Abstract
The decision to move forward with three clinical trials of IL-1 blockade for treatment of acute Kawasaki disease is a case study in translational science. These trials were born on the one hand from transcriptome studies of host response during the acute disease coupled with animal model investigations of key immune signaling pathways and, on the other hand, out of clinical desperation to intervene in patients with severe inflammation in the setting of acute Kawasaki disease. The convergence of laboratory science and clinical observations led to the clinical trials described here and serves as a model for how such observations can be translated into new therapies.
Keywords: vasculitis, interleukin-1, coronary artery aneurysms, pediatrics, autoinflammatory disease, Kawasaki disease
The successful translation of bench research into clinical practice requires physician scientists who understand both the unmet clinical needs as well as the experimental gaps in knowledge. In the case of Kawasaki disease (KD), a self-limited vasculitis of unknown etiology and the most common cause of acquired heart disease in children, communication between research teams achieved the necessary coalescence of science and clinical medicine to rapidly translate experimental mouse experiments and human transcriptomic data and into three separate clinical trials on two continents. What follows is a story of how research teams in the Europe and the U.S. capitalized on experimental findings to devise clinical trials to test a new paradigm: KD is a systemic inflammatory condition in which activation of the IL-1 signaling pathway is an essential driver of disease pathogenesis.
The current understanding of KD is that an immunologic reaction is elicited in genetically susceptible hosts upon exposure to the KD trigger or triggers, which are thought to be stimuli that enter through the upper respiratory tract (1). Both mystery and controversy surround the exact nature of the trigger with some groups advocating a novel virus and others suggesting an antigenic exposure without an infectious agent. The subset of KD children who suffer damage to the coronary arterial wall (2–4) and aneurysms in these vessels comprise only 25% of total population of KD-susceptible children(5). Therapy with intravenous immunoglobulin (IVIG) reduces the prevalence of aneurysms to 3–6% and rates of aneurysms are highest among patients who fail to become afebrile after the first dose of 2g/kg of IVIG (6, 7).
The mechanism by which IVIG reduces inflammation in the majority of patients is only now being elucidated despite the widespread clinical use of this treatment for over three decades. In vitro studies have documented that IVIG increases IL-1R antagonist expression in human monocytes, which may be relevant in KD (8). Recent immunologic studies have established that secretion of IL-10 by tolerogenic myeloid dendritic cells (mDC) and natural regulatory T cells (nTreg) is a critical event in down regulation of inflammation in acute KD(9, 10). Incubation of peripheral blood mononuclear cells with the constant region of the IgG molecule (Fc) in vitro leads to the expansion of a population of Fc-specific nTreg and mDC and to increased secretion of IL-10. Immune monitoring of patients at the subacute stage has established that these populations expand after IVIG treatment in vivo (10). However, patients who develop coronary artery aneurysms (CAA) have a reduced capacity to expand these populations due to mechanisms that are currently under study. Other mechanisms of action of IVIG in KD that lead to the very rapid downregulation of systemic signs of inflammation have yet to be elucidated.(11) Clinicians who care for acute KD patients have been aware of the need for adjunctive therapies that could be used with IVIG to reduce inflammation in the subsets of patients with early aneurysms, severe myocarditis, and in those who had persistent fever following IVIG infusion.
Role of TNFα in acute KD
The pro-inflammatory cytokine TNFα was known to be markedly elevated during the acute phase of KD and levels were highest in patients who developed CAA(12). Thus, it was a logical choice to try TNFα inhibition with infliximab following the approval of this monoclonal antibody for pediatric patients with inflammatory bowel disease in 2006. After publications reporting the compassionate use of infliximab for IVIG-resistant KD patients, the results of the first proof of concept trial were published in 2008 (13–16). This trial enrolled 24 acute KD subjects who had persistent fever 36h after completion of their IVIG infusion and randomized them to either second IVIG infusion or infliximab 5mg/kg. The results were promising with 11 of the 12 subjects randomized to infliximab becoming afebrile and not requiring further treatment compared with eight of the 12 subjects who received a second infusion of IVIG. There was no adverse outcome signal and the pharmacokinetics of the antibody appeared to be similar to adult studies. However, clinicians realized that waiting until a patient had failed therapy to initiate additional treatment left the patient with unchecked inflammation for a prolonged period and put the coronary arteries at risk from on-going destructive processes. The shift in interest then focused on intensification of initial IVIG therapy to more rapidly bring inflammation under control.
Clinical trials of intensification of initial therapy
Three sentinel trials that attempted to identify adjuvant therapies that could benefit KD patients took place in the U.S. and Japan. In the Pediatric Heart Network trial of a placebo-controlled, randomized study of a single IV dose of methylprednisolone (30 mg/kg) plus standard therapy (IVIG plus aspirin), no difference was seen in the coronary artery outcome or rate of IVIG resistance (17). In the Japanese RAISE trial, KD patients at greatest risk of IVIG resistance and CAA were selected using a clinical scoring system that had been validated in the Japanese population, but had shown poor predictive performance for U.S multiethnic KD patients (7, 18, 19). Japanese subjects were randomized to either IV followed by oral methylprednisolone (2 mg/kg) or placebo in addition to IVIG and aspirin and were treated with study drug for approximately three to five weeks. Results showed a significant reduction in IVIG resistance and coronary artery Z score (internal diameter of the coronary artery normalized for body surface area and expressed as standard deviations from the mean) between the groups in favor of steroids. In a U.S. trial of intensification of initial therapy with TNFα blockade, unselected KD patients were randomized to a single dose of infliximab (5 mg/kg) or placebo in addition to IVIG and aspirin (20). Results showed a significant reduction in measures of inflammation, coronary artery Z score, and duration of fever. However, addition of infliximab to initial IVIG therapy failed to prevent IVIG resistance. Current evidence supports the use of infliximab as rescue therapy for patients with IVIG-resistance, but trials have not evaluated its use as adjunctive therapy in patients with early evidence of coronary artery damage.(21) Although some benefit was demonstrated in each of these trials, there remained highly resistant KD patients whose course was not altered by either steroids or TNFα blockade and whose arteries underwent progressive damage despite timely treatment. Thus, clinicians were aware of an unmet clinical need to devise specific and more effective therapies for this subset of patients.
