paul J, Froster-Iskenius U, Moje W, Schwinger E. Heterozygous female carriers of the marker-X-chromosome: IQ estimation and replication status of fra(X)(q) Hum Genet. 1984;66(4):344–6. doi: 10.1007/BF00287638. [DOI] [PubMed] [Google Scholar]
- 44.UK Biobank Array Design Group UK Biobank, editor. UK Biobank Array Content Summary Brochure. 2014 [Google Scholar]
- 45.Axiom 2.0 Assay Automated Workflow User Guide. Santa Clara, CA: 2016. Affymetrix. [Google Scholar]
- 46.Analysis Guide: Axiom Genotyping Solution Data Analysis Guide. Santa Clara, CA: 2015. Affymetrix. [Google Scholar]
- 47.Ware JE, Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–83. [PubMed] [Google Scholar]
- 48.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 49.Neff KL, King AC. Exercise Program Adherence in Older Adults: The Importance of Achieving One's Expected Benefits, Medicine, Exercise. Nutrition and Health. 1995;4:355–362. [Google Scholar]
- 50.Garcia AW, King AC. Predicting long-term adherence to aerobic exercise: a comparison of two models. Journal of Sport and Exercise Psychology. 1991;13:394–410. [Google Scholar]
- 51.Sallis JF, Grossman RM, Pinski RB, Patterson TL, Nader PR. The development of scales to measure social support for diet and exercise behaviors. Prev Med. 1987;16(6):825–36. doi: 10.1016/0091-7435(87)90022-3. [DOI] [PubMed] [Google Scholar]
- 52.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–96. [PubMed] [Google Scholar]
- 53.French SA, Jeffery RW. Current dieting, weight loss history, and weight suppression: behavioral correlates of three dimensions of dieting. Addict Behav. 1997;22(1):31–44. doi: 10.1016/s0306-4603(96)00002-0. [DOI] [PubMed] [Google Scholar]
- 54.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29(1):71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 55.Williams GC, Deci EL, Ryan DH. Building health-care partnerships by supporting autonomy: promoting maintainted health behavior change and positive health outcomes. In: Suchman AL, Hinton-Walker P, Botelho R, editors. Partnerships in healthcare: Transforming relational process. University of Rochester Press; Rochester, NY: 1998. pp. 67–87. [Google Scholar]
- 56.Garner DM, Garfinkel PE. The Eating Attitudes Test: an index of the symptoms of anorexia nervosa. Psychol Med. 1979;9(2):273–9. doi: 10.1017/s0033291700030762. [DOI] [PubMed] [Google Scholar]
- 57.Frank RA, van der Klaauw NJ. The contribution of chemosensory factors to individual differences in reported food preferences. Appetite. 1994;22(2):101–23. doi: 10.1006/appe.1994.1011. [DOI] [PubMed] [Google Scholar]
- 58.Brug J, Lechner L, De Vries H. Psychosocial determinants of fruit and vegetable consumption. Appetite. 1995;25(3):285–96. doi: 10.1006/appe.1995.0062. [DOI] [PubMed] [Google Scholar]
- 59.Garner DM, Olmstead MP, Polivy J. Development and Validation of a Multidimensional Eating Disorder Inventory for Anorexia-Nervosa and Bulimia. Int J Eat Disorder. 1983;2(2):15–34. [Google Scholar]
- 60.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 61.Gearhardt AN, Corbin WR, Brownell KD. Preliminary validation of the Yale Food Addiction Scale. Appetite. 2009;52(2):430–6. doi: 10.1016/j.appet.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 62.Saelens BE, Sallis JF, Black JB, Chen D. Neighborhood-based differences in physical activity: an environment scale evaluation. Am J Public Health. 2003;93(9):1552–8. doi: 10.2105/ajph.93.9.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sampson RJ, Raudenbush SW, Earls F. Neighborhoods and violent crime: a multilevel study of collective efficacy. Science. 1997;277(5328):918–24. doi: 10.1126/science.277.5328.918. [DOI] [PubMed] [Google Scholar]