Interleukin-1 (IL-1) as a master cytokine
IL-1 is a central mediator of innate immunity and inflammation and is considered a master cytokine of local and systemic inflammation (22). IL-1α and IL-1β are encoded by distinct genes, bind to the same receptor (IL-1R1), and have similar biological properties. IL-1β mediates the unchecked inflammation in many autoinflammatory diseases (23). IL-1β also enhances antigen-driven CD8+ T cell differentiation, proliferation, memory, and migration into tissues (24). This is important with respect to the pathogenesis of acute KD as CD8+ T-cells infiltrate the coronary artery wall and likely contribute to a destructive process associated with aneurysm formation (25).
IL-1β is transcribed as an inactive precursor that requires assembly of the NLRP3 inflammasome to activate caspase 1, the enzyme that cleaves pro-IL-1β to release the biologically active form of the cytokine. In contrast, the IL-1α precursor is functional and when released from necrotic cells in a site of injury can bind to the IL-1R1 of neighboring cells, thus initiating a cascade of inflammatory cytokines and chemokines (26). Circulating IL-1α is rarely detected in patient sera but is found within endothelial cells and apoptotic bodies released from these cells. (27).
The relative contributions of IL-1α and β to the vasculitis of KD have yet to be defined. It is intriguing that IL-1β signaling prolongs neutrophil survival and drives proliferation of smooth muscle cells (SMC) and myofibroblast formation (28–31), all pathologic hallmarks of the arteriopathy seen in KD(32, 33). IL-1 induced SMC proliferation is driven by matrix metalloproteinases, including MMP3 and MMP9, both of which are implicated in human KD (30, 34–38). Indeed, SMC-derived myofibroblasts can actively proliferate in an uncontrolled fashion in the arterial wall after KD leading to luminal myofibroblastic proliferation (LMP), which can progress to life-threating coronary artery stenosis and infarction (39). Although therapies are available to reduce risk of thrombosis in CAA, no therapies have been tested to reduce LMP, which can lead to myocardial ischemia. IL-1α signaling may be responsible for amplifying inflammatory cell infiltration into the arterial wall in regions of vascular SMC and endothelial cell necrosis.
Experience with IL-1 blockade in pediatric inflammatory diseases
Cryopyrin-associated periodic fever syndrome (CAPS) is the only disease with FDA and EMA approval for anakinra in infants (≥8 months, and 10 kg), but anakinra is widely used off-label in systemic autoinflammatory and autoimmune syndromes in children including systemic juvenile idiopathic arthritis (sJIA), macrophage activations syndrome, colchicine-resistant familial Mediterranean fever (FMF) and idiopathic pericarditis, and TNFα receptor-associated periodic syndrome (TRAPS), (40–44). In a recent study of 26 patients with the autoinflammatory syndrome neonatal-onset multisystem inflammatory disease (NOMID), treatment with anakinra for 36 months had a low adverse event rate, with upper respiratory infection as the most commonly reported complication (45). A similar study using anakinra in another CAPS phenotype, Muckle-Wells syndrome, did not demonstrate any serious adverse events in the 12 patients treated with anakinra, of whom 5 were children (46). In most of these studies in young children, the dose of anakinra needed to achieve complete anti-inflammatory effect was between 4 and 10mg/kg/day.
Canakinumab is a selective monoclonal antibody against IL-1β with a long half-life of 22 days (47). In patients with CAPS, a single subcutaneous dose of 2mg/kg for pediatric patients and 150 mg in adult patients every eight weeks provided rapid resolution of clinical and biological signs of inflammation (48). Younger patients and those with the most severe phenotypes require higher doses (or shorter intervals between doses). The use of subcutaneous doses of 2 mg/kg of canakinumab every 8 weeks for 24 weeks was associated with complete control of clinical manifestations and laboratory parameters in patients with a Muckle Wells syndrome phenotype, but those who were younger or manifested more of a chronic infantile neurological, cutaneous and articular (CINCA) syndrome phenotype needed higher or more frequent dosing (49). In sJIA, the approved dose is 4mg/kg every 4 weeks (50).
Specific inhibitors of IL-1α have not been tested in pediatric inflammatory diseases. It is intriguing that a human antibody specific for IL-1α, MABp1, is now in early clinical trials for different advanced cancers in adultsand has improved survival and well-being of patients.(51) The availability of selective IL-1 blocking agents will further our understanding of the relative roles of IL-1α and β in different diseases involving both local and systemic inflammation.
Insights from KD genetic studies: Calcium signaling and the IL-1 pathway
Genetic studies have identified several risk alleles that influence both susceptibility to KD and development of CAA (52). Among these, a functional polymorphism in the inositol 1,4,5-trisphosphate 3-kinase C (ITPKC) gene was reproducibly associated with KD susceptibility and CAA across diverse populations (53–56). Another polymorphism that influenced KD susceptibility was private to the Japanese population and was located the ORAI1 gene that encodes for a calcium channel (57). Importantly, both the ITPKC and ORAI1 genes regulate calcium flux in the cell, and these polymorphisms lead to sustained elevation of intracellular Ca2+, which can both induce NLRP3 inflammasome activation and secretion of biologically active IL-1β as well as the release of IL-1α (58–61).
Insights from KD proteomic and transcriptomic studies: Role of IL-1 in acute KD
Several observations suggested that IL-1plays an important role in KD vasculitis. In children with KD, the IL-1 pathway is upregulated compared to pediatric febrile controls, as demonstrated by increased transcript abundance by microarray and by increased levels of pathway proteins in the plasma (62–66). Early studies determined that IVIG treatment was associated with a reduction in IL-1β secretion by peripheral blood mononuclear cells from KD patients with no CAA as compared to persistently elevated levels in IVIG-treated patients with CAA(63). In a microarray study of acute and convalescent whole blood samples collected in PAXgene® tubes from 146 KD subjects, KD transcript abundance profiles were compared to pediatric subjects with different acute infectious diseases and to pediatric healthy controls (65). Differentially expressed transcripts were analyzed according to their participation in different biologic pathways. Common features of the top three pathways for KD were the abundance of transcripts related to the NLRP3 inflammasome, IL-1α and β, and caspase-1. The upregulation of key genes in the IL-1 pathway was confirmed using qRT-PCR in an independent cohort of 20 KD patients and 10 healthy controls (65). In a different microarray study, IVIG-resistant KD patients, when compared to IVIG-responsive patients, had increased expression of the following IL-1 pathway genes: IL-1R1, IL-1R2, IL-1R associated kinase 3 (IRAK3), and TIFA and decreased expression of IL-1Ra (66).
Insights from the KD vasculitis mouse model: Role of IL-1 signaling
The limited availability of tissue samples from patients with KD has significantly impeded progress in understanding the etiology and pathogenesis of the disease, thus making the availability of a relevant animal model extremely valuable. A well-described mouse model of coronary arteritis closely mimics the important histological as well as immune-pathological features of the cardiovascular lesions of acute KD (67–69). A single intraperitoneal injection of a cell wall extract from Lactobacillus casei (LCWE) reproducibly induces proximal coronary arteritis that is histologically similar to the coronary arteritis observed in human KD. In the LCWE mouse model, a subacute coronary arteritis with luminal myofibroblast proliferation (LMP) and stenosis mimics the lesions found in human arteries(70).
The mouse model of KD vasculitis is also associated with systemic inflammation and increased body temperature. Intact TLR2 and IL-1R signaling via MyD88 is required for this LCWE-induced vasculitis (71, 72). Moreover, this KD mouse model also predicts efficacy of treatment in children with KD. Currently used treatments in humans such as IVIG and anti-TNFα antibodies, have been shown to be beneficial in preventing coronary arteritis and aortitis in the LCWE-induced KD vasculitis mouse model (73, 74).
Using this model, Arditi and colleagues demonstrated the key role of IL-1 signaling pathways in the LCWE-induced mouse vasculitis model (75). Anakinra also prevented LCWE-induced abdominal aortic dilation and aneurysms (76). Of interest, anakinra was even more effective than IVIG or TNF-α blockade in reducing LCWE-induced myocarditis and improved heart function (ejection fraction) measured using a small animal MRI (70, 72). Thus, genetic and transcriptomic data from KD patients and data from the experimental mouse model of KD vasculitis have converged on the likely role of IL-1 signaling in the pathogenesis of KD vasculitis (Figure).
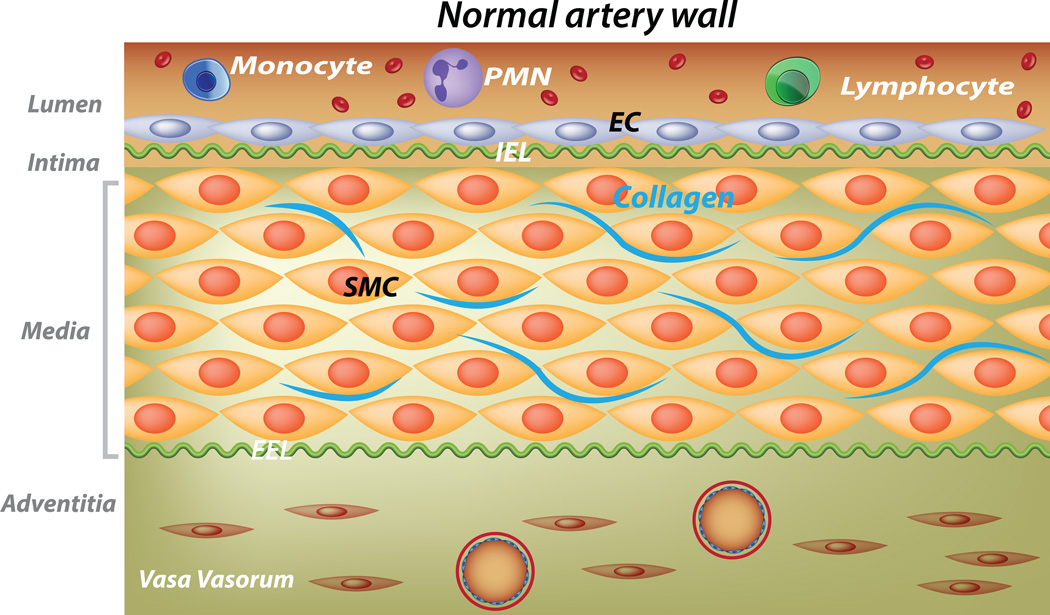
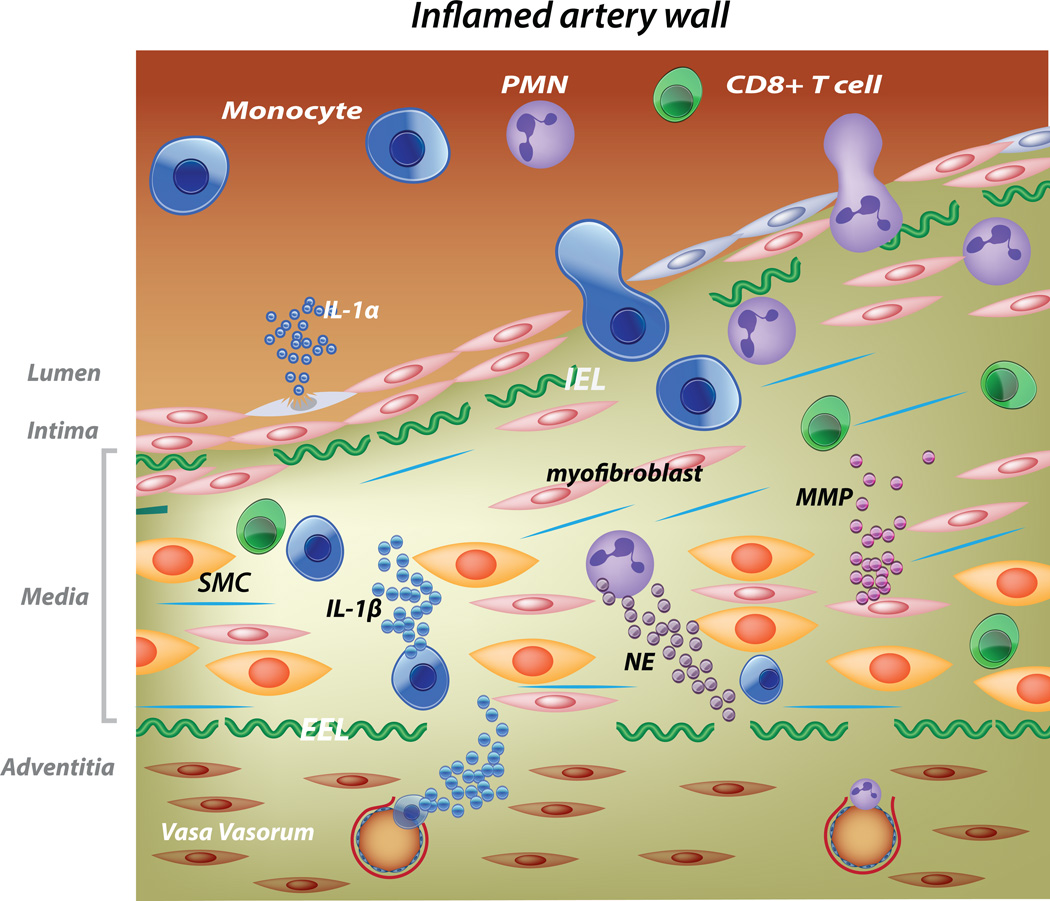
Figure.