- 64.Treadwell T, Lavertue N, Kumar VK, Veeraraghavan V. The group cohesion scale-revised: reliability and validity. International Journal of Action Methods. 2001;54(1):3. [Google Scholar]
- 65.Mezo PG. The self-control and self-management scale (SCMS): Development of an adaptive self-regulatory coping skills instrument. Journal of Psychopathology and Behavioral Assessment. 2008;31:83–93. [Google Scholar]
- 66.Arnow B, Kenardy J, Agras WS. The Emotional Eating Scale: the development of a measure to assess coping with negative affect by eating. Int J Eat Disord. 1995;18(1):79–90. doi: 10.1002/1098-108x(199507)18:1<79::aid-eat2260180109>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 67.Bassett DR, Jr., Howley ET, Thompson DL, King GA, Strath SJ, McLaughlin JE, Parr BB. Validity of inspiratory and expiratory methods of measuring gas exchange with a computerized system. J Appl Physiol (1985) 2001;91(1):218–24. doi: 10.1152/jappl.2001.91.1.218. [DOI] [PubMed] [Google Scholar]
- 68.Crouter SE, Antczak A, Hudak JR, DellaValle DM, Haas JD. Accuracy and reliability of the ParvoMedics TrueOne 2400 and MedGraphics VO2000 metabolic systems. Eur J Appl Physiol. 2006;98(2):139–51. doi: 10.1007/s00421-006-0255-0. [DOI] [PubMed] [Google Scholar]
- 69.Macfarlane DJ, Wu HL. Inter-unit variability in two ParvoMedics TrueOne 2400 automated metabolic gas analysis systems. Eur J Appl Physiol. 2013;113(3):753–62. doi: 10.1007/s00421-012-2483-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Draper C, Wilkins L, Breton G, Perusse L, Debusk R, Ramakrishnan S, Krempin D. Genetic markers for weight management and methods of use thereof. Google Patents. 2012 [Google Scholar]
- 71.Hindorff LA, MacArthur J, Morales J, Junkins HA, Hall PN, Klemm AK, Manolio T. E.M.B.L.-E.B. Institute, editor. A Catalog of Published Genome-Wide Association Studies. 2015 [Google Scholar]
- 72.Cornier MA, Donahoo WT, Pereira R, Gurevich I, Westergren R, Enerback S, Eckel PJ, Goalstone ML, Hill JO, Eckel RH, Draznin B. Insulin sensitivity determines the effectiveness of dietary macronutrient composition on weight loss in obese women. Obes Res. 2005;13(4):703–9. doi: 10.1038/oby.2005.79. [DOI] [PubMed] [Google Scholar]
- 73.Ebbeling CB, Leidig MM, Feldman HA, Lovesky MM, Ludwig DS. Effects of a low-glycemic load vs low-fat diet in obese young adults: a randomized trial. JAMA. 2007;297(19):2092–102. doi: 10.1001/jama.297.19.2092. [DOI] [PubMed] [Google Scholar]
- 74.Gardner CD, Offringa LC, Hartle JC, Kapphahn K, Cherin R. Weight loss on low-fat vs. low-carbohydrate diets by insulin resistance status among overweight adults and adults with obesity: A randomized pilot trial. Obesity (Silver Spring) 2016;24(1):79–86. doi: 10.1002/oby.21331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pittas AG, Das SK, Hajduk CL, Golden J, Saltzman E, Stark PC, Greenberg AS, Roberts SB. A lowglycemic load diet facilitates greater weight loss in overweight adults with high insulin secretion but not in overweight adults with low insulin secretion in the CALERIE Trial. Diabetes care. 2005;28(12):2939–41. doi: 10.2337/diacare.28.12.2939. [DOI] [PubMed] [Google Scholar]
- 76.Jo B. Causal inference in randomized experiments with mediational processes. Psychol Methods. 2008;13(4):314–36. doi: 10.1037/a0014207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kraemer HC, Wilson GT, Fairburn CG, Agras WS. Mediators and moderators of treatment effects in randomized clinical trials. Arch Gen Psychiatry. 2002;59(10):877–83. doi: 10.1001/archpsyc.59.10.877. [DOI] [PubMed] [Google Scholar]
- 78.Little RJA, Rubin DB. Statistical analysis with missing data. Wiley; New York: 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.