Schematic of the vasculitis in acute KD and potential roles of IL-1α and β in disease pathogenesis. In the inflamed artery, endothelial cells (blue spindle-shaped cells) and vascular smooth muscle cells (SMC) transition to myofibroblasts (pink spindle-shaped cells) that secrete matrix metalloproteinases (MMPs) and disordered collagen (blue lines). Neutrophils recruited to the media secrete neutrophil elastases (NE) resulting in breaks in the internal (I) and external (E) elastic lamina (EL). Mononuclear cells trafficking into the media from the lumen and vasa vasorum secrete IL-1β. EC and SMC undergoing necrosis release Il-1α. The structural integrity of the arterial wall is diminished and hydrostatic pressure results in aneurysm formation.
As the use of IL-1 blockade became more widespread for control of a diverse array of inflammatory conditions, clinicians were emboldened to try to IL-1 blockade in the treatment of desperately ill KD patients. In the first report of such use, Kuijpers and colleagues reported treatment of an infant with uncontrolled inflammation and giant aneurysms who finally responded to IL-1 blockade with anakinra after failing multiple other anti-inflammatory therapies (77). The patient experienced a dramatic clinical improvement but required prolonged treatment with anakinra. Subsequently, another severe KD patient who was resistant to IVIG treatment was treated with anakinra, which was well-tolerated and associated with resolution of systemic and coronary artery inflammation (78). So a convergence of genetic and genomic studies with experimental animal data and human clinical data set the stage for further investigation. The body of evidence supporting the importance of the IL-1 pathway in KD pathogenesis was now compelling enough for the launch of three different clinical trials of IL-1 blockade for treatment of acute KD.
Pediatric trials of IL-1 blockade in acute Kawasaki disease
There are currently three clinical trials of IL-1 blockade that enrolling patients with KD in Western Europe and the U.S. (Table 1).
Table 1.
Summary of international trials of IL-1 blockade in acute Kawasaki disease subjects.
| Name of trial and study medication |
Participating centers and country; Target enrollment |
Study design |
Enrollment criteria |
Primary outcome measures |
Secondary outcome measures |
|---|---|---|---|---|---|
| KAWAKINRA anakinra |
6 centers in France, 1 center in Spain, 3 in Italy 20 subjects |
Phase II, within subject dose escalation from 2–6 mg/kg/day |
IVIG- resistance |
Safety, cessation of fever |
Coronary artery Z score, changes in gene expression and protein levels of inflammatory biomarkers |
| ANAKID anakinra |
Rady Children’s Hospital San Diego and Boston Children’s Hospital, USA 30 subjects |
Phase I/IIa 3 + 3 dose escalation study from 4– 8 mg/kg/day |
Z score of RCA and/or LAD ≥ 3.0 and within 20 days of fever onset |
Safety, tolerability, PK |
Cytokine and oxidative stress measurements, change in Z score of RCA and LAD by echocardiogram |
| Canakinumab | 7 European countries 26 subjects |
Phase II 6 mg/kg IV with repeat dose at 4 and 8 weeks |
2-arms: treatment- naïve and IVIG resistant |
Safety, cessation of fever |
Changes in gene expression and protein levels of inflammatory biomarkers |
Kawakinra trial (Europe)
The Kawakinra trial (Eudract Number: 2014-002715-41) is a Phase IIa, single dose, multi-center trial of anakinra in Europe. The primary objective is to assess the efficacy and safety of anakinra in patients with IVIG-resistant KD. The secondary objectives are to assess the effect of anakinra on disease activity, coronary artery Z score, and changes in gene expression and protein levels of inflammatory biomarkers. Eligible patients are those aged eight months (10 Kg) to 18 years with a diagnosis of acute KD according to American Heart Association (AHA) criteria, and who have persistent or recrudescent fever at least 48 hours after the initiation of the IVIG infusion. After parental informed consent, subjects enter the screening period between the fourth to the thirteenth day of fever. Subjects enter the treatment phase if they develop recrudescent fever and receive a first dose of 2 mg/kg of anakinra subcutaneously. In case of fever persistence or recrudescence within 24h after the first dose, the next dose is increased to 4mg/kg. Persistence or recrudescence of fever within 24h is followed by a further increase to 6mg/kg. If a subject fails to respond within 48h at a dose of 6mg/kg, alternative treatment is administered at the center PI’s discretion. The highest daily dose of anakinra (from 2–6mg/kg) is continued to complete 14 days of treatment.
ANAKID Trial (USA)
This is a Phase I/IIa dose escalation study to determine the safety, tolerability, and pharmacokinetics (PK) of anakinra in acute KD (clinicaltrials.gov # NCT 2179853)(79). Study eligible subjects are KD patients meeting AHA criteria who are within the first 20 days after fever onset, at least eight months of age, and have a Z score ≥3.0 for the right coronary artery (RCA) or left anterior descending coronary artery (LAD). All subjects will receive at least two weeks of therapy. Only subjects with an echocardiogram at two weeks that shows either an RCA or LAD Z score ≥ 2.5 or an aneurysm (≥ 1.5 × the adjacent segment) of one of the coronary arteries will complete a six-week course of anakinra. All subjects will remain on study for the full 6 weeks whether or not they are receiving anakinra.
Canakinumab Trial (Europe)
This is an industry-sponsored, Phase II multi-center trial in seven countries that is open for enrollment. The trial has two treatment arms and will enroll not only IVIG-resistant subjects, but also treatment-naïve subjects with complete KD diagnosed early in the course of their disease who will receive canakinumab as initial monotherapy. Treatment-naïve subjects will receive a single 6 mg/kg intravenous dose of canakinumab. The primary outcome measure will be the presence or absence of fever after drug administration. Subjects who respond with complete fever resolution will not receive IVIG, and may be selected to receive one or two follow-up subcutaneous injections at 4 and 8 weeks depending on the CRP level and clinical follow-up. If the subject remains febrile at 48–72h after the initial canakinumab dose, then the subject will receive the standard 2g/kg IVIG.
A second canakinumab treatment arm will enroll IVIG-resistant subjects with KD who have failed to become afebrile following one or two IVIG infusions. Subjects in this arm will receive the same initial canakinumab IV dose and a second and third dose of canakinumab at 4 weeks and 8 weeks after their initial study treatment.
Conclusion
The convergence of human genetic and transcriptomic data from KD patients with animal experimental data from the LCWE KD mouse model has led to three Phase I/II clinical trials that should rapidly determine the safety and tolerability of IL-1 blockade in the treatment of KD. The simultaneous testing of different entrance criteria (persistent fever, CAA, or uncomplicated treatment-naïve KD), different endpoints (body temperature, coronary artery status by echocardiogram), and different study medications (anakinra, canakinumab) will accelerate our understanding of the role of IL-1α and β in KD pathogenesis and the benefits and risks of IL-1 blockade in these diverse study populations.
Acknowledgments
This work supported in part by a grant from the Gordon and Marilyn Macklin Foundation to JCB and by NIH grants (AI072726 and AI070162 to MA).
References
- 1.Rowley AH. Kawasaki disease: novel insights into etiology and genetic susceptibility. Annual review of medicine. 2011;62:69–77. doi: 10.1146/annurev-med-042409-151944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shimizu C, Jain S, Davila S, Hibberd ML, Lin KO, Molkara D, et al. Transforming growth factor-beta signaling pathway in patients with Kawasaki disease. Circ Cardiovasc Genet. 2011;4(1):16–25. doi: 10.1161/CIRCGENETICS.110.940858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shimizu C, Matsubara T, Onouchi Y, Jain S, Sun S, Nievergelt CM, et al. Matrix metalloproteinase haplotypes associated with coronary artery aneurysm formation in patients with Kawasaki disease. J Hum Genet. 2010;55(12):779–784. doi: 10.1038/jhg.2010.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mamtani M, Matsubara T, Shimizu C, Furukawa S, Akagi T, Onouchi Y, et al. Association of CCR2–CCR5 haplotypes and CCL3L1 copy number with Kawasaki Disease, coronary artery lesions, and IVIG responses in Japanese children. PLoS ONE. 2010;5(7):e11458. doi: 10.1371/journal.pone.0011458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suzuki A, Kamiya T, Kuwahara N, Ono Y, Kohata T, Takahashi O, et al. Coronary arterial lesions of Kawasaki disease: cardiac catheterization findings of 1100 cases. Pediatr Cardiol. 1986;7(1):3–9. doi: 10.1007/BF02315475. [DOI] [PubMed] [Google Scholar]
- 6.Ogata S, Tremoulet AH, Sato Y, Ueda K, Shimizu C, Sun X, et al. Coronary artery outcomes among children with Kawasaki disease in the United States and Japan. Int J Cardiol. 2013;168(4):3825–3828. doi: 10.1016/j.ijcard.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tremoulet AH, Best BM, Song S, Wang S, Corinaldesi E, Eichenfield JR, et al. Resistance to intravenous immunoglobulin in children with Kawasaki disease. J Pediatr. 2008;153(1):117–121. doi: 10.1016/j.jpeds.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruiz de Souza V, Carreno MP, Kaveri SV, Ledur A, Sadeghi H, Cavaillon JM, et al. Selective induction of interleukin-1 receptor antagonist and interleukin-8 in human monocytes by normal polyspecific IgG (intravenous immunoglobulin) Eur J Immunol. 1995;25(5):1267–1273. doi: 10.1002/eji.1830250521. [DOI] [PubMed] [Google Scholar]
- 9.Franco A, Touma R, Song Y, Shimizu C, Tremoulet A, Kanegaye J, et al. Specificity of regulatory T cells that modulate vascular inflammation. Autoimmunity. 2014;47:95–104. doi: 10.3109/08916934.2013.860524. [DOI] [PubMed] [Google Scholar]
- 10.Burns JC, Touma R, Song Y, Padilla RL, Tremoulet AH, Sidney J, et al. Fine specificities of natural regulatory T cells after IVIG therapy in patients with Kawasaki disease. Autoimmunity. 2015:1–8. doi: 10.3109/08916934.2015.1027817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burns JC, Franco A. The immunomodulatory effects of intravenous immunoglobulin therapy in Kawasaki disease. Expert Rev Clin Immunol. 2015;11(7):819–825. doi: 10.1586/1744666X.2015.1044980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsubara T, Furukawa S, Yabuta K. Serum levels of tumor necrosis factor, interleukin 2 receptor, and interferon-gamma in Kawasaki disease involved coronary-artery lesions. Clinical immunology and immunopathology. 1990;56(1):29–36. doi: 10.1016/0090-1229(90)90166-n. [DOI] [PubMed] [Google Scholar]
- 13.Weiss JE, Eberhard BA, Chowdhury D, Gottlieb BS. Infliximab as a novel therapy for refractory Kawasaki disease. J Rheumatol. 2004;31(4):808–810. [PubMed] [Google Scholar]
- 14.Burns JC, Mason WH, Hauger SB, Janai H, Bastian JF, Wohrley JD, et al. Infliximab treatment for refractory Kawasaki syndrome. J Pediatr. 2005;146(5):662–667. doi: 10.1016/j.jpeds.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 15.Saji T, Kemmotsu Y. Infliximab for Kawasaki syndrome. J Pediatr. 2006;149(3):426. doi: 10.1016/j.jpeds.2005.07.039. author reply. [DOI] [PubMed] [Google Scholar]
- 16.Burns JC, Best BM, Mejias A, Mahony L, Fixler DE, Jafri HS, et al. Infliximab treatment of intravenous immunoglobulin-resistant Kawasaki disease. J Pediatr. 2008;153(6):833–838. doi: 10.1016/j.jpeds.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newburger JW, Sleeper LA, McCrindle BW, Minich LL, Gersony W, Vetter VL, et al. Randomized trial of pulsed corticosteroid therapy for primary treatment of Kawasaki disease. The New England journal of medicine. 2007;356(7):663–675. doi: 10.1056/NEJMoa061235. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi T, Saji T, Otani T, Takeuchi K, Nakamura T, Arakawa H, et al. Efficacy of immunoglobulin plus prednisolone for prevention of coronary artery abnormalities in severe Kawasaki disease (RAISE study): a randomised, open-label, blinded-endpoints trial. Lancet. 2012;379(9826):1613–1620. doi: 10.1016/S0140-6736(11)61930-2. [DOI] [PubMed] [Google Scholar]
- 19.Sleeper LA, Minich LL, McCrindle BM, Li JS, Mason W, Colan SD, et al. Evaluation of Kawasaki disease risk-scoring systems for intravenous immunoglobulin resistance. The Journal of Pediatrics. 2011;158(5):831 e3–835 e3. doi: 10.1016/j.jpeds.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tremoulet AH, Jain S, Jaggi P, Jimenez-Fernandez S, Pancheri JM, Sun X, et al. Infliximab for intensification of primary therapy for Kawasaki disease: a phase 3 randomised, double-blind, placebo-controlled trial. Lancet. 2014;383(9930):1731–1738. doi: 10.1016/S0140-6736(13)62298-9. [DOI] [PubMed] [Google Scholar]
- 21.Son MB, Gauvreau K, Burns JC, Corinaldesi E, Tremoulet AH, Watson VE, et al. Infliximab for intravenous immunoglobulin resistance in Kawasaki disease: a retrospective study. The Journal of pediatrics. 2011;158(4):644 e1–649 e1. doi: 10.1016/j.jpeds.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 22.Schett G, Dayer JM, Manger B. Interleukin-1 function and role in rheumatic disease. Nat Rev Rheumatol. 2016;12(1):14–24. doi: 10.1038/nrrheum.2016.166. [DOI] [PubMed] [Google Scholar]
- 23.Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11(8):633–652. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ben-Sasson SZ, Hogg A, Hu-Li J, Wingfield P, Chen X, Crank M, et al. IL-1 enhances expansion, effector function, tissue localization, and memory response of antigen-specific CD8 T cells. J Exp Med. 2013;210(3):491–502. doi: 10.1084/jem.20122006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown TJ, Crawford SE, Cornwall ML, Garcia F, Shulman ST, Rowley AH. CD8 T lymphocytes and macrophages infiltrate coronary artery aneurysms in acute Kawasaki disease. J Infect Dis. 2001;184(7):940–943. doi: 10.1086/323155. [DOI] [PubMed] [Google Scholar]
- 26.Rider P, Carmi Y, Guttman O, Braiman A, Cohen I, Voronov E, et al. IL-1alpha and IL-1beta recruit different myeloid cells and promote different stages of sterile inflammation. J Immunol. 2011;187(9):4835–4843. doi: 10.4049/jimmunol.1102048. [DOI] [PubMed] [Google Scholar]
- 27.Berda-Haddad Y, Robert S, Salers P, Zekraoui L, Farnarier C, Dinarello CA, et al. Sterile inflammation of endothelial cell-derived apoptotic bodies is mediated by interleukin-1alpha. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(51):20684–20689. doi: 10.1073/pnas.1116848108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonin PD, Fici GJ, Singh JP. Interleukin-1 promotes proliferation of vascular smooth muscle cells in coordination with PDGF or a monocyte derived growth factor. Exp Cell Res. 1989;181(2):475–482. doi: 10.1016/0014-4827(89)90104-3. [DOI] [PubMed] [Google Scholar]
- 29.Sasu S, Beasley D. Essential roles of IkappaB kinases alpha and beta in serum- and IL-1-induced human VSMC proliferation. Am J Physiol Heart Circ Physiol. 2000;278(6):H1823–H1831. doi: 10.1152/ajpheart.2000.278.6.H1823. [DOI] [PubMed] [Google Scholar]
- 30.Alexander MR, Moehle CW, Johnson JL, Yang Z, Lee JK, Jackson CL, et al. Genetic inactivation of IL-1 signaling enhances atherosclerotic plaque instability and reduces outward vessel remodeling in advanced atherosclerosis in mice. The Journal of Clinical Investigation. 2012;122(1):70–79. doi: 10.1172/JCI43713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colotta F, Re F, Polentarutti N, Sozzani S, Mantovani A. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood. 1992;80(8):2012–2020. [PubMed] [Google Scholar]
- 32.Orenstein JM, Shulman ST, Fox LM, Baker SC, Takahashi M, Bhatti TR, et al. Three linked vasculopathic processes characterize Kawasaki disease: a light and transmission electron microscopic study. PLoS ONE. 2012;7(6):e38998. doi: 10.1371/journal.pone.0038998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimizu C, Oharaseki T, Takahashi K, Kottek A, Franco A, Burns JC. The role of TGF-beta and myofibroblasts in the arteritis of Kawasaki disease. Hum Pathol. 2013;44(2):189–198. doi: 10.1016/j.humpath.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chua PK, Melish ME, Yu Q, Yanagihara R, Yamamoto KS, Nerurkar VR. Elevated levels of matrix metalloproteinase 9 and tissue inhibitor of metalloproteinase 1 during the acute phase of Kawasaki disease. Clin Diagn Lab Immunol. 2003;10(2):308–314. doi: 10.1128/CDLI.10.2.308-314.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Senzaki H. The pathophysiology of coronary artery aneurysms in Kawasaki disease: role of matrix metalloproteinases. Arch Dis Child. 2006;91(10):847–851. doi: 10.1136/adc.2005.087437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shimizu C, Matsubara T, Onouchi Y, Jain S, Sun S, Nievergelt CM, et al. Matrix metalloproteinase haplotypes associated with coronary artery aneurysm formation in patients with Kawasaki disease. J Hum Genet. 2010;55(12):779–784. doi: 10.1038/jhg.2010.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hong YM, Jin H-S, Park IS, Hong S-J. Association of the matrix metalloproteinase-3 (−439C/G) promoter polymorphism with Kawasaki disease in Korean children. Heart Vessels. 2008;23(5):341–347. doi: 10.1007/s00380-008-1041-1. [DOI] [PubMed] [Google Scholar]
- 38.Takeshita S, Tokutomi T, Kawase H, Nakatani K, Tsujimoto H, Kawamura Y, et al. Elevated serum levels of matrix metalloproteinase-9 (MMP-9) in Kawasaki disease. Clinical & Experimental Immunology. 2001;125(2):340–344. doi: 10.1046/j.1365-2249.2001.01608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee A, Shimizu C, Oharaseki T, Takahashi K, Daniels LB, Kahn A, et al. Role of TGF-beta Signaling in Remodeling of Non-coronary Artery Aneurysms in Kawasaki disease. Pediatr Dev Pathol. 2015 doi: 10.2350/14-12-1588-OA.1. [DOI] [PubMed] [Google Scholar]
- 40.Ringold S, Weiss PF, Beukelman T, DeWitt EM, Ilowite NT, Kimura Y, et al. 2013 update of the 2011 American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: recommendations for the medical therapy of children with systemic juvenile idiopathic arthritis and tuberculosis screening among children receiving biologic medications. Arthritis Rheum. 2013;65(10):2499–2512. doi: 10.1002/art.38092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoffman HM. Therapy of autoinflammatory syndromes. The Journal of allergy and clinical immunology. 2009;124(6):1129–1138. doi: 10.1016/j.jaci.2009.11.001. quiz 39–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldbach-Mansky R, Dailey NJ, Canna SW, Gelabert A, Jones J, Rubin BI, et al. Neonatal-onset multisystem inflammatory disease responsive to interleukin-1beta inhibition. N Engl J Med. 2006;355(6):581–592. doi: 10.1056/NEJMoa055137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.ter Haar NM, Oswald M, Jeyaratnam J, Anton J, Barron KS, Brogan PA, et al. Recommendations for the management of autoinflammatory diseases. Ann Rheum Dis. 2015;74(9):1636–1644. doi: 10.1136/annrheumdis-2015-207546. [DOI] [PubMed] [Google Scholar]
- 44.Kone-Paut I, Galeotti C. Anakinra for cryopyrin-associated periodic syndrome. Expert Rev Clin Immunol. 2014;10(1):7–18. doi: 10.1586/1744666X.2014.861325. [DOI] [PubMed] [Google Scholar]
- 45.Sibley CH, Plass N, Snow J, Wiggs EA, Brewer CC, King KA, et al. Sustained response and prevention of damage progression in patients with neonatal-onset multisystem inflammatory disease treated with anakinra: a cohort study to determine three- and five-year outcomes. Arthritis Rheum. 2012;64(7):2375–2386. doi: 10.1002/art.34409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuemmerle-Deschner JB, Tyrrell PN, Koetter I, Wittkowski H, Bialkowski A, Tzaribachev N, et al. Efficacy and safety of anakinra therapy in pediatric and adult patients with the autoinflammatory Muckle-Wells syndrome. Arthritis Rheum. 2011;63(3):840–849. doi: 10.1002/art.30149. [DOI] [PubMed] [Google Scholar]
- 47.Sun H, Van LM, Floch D, Jiang X, Klein UR, Abrams K, et al. Pharmacokinetics and Pharmacodynamics of Canakinumab in Patients With Systemic Juvenile Idiopathic Arthritis. J Clin Pharmacol. 2016 doi: 10.1002/jcph.754. [DOI] [PubMed] [Google Scholar]
- 48.Lachmann HJ, Kone-Paut I, Kuemmerle-Deschner JB, Leslie KS, Hachulla E, Quartier P, et al. Use of canakinumab in the cryopyrin-associated periodic syndrome. The New England journal of medicine. 2009;360(23):2416–2425. doi: 10.1056/NEJMoa0810787. [DOI] [PubMed] [Google Scholar]
- 49.Kuemmerle-Deschner JB, Hachulla E, Cartwright R, Hawkins PN, Tran TA, Bader-Meunier B, et al. Two-year results from an open-label, multicentre, phase III study evaluating the safety and efficacy of canakinumab in patients with cryopyrin-associated periodic syndrome across different severity phenotypes. Ann Rheum Dis. 2011;70(12):2095–2102. doi: 10.1136/ard.2011.152728. [DOI] [PubMed] [Google Scholar]
- 50.Ruperto N, Brunner HI, Quartier P, Constantin T, Wulffraat N, Horneff G, et al. Two randomized trials of canakinumab in systemic juvenile idiopathic arthritis. The New England journal of medicine. 2012;367(25):2396–2406. doi: 10.1056/NEJMoa1205099. [DOI] [PubMed] [Google Scholar]
- 51.Hong DS, Hui D, Bruera E, Janku F, Naing A, Falchook GS, et al. MABp1, a first-in-class true human antibody targeting interleukin-1alpha in refractory cancers: an open-label, phase 1 dose-escalation and expansion study. Lancet Oncol. 2014;15(6):656–666. doi: 10.1016/S1470-2045(14)70155-X. [DOI] [PubMed] [Google Scholar]
- 52.Onouchi Y. Genetics of Kawasaki disease: what we know and don't know. Circ J. 2012;76(7):1581–1586. doi: 10.1253/circj.cj-12-0568. [DOI] [PubMed] [Google Scholar]
- 53.Kuo H-C, Hsu Y-W, Lo M-H, Huang Y-H, Chien S-C, Chang W-C. Single-Nucleotide Polymorphism rs7251246 in ITPKC Is Associated with Susceptibility and Coronary Artery Lesions in Kawasaki Disease. PLoS ONE. 2014;9(3):e91118. doi: 10.1371/journal.pone.0091118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Onouchi Y, Gunji T, Burns JC, Shimizu C, Newburger JW, Yashiro M, et al. ITPKC functional polymorphism associated with Kawasaki disease susceptibility and formation of coronary artery aneurysms. Nature Genetics. 2008;40(1):35–42. doi: 10.1038/ng.2007.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lou J, Xu S, Zou L, Zhong R, Zhang T, Sun Y, et al. A functional polymorphism, rs28493229, in ITPKC and risk of Kawasaki disease: an integrated meta-analysis. Mol Biol Rep. 2012 doi: 10.1007/s11033-012-2022-0. [DOI] [PubMed] [Google Scholar]
- 56.Khor CC, Davila S, Breunis WB, Lee YC, Shimizu C, Wright VJ, et al. Genome-wide association study identifies FCGR2A as a susceptibility locus for Kawasaki disease. Nature genetics. 2011;43(12):1241–1246. doi: 10.1038/ng.981. [DOI] [PubMed] [Google Scholar]
- 57.Onouchi Y, Fukazawa R, Yamamura K, Suzuki H, Kakimoto N, Suenaga T, et al. Variations in ORAI1 Gene Associated with Kawasaki Disease. PLoS ONE. 2016;11(1):e0145486. doi: 10.1371/journal.pone.0145486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rada B, Park JJ, Sil P, Geiszt M, Leto TL. NLRP3 inflammasome activation and interleukin-1β release in macrophages require calcium but are independent of calcium-activated NADPH oxidases. Inflamm Res. 2014 doi: 10.1007/s00011-014-0756-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Horng T. Calcium signaling and mitochondrial destabilization in the triggering of the NLRP3 inflammasome. Trends in Immunology. 2014 doi: 10.1016/j.it.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Murakami T, Ockinger J, Yu J, Byles V, McColl A, Hofer AM, et al. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proceedings of the National Academy of Sciences. 2012 doi: 10.1073/pnas.1117765109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee G-S, Subramanian N, Kim AI, Aksentijevich I, Goldbach-Mansky R, Sacks DB, et al. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca(2+) and cAMP. Nature. 2012 doi: 10.1038/nature11588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maury CP, Salo E, Pelkonen P. Circulating interleukin-1 beta in patients with Kawasaki disease. The New England journal of medicine. 1988;319(25):1670–1671. doi: 10.1056/NEJM198812223192515. [DOI] [PubMed] [Google Scholar]
- 63.Leung DY, Cotran RS, Kurt-Jones E, Burns JC, Newburger JW, Pober JS. Endothelial cell activation and high interleukin-1 secretion in the pathogenesis of acute Kawasaki disease. Lancet. 1989;2(8675):1298–1302. doi: 10.1016/s0140-6736(89)91910-7. [DOI] [PubMed] [Google Scholar]
- 64.Popper SJ, Shimizu C, Shike H, Kanegaye JT, Newburger JW, Sundel RP, et al. Gene-expression patterns reveal underlying biological processes in Kawasaki disease. Genome Biol. 2007;8(12):R261. doi: 10.1186/gb-2007-8-12-r261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hoang LT, Shimizu C, Ling L, Naim AN, Khor CC, Tremoulet AH, et al. Global gene expression profiling identifies new therapeutic targets in acute Kawasaki disease. Genome Med. 2014;6(11):541. doi: 10.1186/s13073-014-0102-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fury W, Tremoulet AH, Watson VE, Best BM, Shimizu C, Hamilton J, et al. Transcript abundance patterns in Kawasaki disease patients with intravenous immunoglobulin resistance. Human immunology. 2010;71(9):865–873. doi: 10.1016/j.humimm.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lehman TJ, Walker SM, Mahnovski V, McCurdy D. Coronary arteritis in mice following the systemic injection of group B Lactobacillus casei cell walls in aqueous suspension. Arthritis & Rheumatism. 1985;28(6):652–659. doi: 10.1002/art.1780280609. [DOI] [PubMed] [Google Scholar]
- 68.Schulte DJ, Yilmaz A, Shimada K, Fishbein MC, Lowe EL, Chen S, et al. Involvement of innate and adaptive immunity in a murine model of coronary arteritis mimicking Kawasaki disease. J Immunol. 2009;183(8):5311–5318. doi: 10.4049/jimmunol.0901395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yeung RS. Lessons learned from an animal model of Kawasaki disease. Clin Exp Rheumatol. 2007;25(1 Suppl 44):S69–S71. [PubMed] [Google Scholar]
- 70.Wakita D, Lee Y, Shimada K, Chen S, Crother TR, Lehman T, et al. Abdominal Aorta Dilatation and Aneurysm in Kawasaki Disease Vasculitis Mouse Model: Role of IL-1 Signaling Circulation. 2015;131:A71. doi: 10.1161/ATVBAHA.115.306475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rosenkranz ME, Schulte DJ, Agle LM, Wong MH, Zhang W, Ivashkiv L, et al. TLR2 and MyD88 contribute to Lactobacillus casei extract-induced focal coronary arteritis in a mouse model of Kawasaki disease. Circulation. 2005;112(19):2966–2973. doi: 10.1161/CIRCULATIONAHA.105.537530. [DOI] [PubMed] [Google Scholar]
- 72.Lee Y, Schulte DJ, Shimada K, Chen S, Crother TR, Chiba N, et al. Interleukin-1beta is crucial for the induction of coronary artery inflammation in a mouse model of Kawasaki disease. Circulation. 2012;125(12):1542–1550. doi: 10.1161/CIRCULATIONAHA.111.072769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Myones BL, Bathoria JM, Lehman TJ, ST S. Human IVIG inhibits Lactobacillus casei-inducible coronary arteritis in a murine model. Proceedings of the V International Kawasaki Disease Symposium. 1995:252–256. [Google Scholar]
- 74.Lehman TJA, Sherry B, Gietl DM, Nguyen HT, A C. Suppression of Lactobacillus casei cell wall-induced coronary arteritis in mice by antibody to murine tumor necrosis factor. Proceedings of the Third International Conference on Kawasaki Disease. 1988:203–206. [Google Scholar]
- 75.Lee Y, Wakita D, Dagvadorj J, Shimada K, Chen S, Huang GY, et al. IL-1 signaling is critically required in stromal cells in Kawasaki Disease Vasculitis Mouse Model. Role of both IL-1α and IL-1β. Arterioscler Thromb Va c Biol. 2015 doi: 10.1161/ATVBAHA.115.306475. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wakita D, Kurashima Y, Crother TR, Noval Rivas M, Lee Y, Chen S, et al. Role of Interleukin-1 Signaling in a Mouse Model of Kawasaki Disease-Associated Abdominal Aortic Aneurysm. Arterioscler Thromb Va c Biol. 2016;36(5):886–897. doi: 10.1161/ATVBAHA.115.307072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cohen S, Tacke CE, Straver B, Meijer N, Kuipers IM, Kuijpers TW. A child with severe relapsing Kawasaki disease rescued by IL-1 receptor blockade and extracorporeal membrane oxygenation. Ann Rheum Dis. 2012;71(12):2059–2061. doi: 10.1136/annrheumdis-2012-201658. [DOI] [PubMed] [Google Scholar]
- 78.Shafferman A, Birmingham JD, Cron RQ. High dose anakinra for treatment of severe neonatal Kawasaki disease: a case report. Pediatric rheumatology online journal. 2014;12:26. doi: 10.1186/1546-0096-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tremoulet AH, Jain S, Kim S, Newburger J, Arditi M, Franco A, et al. Rationale and study design for a phase I/IIa trial of anakinra in children with Kawasaki disease and early coronary artery abnormalities (the ANAKID trial) Contemp Clin Trials. 2016;48:70–75. doi: 10.1016/j.cct.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]